Imaging in the COVID-19 era: Lessons learned during a pandemic
Georgios Antonios Sideris, Melina Nikolakea, Aikaterini-Eleftheria Karanikola, Sofia Konstantinopoulou,Dimitrios Giannis, Lucy Modahl
Georgios Antonios Sideris, Lucy Modahl, Department of Radiology, University of Massachusetts Medical School, Baystate Medical Center, Springfield, MA 01199, United States
Georgios Antonios Sideris, Melina Nikolakea, Aikaterini-Eleftheria Karanikola, Radiology Working Group, Society of Junior Doctors, Athens 11527, Greece
Sofia Konstantinopoulou, Division of Pulmonary Medicine, Department of Pediatrics, Sheikh Khalifa Medical City, Abu Dhabi W13-01, United Arab Emirates
Dimitrios Giannis, Institute of Health Innovations and Outcomes Research, The Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY 11030, United States
Abstract The first year of the coronavirus disease 2019 (COVID-19) pandemic has been a year of unprecedented changes, scientific breakthroughs, and controversies. The radiology community has not been spared from the challenges imposed on global healthcare systems. Radiology has played a crucial part in tackling this pandemic,either by demonstrating the manifestations of the virus and guiding patient management, or by safely handling the patients and mitigating transmission within the hospital. Major modifications involving all aspects of daily radiology practice have occurred as a result of the pandemic, including workflow alterations, volume reductions, and strict infection control strategies. Despite the ongoing challenges, considerable knowledge has been gained that will guide future innovations. The aim of this review is to provide the latest evidence on the role of imaging in the diagnosis of the multifaceted manifestations of COVID-19,and to discuss the implications of the pandemic on radiology departments globally, including infection control strategies and delays in cancer screening.Lastly, the promising contribution of artificial intelligence in the COVID-19 pandemic is explored.
Key Words: COVID-19; Infectious diseases; Diagnostic imaging; Radiography; Computed tomography; Artificial intelligence
INTRODUCTION
The year 2020 has been marked by the worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel SARS-CoV-2 that emerged in Wuhan, China in December 2019 belongs to the same genus as SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS), which caused epidemics with high mortality rates in 2002 and 2012 respectively[1]. Despite the similarities in genomic identity, SARS-CoV-2 has a higher infectivity rate and a lower case-fatality rate, contributing to its much wider spread[2]. Since the first official SARS-CoV-2 case was documented, the virus has resulted in 173.4 million infections and 3.7 million deaths worldwide as of June 4, 2021[3].
SARS-CoV-2 causes multiorgan damage by attaching to the angiotensin-converting enzyme 2 receptors expressed by epithelial cells and microvascular pericytes. It primarily targets the lung parenchyma, resulting in acute respiratory distress syndrome (ARDS). Multiorgan dysfunction can occur either by direct viral-mediated damage, or indirectlyviathe systemic inflammatory response syndrome and the hypercoagulable state induced by the virus[4]. The constellation of end-organ injuries stemming from SARS-CoV-2 infection has been referred to as coronavirus disease 2019(COVID-19). Around 80% of infected individuals develop mild-moderate disease,whereas 14% have severe symptoms and 5% patients become critically ill[5]. Factors associated with worse outcomes and higher mortality include hypertension, diabetes mellitus, coronary artery disease, older age and severe obesity[6-8].
Similar to previous pandemics, imaging has played a pivotal role in the management of patients with acute respiratory illness. In 1918, just 23 years after the discovery of X-rays, the deadly H1N1 influenza A pandemic was the first ever largescale application of radiology. Although plain radiography could not provide a specific diagnosis, it was the only available modality that could visualize the presence,the extent and complications of a pulmonary infection[9]. Nearly a century later,advanced imaging technologies, including ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) have dramatically increased the value of imaging in patient care.
The radiology community has faced numerous challenges as a result of the new global public health emergency. The initially unknown imaging manifestations of the novel virus were described and made available to the scientific community soon after the onset of the pandemic, although studies reporting on radiologic-pathologic correlation were not released till later[10,11]. Unlike previous pandemics, COVID-19 has been the subject of an extraordinary number of publications, the majority of which were commentaries and opinion papers[12]. Many medical journals initiated a rapid peer-review process for COVID-19- related submissions in order to make the latest advances quickly available to the scientific community[13]. However, the lack of meticulous review has led to the publication of biased studies, some of which were later retracted even from top medical journals. This so-called “infodemic” contributes to confusion and dilutes the pool of legitimate original research investigations[12,14].
Radiology departments worldwide implemented drastic policies to minimize transmission of the virus between patients and staff. Some of the strategies that were enforced include efficient triaging of patients, designation of imaging rooms specifically for suspected cases, meticulous disinfection, enforcement of personal protective equipment (PPE) and shortening of scanning protocols whenever possible.Elective imaging studies were postponed during the first surge of the pandemic,which caused a significant drop in case volumes and a delay in cancer detection[15,16].
The aim of this review is to summarize the current knowledge of the multi-organ imaging manifestations of COVID-19 and to describe the infection control strategies that were enforced in radiology departments. Moreover, the repercussions of delayed screening studies and the promise of artificial intelligence (AI) are explored.
PULMONARY MANIFESTATIONS
CT
Diagnostic value:The gold standard for the diagnosis of SARS-CoV-2 is the real-time reverse transcriptase polymerase chain reaction (RT-PCR) using nasopharyngeal samples[17]. Although it has excellent specificity, sensitivity may vary between 42%-83%[18,19]. This wide range in reported sensitivities can be attributed to inadequate or improper tissue sampling, early timing of sampling (when the viral load is low), or laboratory errors[20]. High false-negative rates can have a crucial impact, as patients who are misdiagnosed as negative can continue transmitting the virus within the hospital or the community. Moreover, the initial RT-PCR test kits had long processing times and were not readily available in certain regions due to high demand.
Given the need for rapid triaging of patients and prevention of transmission, chest CT was proposed as a rapid, reproducible and widely available screening tool[21-23].However, many methodologic concerns and shortcomings are present in studies reporting on the performance of chest CT as a diagnostic tool[24]. The majority of published studies based their findings on populations with high disease prevalence or with only symptomatic patients, introducing a selection bias[25]. Furthermore, some studies used CT as a binary test with a low threshold for determining a positive examination, which may also overestimate sensitivity and compromise specificity[26,27]. Several meta-analyses have attempted to generate an estimated sensitivity;however, many of them did not assess the risk of bias in the included studies[28].
As a result of these discrepancies, there is great variability in the reported sensitivities (60%-98%) and specificities (25%-53%) of chest CT in the detection of COVID-19 pneumonia [19]. A Cochrane meta-analysis including data from 31 studies with low risk of bias and 8014 participants, 53% of which were COVID-19 positive, showed that chest CT has 89.9% sensitivity and 61.1% specificity[29]. The use of CT as a screening tool has multiple limitations, including high cost, radiation exposure, and transmission risk within the radiology department due to clustering of patients[30]. It has poor specificity due to overlap with other pulmonary diseases (i.e., viral pneumonias,pulmonary edema, interstitial lung disease), and therefore cannot be used as a confirmatory test[31]. Moreover, with a reported negative predictive value of 42%, CT chest can lead to false negative results in patients early in the course of the disease. In view of these limitations, the World Health Organization (WHO), the American College of Radiology (ACR) and other societies have released statements urging against the use of chest CT as a screening tool[32,33].
Indications:Chest CT is a valuable imaging modality that can provide an accurate assessment of the severity and extent of disease, detect complications, evaluate treatment efficacy and rule out alternative diagnoses[34].
Based on the guidelines released by the Fleischner Society, chest imaging is indicated in patients with worsening clinical status and for rapid triage of patients with moderate-severe respiratory symptoms in a setting of high pre-test probability and low RT-PCR availability[35]. Additionally, the WHO recommends chest imaging when RT-PCR is negative but clinical suspicion for COVID-19 remains high, and to help guide admission to the medical floorvsintensive care unit (ICU) in patients with moderate-severe illness[32]. Although imaging is not indicated in suspected cases with mild symptoms based on the Fleischner Society guidelines, the WHO recommends chest imaging in suspected or confirmed mild cases to help decide on hospital admissionvsdischarge, especially in patients at high risk of disease progression.Neither of the aforementioned guidelines clarify which chest imaging modality needs to be used on each clinical scenario or provide guidance on follow-up imaging intervals and scanning protocols.
Imaging findings:COVID-19 pneumonia causes a wide spectrum of acute lung injury ranging from mild inflammation to diffuse alveolar damage[36]. Ground-glass opacities (GGOs) are the most common imaging manifestation, seen in 65% of patients[37]. GGOs are bilateral in 88%, although they may remain unilateral throughout the course of the disease in 17% of cases[38,39]. They typically have a peripheral/subpleural distribution with a predilection for the posterior segments of the lower lobes, but may be diffuse in 29% of cases[39]. GGOs may be pure (more commonly) or be accompanied by consolidations (mixed pattern). Intralobular and interlobular septal thickening, likely a combination of interstitial inflammation and fluid, is seen in 27% of cases. Superimposed GGOs giving a crazy-paving pattern is seen in 12% and may be a sign of more severe lung injury and disease progression[37]. Consolidations, seen in up to 32% of cases, have a subpleural or peribronchovascular distribution and may or may not have air-bronchograms[37,40]. They are associated with more severe disease requiring management in the ICU[41]. Cavitations are not typically seen.
Subsegmental vascular enlargement (greater than 3 mm) within parenchymal abnormalities has been described in up to 64%-89% of patients[42,43]. Although the exact pathogenesis is uncertain, it is thought to be related to hyperemia or thrombotic microangiopathy[43,44]. Pulmonary nodules are considered atypical, as they are seen in only 9% of cases. The halo sign (consolidation surrounded by GGO) and the reverse halo sign (GGO surrounded by a rim of consolidation) have been described late in the disease course of COVID-19 pneumonia but are considered non-specific[4]. Pleural thickening has been described more commonly than pleural effusions (1.6%).Mediastinal adenopathy is rarely seen in COVID-19 pneumonia (0.7%) and when seen it should point to another process such as concurrent chronic congestive heart failure or even bacterial superinfection. The presence of pericardial effusion should raise concern for COVID-19 related cardiac injury.
Differential diagnosis:GGOs are a non-specific finding that may occur in numerous other entities. However, the distribution and pattern of GGOs as well as accompanying features can aid in the differential diagnosis. Pulmonary edema can manifest with GGOs in a central distribution and peripheral sparing, along with smooth interlobular septal thickening, peribronchial cuffing, cardiomegaly, dilated pulmonary veins, and pleural effusions. Diffuse alveolar hemorrhage is characterized by diffuse peribronchovascular GGOs but no subpleural predominance. Drug-induced pneumonitis can present as non-specific interstitial pneumonia with subpleural sparing. E-cigarette vaping induce lung injury (EVALI) can present with peribronchovascular GGOs with subpleural sparing. Bronchiolitis is characterized by centrilobular opacities, as well as bronchial wall thickening, bronchiectasis and airtrapping[45,46].
Pulmonary manifestations of SARS-CoV-2 on CT overlap with other viral infections.In SARS-CoV-1 and MERS infection, GGOs are typically unifocal and less extensive,and the halo and reverse halo signs are atypical[47,48]. Influenza can also manifest as bilateral GGOs, with or without consolidations, with a lower lobe predilection;bronchiectasis and pleural effusions are, however, more common[49]. In parainfluenza, centrilobular nodules and bronchial wall thickening are typical. Respiratory syncytial virus infection is characterized by centrilobular nodules (tree-in-bud) and asymmetric consolidations. In adenovirus infection, bilateral multifocal GGOs and consolidations are seen in a lobar or segmental distribution, frequently with pleural effusion[46,50].
Lobar bacterial pneumonia (primarily caused byStreptococcus pneumoniae,Legionella pneumophila,Mycoplasma) manifests as lobar or multilobar consolidations, typically with regional adenopathy and pleural effusions. Bronchopneumonia (Staphylococus aureus,Pseudomonas,Klebsiella,Haemophilus influenzae) manifests as confluent peribronchial consolidations, GGOs, centrilobular nodules, bronchial wall thickening,and mucoid impaction. Lymphadenopathy, pleural effusions and cavitations are common with some of these organisms. Interstitial pneumonia (caused byMycoplasmaand other atypical agents) presents with patchy GGOs, consolidations and centrilobular nodules[46].
Structured reporting:The Fleischner society recommends RT-PCR in patients with CT findings suggestive of COVID-19[35]. The Radiological Society of North America(RSNA) suggested the use of a structured reporting system to decrease reporting variability among radiologists and to reduce uncertainty about the findings that should raise concern for COVID-19[19]. It has been validated by several studies and appears to be useful in clinical decision making[18,51]. According to this system,findings on CT are categorized in 4 categories: typical, indeterminate, atypical and negative for COVID-19 pneumonia.
Typical findings are the bilateral multifocal peripheral GGOs, which may or may not be accompanied by consolidations and thickened interlobular septa. Additionally,typical findings include signs of organizing pneumonia (OP), such as the reverse halo sign. When applied to chest CTs of 211 patients positive for SARS-CoV-2 and 249 negative patients in Italy, the “typical” pattern had a 71.6% sensitivity, 91.6%specificity and 87.8% PPV for COVID-19 infection, although the PPV varied by disease prevalence. In negative patients with a typical pattern (8.4%), the final diagnosis was viral pneumonia other than COVID-19 (81.0%), bacterial infection (9.5%) and drug toxicity (9.5%)[51].
The “indeterminate” category includes findings with a lower specificity for COVID-19 pneumonia. These include GGOs that are non-peripheral, multifocal, diffuse,perihilar, unilateral, with or without consolidations. The “indeterminate” category imposes a diagnostic challenge as there is marked overlap with other infectious and non-infectious diagnoses, such as acute hypersensitivity pneumonitis, Pneumocystis infection and diffuse alveolar hemorrhage.
Atypical findings include lobar or segmental consolidations without GGOs,cavitations, small discrete centrilobular nodules, smooth interlobar septal thickening and pleural effusions. These findings are uncommonly reported in association with COVID-19 pneumonia, and are associated with bacterial pneumonia, necrotizing pneumonia, or aspiration, among others. The “negative” category includes cases with no evidence of pneumonia on CT. The atypical and negative patterns were more frequently observed in SARS-CoV-19 - negative patients[51].
Similar to other reporting and data systems widely used primarily for cancer reporting, the COVID-19 Reporting and Data System (CO-RADS) was designed by the Dutch Radiological Society to provide a standardized assessment of the level of suspicion for COVID-19 on chest CT. Seven categories were created, with a considerable overlap with the RSNA reporting system: CO-RADS 0 (technical limitations,uninterpretable), CO-RADS 1 (negative or very low suspicion), CO-RADS 2 (low suspicion), CO-RADS 3 (equivocal), CO-RADS 4 (high suspicion), CO-RADS 5 (very high suspicion) and CO-RADS 6 (confirmed by RT-PCR)[52]. The pilot study that assessed the performance of CO-RADS included 105 suspected COVID-19 cases, 51%of which were confirmed by a positive RT-PCR. Highest interobserver agreement was seen with the CO-RADS 1 and 5 categories. Performance, however, was tested in a setting of high prevalence of SARS-CoV-2 and low prevalence of other viral pneumonias, which may overestimate the positive predictive value[52]. Further studies have investigated the utility of CO-RADS in larger samples. A study included 859 suspected cases (42% of which were positive by RT-PCR), as well as 1138 controls who presented to the emergency room for other reasons within the same time period(5% of which were incidentally found to be COVID-19 positive). In the symptomatic cohort, when CO-RADS 4 was used as a threshold, sensitivity and specificity were 85%and 85% respectively, whereas for CO-RADS 5 rates were 78% and 93%, respectively.In asymptomatic patients, a threshold of CO-RADS 3 had a very poor sensitivity (45%)but high specificity (89%), suggesting that incidental CO-RADS 3 findings should prompt RT-PCR testing[53]. The high sensitivity of CO-RADS 4 and 5 suggests that patients who belong to these categories and have a negative initial RT-PCR need to remain in isolation until a repeat RT-PCR is negative, quarantine has lapsed or an alternate diagnosis is made[29,54].
Various severity scoring systems have been created in order to provide a quantified assessment of pulmonary involvement on chest CT. They usually divide each lung into segments and assign a score for the extent of involvement and nature of opacities. A final score is created by summing the scores for each individual segment. Higher CT severity scores are seen in patients with critical disease compared to those with milder disease[34] and have been associated with worse long-term outcomes[55]. No association has been found between the extent of CT findings and infectivity[56].
Disease phases:The findings on chest CT follow the temporal changes of COVID-19 pneumonia. Chest CT has a limited value during the first 48 h from symptom onset, as up to 56% of patients have no lung abnormalities[54,57]. Within 4 d, pure GGOs develop, which may have rounded margins or may outline adjacent secondary pulmonary lobules[37]. In the progressive phase of the disease (5-14 d), the GGOs become more extensive and may coalesce into multifocal consolidations. Septal thickening and crazy-paving are more frequent. Findings reach their peak at day 9-13 after symptom onset[38,58]. In the late or absorption phase (after day 14), there is a gradual clearance of GGOs and consolidations. Signs of fibrosis and parenchymal remodeling may develop, which can manifest as parenchymal bands, subpleural lines,interlobular septal distortion and traction bronchiectasis[39,59].
OP has been described as a pattern of response to acute lung injury caused by SARS-CoV-2, similar to other viral infections such as SARS-CoV-1, MERS and influenza[60,61]. It is histologically characterized by fibrous plugs within the alveoli and respiratory bronchioles. The transformation of GGOs into linear consolidations is typical for OP (Figure 1). Consolidations can be single or diffuse in a peripheral or peribronchial distribution. A reverse halo sign and spontaneous migration of infiltrates are commonly seen[62]. Treatment with corticosteroids shows dramatic improvement, as evidenced by the decreased mortality rates in COVID-19 patients on oxygen or mechanical ventilation (MV) receiving corticosteroids for 10 d in the RECOVERY trial[63]. However, this treatment duration may be insufficient, as longer duration and higher doses are typically needed for OP[64].
ARDS occurs in 31% of hospitalized patients with COVID-19 pneumonia and is the third most common complication following sepsis and respiratory failure[6]. Unlike typical ARDS which occurs within 1 wk based on the Berlin definition, ARDS in COVID-19 pneumonia develops within 8-15 d of disease onset[6,65]. ARDS is a clinical diagnosis encompassing features of non-cardiogenic pulmonary edema, and stems from the disordered vasoregulation in the setting of an acute systemic inflammatory response. Autopsies of COVID-19 patients revealed diffuse alveolar damage, as well as microvascular thrombosis[66]. Although ARDS is a clinical diagnosis, imaging can play a supportive role in diagnosis and monitoring of treatment response. In the early exudative phase, diffuse ground glass opacities and consolidations develop primarily in a posterior/basal distribution (Figure 1B and Figure 2B). Perfusion abnormalities may be seen on dual-energy CT as a result of ventilation/perfusion mismatches. In the late phase, 2 wk following the symptom onset, fibrotic changes may occur[4].
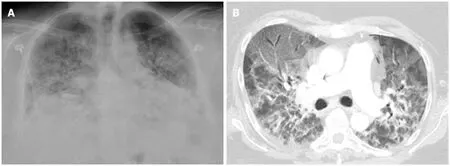
Figure 1 Severe coronavirus disease 2019 pneumonia. Portable chest X-ray and axial image from a computed tomography of the chest in a 52-yr-old female with a history of morbid obesity who was admitted for acute hypoxic respiratory failure secondary to severe acute respiratory syndrome coronavirus 2. A:Chest X-ray shows low lung volumes with diffuse bilateral alveolar and interstitial opacities; B: Chest computed tomography shows diffuse ground glass opacities anteriorly, typical of acute lung injury. The peribronchial and perilobular opacities posteriorly are typical of acute lung injury that has entered a healing phase. The patient subsequently expired.
Patients with critical disease are at increased risk for complications[10]. Secondary bacterial or fungal infection occurs in up to 15% of inpatients with COVID-19 pneumonia and is a major cause of mortality[6]. Moreover, an increased incidence of barotrauma events (pneumothorax, pneumomediastinum, pneumopericardium,subcutaneous emphysema) has been observed in COVID-19 patients on MV, particularly in younger age groups (Figure 2). A study showed that 15% ventilated patients with COVID-19 experienced one or more barotrauma events and that the rate was significantly higher compared to ventilated non-COVID-19 patients. Barotrauma was associated with higher mortality rates and longer hospital stay[67].
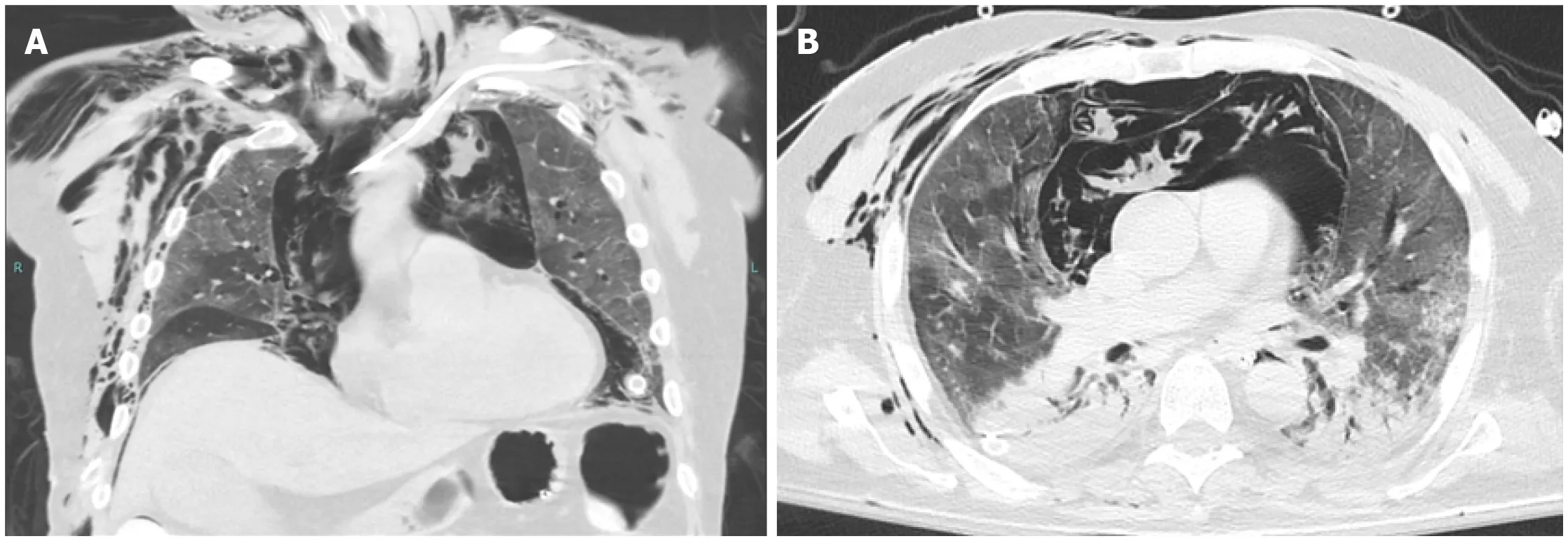
Figure 2 Barotrauma. A and B: Coronal (A) and axial (B) images from an unenhanced computed tomography of the chest of a 75-yr-old male with no significant past medical history, who was intubated for acute hypoxic respiratory failure secondary to coronavirus disease 2019 pneumonia. There are ground glass opacities anteriorly, as well as consolidations with air bronchograms posteriorly, a pattern typical for acute respiratory distress syndrome. There is a massive amount of air within the mediastinum resulting from alveolar rupture (Macklin effect). The arrow on image (A) points to interstitial emphysema, surrounding a pulmonary vein. The arrow on image (B) points to nonanatomic air within a pulmonary lobule, which may represent the initial barotrauma event. There is extensive soft tissue emphysema in the lower neck and lateral chest wall, as well as in the extraperitoneal space of the abdominal cavity. Bilateral thoracostomy tubes and a peripherally-inserted central catheter are in place. The patient could not be weaned from ventilation and subsequently expired.
Both OP and ARDS have the potential to progress into pulmonary fibrosis (Figures 3 and 4). However, there is a paucity of data with regards to the long-term pulmonary sequela of COVID-19 pneumonia. A study prospectively followed 114 patients who were admitted for severe COVID-19 pneumonia. Follow-up CT scans after 6 mo showed fibrotic changes in 35% of patients. Factors associated with a higher risk of fibrosis were older age (> 50 years), longer hospital stay (> 17 d), ARDS, non-invasive ventilation, tachycardia on admission and high CT severity scores on the initial CT scans[68]. Another study prospectively followed a cohort of 83 patients with no pulmonary or cardiovascular comorbidities, who were admitted for severe COVID-19 pneumonia that was managed without the use of MV. Although there was a temporal improvement in pulmonary function tests and imaging findings in most patients, 33%had impaired diffusing capacity of the lungs for carbon monoxide (DLCO) and 24%had residual lung findings on high-resolution CT 12 mo after discharge, including GGOs in 23%, interlobular septal thickening in 5% and reticular opacities in 4% of patients. Patients with a longer hospital stay, higher peak CT severity scores and those who required high-flow oxygen therapy or non-invasive ventilation were more likely to have residual lung abnormalities on follow-up CT[55]. Studies reporting on longterm outcomes are limited by small sample sizes and lack of histologic correlation.Ongoing trials with larger samples and longer follow-up intervals will help elucidate the long-term outcomes of COVID-19 pneumonia (NCT04483752, NCT04376840,NCT04376840).
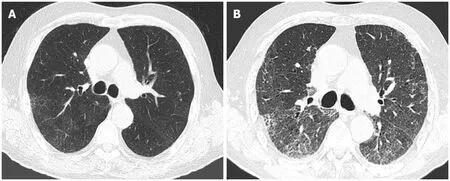
Figure 3 Pulmonary fibrosis. Axial images from computed tomographies of the chest performed 2 yr apart in an 83-yr-old male with a history of silicosis. A: In June 2018, there was mild lung hyperaeration with mild reticulation; B: In August 2020, 4 mo after recovering from coronavirus disease 2019 pneumonia, there is extensive fibrosis, with areas of honeycombing, traction bronchiectasis/bronchiolectasis and architectural distortion.
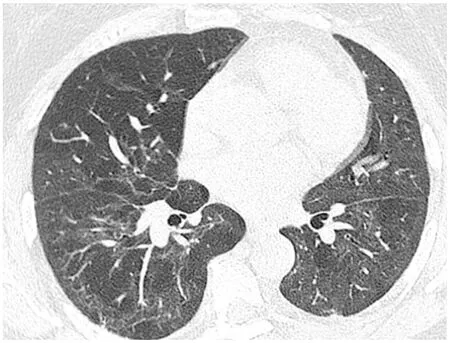
Figure 4 Non-specific interstitial pneumonia. Axial image from a computed tomography of the chest in a 59-yr-old female 6 mo after recovering from acute hypoxic respiratory failure secondary to coronavirus disease 2019. Mild fibrosis in a peribronchial distribution and subpleural sparing in the right lower lobe is in keeping with mild fibrotic non-specific interstitial pneumonia. There is also a mosaic pattern caused by obstructive small airways disease (confirmed on expiration views, not shown), with altered perfusion in the lungs.
Pulmonary embolism:SARS-CoV-2 causes prothrombotic endothelial injury leading to thromboembolic phenomena, which are further propagated by hypoxia[10,69].Pulmonary embolism (PE) is seen in 22%-30% of COVID-19 patients who undergo a CT pulmonary angiogram (CTPA) (Figure 5), a rate that is markedly higher than critically-ill patients without COVID-19[70-72]. In a multicenter study of 1042 COVID-19 patients, PE was found in 5.6%. PE was diagnosed on the day of admission in 47%,and was proximal in 46%, segmental in 41%, and sub-segmental in 14%. Of patients with PE, 42% required ICU management and MV, while 20% of PE patients died.Patients on MV were at higher risk for developing PE, irrespective of the extent of lung abnormalities on chest CT[72]. Other risk factors include severe obesity and African-American decent [71].
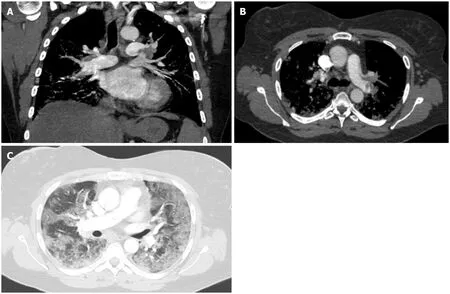
Figure 5 Pulmonary embolism. A-C: Coronal (A) and axial (B and C) images from a computed tomography angiography of the chest in a 53-year-old female with a history of chronic lymphocytic leukemia and asthma who was admitted for acute hypoxic respiratory failure secondary to coronavirus disease 2019 pneumonia,complicated by pulmonary emboli. A large filling defect is seen in the left pulmonary artery extending into lobar and segmental branches (A and B). Diffuse ground glass opacities are noted on the lungs bilaterally (C).
Patients with COVID-19 may have elevated D-dimer levels even in the absence of PE, due to the prothrombotic state induced by the virus. Higher D-dimers are associated with more severe disease[73]. However, COVID-19 patients with PE have significantly higher CRP and D-dimer levels compared to those without PE[71]. A Ddimer value of 2600 ng/mL has been suggested by some studies as the threshold to prompt suspicion for PE[70,72]. The Dutch National institute of Public Health recommends routine D-dimer testing on admission and serial testing during hospital stay. If initial D-dimers are < 1000 μg/L and a significant increase to > 2000-4000 occurs, imaging for deep venous thrombosis or PE should be pursued[43].
Apart from the D-dimer trend, other clinical factors that should prompt a CTPA are:worsening hypoxia not explained by the extent of lung involvement, hemoptysis,tachycardia, deep venous thrombosis, and acute deterioration upon mobilization[56].Presence of kidney disease should not preclude investigation with CTPA, as no significant increase in the risk for acute kidney injury (AKI) has been shown in patients receiving iodinated contrast compared to controls[74]. Dual-energy CT is useful in visualizing perfusion abnormalities, even in the absence of PE. It can demonstrate perfusion defects within lung opacities, and halos of increased perfusion surrounding consolidations[75], although the significance of these findings has not been determined. The use of pulmonary scintigraphy has been discouraged[76]. Due to the risk of aerosolization with the ventilation component of a V/Q scan, perfusiononly scans have been performed when clinically mandated since the onset of this pandemic, which may lack specificity. Combining Q- SPECT with a low-dose CT has been shown to increase the diagnostic performance of the perfusion scan, achieving higher accuracy than planar V/Q[77,78]. Optical coherence tomography may provide a novel means of assessing for microvascular thrombosis in patients with elevated Ddimer levels and a negative CTPA (NCT04410549).
CT scanning protocols:There is a paucity of guidance regarding the optimal CT techniques and protocols for patients with suspected or proven COVID-19 pneumonia.The Fleischner Society guidelines do not provide recommendations regarding scanning protocols and the need for dose-reduction.
A single-phase, unenhanced chest CT performed with volumetric acquisitions in deep inspiration and a < 3 mm thickness is preferred[79]. Expiratory phase is not considered of value as air trapping has not been associated with the acute phase of COVID-19 pneumonia[10]. Motion artifacts may be present in patients who are short of breath or have cough. Faster scanning by means of faster gantry rotation time and higher pitch can prevent suboptimal imaging[80]. High-resolution CT is not required unless there is concern for interstitial lung disease; it may, however, play a role on follow up to characterize fibrosis.
There is no value in obtaining post-contrast images as the findings of uncomplicated COVID-19 are confined to the lung parenchyma. Contrast-enhanced CT is justified when assessing for complications (e.g., abscess, necrotizing infection) or other diagnoses (such as PE or aortic dissection). If contrast-enhanced imaging is needed,there is no need for pre-contrast imaging[81].
A study collected data of CT acquisition protocols and dosimetry across 54 healthcare centers worldwide. It demonstrated wide variations in the median volumetric CT Dose index (CTDIvol) and in the median dose length product[82]. It also showed that 30% of COVID-19 patients underwent 2-8 chest CT examinations within one month[82]. Even though the majority of patients affected by COVID-19 pneumonia are adults and the risk for radiation-induced cancer in this demographic is low, there is a tendency to reduce the overall radiation burden. Low-dose protocols have been recommended for COVID-19 patients by very few studies, with a diagnostic quality comparable to that of standard protocols. A dose reduction of up to 90% has been reported, without significant reduction in the signal-to-noise or contrast-to-noise ratios[83,84]. Spectral shaping with a tin filter has been applied to reduce radiation dose[84]. Low tube voltage (≤ 100 kV) and low tube current are desired for low-dose scanning. Automatic tube current modulation technique is preferred as it accounts for body habitus. Iterative reconstruction can further reduce radiation dose. A target of CTDIvol less than 3 mGy should be selected. A multicenter study revealed that only 1 out of 28 countries reported a median CTDIvol of less than 3 mGy, indicating that lowdose imaging has not been broadly adopted yet[82]. Whether the use of low-dose CT should be a standard for baseline imaging or for follow-up of COVID-19 cases has yet to be determined.
Chest radiography
Chest X-ray (CXR) is the most widely used imaging modality in the workup of patients presenting with respiratory symptoms. It is a cost-effective, widely available examination that is easy to repeat sequentially to monitor disease progression or to evaluate for alternate diagnoses. Portable CXR has been favored as it can be performed on the bedside in isolation rooms, minimizing the risk for transmission[32]. However,routine daily CXR are not recommended in stable intubated patients according to Fleischner Society guidelines[35].
Chest radiography has low sensitivity in the early stages of the disease, as initial imaging can be normal[85]. Diagnostic accuracy increases 6 d after symptom onset[85]. Sensitivity and specificity of CXR in detecting COVID-19 pneumonia vary greatly in the literature, ranging between 57%-89% and 11%-89% respectively and, therefore,its real diagnostic performance is unknown[29].
Similar to CT, findings on CXR reflect the various stages of COVID-19 pneumonia.The typical findings are multifocal, usually bilateral, GGOs and/or consolidations in a peripheral distribution. Lung opacities may coalesce creating a diffuse pattern,peaking at 6-12 d from symptom onset. Reticular opacities may be seen accompanying the GGO. Pleural effusions, cavitations and pneumothorax are considered atypical for COVID-19 pneumonia[4,86,87].
Structured reporting, similar to CO-RADS for CT, has been proposed for chest radiography as well[56]. In a mixed cohort of 582 suspected COVID-19 cases, patients with “characteristic” or “highly suspicious” features on CXR had an 88% probability of having a positive RT-PCR. “Characteristic” pattern included bilateral subpleural opacities, relatively symmetrical, involving > 20% of the lung, predominantly in the outer 1/3 of the lung parenchyma. Highly suspicious findings included unilateral subpleural opacities involving > 20% of the lung, or bilateral large-volume patchy or ill-defined opacities[88].
Severity scores quantifying lung opacities on CXR have been used to predict patient outcomes and track disease progression. The most widely used scoring system is the radiographic assessment of lung edema (RALE) score, whereby each lung is divided in upper and lower parts, and each part is graded based on the extent (0 = no involvement, 1 = < 25%, 2 = 25%-50%, 3 = 50%-75%, 4 = > 75%) and density (1 = hazy,2 = moderate, 3 = dense) of opacities. The 2 scores for each segment are multiplied and the final scores of all segments are added[89]. A modified version of the RALE score assigns a score to each lung as a whole instead of dividing the lung in two segments[90]. Higher RALE scores in patients with COVID-19 pneumonia have been associated with higher probability of ICU admission, MV, and death[85].
US
US of the chest is a widely used modality in the emergency department, ICU or wards as it can be performed in a portable manner at the patient’s bedside, limiting the need for patient transport. US is used to assess the extent and severity of lung disease, and to monitor disease progression and treatment response[91]. Given the predilection of COVID-19 pneumonia for the lung periphery, findings are often within the US beam’s reach. Deeper lesions, however, cannot be identified as the aerated lung parenchyma impedes acoustic waves. Performance of US is also limited in obese patients. A convex or linear probe with a bandwidth of 2-6 MHz with the depth set at 15cm and a single focal point on the pleural line is used to scan all intercostal areas[92,93]. Pocket-sized handheld US probes have also been utilized due to their easier handling, with similar results[94].
The reported sensitivity and specificity of lung US in COVID-19 pneumonia ranges between 89%-97% and 59%-62%, respectively[95,96]. Studies, however, often exclude patients with known heart failure or interstitial lung disease and, therefore, values are likely overestimated[93]. Diagnostic performance of US is higher in severe disease[97].It is considered superior to CXR in detecting lung lesions and has a good correlation with findings on CT[56,91,98]. The lack of non-ionizing radiation makes lung US a favorable imaging modality in pregnant or pediatric patients[32,93]. Its diagnostic performance and prognostic value are topics of ongoing research (NCT04353141,NCT04513210, NCT04338568).
Specificity of lung US is poor due to overlap with other pathologies. A commonly encountered US manifestation are the B-lines, which are vertical echogenic comet-tail artifacts originating from the pleura and extending to the bottom of the screen. They represent thickened subpleural interlobular septa (e.g., interstitial pneumonia, fibrosis)or fluid-filled alveoli (e.g., cardiogenic or non-cardiogenic pulmonary edema)[99]. In early mild-moderate disease, discrete scattered B-lines are observed, along with small(< 1 cm) consolidations. Consolidations appear as subpleural hypoechoic areas with an irregular border (shred sign) and a “white lung” pattern posteriorly. As the disease progresses, the B-lines coalesce and become more confluent and multifocal. The consolidations increase in size, and the pleural lines becomes irregular and thickened[93,100]. In critical disease consolidations may assume a tissue-like pattern. During recovery, consolidations and B-lines gradually disappear, while normal horizontal reverberation artefacts (A-lines) become prominent. Absence of lung sliding indicates pneumothorax. Pleural effusions are usually absent. Various scoring systems have been suggested to quantitate the severity of findings[91].
MRI
Though not a first-line modality, MRI of the chest may be considered when exposure to ionizing radiation should be avoided, such as in pregnant or pediatric patients, or in patients with an increased overall radiation burden due to frequent serial imaging[101]. T2 sequences that have been used in published studies include: HASTE (halffourier acquisition single-shot turbo spin echo), TSE (turbo spin echo), FISP (fast imaging with steady-state precession), TIRM (turbo inversion recovery magnitude)[102]. Lung infiltrates are characterized by increased proton density and therefore demonstrate high signal intensity. There is a high concordance with CT in the assessment of the typical lung findings (GGOs, consolidations, reticulations)[102].However, due to the lower tissue resolution, more detailed findings such as airbronchograms and crazy-paving cannot be readily demonstrated[103]. Chest MRI has a limited practical role due to its considerably higher cost and due to various artifacts that may limit the diagnostic quality (e.g., cardiac and respiratory motion artifacts, low proton density of lung parenchyma, susceptibility artifacts, fast T2* decay)[104].Ultrashort echo-time (UTE) and respiratory-gating have been shown to curb some of the limitations[103]. The value of chest MRI will be further elucidated by several ongoing trials. (NCT04424355, NCT04369807, NCT04510025).
Positron emission tomography/CT
Positron emission tomography/CT (PET/CT) is a highly sensitive modality that can demonstrate metabolically active end-organ damages caused by SARS-CoV-2. It also offers a quantifiable assessment of disease progression and treatment response[48].Due to its low specificity, PET/CT has a limited added benefit in the diagnostic process and may impose the nuclear medicine personnel at risk of infection due to the prolonged acquisition times[104]. Increased activity of pulmonary lesions has been shown with multiple radiopharmaceuticals, including18F-FDG (fluorodeoxyglucose),18F-chlorine,68Ga-PSMA (prostate-specific membrane antigen). The incidental detection of hypermetabolic areas in the lungs of asymptomatic SARS-CoV-2-positive patients shows the potential of FDG-PET to detect early parenchymal changes and prevent disease spread[104]. A systematic review reported a mean SUV of 4.9 ± 2.3 in pulmonary lesions on18F-FDG PET[105]. Increased18F-FDG uptake in mediastinal and hilar lymph nodes has been observed, in the absence of enlargement by CT size criteria[105]. PET/CT using18F selectively binding to the ανβ6 integrin binding protein will be used in future studies to determine the degree of fibrosis in patients with active or resolved COVID-19 pneumonia (NCT04376593, NCT03183570).
EXTRAPULMONARY MANIFESTATIONS
Cardiac manifestations
The vulnerability of cardiac tissue to SARS-CoV-2 has been alarming given the potentially dire consequences. SARS-CoV-2 can cause acute cardiac injuryviamultiple mechanisms: direct ACE-2 mediated myocardial cell damage, hypoxic vasoconstriction-mediated myocardial ischemia and microvascular damage[106]. Cardiac complications are seen in 20%-30% of hospitalized patients with COVID-19 and are associated with high mortality rates reaching 37%[107,108]. Patients with more extensive lung opacities, cardiovascular comorbidities and older age are more prone to myocardial injury[107]. Cardiac complications (including myocarditis, arrhythmia,cardiomyopathy, cardiogenic shock, and cardiac arrest) account for 7% of deaths in COVID-19 patients[109]. Patients who died of COVID-19 had significantly elevated levels of high-sensitivity Troponin I[6]. Troponin elevations have been associated with elevated C-reactive and pro- B-type natriuretic peptide levels, suggesting an interplay between myocardial injury and systemic inflammation[108,110].
Imaging plays a significant role in the early diagnosis of cardiac abnormalities and their potential complications. Echocardiography is a first-line modality in the work-up of patients with suspected cardiac injury. It is an invaluable bedside tool that can reveal structural and functional damage, such as wall-motion abnormalities, chamber dilation, valvular disease, pericardial effusion, decreased ejection fraction and cardiac thrombi. Right ventricular systolic dysfunction may indicate PE or pulmonary hypertension. Transesophageal echocardiography is considered an aerosol-generating procedure and therefore should be avoided during the pandemic, unless there is an absolute indication such as suspected endocarditis[111].
Myocarditis is a potentially threatening complication that can present in a wide spectrum of ways, ranging from mild disease to fulminant heart failure and cardiogenic shock[112]. Although invasive, endomyocardial biopsy is the gold standard for the diagnosis of myocarditis and should not be delayed in suspected cases. Cardiac MRI (cMRI) is the most sensitive imaging modality in detecting myocardial injury. Late gadolinium enhancement (LGE) has high sensitivity in detecting areas of myocardial fibrosis or necrosis and can also have prognostic value as it is associated with worse outcomes[113]. A typical cMRI protocol includes a shortaxis CINE sequence for size and functional assessment, T1/T2 mapping for edema assessment, a delayed post-contrast scan for scar assessment and a T2 sequence.Certain modifications have been proposed to abbreviate the CMR protocol and decrease acquisition times[114].
Imaging manifestations of myocarditis on cMRI include diffuse myocardial edema,pseudo- wall hypertrophy, non-ischemic pattern of LGE, and increased signal on STIR,T1 mapping and T2 mapping. Regional or global wall-motion abnormalities may be present. cMRI aids in the differentiation from alternate diagnoses, such as myocardial infarction (regional wall-motion abnormalities, LGE in sub-endocardial or transmural distribution), Takotsubo cardiomyopathy (diffuse wall edema without arterial territory distribution, transient apical dyskinesias/akinesis, mild LGE only in areas of wall-motion abnormalities) and myocardial infarction with non-obstructive coronary arteries (MINOCA) (edema in non-coronary pattern). cMRI is applied by several ongoing trials for the evaluation of long-term myocardial damage 1-2 years after recovery from COVID-19 (NCT04375748, NCT04625075, NCT04636320, NCT04661657).
Contrast-enhanced cardiac CT (CCT) with electrocardiographic gating is a valuable alternative when cMRI is not feasible, with the advantage of shorter scanning times[115]. Unenhanced, early and delayed post-contrast scans, as well as extracellular volume mapping, are required for the diagnosis of myocarditis. CCT can rule out coronary artery disease in patients with acute chest pain without ST-elevation, and can assess for possible myocarditis, pericarditis, cardiomyopathy or left atrial thrombus[114].
CXR has low sensitivity and specificity in detecting cardiac injury. It may reveal pulmonary edema superimposed on lung opacities, cardiomegaly in the setting of cardiomyopathy, and pericardial effusion.
Abdominal manifestations
The abundance of ACE-2 receptors in epithelial cells along the gastrointestinal (GI)tract, in cholangiocytes and in the intraabdominal vasculature explains the multifaceted abdominal implications of SARS-CoV-2[116,117]. The pooled prevalence of symptoms from the GI tract is 15%-18%[118,119]. Most common complaints are vague abdominal pain, nausea, vomiting, diarrhea and appetite loss. Abdominal complaints may be present even in the absence of pulmonary symptoms and may be the sole complaint in 16% of COVID-19 patients[119-121]. The prevalence of GI symptoms was 12% in patients with non-severe COVID-19 and 17% in patients with severe disease, a difference that was not statistically significant[118]. No increase in mortality has been observed in patients with abdominal manifestations[119].
In a study of 1057 patients with COVID-19 and GI complaints, abdominal CTs were warranted in just 4%, the majority of which (63%) showed no acute abnormality[120].Bowel wall abnormalities have been observed in 3% of hospitalized patients with COVID-19[122]. On contrast-enhanced CT, enterocolitis can manifest as small or large bowel wall thickening with mucosal enhancement, fluid-filled bowel lumen and mesenteric inflammation[106]. The presence of non-enhancing bowel wall,pneumatosis intestinalis or portal venous gas is suggestive of ischemia and bowel infarction. Discontinuity of bowel wall and pneumoperitoneum indicate bowel perforation[106,123]. The etiology of acute mesenteric ischemia is multifactorial. It may occur as a result of hypercoagulability, direct viral-mediated damage on the bowel,and hemodynamic compromise due to shock[123]. Patients may present with abdominal pain, nausea, vomiting, diarrhea, or more severe symptoms suggestive of sepsis. CT angiography is the imaging of choice as it can readily detect filing defects in the mesenteric vasculature. However, non-occlusive ischemia may occur secondary to systemic vasodilation and intestinal hypoperfusion in the setting of sepsis (Figure 6).
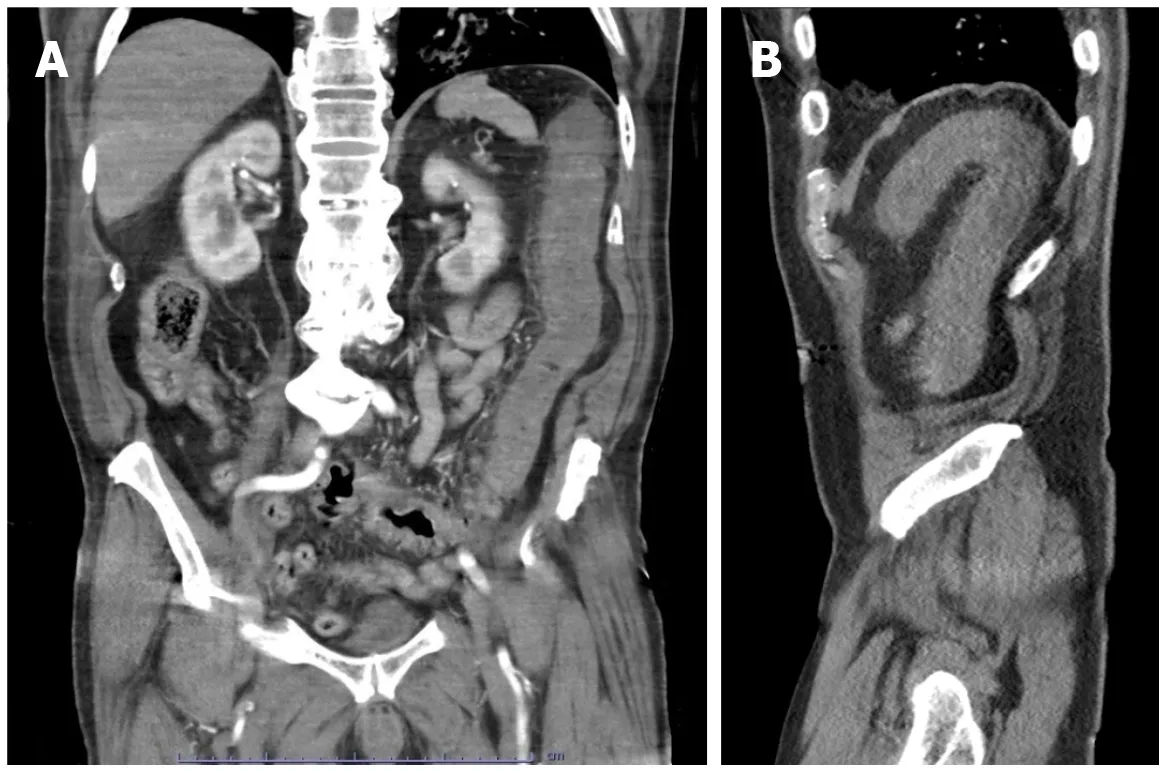
Figure 6 Acute ischemic colitis. A and B: Coronal (A) and sagittal (B) reconstructed images from a computed tomography angiogram of the abdomen and pelvis in an 80-yr-old male admitted for sepsis and lactic acidosis. There is bowel wall thickening and hypoenhancement involving the descending colon, with surrounding inflammatory changes. There is no evidence of pneumatosis coli, pneumoperitoneum or proximal vessel occlusion. Bilateral ground-glass opacities were visualized at the lung bases (not shown). The patient was positive for severe acute respiratory syndrome coronavirus 2.
Hepatobiliary manifestations are more common in patients with severe disease[119]. Liver function tests may be abnormal in 37% of hospitalized patients and are associated with a longer hospital stay[124]. Cholestasis has been observed in up to 54%of hospitalized patients with COVID-19[122]. It may manifest as a sludge-filled gallbladder and intra- and extrahepatic biliary ductal dilation in the absence of an obstructing gallstone or mass. Biliary stasis can predispose to acute cholecystitis. As a result of the prothrombotic state induced by SARS-CoV-2, hepatic or portal venous thrombosis may occur, which can manifest as absent flow on color Doppler or as filling defects on contrast-enhanced CT. Pancreatic inflammation has been described in patients with COVID-19 and is thought to occur secondary to direct cytotoxicity of the virus or systemic inflammatory response[125]. US has low sensitivity for pancreatitis;it may, however, demonstrate an enlarged pancreas with decreased echogenicity and blurred margins. On CT, the pancreatic parenchyma has an edematous and hypoattenuating appearance, with associated peripancreatic fat stranding[106]. Necrotizing pancreatitis in the setting of COVID-19 is uncommon[125].
Renal damage occursviavarious mechanisms including direct endothelial and podocyte injury, glomerular injury by immune complexes, capillary obstruction by aggregated erythrocytes, and disruption of the renin-angiotensin-aldosterone system[126]. AKI is a frequent complication of COVID-19 occurring in up to 37% of hospitalized patients[127]. Electrolyte imbalances (primarily hyperkalemia and alkalosis),hematuria and proteinuria can be observed even in patients without AKI[116]. On US,AKI may manifest as cortical echogenicity and loss of corticomedullary differentiation.Focal areas of decreased vascularity on color Doppler indicate renal infarcts. Contrastenhanced CT may demonstrate infarcts as wedge-shaped areas of hypoenhancement involving both the cortex and medulla as well as renal vascular thrombosis[106].Infarcts, either single or multifocal, can also occur in the spleen secondary to microangiopathy, hypercoagulopathy or thromboembolism[128]. The extent to which contrastenhanced US can demonstrate microvascular perfusion deficits in patients with COVID-19 is under investigation (NCT04640038).
Central nervous system manifestations
The neurotropism of SARS-CoV-2 accounts for some of its potentially fatal sequela, as evidenced by the presence of viral RNA in brain tissues of deceased patients[129].SARS-CoV-2 enters the central nervous system (CNS)viathe hematogenous or the transneuronal route, causing neurological damageviamultiple mechanisms.Endothelial cell injury can cause disruption of the blood-brain barrier, facilitating the penetration of the virus into the CNS as well as the crossing of immune cells and cytokines. The direct micro- and macrovascular injury, combined with the prothrombotic state, can lead to ischemic phenomena. Moreover, prolonged hypoxia and acidosis associated with ARDS can promote cerebral vasodilation and edema[130,131].
Up to 12% of hospitalized patients with COVID-19 undergo neuroimaging[132]. The most common neurological symptoms are: anosmia, ageusia, altered mental status,headache, dizziness and focal neurological deficits[116]. Patients with severe disease are more likely to develop neurological abnormalities[133]. Among critically-ill patients with COVID-19, neurological symptoms were observed upon admission to the ICU in 14% and upon weaning from sedation in 67%[134]. Patients with altered mentation were more likely to be hypotensive, hypoxic, and have elevated creatinine,D-dimers and inflammatory markers, suggesting an interplay between neurological damage and multi-system failure[132]. Neuroimaging may be revealing in up to 23%of patients; however, none of the reported abnormalities is specific for COVID-19[135].
Three non-specific imaging patterns of leukoencephalopathy have been observed in patients with severe COVID-19[136]. A commonly reported finding pertains to signal abnormalities in the mesial temporal lobe seen in up to 43%[136]. These are characterized by hyperintensity on fluid-attenuated inversion-recovery (FLAIR) and diffusion-weighted imaging (DWI), which may also be seen in infectious (e.g., herpes simplex virus) or autoimmune encephalitis (Figure 7). Another pattern involves multifocal supratentorial white matter lesions that are hyperintense on FLAIR and DWI and may be seen in 30% of critically ill patients. These may be related to postinfectious demyelination secondary to the hypoxic-ischemic damage of oligodendrocytes. Other potential causes include delayed post-hypoxic leukoencephalopathy, metabolic or toxic encephalopathy, and posterior reversible encephalopathy syndrome[135]. White matter lesions may be associated with microhemorrhages,resembling acute disseminated encephalomyelitis or acute hemorrhagic leukoencephalitis[137,138]. Isolated, yet extensive, microhemorrhages in the subcortical and deep white matter may also be seen in 24% of patients, in a pattern similar to diffuse intravascular coagulation. This finding has been attributed to hypoxia or small-vessel vasculitis[137]. The splenium of the corpus callosum is one of the predominantly affected areas[135]. There are rare reports of acute necrotizing encephalopathy which presents as rim-enhancing lesions in the thalami, temporal lobes and subinsular regions[137]. Leptomeningeal enhancement suggestive of meningoencephalitis is frequently seen[138].
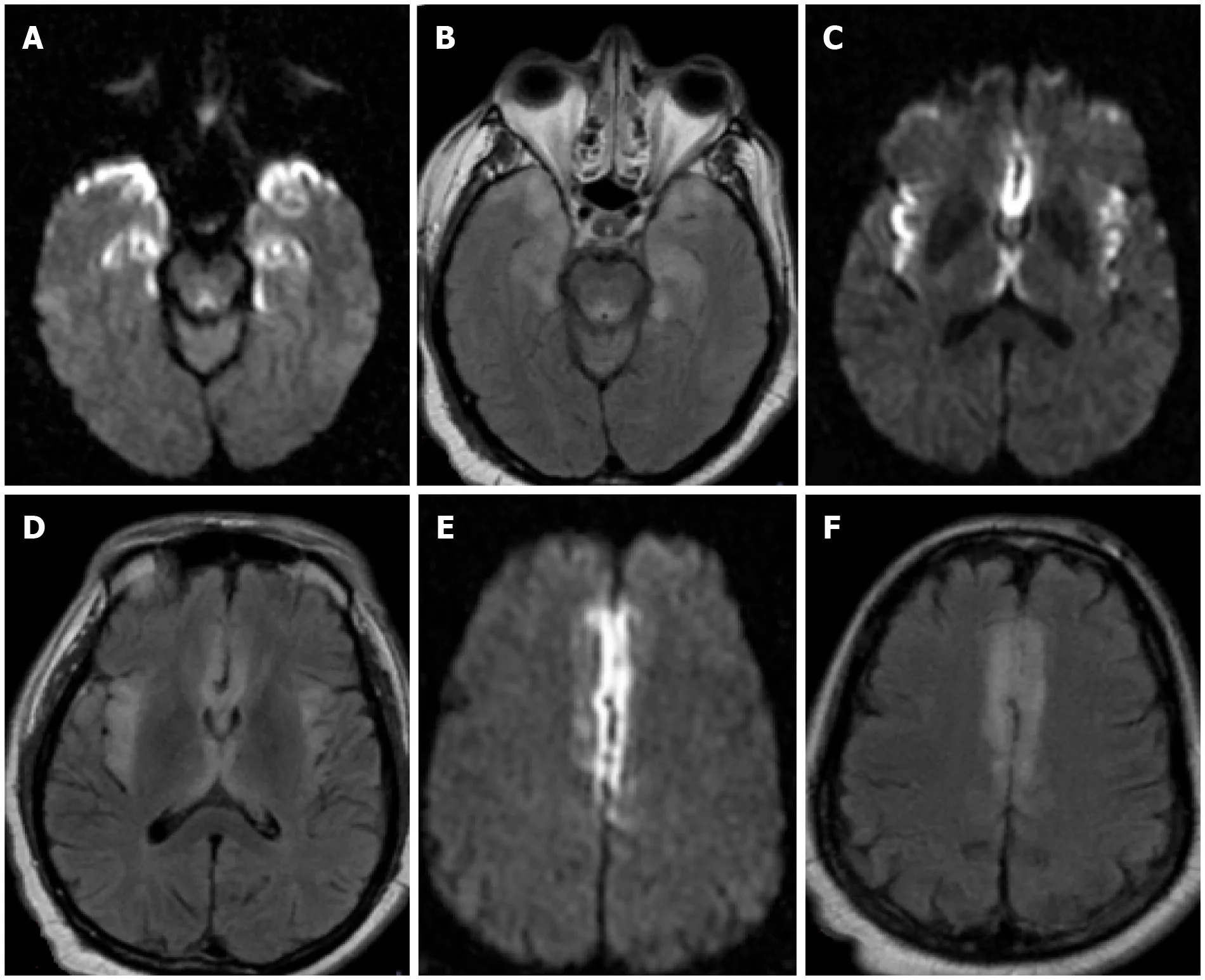
Figure 7 Encephalopathy. A-F: Axial DWI (A, C and E) and FLAIR (B, D and F) sequences from a brain MRI in a 49-year-old - male who was admitted for acute hypoxic respiratory failure secondary to severe acute respiratory syndrome coronavirus 2. A brain MRI was ordered 3d after presentation for progressive lethargy.There were multifocal symmetric areas of restricted diffusion and T2/FLAIR prolongation in bilateral mesial temporal lobes (A and B), insular cortex (C and D), and cingulate cortex (E and F). Cerebrospinal fluid analysis was negative. The patient’s mental status gradually returned to baseline after medical management. Findings were attributed to COVID-19 - related encephalopathy.
Acute ischemic infarcts have been reported in 9% of patients with neurological symptoms and in 1% of all hospitalized patients with COVID-19, even in the absence of underlying risk factors. Among ICU patients undergoing neuroimaging, acute infarcts were identified in 23%[134]. The occurrence of a stroke in COVID-19 patients has been associated with a higher mortality rate [132]. An unenhanced CT of the head is usually the first-line imaging modality, as it can identify acute infarcts (ischemic,embolic or venous), large vessel occlusion, hemorrhagic transformation, and venous sinus thrombosis[106]. Abbreviated MRI protocols with DWI, apparent diffusion coefficient mapping and T2/FLAIR have been recommended for the definitive assessment of infarcts in order to decrease acquisition times[106].
Long-term neurologic morbidity after recovery from SARS-CoV-2 has yet to be determined. Several ongoing trials will examine the presence of structural and cognitive impairment in patients who suffered from acute neurological damage related to COVID-19 infection (NCT04564287, NCT04476589).
Peripheral nervous system and ocular manifestations
Rare cases of acute polyneuropathy in the spectrum of Guillain-Barré syndrome (GBS)have been reported as a result of SARS-CoV-2 infection. Symptoms reportedly occur within 8-24 d after the onset of respiratory symptoms[139]. The most frequently reported manifestation is the classic form of GBS (acute inflammatory demyelinating polyradiculoneuropathy) which is characterized by ascending sensorimotor deficits,with varying degrees of facial nerve involvement, dysphagia and dysautonomia.Other less common variants include the Miller Fisher syndrome (characterized by opthalmoplegia, ataxia, areflexia), pure motor or pure sensory variants, bilateral facial palsies, the pharyngeal-cervical-brachial motor variant, and others[140]. MRI can reveal thickening of the affected nerve roots and avid contrast enhancement of the conus medullaris and cauda equina, with preferential enhancement of the ventral nerve roots[141]. The fact that viral RNA has not been identified on cerebrospinal fluid analysis in affected patients suggests that injury occursviaan immune-mediated mechanism, such as molecular mimicry or antibody precipitation, rather than by direct viral insult[142]. A similar mechanism has been proposed for rare cases of new-onset myasthenia gravis developing in the setting of COVID-19[143].
Patients with severe disease and a prolonged stay in the ICU are at risk for critical illness polyradiculopathy and myopathy, which is characterized by degeneration of sensory and motor axons. The pathophysiology for this disease has not been elucidated yet, but it is thought to involve microvascular alterations, metabolic abnormalities, ion-channel dysfunction[144]. It may lead to ventilator dependence and chronic disability, which are associated with high morbidity rates[145]. Positioning maneuvers in the ICU may also affect the peripheral nerves by causing compression or entrapment[140].
Cranial nerves can also be affected by the immune dysregulation propagated by the viral infection. Anosmia and ageusia occur in up to 88% of patients, even in the absence of upper or lower respiratory symptoms[146]. SARS-CoV-2 causes a direct viral insult to the nasoepithelial cells by directly attaching to olfactory and gustatory receptors, potentially creating a route for retrograde entry into the CNS. Olfactory cleft widening and FLAIR hyperintensities in bilateral olfactory bulbs have been observed,which resolved after recovery of the acute illness[147,148]. Other cranial neuropathies,either single or multiple, have also been reported, which manifest on MRI with signal hyperintensity on T2 and enhancement on post-contrast images[140,149].
As a mucosal surface, the conjunctiva can be exposed to respiratory droplets and act as a potential port of entry for SARS-CoV-2. Ocular manifestations occur in up to 7% of COVID-19 patients, with conjunctivitis being the most prevalent[150]. Less frequently,retinal abnormalities may occur as a result of the microangiopathic damage caused by the virus, which can lead to ischemia[151]. Conditions related to ICU stay (including sedation, MV, neuromuscular blockade and prone positioning) may potentiate keratopathy, acute angle-closure, ischemic optic neuropathy and retinal vascular occlusion[152,153]. A study revealed the incidental presence of nodules on the posterior pole of the globes in patients with severe COVID-19 undergoing brain MRI for altered mental status. The nodules were located in bilateral macular regions, were T2/FLAIR hyperintense, non-enhancing and showed no susceptibility artifacts. No correlate was identified on fundoscopy or optical coherence tomography. The nature of these nodules remains unclear[154].
Musculoskeletal and cutaneous manifestations
Myalgia is a fairly common constitutional symptom that is present in up to 44% of SARS-CoV-2-infected patients upon presentation[155]. It stems from intramuscular inflammation and is typically self-limiting. More severe viral myositis manifesting with pain, tenderness, weakness and elevated creatine kinase may occur in a small percentage of patients. Rhabdomyolysis is a potentially life-threatening complication caused by the breakdown of muscular tissue, which may lead to AKI, compartment syndrome or superimposed infection[141].
Damaged muscles appear enlarged and hypoattenuating on CT. Rim-enhancement may be seen, although intravenous contrast is avoided. Intramuscular calcifications may be seen in the subacute and chronic phase. MRI is the preferred imaging modality as it can distinguish two different types of rhabdomyolysis based on the presence or absence of myonecrosis. In type 1, there is homogeneously increased signal intensity on T2/STIR representing edema, as well as homogeneous hyperenhancement.Increased T1 signal indicates the presence of methemoglobin. In chronic rhabdomyolysis, focal T1 hyperintensity or blooming artifact on susceptibility weighted imaging may be present indicating hemosiderin deposition. In type 2, there is heterogeneously increased signal intensity on T2/STIR, as well as non-enhancing necrotic areas. Rim enhancement may be present in subacute myonecrosis and should not be mistaken for an abscess[106,141,156].
Viral arthritis presents with acute arthralgia that is self-limiting and responds to non-steroidal anti-inflammatory medications. Other causes of arthritis, such as reactive or crystalline arthritis, should be considered in the differential as they may present with similar symptoms. Rare cases of acute exacerbations of chronic rheumatologic diseases (such as systemic lupus erythematosus and rheumatoid arthritis) have been reported. MRI may demonstrate thickened and hyperenhancing synovium, as well as features specific for the underlying autoimmune process[141].
Skin involvement has been reported in up to 8% of patients with SARS-CoV-2[157].Microthrombi, small-vessel vasculitis, immune response and drug reaction (secondary to remdesivir, toclizumab, hydroxychloroquine,etc.) are some of the suspected mechanisms of injury[116]. The most common manifestation is an acrocutaneous lesion similar to chilblain or frostbite. Other possible phenotypes include: uriticarial rash, maculopapular rash, papulovesicular rash, livedo reticularis and purpuric rash[158]. Dry gangrene has been reported in severe cases, likely exacerbated by pressors and coagulopathy in vulnerable patients with diabetes mellitus or peripheral arterial disease. Imaging may reveal skin ulcerations, high signal intensity on T2 and lack of enhancement[106]. Acute soft tissue hematomas may develop secondary to disordered coagulation. They appear as heterogeneous hypoechoic collections on US, and, if large,they may cause compartment syndrome and compressive neuropathy[141].
PEDIATRIC MANIFESTATIONS
There is evidence that children of all ages are susceptible to SARS-CoV-2[159].Pediatric patients are more likely to have a milder disease course or to be asymptomatic carriers compared to adults, likely due to immature immune response and ACE-2 receptors[5,160,161]. Fever and cough are the most common complaints in symptomatic children[162].
There is no indication for routine chest imaging in children with suspected COVID-19 infection, due to the high rate of false negative examinations[162]. If imaging is clinically warranted, chest radiography is the first-line modality. CT should be reserved for more complex cases, suspected complications or to rule out alternative diagnoses, particularly in children with underlying medical conditions[163]. No imaging differences have been demonstrated among age groups[161]. Bilateral GGOs,pure or mixed with consolidations, in a peripheral/subpleural distribution are the predominant findings on chest imaging in the pediatric population[164,165]. Crazypaving pattern and halo signs are also observed indicating a common response to acute lung injury[166]. Airway inflammation, as evidenced by peribronchial thickening has been very frequently observed in pediatric patients[164]. Pleural effusion and lymphadenopathy are atypical. PE is significantly less prevalent than in the adult population. Lung US is being increasingly applied due to the lack of ionizing radiation and higher sensitivity compared to CXR[167,168].
Although the risk of severe illness is significantly lower than in adults, critical cases have been observed in the pediatric population, particularly in patients with underlying medical conditions[160,169]. The multisystem inflammatory syndrome in children (MIS-C) is a worrisome late complication that presents with multiorgan damage in children previously exposed to COVID-19. Its distinction from Kawasaki disease and toxic shock syndrome can be challenging. MIS-C is diagnosed based on clinical and laboratory criteria, such as those established by the WHO. These include:fever, rash, conjunctivitis, shock, end-organ damage (e.g., respiratory, cardiac, renal,neurological or GI), coagulopathy, elevated inflammatory markers, laboratory evidence of recent SARS-CoV-2 infection or contact with a known case, and absence of an alternative diagnosis[170]. Patients typically present with fever, rash, conjunctivitis,vomiting, diarrhea, abdominal pain mimicking appendicitis. Shock may be present on admission in 60% of patients[171]. Although 71% of children may require management in the ICU, mortality rates are relatively low (1.7%)[172].
In patients with MIS-C, chest imaging may reveal bilateral airspace opacities,peribronchial thickening, interstitial thickening, cardiomegaly, pulmonary edema and pleural effusions. 46% of patients may have normal CXRs. CCT may demonstrate myocarditis, pericarditis and coronary aneurysms. Bowel wall thickening involving the terminal ileum or the cecum accompanied by mesenteric inflammation is present in 23% of patients (Figure 8). These findings most likely represent bowel ischemia secondary to small vessel vasculitis or shock. Other findings on abdominal imaging include small-volume ascites, lymphadenopathy, periportal and pericholecystic edema and a normal appendix[171,173]. Although most patients recover after the acute phase with medical management, the long-term morbidity remains unclear. Future studies will attempt to identify the potential long-term complications by prospectively following patients for 2-5 yearsafter recovery from MIS-C (NCT04455347,NCT04757831).
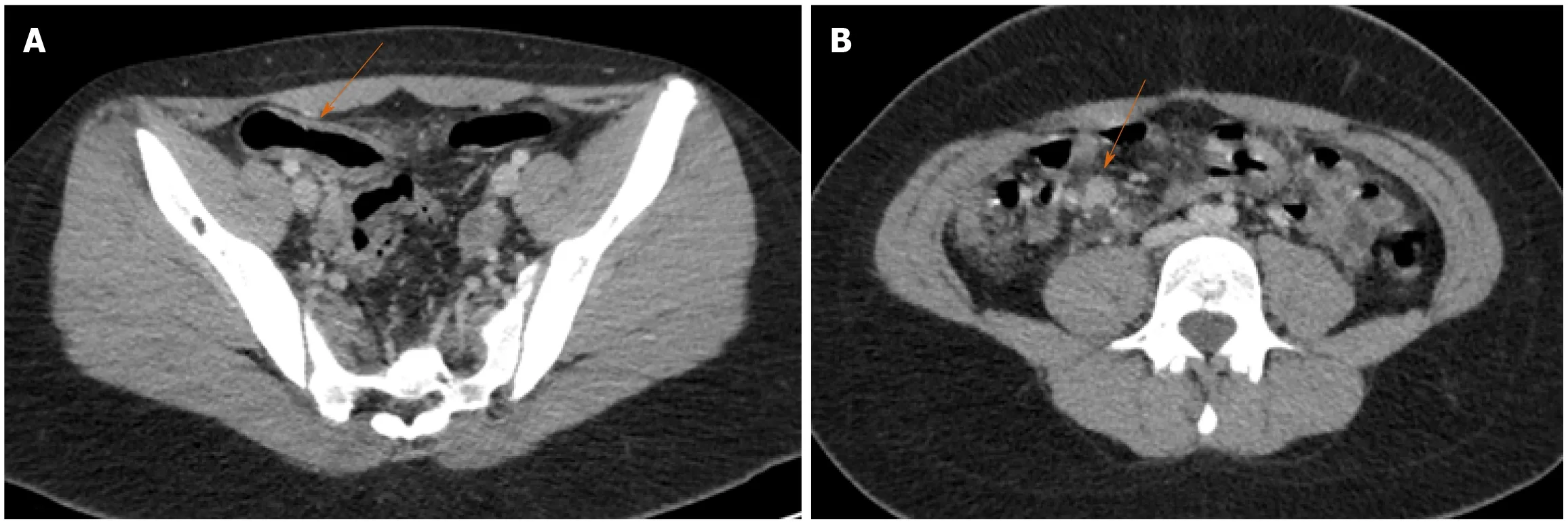
Figure 8 Terminal ileitis in the setting of multisystem inflammatory syndrome in a child. Axial images of a contrast-enhanced computed tomography of the abdomen and pelvis in a 13-yr-old female who presented with a 4d history of abdominal pain. A: There is wall thickening in the terminal ileum with adjacent fat stranding; B: Multiple reactive lymph nodes are seen in the right lower quadrant. Clinical and laboratory findings met criteria for multisystem inflammatory syndrome in children. Of note, the patient had tested positive for severe acute respiratory syndrome coronavirus-2 4 wk prior to presentation. The patient recovered after medical treatment including intravenous immunoglobulin, prednisone and dopamine.
INFECTION CONTROL IN RADIOLOGY DEPARTMENTS
Since the onset of the pandemic, drastic measures have been implemented by radiology departments in order to curb the transmission of SARS-CoV-2 within the hospital. As an initial step, the radiology staff underwent training by a team of infection control specialists regarding the safe handling of patients, use of PPE and disinfection of imaging devices[174]. The dramatic drop in case load as a result of the suspension of all non-urgent imaging examinations in the initial surge of the pandemic allowed for radiology departments to function with limited staff capacities. Division of staff into groups alternating between 2 wk of self-quarantine and 2 wk of on-site work was widely implemented. Remote working of radiologists was also encouraged[175].
Given the long processing times of RT-PCR, triaging of patients in the emergency department based on typical clinical symptoms and known exposure to SARS-CoV-2 was essential in order to provide timely isolation and implement appropriate protective measures. Temperature measurement was not a reliable triaging method as patients may be afebrile in the early phase of the disease[174]. Patients were classified into 3 categories: confirmed and highly suspected cases, suspected cases, and confirmed negative cases. Dedicated imaging rooms, waiting areas and routes of transportation were designated for suspected or confirmed cases. Different levels of PPE and disinfection were applied after interaction with each patient category[176].The use of portable imaging equipment (for radiography or US) has been preferred as it can be brought to the patient’s bedside and can be more easily disinfected[177].Scanning protocols were abbreviated so that the clinical question can be addressed while limiting the duration of the scan and the contact between staff and patients.
CANCER SCREENING DELAYS
Soon after the onset of the pandemic, multiple medical societies (including the American Cancer Society, the American Society of Breast Surgeons, the ACR and the American College of Chest Physicians) released statements suggesting the suspension of all non-urgent cancer screening studies in order to mitigate the spread of SARSCoV-2[178-180].
Between March and May 2020, a 39%-85% decrease in mammograms and a 52%decline in novel breast cancer diagnoses were observed in the United States compared to the previous year[16,181]. Similar rates were reported in other countries[182,183].Given that breast cancer screening can reduce mortality by 40% in females aged 50-69,cancer upstaging due to missed screening appointments could be a serious threat[184]. It is estimated that deaths from breast cancer could increase by up to 10% within the next 5 years[185]. Low-dose CT examinations for lung cancer screening dropped by 72%-78% between March and May 2020[186]. The mortality rate of lung malignancies is projected to increase by up to 5% in the next 5 years[185]. Hepatocellular carcinoma (HCC) surveillance in at-risk individuals by abdominal US, CT or MRI scans demonstrated a declining trend across two centers in the United States and Singapore[187]. Guidelines that were published during the early phase of the pandemic recommended that HCC screening should be limited to high-risk patients[188]. A marked drop was also observed in colorectal cancer screening tests[189]. It was suggested that CT colonography could be a substitute for colonoscopy during the early phase of the pandemic due to its shorter in-hospital stay, limited patient contact and lower risk of complications[190].
As a result of the universal cancer screening interruptions, a large backlog of cancelled appointments emerged. As the first pandemic wave subsided, healthcare institutions implemented a phased reopening, prioritizing patients with acute complaints suspicious for disease progression, those with newly diagnosed cancer and those undergoing treatment[191]. Despite the fact that hospitals returned to their prepandemic capacity, patients were reluctant to reschedule their appointments due to fear of exposure within the hospital or due to lack of health insurance and unemployment[192]. Outreach programs have made significant efforts to address patients’ concerns and to facilitate their access to screening facilities, aspiring to limit the long-term repercussions of these unprecedented screening delays[193].
The emergency authorization of vaccines against SARS-CoV-2 in late 2020 initiated a widescale vaccination program, prioritizing the vulnerable populations and the healthcare workers. As of June 4, 2021, 447.9 million people have fully vaccinated and 424 million people have been partially vaccinated globally[194]. Vaccine-induced axillary lymphadenopathy following COVID-19 vaccination has been reported by multiple studies and is thought to occur at a higher rate compared to other vaccines such as the influenza vaccine[195]. The presence of post-vaccine axillary lymphadenopathy on imaging studies may lead to false-positive results and may provoke unnecessary anxiety especially in patients undergoing cancer screening or surveillance[196,197].
To address the issue, radiological societies have released statements regarding cancer screening. The Radiology scientific expert panel recommends that routine screening imaging studies be scheduled at least 6 wk after the final dose of the vaccine.In patients with cancer history, the vaccine should be administered on the contralateral side of the primary or suspected cancer or in the thigh[198]. If axillary adenopathy is present on a low-dose CT for lung cancer screening, the “S” modifier should be added on the Lung-RADS reporting system and no further imaging should be pursued[199].Based on the Society of Breast Imaging, the presence of unilateral axillary lymphadenopathy should warrant a BI-RADS 0 and prompt further assessment and documentation of the patient’s vaccination history. In women with a recent (4-wk)history of vaccination, short-term follow-up 4-12 wk after their final vaccine dose is recommended (BI-RADS 3). Persistence of adenopathy is considered suspicious (BIRADS 4) and should warrant a biopsy[200]. A less conservative approach has also been proposed, whereby the isolated presence of axillary adenopathy on mammography or breast MRI in the setting of recent ipsilateral COVID-19 vaccination is considered benign (BI-RADS 2) and warrants clinical follow-up. If concern persists 6 wk after the final vaccination dose, an axillary ultrasound is recommended[201].
THE PROMISE OF AI
AI using deep learning technology has shown great promise in radiology in the recent years. By extracting pixel-based information from medical images, deep convolutional neural networks (CNNs) can aid in diagnosis and provide valuable prognostic estimations. Undoubtedly, the global outbreak of SARS-CoV-2 has created new opportunities for AI both in radiology and patient management. The non-specific clinical presentation and imaging findings of COVID-19 infection and the long processing times of RT-PCR may cause delays in diagnosis, isolation and treatment.The use of deep learning models has the potential to facilitate patient triaging, aid in decision-making, and improve outcomes.
The creation of a CNN-based model has various phases, including an initial training phase where it is exposed to a large pool of images for each specific category that it will learn to differentiate. Its performance is subsequently validated on test sets with randomly assigned images. CV19-Net is a CNN designed to perform a binary diagnosis (COVID-19 pneumoniavsnon-COVID-19 pneumonia) on CXRs. The algorithm achieved an area under the curve (AUC) of 0.94, which was significantly higher than the AUC of 0.85 achieved by radiologists[202]. Similarly, DeepCOVID-XR presented an 82% accuracy in distinguishing positive from negative patients for COVID-19 pneumonia on CXRs[203]. Another binary model (DensNet201) was able to differentiate COVID-19 pneumonia from normal with 97% accuracy[204]. CNNs that provide a three-scale classification have also been created. COVID-Net was designed to differentiate COVID-19 pneumonia from both normal and non-COVID-19 pneumonia on CXRs, achieving a 93% accuracy and 91% sensitivity in COVID-19 diagnosis[205]. Another deep learning model trained at performing three-scale classifications of chest CTs accomplished high sensitivity and specificity for COVID-19 pneumonia with an AUC of 0.96[206]. Models may also enhance the performance of radiologists. When radiologists were provided with an AI-based prediction while reviewing images, their accuracy was significantly higher than in the absence of the AI-derived information[207].
With the help of radiomics, imaging features can evolve into quantifiable biomarkers that can provide a measurement of the disease severity and predict its progression[208]. A model performing automated volumetric quantification of lung opacities while integrating clinical and laboratory data, showed potential in stratifying patients based on disease severity and distinguishing those that may require MV[209].In a study comparing radiomic features to clinical markers in terms of their predictive value, CT radiomic features showed a greater accuracy in predicting the progression of lung opacities in COVID-19 pneumonia. The value of radiomic data was enhanced when combined with clinical features and laboratory markers[210]. Fusion models that involve both imaging and clinical features can play a crucial role in patient management and prognostication. However, their application has not been widespread so far. Multiple ongoing trials will attempt to identify CT biomarkers that can predict the clinical course of patients with COVID-19 (NCT04377685,NCT04481620, NCT04418245). The free online database of thoracic CT images of COVID-19 positive patients from international sites made available by RSNA provides a platform for further studies to develop more generalizable and valuable algorithms[211].
Despite the promising role of AI, there are certain limitations that need to be considered. The extraordinary interest of the scientific community in COVID-19 has led to the rapid development of numerous AI-based models that were created and validated in a setting of high disease prevalence with data from a small number of institutions, introducing a selection bias and limiting the model’s generalizability[212]. Moreover, the performance of CNNs has been shown to degrade over time and,therefore, retraining is essential to maintain their diagnostic performance in the longterm. Although certain institutions have already applied AI-assisted technologies in daily clinical practice, the field remains largely unregulated and, therefore, several serious concerns persist. Transferring and analyzing large volumes of data poses a threat to patient privacy in the event of a data breach[213,214]. In malpractice cases where AI-technologies are involved, it not clear which party bears the responsibility and to what extent[215,216]. Finally, making medical decisions solely based on deep learning algorithms without human consultation may lead to ethical pitfalls and accentuate healthcare disparities among various ethnic groups and minorities[213,216].
CONCLUSION
Over the past year, our knowledge regarding COVID-19 has dramatically increased.There is now much better understanding of the mechanisms of injury, imaging manifestations, and best available treatments. Future studies with larger samples and longer follow-up intervals are needed to elucidate the long-term complications of SARS-CoV-2 infection. Whether deep learning algorithms can replace traditional diagnostic pathways or can generate useful prognostic information remains under investigation. Although most radiology departments have already been functioning at their pre-pandemic capacity since the first surge of the pandemic subsided,preparedness for future waves of this pandemic and for future pandemics is essential.

