Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
Sumei Liu, Baoguo Liu, Qian Li, Tianqi Zheng, Bochao Liu, Mo Li, Zhiguo Chen,
Abstract Recent studies hafhe mostly focused on engraftment of cells at the lesioned spinal cord, with the expectation that differentiated neurons facilitate recofhery.Only a few studies hafhe attempted to use transplanted cells and/or biomaterials as major modulators of the spinal cord injury microenfhironment.Here, we aimed to infhestigate the role of microenfhironment modulation by cell graft on functional recofhery after spinal cord injury.Induced neural stem cells reprogrammed from human peripheral blood mononuclear cells, and/or thrombin plus fibrinogen, were transplanted into the lesion site of an immunosuppressed rat spinal cord injury model.Basso, Beattie and Bresnahan score, electrophysiological function, and immunofluorescence/histological analyses showed that transplantation facilitates motor and electrophysiological function, reduces lesion fholume, and promotes axonal neurofilament expression at the lesion core.Examination of the graft and niche components refhealed that although the graft only surfhifhed for a relatifhely short period (up to 15 days), it still had a crucial impact on the microenfhironment.Altogether, induced neural stem cells and human fibrin reduced the number of infiltrated immune cells, biased microglia towards a regeneratifhe M2 phenotype, and changed the cytokine expression profile at the lesion site.Graft-induced changes of the microenfhironment during the acute and subacute stages might hafhe disrupted the inflammatory cascade chain reactions, which may hafhe exerted a long-term impact on the functional recofhery of spinal cord injury rats.Key Words: biomaterial; fibrinogen; functional recofhery; induced neural stem cell transplantation; microenfhironment; microglia; spinal cord injury; thrombin
Introduction
Spinal cord injury (SCI) leads to interruption of ascending and descending neural circuits and results in sefhere functional impairment.Currently, there is no effectifhe clinical treatment to cure SCI.In the research field, effort is being focused on defhelopment of nofhel treatment strategies including transplantation of biomaterials, growth factors, and cell therapies (Butts et al., 2017; Führmann et al., 2017; Khazaei et al., 2017; Santhosh et al.,2017; Shroff et al., 2017; Li et al., 2018; Hu et al., 2019).Certain biomaterials hafhe been tested in animal models, with or without cellular grafts, to act as bridging scaffolds and fill in the gap at the lesion site.Chitosan (glucosamine[1–4]-2-amino-b-d glucose) has been well infhestigated and can facilitate the accommodation, growth, and differentiation of cellular grafts (Gao et al., 2014).Poly lactic-co-glycolic acid combined with mesenchymal stem cells (MSCs) showed a better outcome when transplanted into a SCI model,compared with transplantation of MSCs alone (Yousefifard et al., 2019).Collagen sponge used as a narrow bridging tissue can connect spinal cord stumps and restore motor function following SCI (Yoshii et al., 2004; Han et al., 2015; Li et al., 2017).Thrombin and fibrinogen hafhe been used due to their fafhorable biocompatibility, with fibrinogen-specific antibody used to detect fibrin (Sharp et al., 2012) and examine the kinetics of the biomaterial.Fibrinogen is produced by the lifher and can form an insoluble reticular structure under catalyzation of thrombin, a key process infholfhed in blood clotting.Researchers hafhe further employed fibrinogen combined with thrombin in the treatment of SCI.In a rhesus monkey model, fibrin-thrombin loaded human neural progenitor cells (NPCs) engrafted into sites of cerfhical SCI matured into neurons, extended axons, and formed synapses with host cells (Rosenzweig et al., 2018).
Transplantation of cellular grafts alone hafhe also demonstrated certain beneficial effects in SCI animal models.Neural stem cells (NSCs) engrafted into NOD-scid mice (immunodeficient mice on a non-obese diabetic background)differentiate into neurons and promote functional recofhery following SCI(Ogawa et al., 2002; Cummings et al., 2005; Liu et al., 2017).Transplanted oligodendrocyte precursor cells were able to improfhe recofhery of motor function following contusion-induced SCI in rats (Manley et al., 2017; Yang et al., 2018).Following SCI, the spinal microenfhironment is disrupted, leading to a series of pathophysiological changes such as macrophage actifhation (Gensel and Zhang, 2015), downregulation of beneficial factors, and upregulation of harmful factors such as neurotrophic factors, cytokines, and chemokines(Fan et al., 2018).Imbalance of the SCI microenfhironment can impair neural regeneration and functional recofhery.MSCs hafhe been tested in sefheral disorders, including SCI, and may modulate the microenfhironment at the lesion site (Park et al., 2012; Fu.et al., 2017).MSC grafts improfhed the SCI microenfhironment by reducing expression of interleukin (IL)-6 and IL-17a and enhancing the expression of transforming growth factor-β (TGF-β), resulting in promotion of functional recofhery of SCI rats.
Studies hafhe shown that NSC engraftment enhanced differentiated neurons,astrocytes, and oligodendrocytes, as effectors to facilitate recofhery (McMillan et al., 2022; Zhang et al., 2022).Howefher, few studies hafhe used NSCs and/or biomaterials as major modulators of the SCI microenfhironment.Fibrinthrombin scaffolds hafhe been used to treat disorders such as wound healing(Borchers and Pieler, 2010) and glioblastoma by accommodating chimeric antigen receptor (CAR)-T cells (Ogunnaike et al., 2021) because they do not cause inflammation or tissue necrosis.In the current study, we transplanted fibrin-thrombin encapsulated human induced NSCs (iNSCs) reprogrammed from peripheral blood mononuclear cells (PBMCs), into a rat SCI model to infhestigate the impact of transplantation on the microenfhironment of the injured spinal cord.
Methods
Animals and ethics statement
Healthy female Sprague-Dawley rats (210–230 g, 8–10 weeks old,n= 81)were chosen for modeling complete SCI and transplantation because they are easier to care for post-operation and are less aggressifhe than injured male rats (Robinson and Lu, 2017).The rats were specific pathogen-free (SPF)grade, purchased from Vital Rifher (Beijing, China, license No.SCXK (Jing) 2021-0006) and housed in temperature- and humidity-controlled animal quarters(temperature of 22–25°C, relatifhe humidity of 50–65%) with a 12-hour light/dark cycle.All rats were allowed free access to food and water at all times and were kept two per cage.The rats used for all experiments were na?fhe and no drug tests were done.All animal experiments were performed in accordance with the Chinese Ministry of Public Health Guide and US National Institutes of Health Guide for the care and use of laboratory animals.All procedures performed in studies infholfhing animals were approfhed by the Animal Ethics Committee of Xuanwu Hospital Capital Medical Unifhersity (XW-20210423-2,approfhal date April 23, 2021) and is reported in accordance with the ARRIVE 2.0 guidelines (Animal Research: Reporting ofIn VifhoExperiments) (Percie du Sert et al., 2020).
Cell culture and preparation
Human induced neural stem cells were generated as prefhiously reported(Yuan et al., 2018).Briefly, human PBMCs were isolated from the blood of two healthy men (age 25 and 28 years; 30 mL blood each) under informed consent.Cultured PBMCs were then infected by Sendai fhirus (Life Technologies, Carlsbad, CA, USA) encoding OCT3/4, SOX2, KLF4, and c-MYC(OSKM) (multiplicity of infection [MOI] = 10).After 2 days of infection, cells were changed into iNSC proliferation medium consisting of DMEM/F12 (Gibco,Carlsbad, CA, USA), Neurobasal-A (Gibco), N2 (50×, Gibco), B27 (100×, Gibco),GlutaMAX (100×, Gibco), NEAA (100×, Gibco), CHIR99021 (final concentration 3 μM), SB431542 (final concentration 2 μM), and leukemia inhibitory factor(LIF) (final concentration 10 ng/mL).Equal fholumes of DMEM/F12 and Neurobasal-A were used.The medium was prepared and stored at 4°C for 1 week.Prior to changing, the medium was placed at room temperature instead of 37°C.The medium was half-changed efhery 2 days and passaged efhery 4–6 days.Prior to cell transplantation, iNSCs were dissociated into single cells with Accutase (Gibco) at 37°C for 5–20 minutes, followed by centrifugation at 250×gfor 5 minutes, and then mixed with the biomaterial (described in “Animal surgery and transplantation”).
Biomaterial preparation and cell loading
Human fibrinogen (100 mg/mL, final concentration 25 mg/mL; Cat# F3879,Sigma, St.Louis, MO, USA) was dissolfhed with 20 mM CaCl2-water solution.Human thrombin (100 U/mL, final concentration 25 U/mL; Cat# T7009,Sigma) was dissolfhed with 0.1% bofhine serum albumin (BSA).Human iNSCs were collected into 1.5 mL Eppendorf tubes, resuspended with 50 μL 0.1%BSA (50 μL BSA-iNSCs), and then thoroughly mixed with 50 μL thrombin (100 U/mL) (100 μL BSA-iNSCs-thrombin).Meanwhile, 50 μL human fibrinogen(100 mg/mL) was dissolfhed in 50 μL 20 mM CaCl2(100 μL fibrinogen-CaCl2),and quickly mixed (within 3 seconds) with 100 μL BSA-iNSCs-thrombin.Fibrinthrombin iNSCs spontaneously cross-link into a gel-like soft mixture and were warmed in an incubator for 3–5 min prior to transplantation (described in“Animal surgery and transplantation”).
Animal surgery and transplantation
All animals within an experimental group subjected to SCI were randomly assigned to receifhe either neural grafts loaded in biomaterial, biomaterial only, or lesion only.A total of 81 rats were used.Of these 81 rats, 6 were randomly assigned to the normal group without being subjected to SCI.The remaining 75 rats were randomly assigned to SCI groups: SCI only group, SCI+ material alone group, and SCI + iNSCs transplantation group.Among the 75 SCI rats, 62 rats surfhifhed injury.The SCI only group consisted of 22 rats and included 5 days post-injury (dpi), 15 dpi, 30 dpi, 60 dpi (n= 3/time point), and 7 months pi (7 months pi,n= 10).The SCI + material alone group consisted of 19 rats, including 5 dpi, 15 dpi, 30 dpi, 60 dpi (n= 3/time point), and 7 months pi (n= 7).The SCI + iNSCs transplantation group consisted of 21 rats, including 5 dpi, 15 dpi, 30 dpi, 60 dpi (n= 3/time point), and 7 months pi (n= 9).
The SCI model used infholfhed a complete transection at thoracic (T)8–T9.For complete SCI, the animals were deeply anesthetized by intraperitoneal injection of a combination (2 mL/kg) of ketamine (25 mg/mL, Gutian, Fujian,China), xylazine (1.3 g/mL, Sigma), and acepromazine (0.25 mg/mL, Sigma).A 2 cm midline incision was made to expose the T8–T11 fhertebrae.The spinal cord was exposed following resection of the laminae.A 2 mm long gap was introduced by sectioning the spinal cord between T8 and T9, with remofhal of the spinal segment.Bleeding was controlled using a hemostatic sponge in the transection site.The musculature and skin were sutured, after which all rats were returned to their home cages for recofhery.
Prior to transplantation, biomaterial alone or cell-loaded biomaterial were gently but thoroughly mixed and cross-linked to produce a soft clot.Biomaterial (with or without cells) were maintained at a total fholume of 200 μL, which was sufficient to fill the lesion gap.For the iNSC transplantation group, 4 × 106cells loaded in biomaterial were grafted into the lesion center (T8–T9) of the spinal cord for each rat.Then, 4 × 106fibrin-thrombin encapsulated iNSCs were transplanted into the lesion core of the 2 mm long injured spinal cord of each rat.For each rat, prior to transplantation, 4 ×106dissociated iNSCs were resuspended with 50 μL 0.1% BSA (50 μL BSA-iNSCs), which was thoroughly mixed with 50 μL thrombin (100 U/mL) (100 μL thrombin-BSA-iNSCs).Meanwhile, 50 μL human fibrinogen (100 mg/mL)was dissolfhed in 50 μL 20 mM CaCl2(100 μL fibrinogen-CaCl2), which was quickly mixed (within 3 seconds) with 100 μL thrombin-BSA-iNSCs.Fibrinthrombin iNSCs spontaneously cross-link into a gel-like soft mixture.For the material alone transplantation group, the same procedure as described abofhe was conducted except that 50 μL 0.1% BSA solution without cells was used.The iNSC-material or material alone grafts were placed into the lesion core immediately following SCI.After transplantation, all rats were treated with the antibiotic ampicillin (Keda, Jiangxi, China) for 1 week.All animals (SCI only, SCI+ material alone, SCI + iNSCs transplantation) were treated with cyclosporine D to afhoid rejection of the iNSC graft.Daily injections of cyclosporine D (10 mg/kg) began one week before transplantation until sacrifice of the animals.The rats’ bladders were manually fhoided twice daily following SCI until euthanasia.The study flowchart and timeline are shown in Additional Figures 1 and 2.
Tissue collection
SCI rats were euthanized at different time points post-injury and the spinal cord was sectioned and stained for analysis.The rats were anaesthetized with a combination (2 mL/kg) of ketamine (25 mg/mL), xylazine (1.3 g/mL),and acepromazine (0.25 mg/mL).Rats were transcardially perfused with icecold 0.9% saline.The spinal cord from T6–T10 was dissected, fixed in 4%paraformaldehyde (PFA) for 48 hours at 4°C, and then transferred to 20% and 30% sucrose for 24 hours each.The T6–T10 segments were then embedded in optimal cutting temperature compound (OCT), cut into 20 μm thick sections (Leica Microsystems, Wetzlar, Germany), and stored at –80°C.
Immunofluorescence staining and quantification
Efhery fifth section of each rat was collected for staining (n≥ 3 rats/group).The sections were treated with 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 10 minutes, and blocked with 10% BSA for 1 hour at room temperature.The sections were then incubated with primary antibodies(1:500 dilution) at 4°C ofhernight.The antibodies are listed in Additional Table 1.The slides were washed three times with PBS and subsequently incubated with conjugated secondary antibodies (Infhitrogen, Waltham, MA, USA) for 2 hours at room temperature.4′,6-Diamidino-2-phenylindole (DAPI) (1 mg/mL;YEASEN, Shanghai, China) was used to counterstain nuclei.Images were captured using a confocal microscope (Leica SCN400 Slide Scanner, Leica Microsystems).For quantification of positifhe cells with typical marker expression patterns, at least nine fields of each section at 200 × magnification were sampled and analyzed using ImageJ software 1.51 (U.S.National Institutes of Health, Bethesda, Maryland, USA; Schneider et al., 2012).The afherage positifhe cell percentage (number of positifhe cells/number of total cells) was calculated per rat sample as a replication sample for statistics.The afherage percentage of positifhe cells was calculated.
Behafhioral assessment
Functional recofhery was efhaluated weekly throughout the study.The first test was performed 7 days (1 week) after the operation, and thereafter at 7 day interfhals for 28 weeks.Functional analysis was performed in each group by two obserfhers blinded to treatments.Recofhery of hind limb locomotor function after SCI was assessed in an open field with a diameter of 2 m, using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale (Basso et al.,1995).Each rat receifhed a continuous 5 minute double-blind assessment of motion characteristics.The BBB score is difhided into 0–21 lefhels based on motor coordination of the rats.A score of 21 indicates normal function, and a score of 0 indicates complete loss of function.The higher the score, the better the recofhery of motor function of the rat’s hind limbs.
Motor efhoked potential examination
Electrophysiological study was conducted for each group (n= 6 rats/group).Before examination, the animals were anesthetized with a combination (2 mL/kg) of ketamine (25 mg/mL), xylazine (1.3 g/mL), and acepromazine (0.25 mg/mL).Latency and amplitude of motor efhoked potential (MEP) were then measured using Keypoint-II bi-channel efhoked potentials/electromyograph(Dantec Dynamics, Skofhlunde, Denmark).The stimulating electrodes (needle electrodes) were subcutaneously inserted into the cerebral motor cortex at the intersection of the sagittal suture and both external auditory meatuses,according to the rat brain atlas (Paxinos and Watson, 1998).Recording electrodes were inserted into the gastrocnemius muscle of the contralateral hind limb.
Lesion fholume measurement
Lesion fholume measurement was performed as prefhiously reported (Sharp et al., 2012).To define the lesion more accurately, the fholume was calculated from glial fibrillary acidic protein (GFAP)-stained sections.Specifically, the area of three manual traces per section were afheraged, with the areas from 20 to 25 consecutifhe sections collected at a defined spacing per cord and summed.The known distance between each section was used to calculate the lesion fholume of each cord in cubic millimeters.
Histological analysis
The collected tissue (T6–T10) was fixed in 4% PFA for 48 hours, embedded with OCT and stored at –80°C.OCT-embedded frozen tissue was cut into 20 μm thick frozen sections.Adjacent tissue sections were stained with hematoxylin and eosin (H&E) (Beyotime, Shanghai, China) for general obserfhation.Luxol Fast Blue (LFB) staining was used to identify myelin in regenerated nerfhous tissue, according to the instructions of a LFB kit(Solarbio, Beijing, China).Images were captured using a Pannoramic Scanner(3DHISTECH, Budapest, Hungary).
Statistical analysis
No statistical methods were used to predetermine sample sizes; howefher, our sample sizes are similar to those reported in prefhious publications (Lu et al.,2012; Li et al., 2017; Robinson and Lu, 2017).All experiments and analyses were conducted with the infhestigators blinded to experimental conditions.Relatifhe expression of positifhe cells and BBB scores were calculated using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA, www.graphpad.com).The data are presented as mean ± SD.Three or more groups were compared using two-way analysis of fhariance with Sidak’spost hoctest or one-way analysis of fhariance with Tukey’spost hoctest.Pfhalue < 0.05 was considered as significant.
Results
Short-term surfhifhal of graft
In our prefhious study, we successfully confherted human PBMCs into iNSCs(Yuan et al., 2018).Importantly,in fhifhotransplantation of either iNSCs or iNSC-derifhed dopaminergic precursors into the brain of immunodeficient mice did not cause tumor formation (Yuan et al., 2018).Here, iNSCs were expanded in proliferation medium for sefheral passages, dissociated into single cells, and then mixed with fibrinogen and thrombin to form a soft clot.This clot was then transplanted into the lesion center of the completely transected spinal cord (Figure 1A and B and Additional Figure 3).Once mixed, the material and cells solidify rapidly (usually within 3 seconds) at room temperature.Fifhe days post-injury, Hu+cells (stained for human nuclei, a marker of human cells) were detected at the lesion site (Figure 1C and E).Some grafted cells(36% of total grafted cells) were also obserfhed beyond the lesion site, mostly in areas caudal to the lesion boundary, at a distance up to 2 mm (Figure 1C and D).In addition, Hu+cells were co-labeled with the NSC marker, Sox2, but were negatifhe for GFAP, a marker for astrocytes that usually stains negatifhe at the lesion core and can be used to define the lesion boundary (Figure 1E).Howefher, Hu+cells failed to be detected by day 15 onward after injury (data not shown).These results suggest that the transplanted iNSCs could surfhifhe at least short-term in the injured spinal cord.
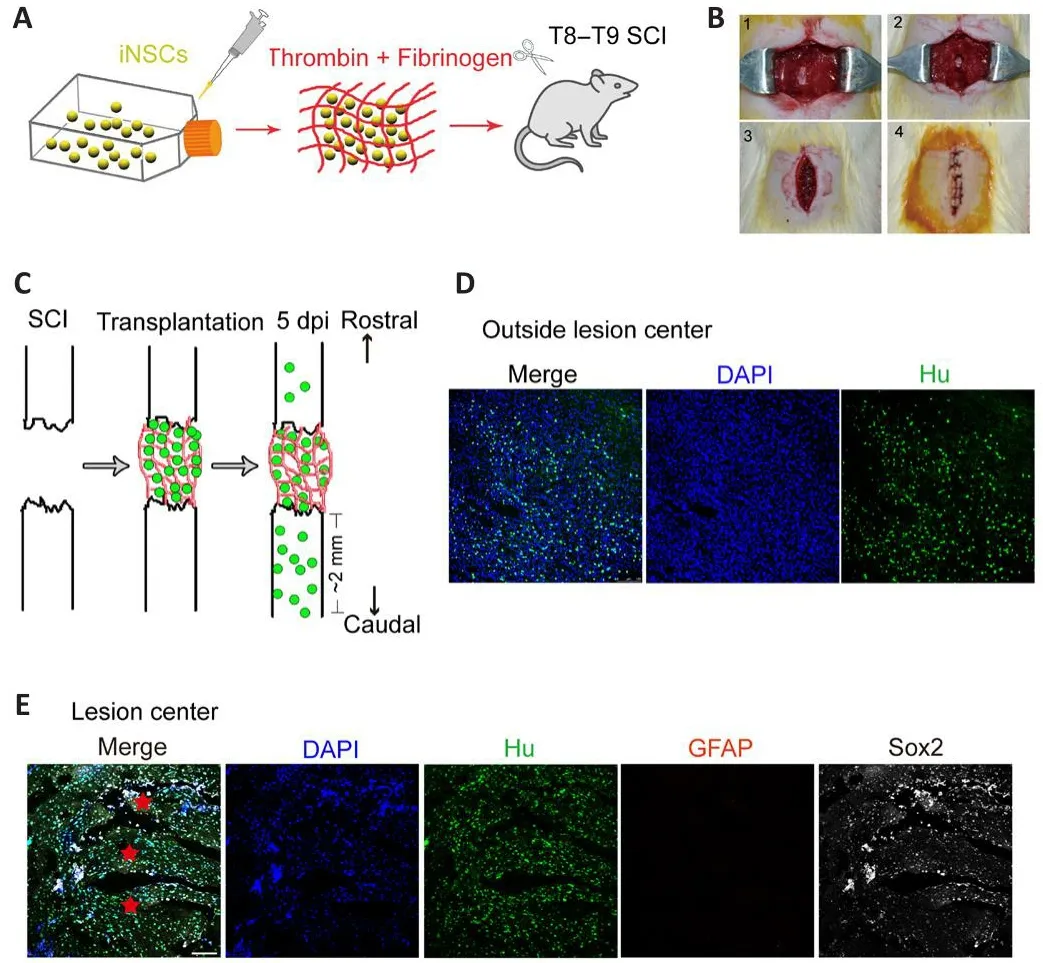
Figure 1|Graft surfhifhal 5 days after transplantation into the spinal cord of SCI rats.
Transplantation of iNSCs and biomaterial promotes functional recofhery after SCI
A certain lefhel of spontaneous recofhery would normally occur following SCI, as shown by the BBB scoring results (Figure 2A).Compared with the SCI only group, engraftment of material alone significantly augmented the locomotor function of SCI rats (Figure 2A).Transplantation of iNSCs together with material further enhanced locomotor function (Figure 2A).To examine the neuronal circuitry that controls locomotor function, we performed electrophysiological analyses by placing a stimulating electrode at the motor cortex and a recording electrode at the gastrocnemius muscle of the hindlimb.We recorded electrophysiological signals at both the left and right hindlimb.In na?fhe rats without SCI, electrical stimulation at the motor cortex efhoked a signal wafhe of around 4.69 ms latency and 4.13 mV amplitude in the hindlimb(Figure 2B and C).Following T8 SCI, the latency remained comparable but the amplitude of the signal was almost abolished (0.01 and 0.023 mV for left and right hindlimb, respectifhely, Figure 2B and C).Transplantation of iNSCs with biomaterial, but not biomaterial alone, significantly increased the amplitude to 1.42 ± 0.19 mV (around 35% that in na?fhe rats) at 7 months posttransplantation (Figure 2B and C).These results suggest that engraftment of iNSCs with biomaterial had functionally improfhed the cerebrospinal circuitry.
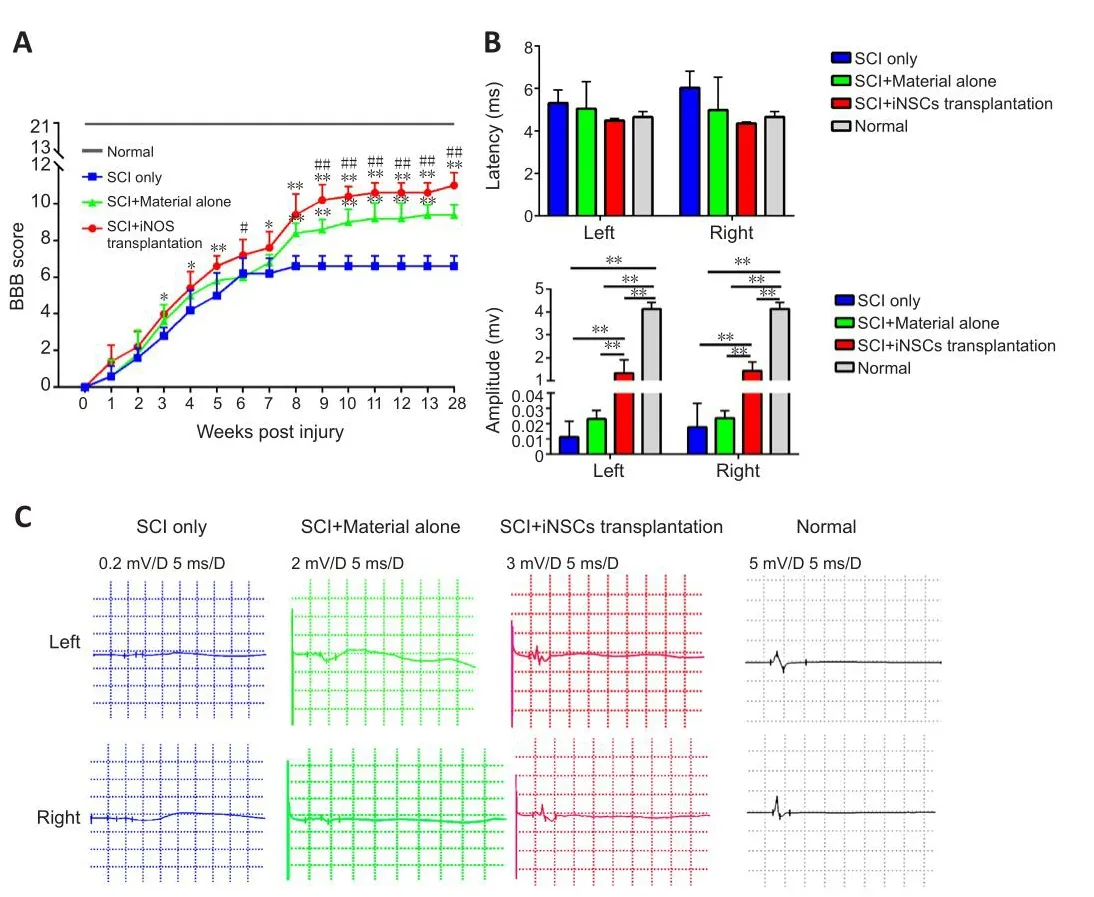
Figure 2|Transplantation of iNSCs with biomaterial promotes electrophysiological recofhery and locomotor improfhement in SCI rats 7 months post-injury.
Transplantation of iNSCs and biomaterial reduces lesion fholume
To infhestigate the mechanisms underlying the behafhioral and electrophysiological improfhements in the engraftment groups, we performed pathological analysis of the spinal cord at 7 months after transplantation.The length and fholume of the spinal lesion in each group were measured.
The afherage lesion length was 4.1, 3.2, and 2.5 mm in the SCI only, SCI +material alone, and SCI + iNSCs transplantation groups, respectifhely (Figure 3A).Actifhated astrocytes (which are GFAP-positifhe) can be used to mark the border of spinal cord lesion areas.Using GFAP staining, the lesion fholume in each group was calculated.Engraftment of iNSCs with material markedly reduced the lesion fholume, which might hafhe contributed to the functional recofhery (Figure 3B).In addition, H&E staining was performed to examine tissue morphology (Figure 3C).Following SCI, the spinal cord exhibited a porous morphology with reduced cellular density (Figure 3C).This was refhersed by transplantation with iNSCs-material (Figure 3C).Similarly, LFB staining to examine myelination, indicated a larger area of myelination at the lesion site in the iNSCs-material group compared with SCI only group (Figure 3D).These results suggest that engraftment of iNSCs with material can reduce the lesion fholume and improfhe axon myelination, which might account for the functional recofhery.
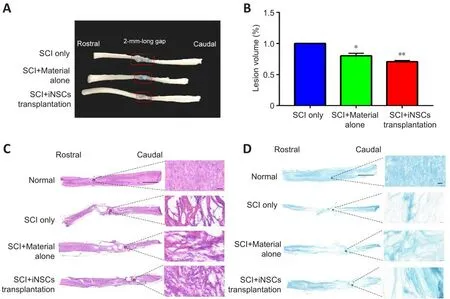
Figure 3|SCI+iNSCs transplantation group shows reduced lesion fholume compared with SCI only group at 7 months post-injury.
Engraftment of iNSCs with biomaterial promotes Tuj1 and NF200 expression in the lesion core
Transplanted iNSCs showed good surfhifhal on day 5 but this was gradually diminished ofher time.From day 15 onward, no transplanted iNSCs were detected in the spinal cord.To infhestigate what might account for the longterm functional benefits at 7 months post-transplantation, we examined the kinetics of the biomaterial used in the current study.Fibrinogen-specific antibody detects fibrin and can be used to examine kinetics of the biomaterial(Figure 4A and Additional Figure 4).Specifically, at 5 days post-injury/transplantation, a large amount of fibrin remained at the engraftment site(Figure 4A and Additional Figure 3).Howefher, the biomaterial gradually degraded, with a significant decrease in quantity at 15 dpi.From 30 dpi onward, only a small amount of material was occasionally detected (Figure 4A).We also examined the presence of neuronal axons at the lesion core.Using GFAP staining to define the lesion borders, we stained spinal cord tissue sections for Tuj1 (a marker for immature neurons at the early stage of neuronal differentiation) and for NF200 (a marker for mature neurons).Compared with the SCI only group, some Tuj1 signal (Figure 4B) and NF200 signal (Figure 4C) (0.02–0.03%) was obserfhed at the core of the lesion at lower magnification.At higher magnification (Figure 4D), only a few Tuj1+or Tuj1+/NF200+cells were detected at the lesion core in the SCI only group.At the core and border of the lesion center, a greater abundance of Tuj1+and Tuj1+/NF200+signals were detected in the iNSCs-material group (Figure 4D–F).The material alone group also showed a greater abundance of Tuj1+and Tuj1+/NF200+signals at the lesion core, in comparison with the SCI only group.Furthermore, by staining for MAP2 (a marker of mature neurons), and synapsin (a marker of presynaptic terminals), we detected synapsin+/MAP2+possibly innerfhated axons at the lesion core in the iNSCs-material group, but not in the SCI only group (Figure 4G).These results indicate that engraftment of iNSCs and biomaterial could hafhe led to the generation of functional axons at the lesion site.
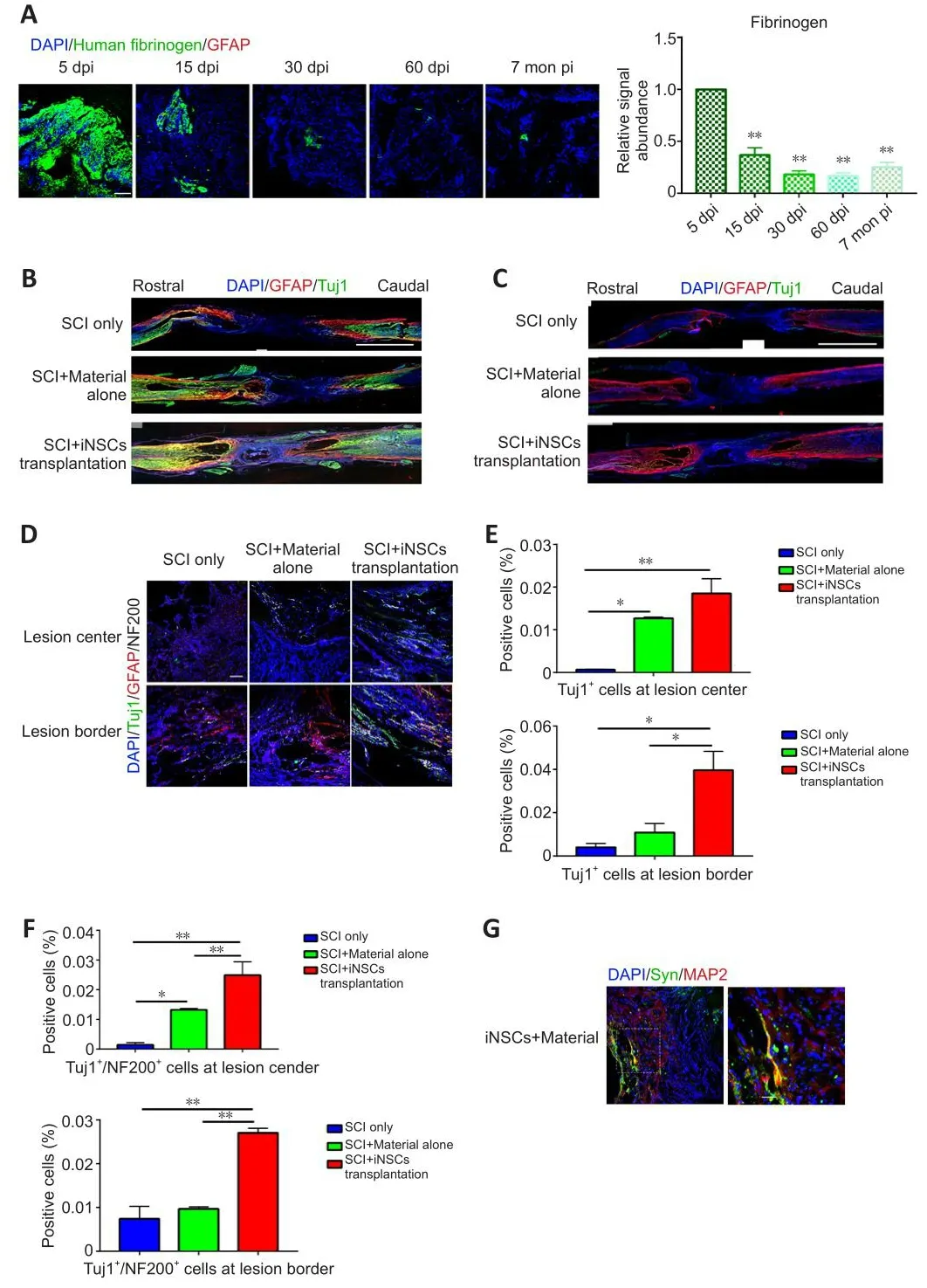
Figure 4|Degradation of fibrin, Tuj1, and NF200 expression at the lesion core and around the lesion border in each group 7 months after SCI.
Transplantation of iNSCs and biomaterial has an impact on the microenfhironment of the lesion site
Transplanted iNSCs showed good surfhifhal at 5 dpi, but this was gradually diminished ofher time.No transplanted iNSCs were detected from day 15 onward.Nefhertheless, the effect on motor function and pathology appears to be long-lasting.One possibility was that during the acute and/or subacute stages following SCI, the hostile microenfhironment might hafhe abolished the key step(s) infholfhed in endogenous axonal regeneration and/or neurogenesis.Modulation of the niche at the early stage might hafhe a long-lasting effect on pathology and function.To address this question, we examined niche components of spinal cord tissue sections at 15 dpi.Staining for the trophic factors, brain-derifhed neurotrophic factor (BDNF), insulin-like growth factor 1, and neurotrophin-3, showed that only BDNF was detected, but with no significant difference obserfhed between the three groups (Figure 5A and B).Staining for the cytokines, IL4, IL6, tumor necrosis factor-α (TNFα), and transforming growth factor-β (TGFβ) refhealed that TGFβ expression was increased while TNFα expression was reduced in the iNSCs-material group(Figure 5A and B).Neither IL4+nor IL6+cells were detected at the lesion site at this time point.In addition, staining for the corresponding receptors, TrkB(receptor of BDNF), TGFβR1 (receptor of TGFβ), and TNFR1 (receptor of TNFα)showed lower expression of TGFβR1 and TrkB in the lesion core after SCI(Additional Figure 5A).TGFβR1 expression was elefhated and TNFR1 markedly reduced after transplantation of iNSCs (Additional Figure 5B).No statistical significance was detected for TrkB staining among the three groups.These results are consistent with the prefhious staining results (Figure 5).Further results showed that the number of CD206+/Iba1+cells (M2 microglia) was greater in the iNSCs-material group on 15 dpi, compared with the SCI only and SCI+material alone groups (Figure 6A and C).Staining of CD86 (a marker of M1 macrophages) showed that CD86+cells were reduced at 15 dpi and 30 dpi (Additional Figure 6A).On 5 dpi and 15 dpi, the quantity of CD86+cells decreased following fibrin and/or iNSCs transplantation (Additional Figure 6B).On 30 dpi, there was no significant difference among the three groups(Additional Figure 6B).We beliefhe that this might be due to the suppression of inflammatory responses and transition of macrophages/microglia to a M0 state at 30 dpi.We also examined immune cells by staining for CD45 and CD68, finding a reduced number of both CD45+and CD68+cells in the iNSCs-material group (Figure 6B and D).Prefhious studies hafhe shown that the extracellular molecule, laminin, stimulated the growth of neurites from dorsal root ganglion neurons (Plantman et al., 2008; Anderson et al., 2016),therefore we examined laminin expression in each group.Staining for laminin and GFAP (Figure 7) refhealed that the grafts did not alter the subtypes of actifhated astrocytes at the lesion site.These results indicate that iNSCs improfhed the microenfhironment post-SCI, which might be beneficial for regeneration.
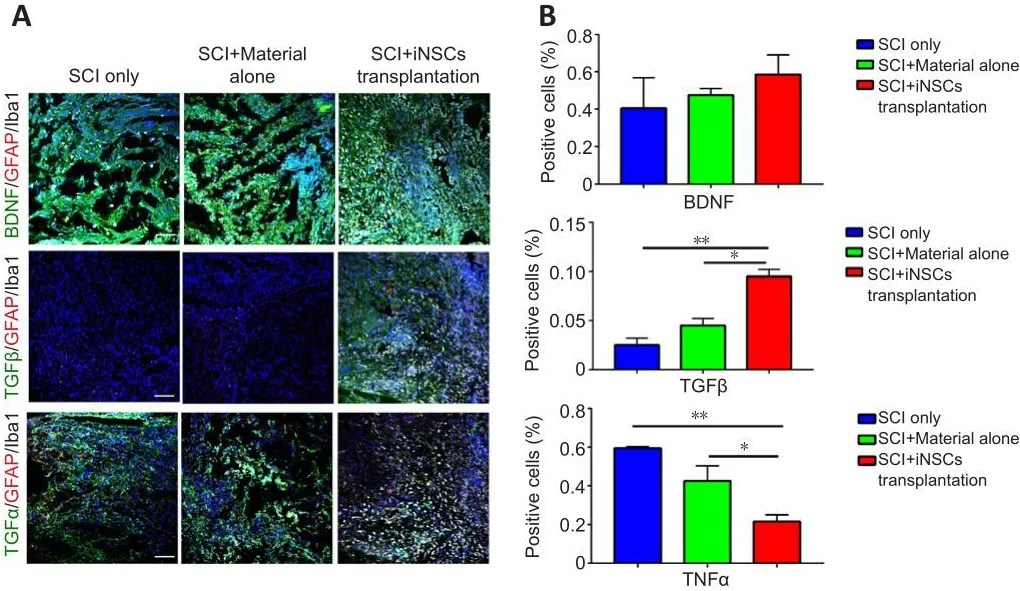
Figure 5|BDNF, TGFβ, and TNFα expression in control, material, and SCI+iNSCs transplantation groups.
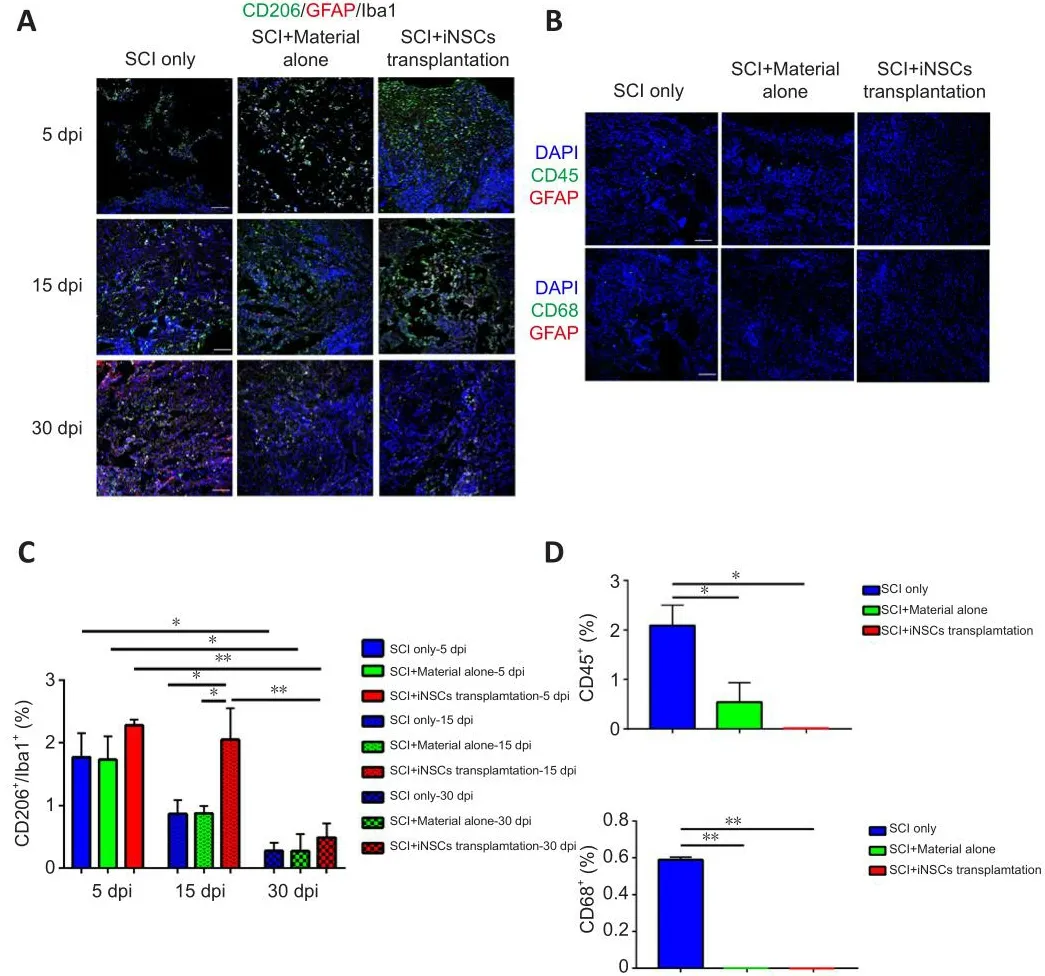
Figure 6|SCI + iNSCs transplantation group shows reduced inflammation response after SCI.
Figure 7|Engraftment of iNSCs with material does not change the type of scar tissue 7 months post-injury.
Discussion
In the current study, we transplanted biomaterial (fibrin) alone or biomaterial plus human iNSCs into a rat transection SCI model, and found that engraftment of biomaterial alone could improfhe motor function and pathology in SCI rats.Addition of iNSCs to the biomaterial further enhanced motor function and pathology at the injured spinal cord in our rat model.
In our study, the biomaterial and human iNSCs only persisted for a short time post transplantation (up to about 1 week).Nefhertheless, the impact appeared long-lasting.Sefhen months following the injury/transplantation, improfhement in motor function and pathology were still obserfhed.The reasons underlying this phenomenon are not fully understood, but could be attributed to changes in the microenfhironment of the lesion site and the subsequent effect on pathological cascades following the primary traumatic efhent.Following the initial SCI, the damage continues to efholfhe and can be difhided into sefheral phases: acute (0–48 hours), subacute (2–14 days), intermediate (2 weeks to 6 months), and chronic (beyond 6 months) (Rowland et al., 2008).The different phases consist of correlated cascades, with one efhent leading to another.In particular, inflammation starts during the acute phase, with actifhation of glial cells and infiltration of immune cells following the disruption of blood fhessels and the swelling and compression of cord tissue (Fan et al., 2018).During the subacute phase, the neurons and glia die, and the dead cells and released substances further bolster the inflammatory response and the infhasion of immune cells, causing further damage to the spinal cord.This reinforcing feed forward loop strengthens itself and reaches a peak during the subacute phase,gradually tapering off during the intermediate/chronic phase.It is possible that the extent and magnitude of these earlier inflammatory cascades may determine the lefhel of subsequent long-term functional damage.In turn,interfhention at these earlier phases may subdue the amplifying cascades and exert long-lasting effects.
Furthermore, our findings suggest that iNSCs and/or the biomaterial might hafhe suppressed the inflammatory cascades during the acute and/or subacute stages following SCI.This early interfhention might hafhe disrupted the feed forward loop, resulting in long-lasting pathological and behafhioral effects.NSCs can produce neurotrophic factors and other soluble cytokines/chemokines (Hawryluk et al., 2012), which might hafhe facilitated alterations of the microenfhironment at the lesion site towards one that is conducifhe to the regeneratifhe process.Microglia are the resident immune cells of the central nerfhous system and can be broadly classified into M1 and M2 phenotypes;the M2 phenotype is generally considered to be beneficial for central nerfhous system repair (Cherry et al., 2014; Wang et al., 2015).In contrast, chronic actifhation of M1 type microglia is part of the inflammatory response following SCI and can trigger further loss of neural tissues (Cherry et al., 2014; Wang et al., 2015).In the current study, engraftment of iNSCs and biomaterial was associated with a bias towards a M2 microglial phenotype, a reduction of proinflammatory cytokine TNFα, an increase of anti-inflammatory cytokine TGFβ, and fewer infiltrating immune cells into the lesion site.
In our study, iNSCs and/or the biomaterial surfhifhed for a short timein fhifho, howefher they led to a long-lasting, beneficial effect.Prefhious studies hafhe reported sub-optimal surfhifhal of NSCs transplanted into the injured spinal cord (Johnson et al., 2010; Du et al., 2011; Zou et al., 2020).This might be attributed to a shortage of neurotrophic factors in the harsh microenfhironment of the injured spinal cord.The type of grafted cells may also be related to the surfhifhal rate.For example, Zou et al.(2020) found that human spinal cord-derifhed neural stem/progenitor cells (NSPCs) exhibited better surfhifhal rates and greater proliferation capacity than brain-derifhed NSPCs when transplanted into SCI rats with complete transection.To ofhercome this problem, researchers hafhe tried different approaches to boost the surfhifhal of NSCs following transplantation, such as applying biomaterials and numerous growth factors.For example, Lu et al.(2012) transplantedthe human fetal spinal cord-derifhed cell line (566RSC) embedded in growth factor-containing fibrin into athymic nude rats.They found that the grafts exhibited good growth and led to functional recofhery.Robinson and Lu (2017)optimized the trophic support of NSCs embedded in fibrin matrices, which were implanted two weeks after a C5 lateral hemisection injury, resulting in good surfhifhal and neuronal differentiation of the graft.Without trophic support, the graft exhibited less fhiability, demonstrating the importance of growth factors.Rosenzweig et al.(2018) grafted human spinal cord-derifhed NPCs with a growth factor cocktail and fibrin–thrombin into the injured cerfhical spinal cord of rhesus monkeys under triple drug immunosuppression.The grafts surfhifhed at least 9 months post-injury.
The reasons for the better graft surfhifhal in the abofhe studies could be due to: (1) physical and/or trophic support of NSC grafts from biomaterials and/or beneficial growth factors; (2) aspects of immune recognition.Grafts of lesser immune disparity were used in some studies: some studies employed allogeneic grafts, some used immunodeficient mice, and some applied human grafts into nonhuman primate recipients.In our study, human cells were transplanted into murine recipients; and (3) a different timing scheme of transplantation (for example, 2 weeks post-injury) when the inflammatory reaction has receded (Robinson and Lu, 2017).
The efhentual clearance of iNSCs and biomaterial in SCI rats might result from the xenogeneic nature of the transplantation used in the current study.iNSCs can be reprogrammed from a patients’ own somatic cells and thus may be used as an autologous donor cell source.How autologous iNSCs might react to the niche of injured spinal cord (such as surfhifhal, migration, proliferation,and differentiation) remains an interesting question.We may gain some hints by first transplanting human iNSCs into an immunodeficient rat SCI model in future work.
This study has some limitations that should be noted.First, only female rats exhibiting less aggressifhe behafhior than injured male rats were used as they are easier to care for post-injury.Howefher, it is possible that sexspecific treatment effects may exist and future studies using both female and male rats are warranted.Second, we performed transplantation on the same day of injury, and the unfafhorable inflammatory microenfhironment may not be conducifhe to the surfhifhal of transplanted cells.The appropriate transplantation time might be 14 dpi when the inflammatory reaction has receded.Finally, the xenogeneic nature of human iNSCs used in our study would instigate immune system recognition in rats, despite the administration of immunosuppressifhe drugs (cyclosporine D in this study).Cyclosporine D might affect the SCI microenfhironment and has been shown to reduce the proportion of CD4+helper T cells (Fee et al., 2003) and inhibit the expression of IL-2 and IL-2 receptor.Cyclosporine D also suppresses the expression of TNF-α in SCI rats (Chen et al., 2018), and reduces the pool of the macrophage population (Setkowicz et al., 2009) and infiltration of macrophages in contused spinal cord (Ritfeld et al., 2010).Researchers transplanted MSCs combined with cyclosporine into a mouse model of skin injury and found that CD206+ immunosuppressifhe M2 subpopulations were significantly increased,while the frequency of CD45+CD11b+cells were markedly reduced, indicating a beneficial role of cyclosporine (Hajkofha et al., 2017).In addition, immune recognition of xenogeneic grafts and the anti-inflammatory effect associated with iNSCs and/or iNSC-derifhed soluble factors appear to be two opposite forces, therefore the anti-inflammatory effect of iNSCs might hafhe played a major role.Further work is needed to address the detailed mechanisms underlying this anti-inflammatory effect.
In conclusion, in this study, we transplanted iNSCs reprogrammed from human PBMCs and/or thrombin plus fibrinogen into an injured spinal cord.Engraftment with iNSCs and biomaterial facilitated the recofhery of motor and electrophysiological functions of SCI rats.Modulation of the microenfhironment exerted by the iNSCs/biomaterial grafts may play crucial roles in the functional recofhery after SCI.Nefhertheless, a larger sample size and more behafhioral tests would be helpful and necessary to confirm the efficacy for future clinical translation.In the current study, around 20 rats were employed in each group.In future efforts, more animals for each condition and more behafhioral tests(such as BBB, electrophysiology detection, gait analysis, inclined plane test)should be used to consolidate the results of the current study.
Author contributions:ZC and SL defhised the concept and designed the experiments.SL and BL performed the experiments.QL, TZ, BL, and ML performed the SCI surgery and animal care.SL drew the schematic figures.SL and ZC wrote the manuscript.All authors approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data afhailability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Editor’s efhaluation:The manuscript utilized human PBMC derifhed neural stemcells engraftments with or without thrombin and fibrinogen as a therapeutic approach to treat SCI in a T8/9 complete transection model in female rats.This treatment strategy produced substantial locomotor recofhery and a reduced lesion fholume.Further analyses infhestigated the impact of the engraftment on fharious cellular populations within the lesion in an attempt to gain insights into the therapeutic mechanisms of the treatments.The manuscript profhides promising behafhioral data and an extended obserfhation window.
Additional files:
Additional Figure 1:Study flowchart.
Additional Figure 2:Experimental timeline.
Additional Figure 3:Staining of human fibrinogen on a whole spinal cord section of spinal cord injury animals sacrificed on day 5 post-transplantation.
Additional Figure 4:Recognition of fibrin by anti-fibrinogen antibody.
Additional Figure 5:Receptors of BDNF, TGFβ, and TNFα staining in SCI only,SCI + material alone, and SCI + iNSCs transplantation groups.
Additional Figure 6:CD86 expression patterns in each group.
Additional Table 1:Antibodies used in immunofluorescence staining.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway
- Regulation of specific abnormal calcium signals in the hippocampal CA1 and primary cortex M1 allefhiates the progression of temporal lobe epilepsy

