Novel pyrimidine-benzimidazole hybrids with antibacterial and antifungal properties and potential inhibition of SARS-CoV-2 main protease and spike glycoprotein
Shruk Khn,Myur Kle,Flk Siddiqui,Nitin Nem
a.Department of Pharmaceutical Chemistry,MUP’s College of Pharmacy(B Pharm),Washim,Maharashtra 444504,India
b.Department of Pharmaceutical Chemistry,Government College of Pharmacy,Aurangabad,Maharashtra 431003,India
c.Department of Pharmacology,Shri Vile Parle Kelavani Mandal’s(SVKM’s)Institute of Pharmacy,Dhule,Maharashtra 424001,India
ABSTRACT Objective The study aimed to synthesize and characterize pyrimidine-linked benzimidazole hybrids,define their antimicrobial and antifungal activities in vitro,and determine their ability to inhibit the main protease and spike glycoprotein of SARS-CoV-2.Methods The ability of the synthesized compounds to inhibit the main protease and spike glycoprotein inhibitory of SARS-CoV-2 was investigated by assessing their mode of binding to the allosteric site of the enzyme using molecular docking.The structures of pyrimidine-linked benzimidazole derivatives synthesized with microwave assistance were confirmed by spectral analysis.Antibacterial and antifungal activities were determined by broth dilution.Results Gram-negative bateria(Escherichia coli and Pseudomonas aeruginosa)were more sensitive than grampositive bateria(Staphylococcus aureus and Streptococcus pyogenes)to the derivatives.Candida albicans was sensitive to the derivatives at a minimal inhibitory concentration(MIC)of 250 μg/mL.The novel derivatives had better binding affinity(kcal/mol)than nelfinavir,lopinavir,ivermectin,remdesivir,and favipiravir,which are under investigation as treatment for SARSCoV-2 infection.Compounds 2c,2e,and 2g formed four hydrogen bonds with the active cavity of the main protease.Many derivatives had good binding affinity for the RBD of the of SARSCoV-2 spike glycoprotein with the formation of up to four hydrogen bonds.Conclusion We synthesized novel pyrimidine-linked benzimidazole derivatives that were potent antimicrobial agents with ability to inhibit the SARS-CoV-2 spike glycoprotein.Understanding the pharmacophore features of the main protease and spike glycoprotein offers much scope for the development of more potent agents.We plan to optimize the properties of the derivatives using models in vivo and in vitro so that they will serve as more effective therapeutic options against bacterial and SARS-CoV-2 infections.
Keywords SARS-CoV-2 inhibitor COVID-19 Molecular docking Pyrimidine-benzimidazole Bacteria Antifungal
1 Introduction
Coronavirus disease 2019(COVID-19)[1]induced by the novel severe acute respiratory syndrome-related coronavirus(SARS-CoV-2)has been declared by the World Health Organization(WHO)as a pandemic[2].Coronaviruses have triggered two other epidemics in addition to COVID-19,namely Middle East respiratory syndrome(MERS;2012),and severe acute respiratory syndrome(SARS;2002)[3].The National Health Commission of China declared in January 20th,2020 that SARS-CoV-2 infection is transmitted by person-to-person contact[4].SARS-CoV-2 belongs to the same familyBetacoronaviruses,as those that caused SARS and MERS[5,6].The novel coronavirus is a single-stranded positive-sense RNA with a diameter of 80-120 nm and 42 large viral RNA genomes[7].Coronaviruses are categorized as alpha-(α-COV),beta-(β-COV),gamma-(γ-COV),and delta-(δ-COV)types[8].Six of them have infected humans,and SARS-CoV-2 is the seventh after SARS-CoV and MERS-CoV[9].Symptoms of SARS-CoV-2 infection include fever,cough,dyspnea,myalgia,fatigue,decreased leukocyte counts,and pneumonia.Although numerous clinical trials have evaluated possible therapies for SARS-CoV-2 infection[10,11],treatment is not yet available for COVID-19[12].
SARS-CoV-2 invades after binding to host cellular receptors[13,14].Host cell receptors and the receptorbinding domain(RBD)of SARS-CoV-2 might be viable targets of interest in treating SARS-CoV-2 infection[15].Nucleocapsid(N),envelope(E),membrane(M),and spike(S)proteins comprise the structural proteins of SARS-CoV-2[16,17].The spike protein consists of an RBD that specifically binds to human angiotensin-converting enzyme-2(hACE-2),which leads to host cell invasion[14,17].Much investigative focus is presently directed towards developing specific novel inhibitors of the RBD or hACE-2.
Remdesivir is an approved treatment for COVID-19[18].Lopinavir and nelfinavir might inhibit SARSCoV-2 viral protease,and a clinical trial of favipiravir is underway for treating pneumonia induced by SARS-CoV-2[17].Favipiravir is a purine nucleoside that disrupts viral RNA synthesis[1],and ivermectin inhibits the replication of SARS-CoV-2in vitro[19].Therefore,we used remdesivir,nelfinavir,lopinavir,favipiravir,and ivermectin along with the native ligand in the crystal structure of SARS-CoV-2 main protease,that is,N3 as reference moieties for molecular docking studies[20].
Heterocyclic compounds provide scaffolds upon which pharmacophores can assemble to yield potent and selective drugs[21].Among these,benzimidazole heterocyclics have attracted attention because they are easy to synthesize and have a wide range of biological activities.The benzimidazole ring is an essential component of vitamin-B12 in the form of 5,6-dimethyl-l-(alpha-D-ribofuranosyl)benzimidazole[22].Various benzimidazole derivatives with human and veterinary anthelmintic[23],anti-ulcer[24],cardiotonic[25],antihypertensive[26],analgesic[27],anticonvulsant[28],anticancer[29]properties have been developed[30,31].Pyrimidines and their derivatives also have anticancer[32],anxiolytic[33],antioxidant[34],antiviral[35],antifungal[36],anticonvulsant[36],antidepressant,and antibacterial properties[37].The United States Food and Drug Administration(USFDA)has approved many purine and pyrimidine derivatives for the management of cancer and viral diseases[38].Pyrimidine-fused bicyclic heterocyclic agents have anticancer,antiviral,and many other biological activities.
To date,147 pyrimidine-fused bicyclic heterocyclic drugs have been approved for clinical application or are currently being clinically administered.The USFDA has authorized 57 of them to treat various diseases,among which,22 are currently being applied to treat various types of cancer[39].The pyrimidine ring system is abundant in nature as substituted and ring-fused compounds and equivalents,such as cytosine,thymine,uracil,thiamine(vitamin B1)and alloxan.It is also found in various synthetic compounds,including barbiturates and the HIV medication,zidovudine.Bacimethrin,a naturally occurring thiamine antimetabolite obtained in 1961 fromBacillus megatherium,is the most basic pyrimidine antibiotic,and it acts against many bacterial infections[40].Pyrimidine-fused bicyclic heterocyclic compounds can serve as scaffolds to find new and effective medicines for specific biological targets.
The present study aimed to synthesize and characterize pyrimidine-linked benzimidazole hybrids with antimicrobial and antifungal activity as well as inhibitory activity against SARS-CoV-2 main protease and spike glycoprotein.We screened their antiviral inhibitory action by molecular dockingin silicoas we were unable to screen them for SARS-CoV-2 activityin vivodue to safety issues.We therefore investigated their antimicrobial and antifungal activitiesin vitroas preliminary evidence of their biological potential.Molecular dockingin silicovalidates the binding affinity of compounds for target molecules as a docking scores(kcal/mol).This allows the prediction of structural activity relationships between compounds and targets.
2 Materials and methods
2.1 Molecular docking
Compounds were screened by molecular docking using the PyRx-Virtual Screening Tool[41]on a Lenovo ThinkPad with a 64-bit operating system,an Intel(R)CoreTMi5-4300M processor with a base frequency of 2.60 GHz and 4GB RAM.
The structures of approved drugs remdesivir,lopinavir,nelfinavir,invermectin,favipiravir,and native ligand(Spatial Data File[SDF])were downloaded from the U.S.National Library of Medicine,Pub-Chem(https://pubchem.ncbi.nlm.nih.gov/),and the structures of 1,2,3,4-tetrahydropyrimidine-2-thiols and novel pyrimidine-linked benzimidazole derivatives were sketched in ChemDraw Ultra 8.0.Energy was minimized using a universal force field(UFF)[42].We investigated the binding affinity of the derivatives for the SARS-CoV-2 main protease(PDB ID:6LU7)and spike glycoprotein(6VSB).The crystal structures of 6LU7(https://www.rcsb.org/structure/6LU7)and 6VSB(https://www.rcsb.org/structure/6VSB)were downloaded from the RCSB Protein Data Bank.The native ligand in 6LU7 was N-[(5-methylisoxazol-3-yl)carbonyl]alanyl-L-valyl-N~1~-((1R,2Z)-4-(benzyloxy)-4-oxo-1-{[(3R)-2-oxopyrrolidin-3-yl]methyl}but-2-enyl)-L-leucinamide[20].The crystal structure of 6VSB did not indicate a native ligand.Molecular docking proceeded as described[43-45].The interacting amino acid residues in the protein were identified using BIOVIA Discovery Studio Visualizer version 19.1.0.182 87(Dassault Systemes,Paris,France)[46].
2.2 Design of novel pyrimidine-linked benzimidazole hybrids
We designed derivatives by merging the 2-(chloromethyl)-1H-benzimidazole moiety with 1,2,3,4-tetrahydropyrimidine-2-thiol pyrimidine derivatives synthesized via the modified Biginelli reaction.Figure 1 shows the approach used to construct the derivatives.We then compared binding affinities of 1,2,3,4-tetrahydropyrimidine-2-thiols and novel pyrimidine-linked benzimidazole derivatives to determine the significance of merging the two moieties.

Figure 1 Synthesis of pyrimidine-linked benzimidazole scaffold
Table 1 shows structures of the pyrimidine derivatives and final novel derivatives obtained by merging benzimidazole with pyrimidine.

Table 1 Structures of 1,2,3,4-tetrahydropyrimidine-2-thiols and novel pyrimidine-linked benzimidazole derivatives
2.3 Laboratory procedures
2.3.1 Synthesis of 2-(chloromethyl)-1H-benzimidazoleThis procedure is described in the Supplementary material.The yield was 85%.A yellowish-brown product recrystallized from dioxane;m.p.,152-154 °C[compared with the literature:147.8-148.2 °C][47].Care was taken while handling 2-(chloromethyl)-1Hbenzimidazole because it is a powerful skin and mucous membrane irritant[48].Figure 2 shows the reaction scheme for the synthesis of this compound.

Figure 2 Synthesis of 2-(chloromethyl)-1H-benzimidazole
2.3.2 Synthesis of pyrimidine derivativesThe modified Biginelli reaction proceeded as described and detailed in the Supplementary material[49]and generated 1,2,3,4-tetrahydropyrimidine-2-thiol from ethyl acetoacetate,aldehyde,and thiourea[37,50]at 75%-95% yield(Figure 3).

Figure 3 Synthesis of 1,2,3,4-tetrahydropyrimidine-2-thiols via modified Biginelli reaction
2.3.3 Merging 2-(chloromethyl)-1H-benzimidazole and 1,2,3,4-tetrahydropyrimidine-2-thiols to synthesize pyrimidine-linked benzimidazole derivativesWe condensed 2-(chloromethyl)-1H-benzimidazole(1.66 g,0.01 mol and 1,2,3,4-tetrahydropyrimidine-2-thiol(0.01 mol)by heating with potassium hydroxide(KOH)and H2O :acetone(2 :1)at 50-60 °C for 45 min.The reaction mixture was chilled to room temperature,decanted into ice-cold water,filtered,and recrystallized from ethanol(Figure 4).The yield was 90%-95%.

Figure 4 Synthesis of novel pyrimidine-linked benzimidazole derivatives
2.4 Calculation of Lipinski rule of five
We applied the Lipinski rule of five that defines the ability of new molecular entities to be useful drugs.In terms of drug development,the rule states that weak absorption or permeation is more likely when the criteria of>5 H-bond donors,10 H-bond acceptors,molecular weight>500,and a measured LogP(MLogP)>5 are met[51-54].The properties of all derivatives were calculated using the SwissADME online tool(http://www.swissadme.ch/index.php).
2.5 Biological activity
Various concentrations of derivatives were prepared in DMSO to assess their antibacterial and antifungal activities against standard strains(Table 2)using broth dilution.Bacteria were maintained,and drugs were diluted in nutrient Mueller Hinton broth.The broth was inoculated with 108colony-forming units(CFU)per milliliter of test strains(Institute of MicrobialTechnology,Chandigarh,India)determined by turbidity.Stock solutions of synthesized derivates(2 mg/mL)were serially diluted for primary and secondary screening.The primary screen included 1 000,500,and 250 μg/mL of synthesized derivatives,then those with activity were further screened at 200,100,50,25,12.5,and 6.250 μg/mL.A control without antibiotic was subcultured(before inoculation)by spreading one loopful evenly over a quarter of a plate of medium suitable for growing test organisms and incubated at 37 °C overnight.The lowest concentrations of derivatives that inhibited bacterial or fungal growth were taken as minimal inhibitory concentrations(MICs).These were compared with the amount of control growth before incubation(original inoculum)to determine MIC accuracy[55-57].The standards for antibacterial activity were gentamycin,ampicillin,chloramphenicol,ciprofloxacin,and norfloxacin served,and those for antifungal activity were nystatin and griseofulvin.

Table 2 Bacterial and fungal strains for activity assay
3 Results
3.1 Molecular docking
Table 3 shows details of the SARS-CoV-2 main protease and spike glycoprotein according to PDB Xray structure validation reports.

Table 3 Crystal structures of SARS-CoV-2 main protease(Mpro)and spike glycoprotein used for molecular docking
Table 4 shows details of the derivatives,their binding affinity(kcal/mol),number of hydrogen bonds formed with targets and active amino acid residues involved in interactions.Data for compounds 1a-1h(1,2,3,4-tetrahydropyrimidine-2-thiols),are provided in Supplementary material.

Table 4 Details of the synthesized derivatives
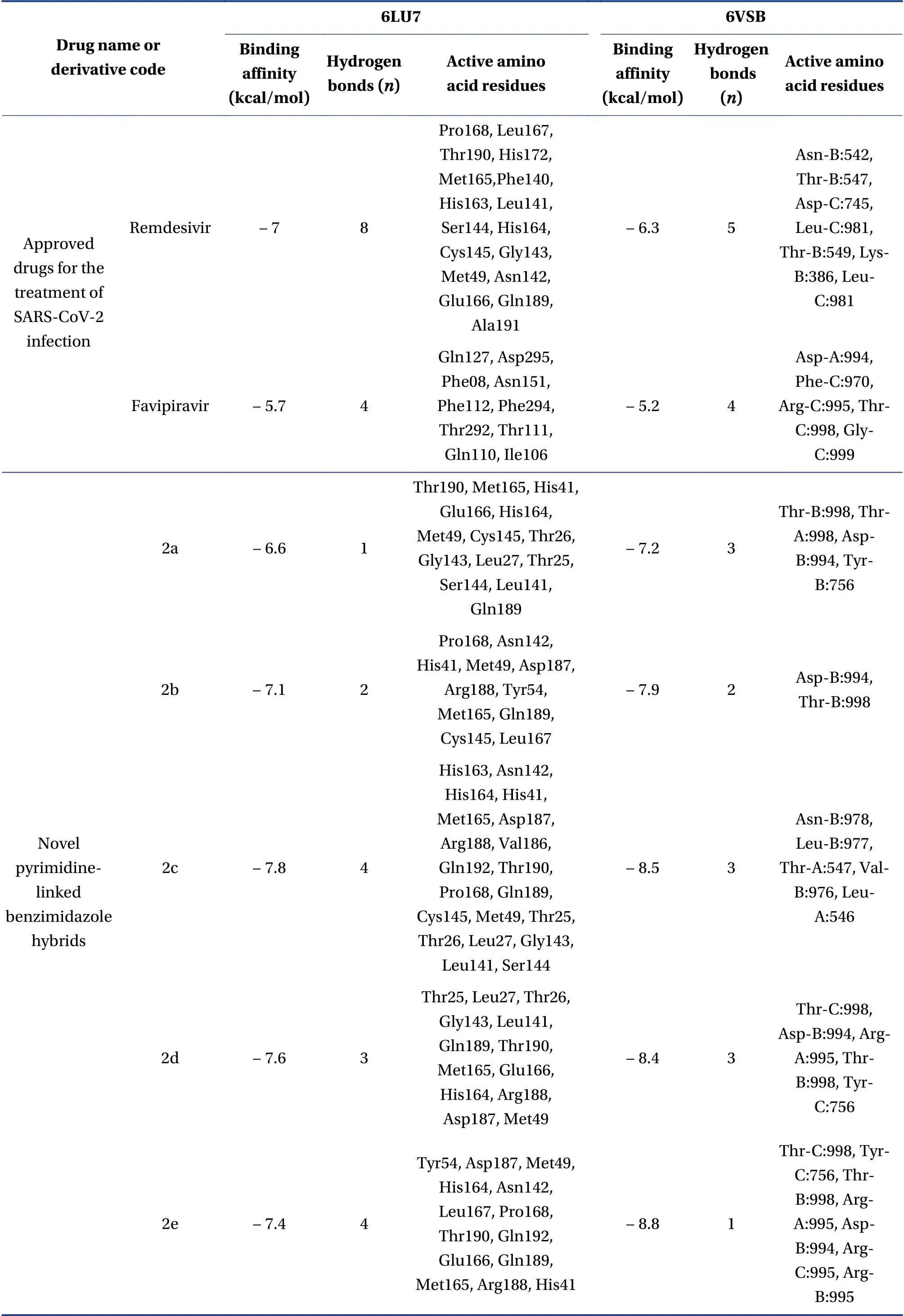
Table 4 Continued

Table 4 Continued
Table 5 shows the two-and three-dimensional(2D and 3D)binding positions of the derivatives.These enabled us to predict which atoms and/or groups in a ligand are involved in interactions with amino acid residues in target derivatives.Details of 2D and 3D-docking of compounds 1a-1h are provided in the Supplementary material.

Table 5 2D and 3D docking positions of drugs targeting SARS-CoV-2 main protease and RBD of spike glycoprotein
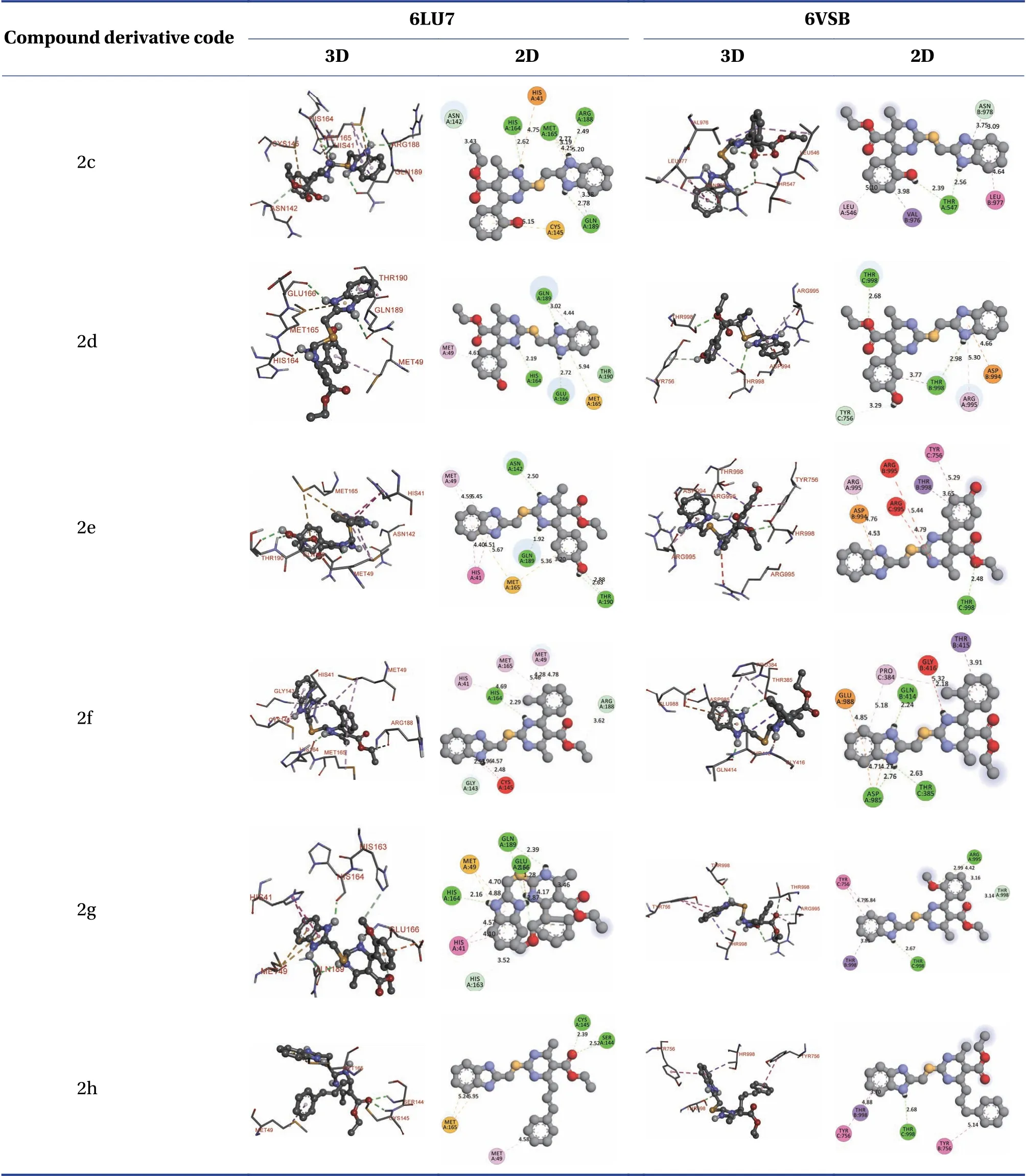
Table 5 Continued
Table 6 shows changes in the number of hydrogen bonds formed and binding affinity before and after merging with benzimidazole.

Table 6 Affinity and hydrogen bonds formed after pyrimidine-linked benzimidazole hybrids bound to SARSCoV-2 main protease
3.2 Chemistry
Spectral characterization revealed the formation of pyrimidine-linked benzimidazole derivatives.The chemistry,melting points,physical properties,and IR spectra are provided in the Supplementary material.
3.2.1 2-(chloromethyl)-1H-benzimidazoleMolecular formula,C8H7ClN2;molecular weight,166.61;appearance,yellowish brown;soluble in ethanol,acetone,benzene;elemental analysis,C,57.67;H,4.23;Cl,21.28;N,16.81;LogP,2.11;yield,90%;m.p.,152-154 °C;IR:aromatic,933 and 842 cm-1;halogen,642 cm-1;NH bending,1 600 cm-1;NH stretching,3 300-3 400 cm-1;CH bending,700 and 842 cm-1;CH stretching,3 084 cm-1;C=C,1 650 cm-1.
3.2.2 Ethyl 1,2,3,4-tetrahydro-2-mercapto-6-methylpyrimidine-5-carboxylate(1a)Molecular formula,C8H14N2O2,S;molecular weight,198.24;appearance,light pink powder;soluble in ethanol,acetone,benzene;m/e ratio,198.05(100.0%),199.05(9.6%),200.04(4.5%);elemental analysis,C,48.47;H,5.08;N,14.13;O,16.14;S,16.17;LogP,1.66;yield,80%;m.p.,213-215 °C;IR:NH bending,1 600 cm-1;NH stretching,3 315 cm-1;CH bending,960 cm-1;CH stretching,3 030 cm-1;ester group,1 710 cm-1;SH stretching,2 524 cm-1;C-S stretching,680 cm-1;aromatic,690 cm-1.
3.2.3 Ethyl-1,2,3,4-tetrahydro-2-mercapto-6-methyl-4-phenylpyrimidine-5-carboxylate(1b)Molecular formula,C14H18N2O2S;molecular weight,274.34;appearance,milky white crystals;soluble in ethanol,acetone,benzene;m/e ratio,274.08(100.0%),275.08(16.2%),276.07(4.5%),276.08(1.7%);elemental analysis,C,61.29;H,5.14;N,10.21;O,11.66;S,11.69;LogP,3.76;yield 85%;m.p.,203-205 °C;IR:NH bending 1 654 cm-1;NH stretching,3 332 cm-1;CH bending,869 cm-1;CH stretching,3 180 cm-1;ester group,1 700 cm-1;aromatic,700 cm-1;SH stretching,2 582 cm-1;C-S stretching 692 cm-1.
3.2.4 Ethyl-1,2,3,4-tetrahydro-4-(2-hydroxyphenyl)-2-mercapto-6-methylpyrimidine-5-carboxylate(1c)Molecular formula,C14H18N2O3S;molecular weight,290.34;appearance,prismatic white crystals;solublein ethanol,acetone,benzene;m/e ratio,290.07(100.0%),291.08(15.4%),292.07(4.6%),292.08(1.8%),291.07(1.5%;elemental analysis,C,57.92;H,4.86;N,9.65;O,16.53;S,11.04;LogP,3.37;yield,77%;m.p.,201-203 °C;IR:NH bending,1 581 cm-1;NH stretching,3 300 cm-1;CH bending,756 cm-1;CH stretching,3 003 cm-1;ester group,1 751 cm-1;hydroxy group,3 600 cm-1;aromatic o-disubstituted,730 cm-1;SH stretching,2 600 cm-1;C-S stretching,650 cm-1.
3.2.5 Ethyl-1,2,3,4-tetrahydro-4-(3-hydroxyphenyl)-2-mercapto-6-methylpyrimidine-5-carboxylate(1d)Molecular formula,C14H18N2O3S;molecular weight,290.34;appearance,light brown powder;soluble in ethanol,acetone,benzene;m/e ratio,290.07(100.0%),291.08(15.4%),292.07(4.6%),292.08(1.8%),291.07(1.5%);elemental analysis,C,57.92;H,4.86;N,9.65;O,16.53;S,11.04;LogP,3.37;yield,79%;m.p.,179-181 °C;IR:-NH bending,1 610 cm-1;NH stretching,3 319 cm-1;CH bending,866 cm-1;CHstretching 3 150 cm-1;ester group 1 700 cm-1;hydroxy group,3 600 cm-1aromatic m-disubstituted,680 and 788 cm-1;SH stretching,2 500 cm-1;C-S stretching,630 cm-1.
3.2.6 Ethyl-1,2,3,4-tetrahydro-4-(4-hydroxyphenyl)-2-mercapto-6-methylpyrimidine-5-carboxylate(1e)Molecular formula,C14H14N2O3S;molecular weight,290.34;appearance,off-white powder;soluble in ethanol,acetone,benzene;m/e ratio,290.07(100.0%),291.08(15.4%),292.07(4.6%),292.08(1.8%),291.07(1.5%);elemental analysis,C,57.92;H,4.86;N,9.65;O,16.53;S,11.04;LogP,3.37;yield,85%;m.p.,225-227 °C;IR:NH bending,1 581 cm-1;NH stretching 3 400 cm-1;SH bending,825 cm-1;SH stretching 3 016 and 3 196 cm-1;ester group 1 689 cm-1;hydroxy group 3 502 cm-1aromatic p-disubstituted,825 cm-1;SH stretching,2 561 cm-1;C-S stretching,642 cm-1.
3.2.7 Ethyl-4-(2-chlorophenyl)-1,2,3,4-tetrahydro-2-mercapto-6-methylpyrimidine-5-carboxylate(1f)Molecular formula,C14H17ClN2O2S;molecular weight,308.78;appearance,yellowish white sticky product;soluble in ethanol,acetone,benzene;m/e ratio,308.04(100.0%),310.04(32.6%),309.04(16.9%),311.04(5.9%),310.03(4.5%),312.03(1.5%),310.05(1.1%);elemental analysis,C,54.46;H,4.24;Cl,11.48;N,9.07;O,10.36;S,10.38;LogP,4.31;yield,87%;m.p.192-194 °C;IR:NH bending,1 580 cm-1;NH stretching,3 350 cm-1;CH bending,767 cm-1;CH stretching,3 100 cm-1;ester group,1 724 cm-1;halogen group,646 cm-1;aromatic o-disubstituted,767 cm-1;SH stretching,2 349 cm-1;C-S stretching,646 cm-1.
3.2.8 Ethyl-1,2,3,4-tetrahydro-2-mercapto-4-(4-methoxyphenyl)-6-methylpyrimidine-5-carboxylate(1g)Molecular formula,C15H20N2O3S;molecular weight,304.36;appearance,white crystals;soluble in ethanol,acetone,benzene;m/e ratio,304.09(100.0%),305.09(18.1%),306.08(4.5%),306.09(2.1%);elemental analysis,C,59.19;H,5.30;N,9.20;O,15.77;S,10.54;LogP,3.63;yield,92%;m.p.,-199-201 °C;IR:NH bending,1 581 cm-1;NH stretching,3 319 cm-1;CH bending,767 cm-1;CH stretching,3 150 cm-1;ester group,1 710 cm-1;ether group,1 186 cm-1;aromatic p-disubstituted,790 cm-1;SH stretching,2 500 cm-1;C-S stretching,653 cm-1.
3.2.9 Ethyl-4-cinnamyl-1,2,3,4-tetrahydro-2-mercapto-6-methylpyrimidine-5-carboxylate(1h)Molecular formula,C17H22N2O2S;molecular weight,314.4;appearance,white crystals;soluble in ethanol,acetone,benzene;m/e ratio,314.11(100.0%),315.11(20.0%),316.10(4.5%),316.12(1.6%);Elemental Analysis,C,64.94;H,5.77;N,8.91;O,10.18;S,10.20;LogP,4.55;yield,82%;m.p.,200-202 °C;IR,NH bending,1 595 cm-1;NH stretching,3 400 cm-1;CH bending,852 cm-1;CH stretching,3 150 cm-1;ester group,1 703 cm-1;C=C,1 670 cm-1;aromatic,monosubstituted,700 and 770 cm-1;SH stretching,2 600 cm-1;SH stretching,661 cm-1.
3.2.10 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-6-methylpyrimidine-5-carboxylate(2a)Molecular formula,C16H20N4O2S;molecular weight,328.39;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,328.10(100.0%),329.10(19.7%),330.10(5.3%),330.11(1.5%);elemental analysis,C,58.52;H,4.91;N,17.06;O,9.74;S,9.76;LogP,3.07;yield,91%;m.p.,172-174 °C;IR:NH bending,1 546 cm-1;NH stretching,3 313 cm-1;CH bending,750 cm-1;CH stretching,3 034 cm-1;ester group,1 700 cm-1;C=C,1 600 cm-1;aromatic,750 cm-1;-C-S-C,750 cm-1;C-S stretching,680 cm-1.
3.2.11 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-6-methyl-4-phenylpyrimidine-5-carboxylate(2b)Molecular formula,C22H24N4O2S;molecular weight,404.48;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,404.13(100.0%),405.13(26.1%),406.13(5.5%),406.14(2.8%),407.13(1.1%);elemental analysis,C,65.33;H,4.98;N,13.85;O,7.91;S,7.93;LogP,5.17;yield,93%;m.p.,142-144 °C;IR,NH bending,1 600 cm-1;NH stretching,3 313 cm-1;CH bending,842 cm-1;CH stretching,3 061 cm-1;ester group,1 700 cm-1;C=C,1 600 cm-1;aromatic,700 and 742 cm-1;C=N group,1 644 cm-1;-C-S-C,742 cm-1;C-S stretching,700 cm-1.
3.2.12 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-4-(2-hydroxyphenyl)-6-methylpyrimidine-5-carboxylate(2c)Molecular formula,C22H24N4O3S;molecular weight,420.48;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,420.13(100.0%),421.13(24.1%),422.12(4.5%),422.13(3.9%),421.12(2.3%),423.12(1.1%);elemental analysis,C,62.84;H,4.79;N,13.32;O,11.41;S,7.63;LogP,4.78;yield,95%;m.p.,152-154 °C;IR:NH bending,1 593 cm-1;NH stretching,3 313 cm-1;CH bending,700 cm-1;CH stretching,3 055 cm-1;ester group,1 764 cm-1;C=C,1 600 cm-1;aromatic o-disubstituted,700 and 746 cm-1;C=N group,1 670 cm-1;C-S-C,746 cm-1;C-S stretching,600 cm-1.
3.2.13 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-4-(3-hydroxyphenyl)-6-methylpyrimidine-5-carboxylate(2d)Molecular formula,C22H24N4O3S;molecular weight,420.48;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,420.13(100.0%),421.13(24.1%),422.12(4.5%),422.13(3.9%),421.12(2.3%),423.12(1.1%);elemental analysis,C,62.84;H,4.79;N,13.32;O,11.41;S,7.63;LogP,4.78;yield,95%;m.p.,223-225 °C;IR:NH bending,1 595 cm-1;NH stretching,3 300 cm-1;CH bending,700 cm-1;CH stretching,3 050 cm-1;ester group,1 700 cm-1;C=C,1 600 cm-1;aromatic m-disubstituted,700 and 742 cm-1;C=N group,1 595 cm-1;-C-S-C,742 cm-1;C-S stretching,700 cm-1.
3.2.14 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-4-(4-hydroxyphenyl)-6-methylpyrimidine-5-carboxylate(2e)Molecular formula,C22H24N4O3S;molecular weight,420.48;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,420.13(100.0%),421.13(24.1%),422.12(4.5%),422.13(3.9%),421.12(2.3%),423.12(1.1%);elemental analysis,C,62.84;H,4.79;N,13.32;O,11.41;S,7.63;LogP,4.78;yield,96%;m.p.,138-140 °C;IR:NH bending,1 598 cm-1;NH stretching,3 400 cm-1;CH bending,850 cm-1;CH stretching,3 062 cm-1;ester group,1 700 cm-1;C=C,1 598 cm-1;aromatic p-disubstituted,742 cm-1;C-S-C,742 cm-1;C-S stretching,690 cm-1.
3.2.15 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-4-(2-chlorophenyl)-1,2,3,4-tetrahydro-6-methylpyrimidine-5-carboxylate(2f)Molecular formula,C22H23ClN4O2S;molecular weight,438.93;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,438.09(100.0%),440.09(37.0%),439.10(24.1%),441.09(9.5%),440.10(3.2%),439.09(2.3%),442.08(1.4%);elemental analysis,C,60.20;H,4.36;Cl,8.08;N,12.76;O,7.29;S,7.31;LogP,5.73;yield,90%;m.p.,106-108 °C;IR:NH bending,1 571 cm-1;NH stretching,3 298 cm-1;CH bending,700 cm-1;CH stretching,2 950 cm-1;ester group,1 700 cm-1;C=C,1 470 cm-1;C=N group,1 691 cm-1;halogen,700 cm-1;aromatic o-disubstituted,752 cm-1;-C-S-C,752 cm-1;C-S stretching,650 cm-1.
3.2.16 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-1,2,3,4-tetrahydro-4-(2-methoxyphenyl)-6-methylpyrimidine-5-carboxylate(2g)Molecular formula,C23H26N4O3S;molecular weight,434.51;appearance,yellowish brown;soluble in ethanol,acetone,benzene;m/e ratio,434.14(100.0%),435.14(27.2%),436.14(5.1%),436.15(3.7%),437.14(1.2%);elemental analysis,C,63.58;H,5.10;N,12.89;O,11.05;S,7.38;LogP,5.04;yield,92%;m.p.,148-150 °C;IR:NH bending,1 590 cm-1;NH stretching,3 300 cm-1;CH bending,833 cm-1;CH stretching,3 150 cm-1;ester group,1 699 cm-1;ether,1 184 cm-1;C=C,1 450 cm-1;C=N group,1 680 cm-1;aromatic p-disubstituted,800 cm-1;C-S-C,744 cm-1;C-S stretching,650 cm-1.
3.2.17 Ethyl-2-((1H-benzo[d]imidazol-2-yl)methylthio)-4-cinnamyl-1,2,3,4-tetrahydro-6-methylpyrimidine-5-carboxylate(2h)Molecular formula,C25H28N4O2S;molecular weight,444.55;appearance,yellowish brown;solubility,ethanol,acetone,benzene;m/e ratio,444.16(100.0%),445.17(27.4%),446.16(5.2%),446.17(4.0%),445.16(2.3%),447.16(1.2%);elemental analysis,C,67.54;H,5.44;N,12.60;O,7.20;S,7.21;LogP,5.04;yield,90%;m.p.,180-182 °C;IR:NH bending,1 564 cm-1;NH stretching,3 250 cm-1;CH bending,850 cm-1;CH stretching,3 059 cm-1;ester group,1 700 cm-1;C=C,1 480 cm-1;C=N group,1 680 cm-1;aromatic mono-substituted,694 cm-1;-CS-C,746 cm-1;C-S stretching,694 cm-1.
3.3 Antimicrobial and antifungal activity
The antimicrobial susceptibility of all synthesized pyrimidine-linked benzimidazole derivatives was tested.Table 7 shows the MIC and minimum fungicidal concentrations(MFCs).The MIC of derivative 2a againstE.coliwas 62.5 μg/mL,which was much more potent than ampicillin,whereas derivatives 2c,2e,and 2f were equipotent at a MIC of 100 μg/mL.Pseudomonas aeruginosawas sensitive to all synthesized derivatives at 62.5,100,and 250 μg/mL,but not to ampicillin.Staphylococcus aureuswas sensitive to derivatives 2a,2b,2d,2e,and 2g at 200,100,100,100,and 200 μg/mL,respectively,indicating that they were more potent than ampicillin,which was active at 250 μg/mL.The MICs of derivatives 2b and 2f were both 100 μg/mL,and these compounds were equipotent againstS.pyogenes.Derivatives 2b,2c,2d,2e,and 2f exerted more effective fungicidal activity againstC.albicanscompared with griseofulvin with MICs of 250 and 500 μg/mL,respectively.
We used nystatin and griseofulvin as the standard antifungals againstA.niger,C.albicans,andA.clavatus.Table 7 shows the MFCs.Derivatives 2b,2c,2d,2e,and 2f exerted fungicidal activity against,C.albicanswas sensitive at a MIC of 250 μg/mL compared with griseofulvin at 500 μg/mL.

Table 7 Minimum inhibitory and fungicidal concentrations of standard drugs and synthesized derivatives(μg/mL)
3.4 Lipinski rule of five
None of the derivatives violated the rule of 5,indicating good absorption or permeation of the derivatives(Table 8).

Table 8 Lipinski rule of five for all synthesized derivatives
4 Discussion
We applied molecular docking to compare the ability of pyrimidine-linked benzimidazole hybrids to inhibit SARS-CoV-2 main protease and the RBD of spike glycoprotein with approved drugs and native ligands.The binding affinity of several derivatives was similar to that of approved drugs.The formation of a hydrogen bonds with target molecules results in inhibition,but binding affinity can be increased by van der Waals forces,Pi-Pi,and hydrophobic interactions.Thus,optimal inhibitors should comprise ligands that form hydrogen bonds with targets.For example,the binding affinity of remdesivir for the main protease is-7 kcal/mol,which is much lower than that of approved drugs,but it forms about eight hydrogen bonds with target,which confers better inhibitory activity than these drugs.This could explain why it has been accepted for clinical trials for the management of COVID-19.Our novel derivatives also formed hydrogen bonds with their targets,indicating inhibitory potency towards the SARS-CoV-2 main protease.
The binding affinity of our novel derivatives for the RBD of the SARS-CoV-2 spike glycoprotein was as good that that of the approved drugs.The binding affinity of ivermectin for the RBD of SARS-CoV-2 spike glycoprotein is-9.1 kcal/mol and it forms four hydrogen bonds.It interacts with Cys-C at 379,Glu-A at 988,Val-C at 382,Pro-A at 987,Val-A at 991,Val-B at 991,and Lys-C at 378.The binding affinity of remdesivir is-6.3 kcal/mol and it forms five hydrogen bonds with the RBD.It interacts with Asn-B at 542,Thr-B at 547,Asp-C at 745,Leu-C at 981,Thr-B at 549,Lys-B at 386,and Leu-C at 981.Favipiravir forms four hydrogen bonds with the RBD and its binding affinity is-5.2 kcal/mol.It interacts with Asp-A at 994,Phe-C at 970,Arg-C at 995,Thr-C at 998,and Gly-C at 999.Ivermectin,remdesivir,and favipiravir are currently applied to treat SARS-CoV-2 infection.Several of our derivatives have good binding affinity and formed up to four hydrogen bonds with the RBD of the SARS-CoV-2 spike glycoprotein.
Antimicrobial screening revealed that compounds with an aromatic ring at the R position were more potent than ampicillin,which is the standard antimicrobial againstP.aeruginosa,S.aureus,andS.pyogenes.This might be attributed to the polar effect of the aromatic rings.Derivatives without substitution at the R position were more potent than ampicillin againstE.coliandS.aureus,which might have been due to being smaller and having a low molecular weight.Compounds with phenyl,hydroxy phenyl,and chlorophenyl substitutions at the R position were more active than griseofulvin againstC.albicans.
The drug-likeliness of ligands was assessed using Lipinski's rule of five in order to determine the pharmacokinetic characteristics of the synthesized ligands.All ligands were recognized as drug-like compounds and without any structural caution the physicochemical filter was passed through.The virtual screening method has the advantage of being able to produce ligands with high predicted binding affinities for completely new protein sequences.Here from the binding affinity,we can choose few potential ligands for the further optimization and development of novel anti-SARS-CoV-2 drugs.Compound 2c,2d,2e,2f,2g,and 2h exhibited good binding affinity with main protease and RBD of spike glycoprotein,also formed enough number of hydrogen bonds.We can choose these ligands for further optimization and validation,in order to search for more novel compounds for the treatment of COVID-19.
We determined changes in the binding affinity of pyrimidines after combining them with benzimidazole to predict the contributions of functional groups.The numbers of hydrogen bonds also changed,indicating the significance of merging benzimidazole with pyrimidine.
The docking scores of almost all derivatives indicated that binding affinity increased when merged with benzimidazole.Compound 1a formed four hydrogen bonds and 2a formed only one with the SARS-CoV-2 main protease.Compounds 2c,2d,2e,2f,and 2 g had better binding affinity and formed more hydrogen bonds than compound 2b,indicating that synthesized derivatives with different substituted benzaldehydes,preferably at the ortho and meta positions,would generate more potent derivatives.The binding affinity of compound 2h increased and it formed two hydrogen bonds,indicating that increasing the chain length of the R group increases potency.We speculated that substitution with cinnamaldehyde will increase binding affinity as well as the number of hydrogen bonds.The information rendered by molecular docking study improved understanding of the structural requirements for developing more novel blockers of SARS-CoV-2 main protease and inhibitors of the RBD of spike glycoprotein.Figure 5 shows the predicted pharmacophore features of each compound.
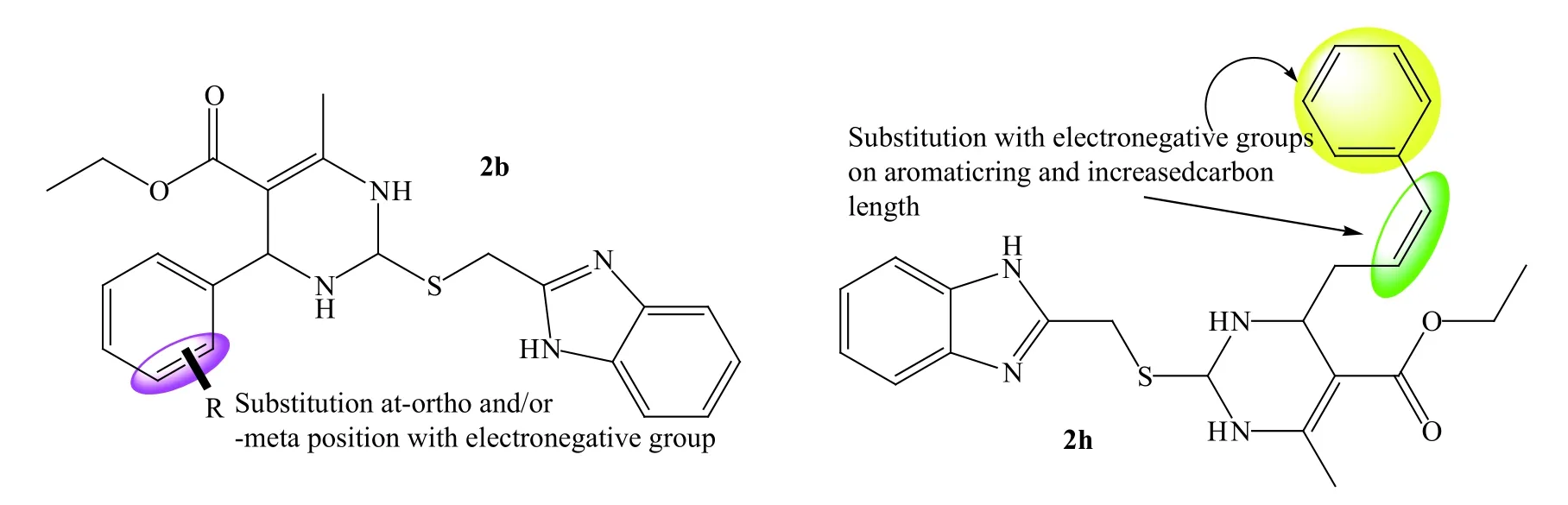
Figure 5 Predicted pharmacophore features of novel derivatives for further optimization
5 Conclusion
We could not assess the ability of our derivatives to inhibit SARS-CoV-2in vitrodue to safety reasons.However,we investigated their antimicrobial and antifungal properties as preliminary biological evidence.We found that pyrimidine-linked benzimidazole derivatives at specific concentrations were more effective than the standard ampicillin against gram-positive and gram-negative bacteria.Some derivatives were more active at higher concentrations than standard drugs.Gram-negative abcteriaE.coliandP.aeruginosawere more sensitive to the novel derivatives than gram-positive bacteriaS.aureusandS.pyogenes.C.albicanswas sensitive to the derivatives at a MFC of 250 μg/mL.
The molecular docking method was used to examine whether any possible ligands had potential interactions with the main protease and RBD of spike glycoprotein.Despite certain disadvantages,such as the use ofin vitroconditions rather thanin vivoconditions,molecular docking enables researchers to make more accurate decisions in a smaller duration.We developed eight of derivatives that had binding affinity and potential anti SARS-CoV-2 activities that exceeded those of currently approved drugs for treating COVID-19 infection.However,understanding the pharmacophore features of the SARS-CoV-2 main protease and the RBD of spike glycoprotein provides much scope to generate more potent derivatives.Optimizing the properties of these derivatives in modelsin vivoandin vitro,will lead to more effective options to fight SARS-CoV-2 infection.Because of the critical global COVID-19 situation,we believe that extensive investigation is imperative to acquire a deeper understanding of SARS-CoV-2 and generate effective agents to treat and prevent infection worldwide.At present,a single lead could be a game changer.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年3期
Digital Chinese Medicine2021年3期
- Digital Chinese Medicine的其它文章
- Instructions for Authors
- Research on classification diagnosis model of psoriasis based on deep residual network
- Comparison of mechanisms and efficacies of five formulas for improving blood circulation and removing blood stasis
- Network pharmacology research and experimental verification of Huangqi(Astragalus Radix)and Jinyingzi(Rosae Laevigatae Fructus)in treating benign prostatic hyperplasia
- Evaluation of Baishao(Paeoniae Radix Alba)and Chishao(Paeoniae Radix Rubra)from different origins based on characteristic spectra of amino acids
- Analysis of the hotspots and trends in traditional Chinese medicine immunomodulation research based on bibliometrics
