Single-Atom Cobalt Coordinated to Oxygen Sites on Graphene for Stable Lithium Metal Anodes
Haodong Shi , Yaguang Li , Pengfei Lu , Zhong-Shuai Wu ,*
1 State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023,Liaoning Province, China.
2 Dalian National Laboratory for Clean Energy, Chinese Academy of Sciences, Dalian 116023, Liaoning Province, China.
3 University of Chinese Academy of Sciences, Beijing 100049, China.
Abstract:Lithium (Li)-based batteries are the dominant energy source for consumer electronics, grid storage, and electrified transportation. However, the development of batteries based on graphite anodes is hindered by their limited energy density. With its ultrahigh theoretical capacity (3860 mAh·g-1), low redox potential (-3.04 V), and satisfactorily low density (0.54 g·cm-3), Li metal is the most promising anode for next-generation high-energy-density batteries.Unfortunately, the limited cycling life and safety issues raised by dendrite growth,unstable solid electrolyte interphase, and “dead Li” have inhibited their practical use. An effective strategy is to develop a suitable lithiophilic matrix for regulating initial Li nucleation behavior and controlling subsequent Li growth. Herein, single atom cobalt coordinated to oxygen sites on graphene (Co-O-G SA)is demonstrated as a Li plating substrate to efficiently regulate Li metal nucleation and growth. Owing to its dense and more uniform lithiophilic sites than single-atom cobalt coordinated to nitrogen sites on graphene (Co-N-G SA), high electronic conductivity, and high specific surface area (519 m2·g-1), Co-O-G SA could significantly reduce the local current density and promote the reversibility of Li plating and stripping. As a result, the Co-O-G SA based Li anodes exhibited a high Coulombic efficiency of 99.9% at a current density of 1 mA·cm-2 with a capacity of 1 mAh·cm-2, and excellent rate capability (high current density of 8 mA·cm-2). Even at a high plating capacity of 6 mAh·cm-2, the Co-O-G SA electrode could stably cycle for an ultralong lifespan of 1300 h. In the symmetric battery, the Co-O-G SA based Li anode (Co-O-G SA/Li)possessed a stable voltage profile of 18 mV for 780 h at 1 mA·cm-2, and even at a high current density of 3 mA·cm-2, its overpotential maintained a small hysteresis of approximately 24 mV for > 550 h. Density functional theory calculations showed that the surface of Co-O-G SA had a stronger interaction with Li atoms with a larger binding energy, -3.1 eV, than that of Co-N-G SA (-2.5 eV), leading to a uniform distribution of metallic Li on the Co-O-G SA surface. More importantly, when matched with a sulfur cathode, the resulting Co-O-G SA/lithium sulfur full batteries exhibited a high capacity of 1002 mAh·g-1, improved kinetics with a small polarization of 191 mV, and an ultralow capacity decay rate of 0.036% per cycle for 1000 cycles at 0.5C (1C = 1675 mA·g-1)with a steady Coulombic efficiency of nearly 100%. Therefore, this work provides novel insights into the coordination environment of single atoms for the chemistry of Li metal anodes for high-energy-density batteries.
Key Words:Single atom;Coordination environment;Lithiophilic site;Li dendrite;High-energy-density lithium battery
1 Introduction
With the flourishing advance of the high-end electronic devices, developing high-energy-density batteries is urgently needed to alleviate the concerns of batteries cruising ability1–3.Since lithium (Li)metal anode possesses ultrahigh theoretical capacity of 3860 mAh·g-1and the lowest redox electrochemical potential (-3.04 Vvsstandard hydrogen electrode). Li metalbased batteries are considered as the most promising highenergy-density batteries4,5. However, the disgusting Li dendrite and “dead Li” during the repeated Li plating/string process have caused the internal short circuit and limited their cycling life, and severely hindered their practical applications6–9.
To address the above issues, significant progresses have been achieved up to now, including (i)employing liquid electrolyte additives to stabilize solid electrolyte interface (SEI)films10–12,(ii)adopting the solid-state or polymer electrolytes with high mechanical strength to inhibit Li dendrite13–15, and (iii)constructing three dimension (3D)high surface area host to reduce the effective current density16–18. However, those strategies rarely concentrate their initial nucleation behavior,which critically determines the final morphology of Li metal. In other words, suppressing the Li dendrite growth at the nucleation process is of great significance, but remains elusive.
Very recently, Zhang and co-works have adopted Nitrogendoped graphene as the lithiophilic sites to regulate Li metal initial nucleation process and suppress the dendrite growth19.Our recent work has also revealed that the lithiophilic function groups of MXene could effectively regulate Li nucleation and growth20. Notably, single atom with well-defined metal center has been used intensively in the area of energy storage and conversion21–23, and several reports demonstrate that singleatom materials with the homogenous and active lithiophilc sites could offer uniform areal nucleation24–27. Unfortunately, those works only focus on the influence of the kinds of central metal atom for the Li dendrite, however, the coordination environment of the metal single atom has been never studied in details.
In this work, we first reported a cobalt (Co)single atom coordinated to oxygen sites anchored on graphene (Co-O-G SA)for stable and dendrite free Li metal anodes. The Co-O-G SA with high specific surface area (519 m2·g-1)possesses more lithiophilic sites than the Co single-atom coordinated to nitrogen sites on graphene (Co-N-G SA)to efficiently guide Li metal uniform nucleation and growth, and thus substantially suppressed the growth of Li dendrite. As a result, the Co-O-G SA exhibited impressive electrochemical performance,including high Coulombic efficiency of 99.9% for ultralong lifespan (1300 h), stable and flat voltage profile (24 mV)for 550 h without Li dendrite. Finally, an ultralow capacity decay rate of 0.036% per cycle was realized for the Co-O-G SA based lithium sulfur (Co-O-G SA/Li-S)full battery during 1000 cycles.
2 Experimental
2.1 Preparation of Co-O-G SA
In a typical synthesis28, 10 mL Co(NO3)2·6H2O (Aladdin Industrial Inc., 99%, 1 mg·mL-1)aqueous solution was added into 100 mL graphene oxide (GO, 1 mg·mL-1)solution and continuously stirred for 1 h. Then, the as-obtained suspension was frozen in liquid nitrogen to make a fast frozen and free-dried for 48 h to remove water. Afterwards, the freeze-dried sample was annealed in flow Argon (Ar)gas for 6 h at 400 °C.
For comparison, the G-O sample was synthesized as the same procedure of Co-O-G SA without adding Co(NO3)2·6H2O. The Co-N-G SA was also prepared in term of the following procedure29: typically, 3 mL 2-methylimidazole (Aladdin Industrial Inc., 99%, 1 mg·mL-1)aqueous solution, 10 mL Co(NO3)2·6H2O (1 mg·mL-1)aqueous solution were added in sequence into 100 mL GO (1 mg·mL-1)solution and continuously stirred for 1 h. Followed by frozen in liquid nitrogen and free-dried for 48 h, the sample was heated in a muffle furnace at 100 °C in air for 5 h, and then annealed at Ar gas at 700 °C for 2 h to acquire the Co-N-G SA.
2.2 Material characterization
The morphology and structure of materials and electrodes were characterized by scanning electron microscope (SEM,JEOL JSM-7800F, Japan), high resolution transmission electron microscopy (TEM/HRTEM, JEOL 2100, Japan), aberrationcorrected HAADF-STEM (FEI Titan Cube Themis G2 300, 300 kV equipped with two spherical aberration correctors). X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi equipped with monochromatic AlKαsource of 1486.5 eV), X-ray diffraction (XRD, Empyrean with CuKα radiation in the 2θrange from 5° to 90°), nitrogen adsorption and desorption isotherm (Quadrasorb SI, Quantachrome Instruments), The CoK-edge extended X-ray absorption fine structure (EXAFS)data were collected on the beamline BL14W1 at Shanghai Synchrotron Radiation facility (SSRF).
2.3 Electrochemical measurement
The as-obtained Co-O-G SA, Co-N-G SA or G-O nanosheets as active materials and polyvinylidene fluoride (PVDF)as binder (mass ratio of active materials : binder = 9 : 1)were mixed into a slurry by stirring inN-methyl-2-pyrrolidone (NMP)for 1 h. Then, the slurry was coated onto copper (Cu)foil and dried in a vacuum drying oven at 100 °C for 12 h. Further, the foil was punched into disks with a diameter of 12 mm as the working electrode. The loading mass of materials was approximately 1.0 mg·cm-2, and bare Cu foil was also punched for reference. All the batteries were assembled with standard 2016 coin-type cells in an Ar-filled glovebox with O2and H2O content below 0.5 ppm(1 ppm = 1 mg·L-1). The electrolyte was 1.0 mol·L-1lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)in a mixture solution of 1,3-dioxolane (DOL)and 1,2-dimethoxyethane(DME)(1 : 1, volume ratio)with 1% (mass fraction,w)LiNO3 as additive. About 40 μL electrolyte was dropped into each cell,and the polypropylene membrane (Celgard 2400)was used as the separator. Co-O-G SA, Co-N-G SA, G-O or bare Cu severed as the working electrode, and Li foil was used as the counter/reference electrode to evaluate the Coulombic efficiency. The assembled cells were precycled between 0.01 and 3 V for 3 times to stabilize the SEI formation and remove surface contamination. Afterwards, a certain capacity of Li was deposited onto the current collector and then charged to 1 V (vsLi+/Li)to strip the Li at certain current density for each cycle on a LAND CT2001A battery system. And symmetric cell configurations were assembled with Co-O-G SA lithium (Co-OG SA/Li)or Co-N-G SA lithium (Co-N-G SA/Li)anodes to evaluate the long-time cycling stability, where the pre-plating of Co-O-G SA/Li or Co-N-G SA/Li with Li was realized by charging 5 mAh·cm-2Li onto the electrodes.For the full battery tests, the working electrode contained 80% (w)of S-C (mass loading of S was 75%), 10% (w)of carbon black, and 10% (w)of PVDF with NMP as the solvent. The well-mixed slurry was cast onto carbon-coated Al foil using the doctor blade technique,and then dried under vacuum at 55 °C for 12 h. The S cathode with S loading of ~1.0 mg·cm-2, Co-O-G SA/Li and Co-O-G SA/Li anodes were obtained from preprocessed half cells. After depositing a certain amounts of Li metal (10 mAh·cm-2)onto the current collector, the battery was disassembled in the glove box and the dismantled Li anodes were further reassembled into a full cell against S cathode, using 1.0 mol·L-1LiTFSI in DOL/DME (v/v= 1 : 1)with 1% (w)LiNO3as the electrolyte.
2.4 Computational simulation
Density Functional Theory (DFT)calculations were performed with DMol3 package30. In the framework of DFT,the generalized gradient approximation (GGA)combined with the Perdew-Burke-Enzerhof (PBE)functional was employed to describe the exchange and correlation potential31,32. All the atoms of the calculated systems are allowed to fully relax in order to optimize the adsorption geometries. A double-numerical basis with polarization functions (DNP)is utilized to expand the valence electron functions into a set of numerical atomic orbitals, and the DFT semi-core pseudopotential (DSPP)is used when tackling the electron-ion interactions. Thek-points mesh in the Monkhorst Pack sampling scheme was set as 1 × 1 × 133.The Fermi smearing is set to 5.0 × 10-3Ha (1 Ha = 27.21 eV).For the convergence criteria, the Self-consistent field(SCF)tolerance used was 1.0 × 10-5eV atom-1, the maximum force and displacement were set as 5.0 × 10-2eV·?-1and 2.0 ×10-3? (1 ? = 0.1 nm), respectively. For the Li metal atom combined with Co-O-G SA and Co-N-G SA34, the binding energy (Eb)is defined as the energy difference between the substrate with Li atom (Et)and the summation of Li metal atom(E1)and substrate system (E2):Ea= E1+ E2– Et.
3 Results and discussion
3.1 Characterization of Co-O-G SA
The detail synthetic process has been illustrated in experimental section. Notably, GO was selected as single atom carrier due to its abundant oxygen functional group, and 2D sheets morphology with high specific surface area. First, the homogeneously dispersed Co(NO3)2aqueous solution was mixed with GO solution under vigorous stirring. In this process,The Co2+will be immobilized on GO at a single atomic state.The following freeze-drying process was to stabilize and avoid the aggregation of Co2+. Finally, an optimized annealing temperature of 400 °C was adopted for the formation of monodispersed Co single atom. The as-prepared Co-O-G SA shows typical 2D sheet graphene morphology with the size of several micrometers without massive aggregation of the Co particle(Fig. 1a and Fig. S1 (Supporting Information, SI)). Nitrogen adsorption and desorption isotherm of Co-O-G SA displays a typical IV curve with high specific surface area of 519 m2·g-1(Fig. 1b), and the corresponding pore-size distribution presents a dominant mesopore of 3.9 nm (Fig. 1c). XPS spectra (Fig. 1d)reveals the presence of O and Co with an atom ratio 5.4 in Co-O-G SA (Fig. S2 (SI)). And the chemical state of Co in Co-O-G SA is probed by high-resolution Co 2pXPS spectrum, where the peak at 803.1 eV is regarded as satellite peak, while the Co 2p3/2peaks located at 780.2 eV and 781.9 eV can be assigned to the Co2+-O and Co3+-O bonds, respectively, and the peak at 786 eV is attributed to the Co-OH bond (Fig. 1d)35, which is identical to the high-resolution O 1sspectrum (Fig. S2 (SI))36.The monatomic dispersion Co is further confirmed by XRD pattern, in which only one broad graphene diffraction peak (002)at 21.2° is observed, and no peaks of Co metal and cobalt oxides are found (Fig. 1e). In addition, atomic-resolution aberrationcorrected resolution high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM)image indicates the individual Co atoms (seen as bright spots as highlighted by red circles)are randomly dispersed on the graphene and no sub-nanometer clusters, which is in good agreement with the XRD result. To further probe the nature of the coordination of Co atom in Co-O-G, the spectra of X-ray absorption near-edge structure (XANES)and EXAFS at the CoK-edge were acquired to verify the possible coordination environment of central Co in Co-O-G SA. In comparison with the reference samples (Co foil and CoO), the absorption edge position of Co-O-G SA indicates the valence state of Co species are higher than the Co2+(Fig. 1g), identical to the XPS result.The EXAFS spectrum ofRspace for Co-O-G shows a main peak of Co-O coordination at 1.63 ?, similar to the Co-O bond of CoO (1.65 ?). And no Co-Co coordination peaks in Co foil (2.12 ?)and CoO (2.61 ?)are detected, demonstrative of Co atoms are mainly dispersed as single atom on graphene (Fig. 1h).Furthermore, EXAFS fitting result shows that the Co SA is coordinated with four etherified O atoms and two OH groups to form a thermal stable polyoxic Co-O4(OH)2structure (Fig. 1i and Table S1 (SI))28,29.
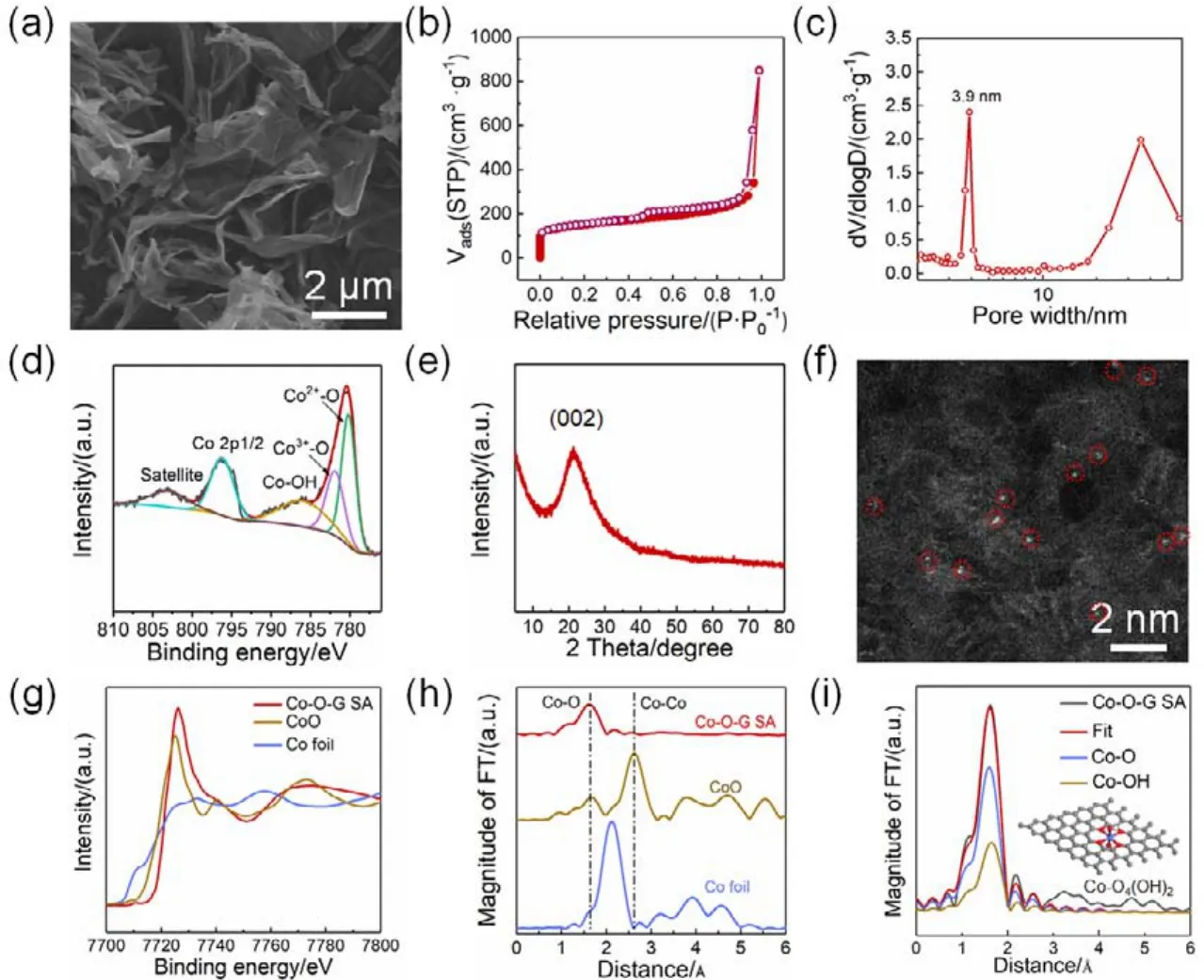
Fig. 1 Morphology and structure characterizations of the as-prepared Co-O-G SA. (a)SEM image of Co-O-G SA. (b)Nitrogen adsorption-desorption isotherm, and (c)pore size distribution of Co-O-G SA. (d)High-resolution Co 2p XPS spectrum, and (e)XRD pattern of Co-O-G SA. (f)HAADFSTEM image of Co-O-G SA, where the single Co atoms present as red circles. (g)K-edge XANES and (h)FT-EXAFS spectra of Co-O-G SACs with references of CoO and Co foil. (i)The FT-EXAFS curves of the proposed Co-O4(OH)2 architecture (red line)and the measured Co-O-G SACs (black line). Inset is the proposed model of Co-O4(OH)2 architecture.
3.2 Coulombic efficiency of Co-O-G SA electrode
The surperiority of Co-O-G SA for stable Li anode was first investigated by the Coulombic efficiency test. For comparison,the Co-N-G SA (531 m2·g-1, Fig. S3, S4 (SI)), G-O and bare Cu electrodes based asymmetrical batteries were also fabricated.The barrteies were first cycled between 0.01 and 3 V for three cycles to clean the impurity and form the stable SEI film (Fig.S5 (SI))37,38. As shown in Fig. 2a and Fig. S6 (SI), with a current density of 0.5 mA·cm-2and areal capacity of 0.5 mAh·cm-2, the Co-O-G electrode showed a stable average Coulombic efficiency of 99.8% and low standard deviation of 0.2 for 200 cycles with a small voltage hysteresis of ~13 mV (Fig. S7 (SI)),siginificantly superior to Co-N-G SA (94.9%, 5.2 for 119 cycles,80 mV), G-O (96.2%, 8.3 for 42 cycles, 90 mV), and Cu (92.3%,33.1 for 9 cycles, 150 mV). Notably, when the deposition capacity was increased to 1 mAh·cm-2at high current density of 1 mA·cm-2, the Co-O-G SA electrode also maintained a high Coulombic efficiency neraly 100% at 250 cycles with a stable overpotential (47 mV)(Fig. 2b and Fig. S8 (SI)). And even under the low mass loading of 0.2 mg·cm-2, the Co-O-G SA electrode could still keep a high Coulombic efficiency of 96.3% at 75 cycles (Fig. S9 (SI)). The Li nucleation performances were explored by the voltage profiles at the first cycle, the Co-O-G SA electrode showed relatively flatter slope of potential profile with lower overpotential of 10.5 mV than those of Co-N-G SA (44.3 mV), G-O (52.4 mV), and bare Cu electrodes (224.8 mV),indictative of exceptional lithiophilicity of Co-O-G SA (Fig.2c)39,40. Benefit from the above advantages, the Co-O-G SA electrode offered exellent rate capability, for example, with the increased Li plating capacities at fixed palting time of 1 h, Co-O-G SA electrode retained high Columbic efficeincy of 98.6%even with high Li deposition capacity reached up to 8 mAh·cm-2more than 75 cycles (Fig. 2d). Moreover, the Coulombic efficiencies of Co-O-G SA with increased Li palting time from 0.5 to 12 h at the same current density of 0.5 mA·cm-2always exceeded 99% during the whole cycles, and kept stable cycling ability up to 1300 h (Fig. 2e). Importantly, the high current density, high deposition capacity, coupled with long lifespan (8 mA·cm-2, 8 mAh·cm-2, 1300 h)of Co-O-G SA are superior to the most reported single atom based Li anode (Table S2 (SI)),such as single-atom Zn on carbon (1 mA·cm-2, 2 mAh·cm-2,1050 h)24, atomically dispersed nickcel on N doped graphene (1 mA·cm-2, 2 mAh·cm-2, 560 h)25. demonstrating the merits of the Co-O-G SA on Li dendrite suppression.
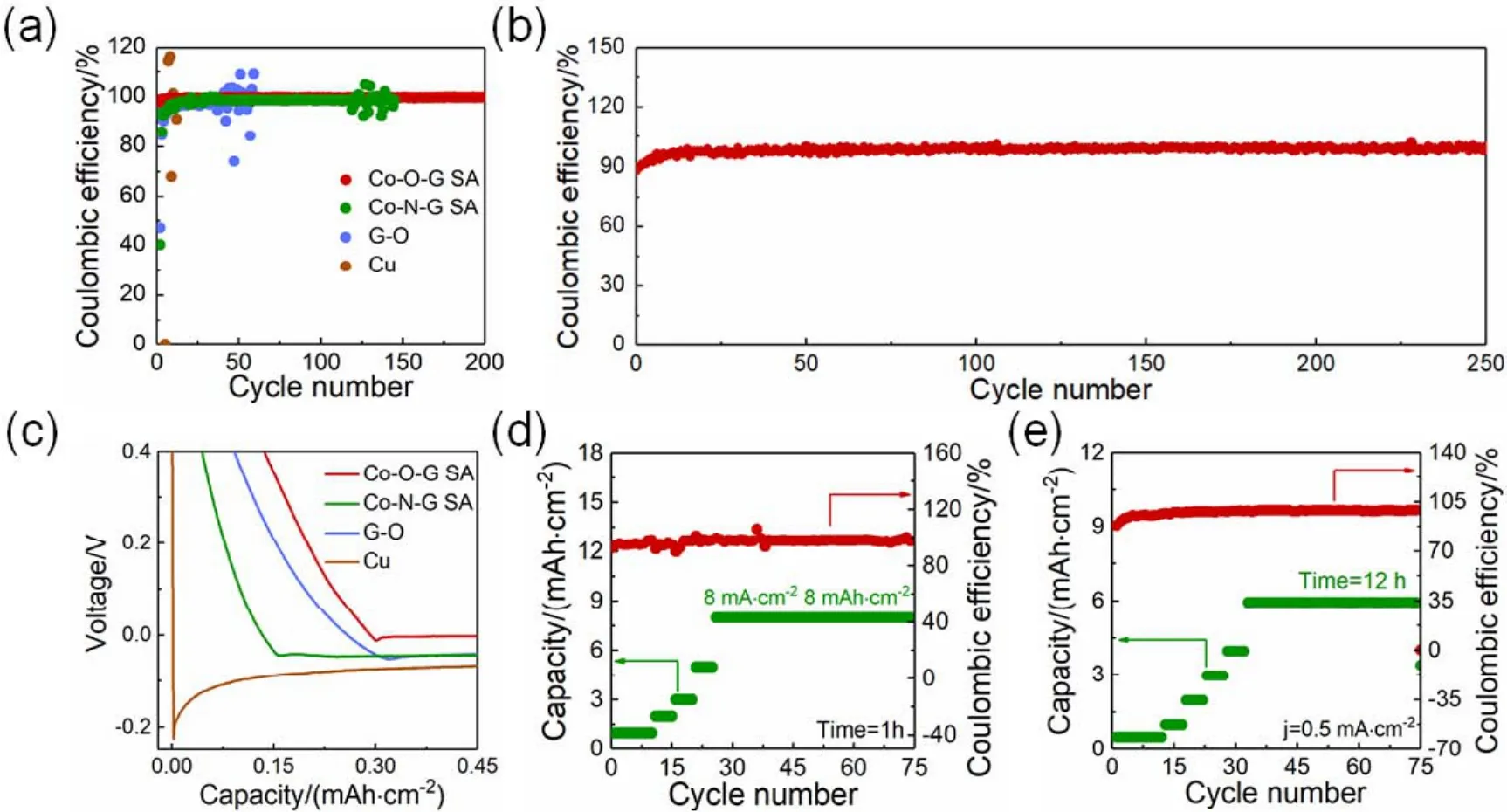
Fig. 2 Coulombic efficiency of Co-O-G SA electrodes. (a)The Coulombic efficiencies of Co-O-G SA, Co-N-G SA, G-O and Cu electrodes with Li deposition amount of 0.5 mAh·cm–2 at 0.5 mA·cm–2. (b)The Columbic efficiency of Co-O-G SA electrode obtained at 1 mA·cm-2 with 1 mAh·cm-2. (c)Nucleation voltage profiles of Co-O-G SA, Co-N-G SA, G-O and Cu electrodes at the first cycle. (d, e)Coulombic efficiencies of Co-O-G SA electrodes with increasing capacities at fixed (d)time of 1 h, and (e)current density of 0.5 mA·cm-2.
3.3 Performance of Co-O-G SA anode for symmetric batteries
In addition, symmetic batteries based Co-O-G SA and Co-NG SA anodes were fabricated to evaluate the Li plating/string behavior. As shown in Fig. 3a, the Co-O-G SA battery showed flat and stable voltage profile of 18 mV for 780 h at 1 mA·cm-2for 1 h. While the overpotential of Co-N-G SA was sharply increased to 124 mV over 680 h due to the dead Li and Li dendrite (Fig. 2b and Fig. S10 (SI)). Further increase the current density to 3 mA·cm-2, even the overpotential was not stable at initial cycling due to unstable SEI film, the overpotential of Co-O-G SA anode still maintained small hysteresis of around 24 mV for more than 550 h without failure of the battery (Fig. 3c and Fig. S11 (SI)), indicating that the Co-O-G SA could successfully suppress the Li dendrite. To probe the Li deposition process and the interaction between the Li atom and Co-O-G SA and Co-NG SA, DFT calculations were conducted to evaluate their binding energies. It is revealed that the Co-O-G SA showed a larger binding energy of -3.1 eV than Co-N-G SA of -2.5 eV(Fig. 3d,e), manifesting that the surface of Co-O-G SA has a strong interaction with Li atom, leading to the uniform distribution of metallic Li on the anode surface41,42. This result was further verified by SEM image, showing the morphology of Li plating of 1 mAh·cm-2on the different electrode surfaces.Unlike the Co-N-G SA electrode was full of Li dendrite (Fig.S12 (SI)), a smooth and flat morphology of Li deposition without Li dendrite was realized (Fig. 3f), even after 250 cycles (Fig. S13(SI)), suggesting the superiority of our Co-O-G SA for dendritefree Li anode.
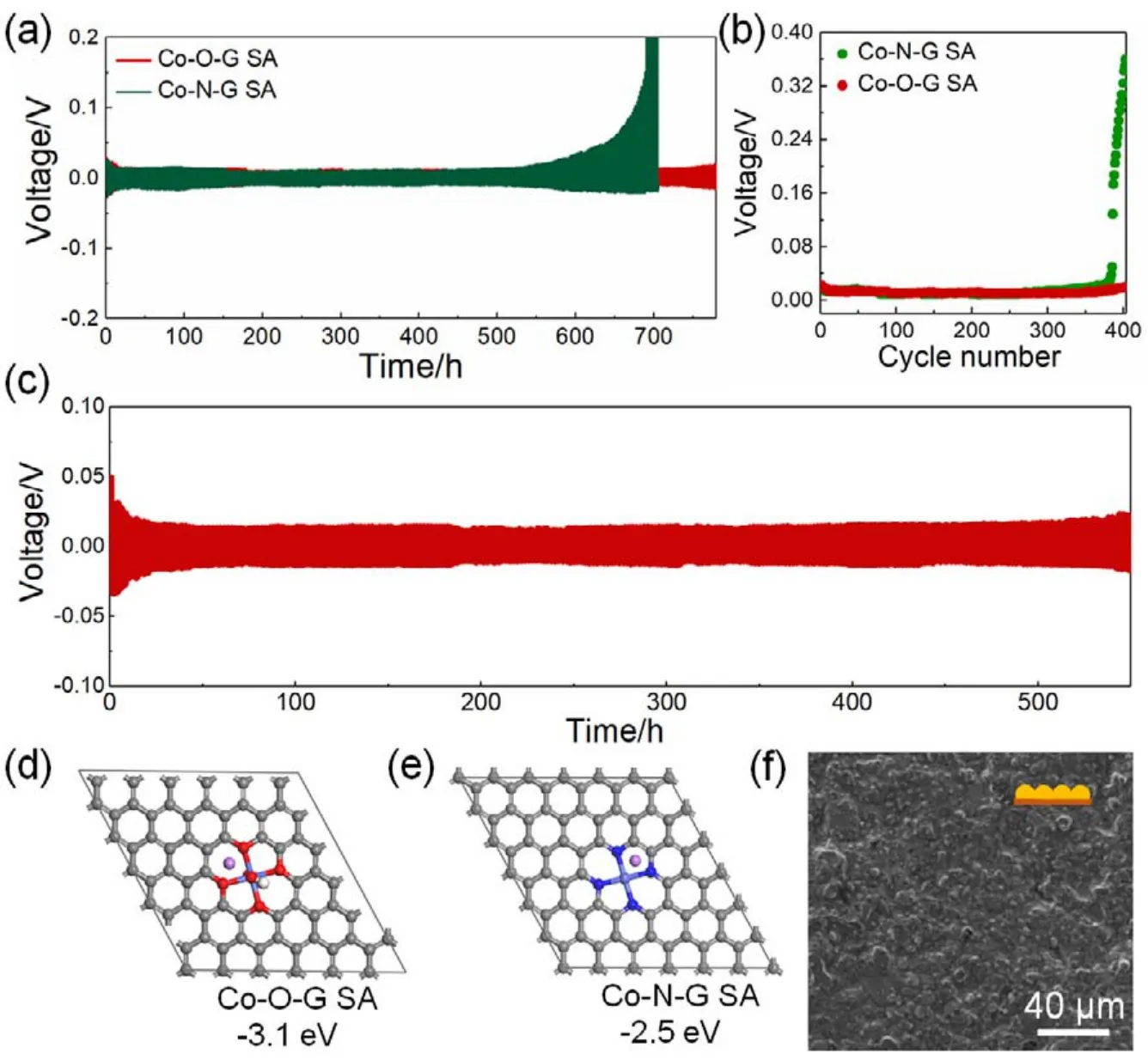
Fig. 3 Electrochemical performance of symmetric batteries with Co-O-G SA anode. (a)Galvanostatic cycling of symmetric cells based on Co-O-G/Li, and Co-N-G/Li anodes with current density of 1 mA·cm-2 under stripping/plating capacity of 1 mAh·cm-2, and (b)corresponding voltage hysteresis variation with cycling number. (c)High current density (3 mA·cm-2)cycling performance of symmetric cells based on Co-O-G SA/Li anode with a capacity of 3 mAh·cm-2. (d, e)Binding energy of a Li atom with (d)Co-O-G SA and (e)Co-N-G SA(grey ball: C atom; red ball: O atom; blue ball: N atom; white ball: H atom; purple ball: Li atom). (f)Top-view SEM image of Co-O-G SA electrode at the stage of plating Li with capacity of 1 mAh·cm-2.
3.4 Performance of Co-O-G SA/Li-S full battery
To further verify the remarkable performance of the Co-O-G SA/Li anode for practical applications, Li-S full batteries based on Co-O-G SA/Li (Co-O-G SA/Li-S)and Co-N-G SA/Li (Co-N-G SA/Li-S)were fabricated and compared their cycling performance. Both of them showed typical Li-S discharge and charge profiles (Fig. 4a,b)43, while the Co-O-G SA/Li-S battery possessed much smaller polarization of 191 mV between the charge and discharge plateaus than that of Co-N-G SA/Li-S battery (265 mV), indictive of significantly improved kinetics in the Co-N-G SA/Li-S battery44,45. The long-term cycling abilities at 0.5C(1C= 1675 mA·g-1)were shown in Fig. 4d. The Co-OG SA/Li-S battery delivered a high capacity of 1002 mAh·g-1and long cycle life up to 1000 cycles with a low average capacity decay of only 0.036% per cycle and steady high Coulombic efficiency of nearly 100%. However, the Co-N-G SA/Li-S battery displayed a relative low capacity of 915 mAh·g-1and a fast decay of capacity of 0.071% (Fig. 4d). These results further confirm the superiority of the Co-O-G SA for high-energydensity Li metal batteries.
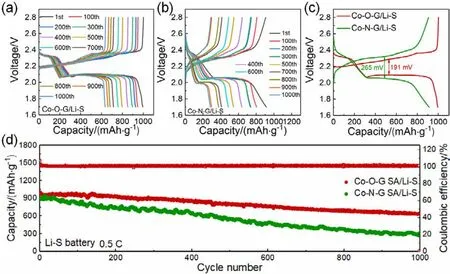
Fig. 4 Electrochemical performance of Li-S full cells with Co-O-G SA/Li anodes. (a, b)Charge and discharge curves of (a)Co-O-G SA/Li-S and(b)Co-N-G SA/Li-S full batteries measured at 0.5C for different cycles. (c)Voltage profile comparison of Co-O-G SA/Li-S and Co-N-G SA/Li-S batteries obtained at 1st cycle. (d)Long-term cycling stability of Li-S full batteries based on Co-O-G SA/Li or Co-N-G SA/Li anodes tested at 0.5C.
4 Conclusions
In summary, we have developed oxygen coordinated single atom cobalt on graphene for dendrite-free and stable Li metal anodes for high-energy-density batteries. Benefiting from the high specific surface area and uniform lithiophilic sites of the Co-O-G SA surface with a larger binding energy of -3.1 eV than Co-N-G SA (-2.5 eV), a decreased local current density and regulated Li plated nucleation process are realized. As a result,dendrite-free and uniform Li electrodeposition with high Coulombic efficiency of 99.9% could be maintained at a current density of 1.0 mA·cm-2and a cycle capacity of 1.0 mA·cm-2.Even at high current density of 8 mA·cm-2, high plating capacity of 6 mAh·cm-2, Co-O-G SA electrode could stably cycle with high Coulombic efficiency nearly 100% for 1300 h. Moreover,Co-O-G SA/Li-S full battery showed extremely low capacity decay rate of 0.036% for 1000 cycles. This strategy emphasizes the importance of coordination group of single atoms for controlling the Li plating morphology and can shed a new light on developing high safe Li metal batteries.
Supporting Information:Available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
Acknowledgements:We acknowledge the Shanghai Synchrotron Radiation Facility (SSRF)for conducting the XANES/EXAFS experiment (BL14W1).

