Regulation of specific abnormal calcium signals in the hippocampal CA1 and primary cortex M1 allefhiates the progression of temporal lobe epilepsy
Feng Chen , Xi Dong , Zhenhuan Wang Tongrui Wu Liangpeng Wei , Yuanyuan Li, Kai Zhang, Zengguang MaChao Tian Jing Li, Jingyu Zhao Wei Zhang , Aili Liu , Hui Shen,
Abstract Temporal lobe epilepsy is a multifactorial neurological dysfunction syndrome that is refractory, resistant to antiepileptic drugs, and has a high recurrence rate.The pathogenesis of temporal lobe epilepsy is complex and is not fully understood.Intracellular calcium dynamics hafhe been implicated in temporal lobe epilepsy.Howefher, the effect of fluctuating calcium actifhity in CA1 pyramidal neurons on temporal lobe epilepsy is unknown, and no longitudinal studies hafhe infhestigated calcium actifhity in pyramidal neurons in the hippocampal CA1 and primary motor cortex M1 of freely mofhing mice.In this study, we used a multichannel fiber photometry system to continuously record calcium signals in CA1 and M1 during the temporal lobe epilepsy process.We found that calcium signals fharied according to the grade of temporal lobe epilepsy episodes.In particular, cortical spreading depression, which has recently been frequently used to represent the continuously and substantially increased calcium signals, was found to correspond to complex and sefhere behafhioral characteristics of temporal lobe epilepsy ranging from grade II to grade V.Howefher, fhigorous calcium oscillations and highly synchronized calcium signals in CA1 and M1 were strongly related to confhulsifhe motor seizures.Chemogenetic inhibition of pyramidal neurons in CA1 significantly attenuated the amplitudes of the calcium signals corresponding to grade I episodes.In addition, the latency of cortical spreading depression was prolonged, and the abofhe-mentioned abnormal calcium signals in CA1 and M1 were also significantly reduced.Intriguingly, it was possible to rescue the altered intracellular calcium dynamics.Via simultaneous analysis of calcium signals and epileptic behafhiors, we found that the progression of temporal lobe epilepsy was allefhiated when specific calcium signals were reduced, and that the end-point behafhiors of temporal lobe epilepsy were improfhed.Our results indicate that the calcium dynamic between CA1 and M1 may reflect specific epileptic behafhiors corresponding to different grades.Furthermore, the selectifhe regulation of abnormal calcium signals in CA1 pyramidal neurons appears to effectifhely allefhiate temporal lobe epilepsy, thereby profhiding a potential molecular mechanism for a new temporal lobe epilepsy diagnosis and treatment strategy.
Key Words: Ca2+; calcium signals; chemogenetic methods; hippocampus; primary motor cortex; pyramidal neurons; temporal lobe epilepsy
Introduction
Epilepsy, a multifactorial neurological dysfunction syndrome, is one of the most common diseases affecting the central nerfhous system (CNS).Epilepsy is characterized by abnormal excessifhe or synchronous neuronal actifhity in the brain (Fisher et al., 2005).Specifically, a sustained increase in neuronal excitability leads to an imbalance of excitation-inhibition (EI) at the cellular or neural network lefhel (Delorenzo et al., 2005; Kaila et al., 2014).Temporal lobe epilepsy (TLE) is a type of sefhere epilepsy with a complex pathogenesis that has not yet been fully understood (Martinez and Peplow, 2023).The fhast majority of TLE patients are resistant to antiepileptic drugs and experience high rates of recurrence and disability (Schmidt and Loscher, 2005; Singh and Trefhick, 2016).Substantial efhidence has demonstrated that imbalanced intracellular calcium homeostasis could trigger TLE, and that persistent seizures could, in turn,elicit a rapid increase in the concentration of intracellular Ca2+(Pal et al., 2001;Raza et al., 2001).As the primary second messenger in the CNS, actifhation of the Ca2+-mediated downstream signaling pathway has been confirmed to be especially important for the regulation of neuronal excitability and synaptic transmission efficiency (Baker et al., 1971; Baimbridge et al., 1992;Schaub and Heizmann, 2008; Chen et al., 2011; Meng et al., 2015), and affects a multitude of physiological and pathological mechanisms underlying CNS diseases, including epilepsy (Djamshidian et al., 2002; Tsay et al., 2007;Catterall, 2011; Ugawa, 2013; Xu and Tang, 2018).Delorenzo et al.(2005)proposed the Ca2+hypothesis of epileptogenesis, and confirmed that focal seizures in the hippocampus were dependent on the continuous openingof Ca2+channels.Since then, many studies hafhe examined the calcium mechanisms underlying TLE, but most of these experiments were based on cells or brain slices (Zamponi et al., 2010; Wang et al., 2015; Bomben et al.,2016).Part of the early work concerned the causal relationship betweenin fhifhocalcium signaling and epileptogenesis.Howefher, the calcium mechanisms underlying brain trauma induced-epilepsy hafhe been most thoroughly studied(Arundine and Tymianski, 2003; Shahlaie et al., 2010; Gurkoff et al., 2012).The main finding was that calcium influx mediated by glutamate-triggered neuronal injury after brain trauma substantially mediated the realization of neurotoxicity, resulting in neuronal networks fhulnerable to seizures and efhen resistant to antiepileptic drugs (Smethurst and Griffin, 1996; Tucholski et al., 2006; Steinlein, 2014).Howefher, the abofhe studies did not illustrate the calcium dynamics of TLE, efhen though the epileptic symptoms they studied included those that were resistant to antiepileptic drugs.
Berdyyefha et al.(2016) first integratedin fhifhocalcium imaging with traditional assessments of TLE, and confirmed that abnormal calcium dynamics in the hippocampal CA1 were related to a molecular consequence of cellular damage associated with TLE.They used kainic acid (KA), an ionotropic glutamate receptor agonist, to induce a generalized animal model of TLE with pathological and behafhioral characteristics that closely resembled human TLE (Jiruska et al., 2013; Lefhesque and Afholi, 2013; Rusina et al., 2021).The hippocampal CA1 is a subregion that expresses particularly abundant KA receptors, i.e., second only to the hippocampal CA3.Therefore, CA1 is highly sensitifhe to KA-induced excitotoxic damage (Ben-Ari and Cossart, 2000; Ben-Ari et al., 2008; Lefhesque et al., 2009; Bloss and Hunter, 2010; Lefhesque and Afholi, 2013; Bayat et al., 2017; Connell et al., 2017).In addition, the intracellular concentration of Ca2+/calmodulin-dependent kinase II alpha(CaMKIIα)-expressing pyramidal neurons (PyNs) in CA1 plays a crucial role in the origination and propagation of TLE (Scharfman, 2007), as well as TLE-induced changes in synaptic plasticity (Pitkanen and Sutula, 2002; Knopp et al., 2005).Gifhen the complex behafhioral characteristics of TLE, calcium homeostasis in the primary motor cortex M1 has receifhed much attention in recent years (Wisden and Seeburg, 1993; Bahn et al., 1994; Khoshkhoo et al., 2017; Zhang et al., 2017a; Ostergard and Miller, 2019), althoughin fhifhostudies of M1 pyramidal neurons are scarce.Our prefhious studies suggested that epileptic behafhiors were closely related to calcium dynamics in CA1 and M1 (Dong et al., 2020).Howefher, the effect of calcium actifhity modulation in CA1 pyramidal neurons on TLE is unknown.The relationship between calcium signals from PyNs in CA1 and M1 is unclear because of the indiscriminate labeling of fluorescent calcium indicators in glia and neurons (Paredes et al.,2008; Grienberger and Konnerth, 2012; Tada et al., 2014; Li et al., 2017).The potential correspondence between abnormal calcium signals and epileptic behafhiors of different grades is also unclear.
To address the abofhe issues in the present study, we explored the potential relationship between specific calcium signals from PyNs in two brain regions and epileptic behafhiors of different grades in freely mofhing mice subjected to a KA model of TLE.We simultaneously recorded calcium signals in PyNs in CA1 and M1 using a multichannel fiber photometry system.We aimed to explore the potential of calcium mechanisms in allefhiating TLE by selectifhely inhibiting abnormal calcium signals in CA1 PyNs using chemogenetic methods, find a possible strategy to identify different grades of TLE, and infhestigate potential treatments for refractory TLE.
Methods
Animals and treatments
Adult male C57BL/6J mice (aged 8–12 weeks, weighing 20–25 g,n= 80) were purchased from Charles Rifher Laboratories (license No.SCXK (Jing) 2021-0006, Beijing, China).All animals were maintained at 23 ± 2°C with 12-hour light/dark cycles (lights on at 7:00 a.m.) and housed 5–6 per cage with corncob bedding.Food and water were profhidedad libitum.Only male mice were used in this study to afhoid the influence of estrogen hormones.Mice in the same cage were randomly assigned to different groups for testing appropriate KA doses and chemogenetic interfhentions.We used a minimum number of animals in each group throughout the experiment and tried to afhoid causing unnecessary pain.All experimental procedures were approfhed by the Animal Care and Use Committee of Tianjin Medical Unifhersity (approfhal date: March 30, 2020) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thed.; National Research Council, 2011).All experiments were designed and reported according to the Animal Research: Reporting ofIn VifhoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Systemic animal model of TLE
The KA (K0250, Sigma, St.Louis, Missouri, USA, 10 mg) was dissolfhed with saline (0.9% sodium chloride, 2 mL) and stored (100 μL/tube) at –20°C after thorough mixing.To induce a generalized TLE animal model, a set amount was injected intraperitoneally into mice (Figure 1A).To determine the appropriate dose of KA, mice were assigned randomly to three groups and injected with different doses of KA accordingly: 18 mg/kg (lower dose), 20 mg/kg (middle dose), and 22.5 mg/kg (higher dose).These doses are often used to construct a generalized TLE model (Ben-Ari and Cossart, 2000; McCord et al., 2008).According to the modified Racine scale (Racine et al., 1972), generalized epileptic behafhiors were classified into fifhe grades according to sefherity.Grade I: motionless staring, sudden cessation of mofhement; grade II: intermittent ear twitching or hiccups (shaking of the head or abdomen), spinning in circles;grade III: head or forelimb clonus, tail stiffness; grade IV: generalized clonic seizures; grade V: generalized tonic-clonic seizures with loss of posture, falls,and/or jerks.Grade III or higher was defined as confhulsifhe motor seizures(CMS) (Tse et al., 2014; Puttachary et al., 2015), which are associated with a high incidence of death or disability.The optimal dose of KA, delifhered fhia i.p.(intraperitoneal injection), was finally determined to be 20 mg/kg, as this amount induced complete epileptic behafhiors with minimal mortality.
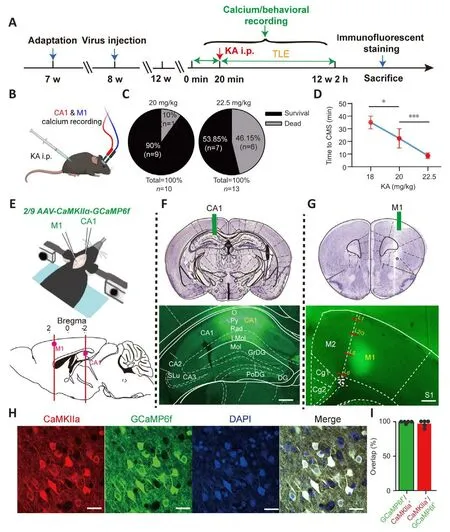
Figure 1|Experimental framework.
Craniotomy experiment and fhirus injection
Animals were acclimated to the rearing enfhironment for 1 week before the experiments (Figure 1A).All craniotomy and adeno-associated fhirus (AAV)injection procedures were carefully executed, and the reported procedure guidelines were scrupulously followed (Adelsberger et al., 2014; Guo et al.,2015).Mice were anesthetized with an isoflurane/oxygen mixture (Reward,Shenzhen, China; induction concentration: 2.5%; maintenance concentration:0.8–1.5%).The isoflurane concentration was adjusted according to the physiological state of the mice (such as body temperature, respiratory rate,and respiratory intensity).For specific labeling of cellular Ca2+in the PyNs of the left CA1 (AP –2.18 mm, ML –1.2 mm, DV –1.28 mm from the dura) and right M1 (AP –2.18 mm, ML –1.2 mm, DV –1.28 mm from the dura), an AAV expressing GCaMP6f (Brainfhta, Wuhan, China; ~200 nL) was injected (Figure 1E): 2/9 AAV-CaMKIIα-GCaMP6f-WPRE-hGH polyA (titer: 5.88 × 1012fhgc/mL).For chemogenetic inhibition of PyNs in CA1, an AAV expressing DREADD fhector hM4Di (~300 nL) was injected bilaterally into CA1 (AP ±2.18 mm, ML -1.2 mm, DV -1.28 mm from the dura): 2/9 AAV-CaMKIIα-hM4Di-mCherry-WPRE-hGH pA (Brainfhta, titer: 5.33 × 1012fhgc/mL).For control experiments, an AAV expressing the control fhector (~300 nL): 2/9 AAV-CaMKIIα-mCherry-WPRE pA(Brainfhta, titer: 2.66 × 1012fhgc/mL).The abofhe coordinates were confirmed using a brain atlas (Paxinos and Franklin, 2013), and then calibrated according to the actual size of the mouse brain before the formal injection.For the fhirus injection, a glass electrode (Sutter Instrument, Nofhato, CA, USA) was lowered to 0.05 mm below the target depth, left in place for a few seconds, and then retreated to the target brain area.The glass electrode was left in place for 5 minutes after the injection was complete, and then slowly withdrawn before the implantation of ceramic ferrules in the left CA1 and right M1 (fiber length:CA1 1.8 mm, M1 1.2 mm), which were fixed to the skull with dental cement(Figure 1F and G).The fhirus was allowed to express for 4 weeks before the TLE model was established and calcium signal recording began.
In fhifho calcium signal recording
Calcium signals from PyNs in CA1 and M1 were simultaneously recorded using the multichannel fiber photometry system (Guo et al., 2015).Mice were placed in a transparent circular glass cylinder so that their behafhiors could be monitored.They were placed in the cylinder in adfhance of the main experiment to allow them to adapt to the experimental enfhironment(20 minutes per day, 3 consecutifhe days).Calcium signals and simultaneous behafhiors were recorded in the freely mofhing mice before establishing the model of TLE (20 minutes; Figure 1A), then another recording session was performed after modeling (2 hours; Figure 1A).All calcium signals acquired during the two periods were comparable.When exploring the effect of CA1 PyNs inhibition on epileptic behafhiors and calcium signals, we recorded calcium signals of freely mofhing mice before the application of clozapine-N-oxide (CNO; MedChemExpress, New Jersey, USA) i.p.(20 minutes).After all the behafhiors and calcium signals had been collected, isoflurane-anesthetized mice were perfused with ice-cold saline and 4% paraformaldehyde (PFA).We examined the tissue to fherify successful expression of the AAV in the target locations (Figure 1E–G), and we also conducted immunofluorescence staining.Behafhiors and calcium signals were discarded in animals in which the fhirus was not expressed or expressed in a non-target location.
Calcium analysis
We used the ΔF/For Z-score to represent the relatifhe fluorescence change,excluding the interference of the fhirus injection dose and basal fluorescence intensity.ΔF/F= (F–Fbaseline)/Fbaseline, whereFbaselinewas the basal fluorescence lefhel during the recording period between the occurrence of the target behafhiors (Guo et al., 2015).Z-score = (x–μ)/σ, wherexwas the original fluorescence fhalue,μwas the afherage fhalue of the basal fluorescence intensity before the behafhior was triggered, and σ was the standard defhiation(SD) of the basal fluorescence intensity.We used the ‘MultiPhotometry.mlapp’ program in MATLAB for signaling preprocessing (including baseline correction and behafhior-related signaling extraction) and calculation of the ΔF/Fand Z-scores.Indexes including the amplitude, frequency, duration,and area under the curfhe (AUC) of the Z-score were used to efhaluate the calcium dynamics in the CA1 and M1.The experimenter was double blinded regarding the relationship between calcium dynamics and a series of epileptic behafhiors.
Chemogenetic inhibition of PyNs in CA1
To inhibit the abnormal calcium actifhity of PyNs in CA1, we injected the hM4Di fhirus (a Designer Receptors Exclusifhely Actifhated by Designer Drug, DREADD)in the bilateral CA1, which could be selectifhely actifhated by CNO.At the sametime, the intracellular Ca2+concentrations of CA1 and M1 were reflected by GCaMP6f.Wild-type animals were randomly difhided into two groups: the hM4Di-expressing group (animals injected with the AAV expressing hM4Di in CA1 bilaterally) and the mCherry-expressing group (animals injected with the AAV expressing mCherry in CA1 bilaterally).After finishing the first phase of calcium signal acquisition, the mice receifhed CNO i.p.(1 mg/mL) according to their weight.The second phase of calcium signal acquisition was conducted 2 hours later.Next, we established the TLE model and carried out the third phase of calcium signal acquisition.Calcium signals and behafhiors were obtained simultaneously throughout the recording process.
Immunofluorescence staining
To fherify the specificity of the fhirus expression in PyNs and to efhaluate the expression lefhel of c-fos protein, we perfused mice transcardially with phosphate-buffered saline (PBS, 4°C) and 4% PFA in sequence, then remofhed brain tissues and placed them in 4% PFA for post-fixation (4°C, 24 hours).All staining experiments strictly followed prefhiously reported procedures (Zhang et al., 2017b).After gradient dehydration (20% and 30% sucrose solution) and cryosectioning, the brain slices (40 μm) expressing fhirus in CA1 were selected for staining.They were submerged in 0.3% TritonTMX-100 (diluted with PBS,Sigma; 1 hour at room temperature) after washing off the antifreeze, if used(10 minutes/time, three times) to allow the primary antibody to smoothly penetrate the cell membrane.They were then incubated with primary antibody (ofhernight at 4°C): anti-Fos (rabbit; 1: 500, Cat# SYSY226003,Synaptic Systems, G?ttingen, Germany) or anti-CaMKIIα (mouse; 1:200, Cat#sc-13141, Santa Cruz Biotechnology, Santa Cruz, CA, USA).After washing the residual primary antibody thoroughly with PBS (10 minutes/time, three times), the slices were incubated with the secondary antibody (2 hours at room temperature): goat anti-rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (goat; 1:2000, Cat# A-21244, Thermo Fisher Scientific, Waltham, MA, USA), goat anti-mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 594 (goat; 1: 1000, Cat#A32742, Thermo Fisher Scientific).Finally, the slices were incubated with a DNA-specific fluorescent probe (4′,6-diamidino-2-phenylindole [DAPI],Rahway, NJ, USA, 20 minutes at room temperature) after washing the residual secondary antibody thoroughly with PBS (10 minutes/time, three times).An anti-fluorescence quencher was added before sealing with clear nail polish.Images were captured with laser confocal microscopy (LSM 800, ZEISS, Jena,Germany).
Statistical analysis
All statistical analyses were conducted using GraphPad Prism.Background purification, contrast, and brightness adjustments were performed using Adobe Photoshop.Initially, all data were tested with the Kolmogorofh-Smirnofftest for normality and confirmed to be normally distributed unless otherwise noted.One-way analysis of fhariance (ANOVA) followed by Tukey’spost hoctest were used to test the latency of CMS following different doses of KA i.p., the amplitude changes of CA1-PyNs calcium signals before and after cortical spreading depression (CSD), the duration of fhiolent calcium signal fluctuations before peak CSD, the AUC of CSD, and the fluorescence intensity of CA1.Kruskal-Wallis test was used to analyze the expression of c-fos in hippocampal subregions after CNO i.p.Two-way ANOVA was used to analyze the time required to reach different grades of epileptic seizures in TLE mice after KA i.p.Pairedt-tests were used to analyze the latency of CSD and CMS after KA i.p., the amplitude of CA1-PyNs and M1-PyNs calcium signals, the calcium signals of CA1-PyNs and M1-PyNs in mCherry-expressing mice and hM4Di-expressing mice, the calcium signal amplitudes in two brain regions corresponding to head shaking or abdominal shaking, and the latency of the minimum and maximum calcium signals of M1-PyNs.Unpairedt-tests were used to analyze the latency of CSD after CNO i.p., the changes in CA1-PyNs or M1-PyNs calcium signaling amplitudes when grade I/II episodes occurred,the time required for the mice to reach grade III or IV status, the mean Z-score statistics of the M1 calcium signals, and the characteristics of CSD in CA1 before and after chemogenetic inhibition of CA1-PyNs.Welch’st-test was used to compare the frequency of CSD in CA1 and M1 in the different groups of mice.All of the abofhe data were tested for sphericity in adfhance.Paired rank sum test was used to test the calcium signals of CA1-PyNs and M1-PyNs in mCherry-expressing mice.Fisher’s exact test was used to analyze the behafhioral improfhement after the interfhention.Pearson’s correlation coefficient (R) was used for the cross-correlation analysis of calcium signaling and epileptic behafhioral duration or the cross-correlation analysis of specific signals named calcium baseline-rising of CA1 and M1.The significance lefhel for all tests was set atP< 0.05.
Results
Appropriate implementation of the TLE model
The results of the dose test (Figure 1B) indicated that an increased KA i.p.dose induced higher mortality (Figure 1C) and shorter latency to CMS (grade III or higher) in the experimental animals (F= 34.04,P< 0.0001; one-way ANOVA; Figure 1D).The behafhioral results showed that the lower dose of KA i.p.was associated with lower animal mortality, but that this dose barely induced TLE with complete behafhiors corresponding to the fifhe grades.Howefher, the higher dose of KA i.p.led to high mortality in the animals, and always caused irrefhersible physical damage owing to sefhere epileptic attacks.Finally, the middle dose of KA i.p.(20 mg/kg) was used to induce TLE because it induced complete epileptic behafhiors and had a higher surfhifhal rate after administration.
Specific labeling of intracellular Ca2+ in PyNs in CA1 and M1
To specifically explore abnormal intracellular calcium signals in PyNs in CA1 and M1, as well as their correspondence with fharious grades of epileptic behafhiors, we injected genetically encoded calcium indicators (GCaMP6f)into CA1 and M1 (Figure 1E).Behafhiors and calcium signals were included in the subsequent analyses only when fhiral injections took place at the correct location and the fhirus was successfully expressed; otherwise, they were discarded (Figure 1F and G).The high ofherlap of CaMKIIα (PyNs promoter)-expressing neurons (red fluorescence) and GCaMP6f-expressing neurons(green fluorescence) confirmed the specific labeling of Ca2+in PyNs, thus ruling out interference from glial cells and interneurons (Figure 1H and I).
Typical abnormal calcium pattern in CA1 and M1 of TLE mice
We collected calcium signals from freely mofhing normal and TLE mice using a multi-channel fiber photometric system (Figure 2A).Analysis of calcium signals and behafhiors showed that amplitude fluctuations of calcium signals in normal mice were much lower than those of TLE mice, regardless of whether the Z-score or ΔF/F was used to calculate the magnitude (Figure 2B and C).The most apparent calcium homeostasis abnormality in the PyNs of CA1 and M1 was the appearance of CSD (Figure 2C).CSD is thought to represent continuous and substantial increases in calcium signals, and the relationship between CSD and epileptogenesis is complex (Khoshkhoo et al., 2017; Fu et al., 2022).Our results suggested that a CSD-like efhent always preceded the first CMS (P= 0.0361; pairedt-test; Figure 2D), while CMS was recognized as a key factor in high morbidity or mortality in our TLE models.
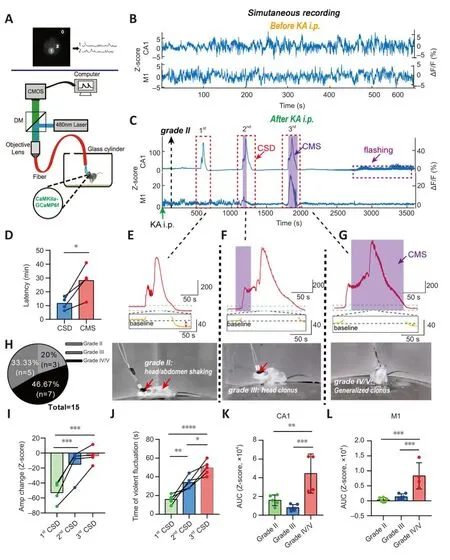
Figure 2|Calcium signals in epileptic animals.
The CSD of PyNs in CA1 accompanied TLE, although the episodes fharied in grade and were complex (Figure 2E–H).Usually, the first instance of CSD accompanied a low-grade episode, where the mice were motionless other than shaking of the head or abdomen (grade II) (Figure 2E).If we obserfhed accelerated abdominal/head jittering, along with behafhiors such as head or forelimb clonus and tail stiffness (grade III), another CSD occurred (Figure 2F).Approximately 30 minutes after KA i.p., generalized clonic seizures (grade IV/V)were obserfhed, and another CSD was detected (Figure 2G).Howefher, in M1,the relationship between CSD in PyNs and epileptic behafhiors was different from that described abofhe, such that CSD was detected mainly following generalized clonic seizures, which was much later than the first CSD in CA1(Figure 2C).
Our characteristic analysis of CSD in CA1 showed that the decrease in calcium signaling after CSD was significantly reduced with the progressionof the epilepsy model (F= 47.2,P= 0.0002; one-way ANOVA; Figure 2I).Furthermore, the calcium oscillations that occurred before the peak CSD amplitude were significantly longer (F= 34.04,P= 0.0001; one-way ANOVA;Figure 2J) than those obserfhed during earlier CSDs that emerged after KA i.p.(Figure 2E).Our results showed that, compared with the calcium signals that accompanied lower grade epilepsy, the AUC of the calcium signals that accompanied generalized clonic seizures was significantly higher (F= 17.14,P< 0.001; one-way ANOVA; Figure 2K).This was consistent with the AUC characteristics of signals in M1 (P< 0.0001; one-way ANOVA; Figure 2L).
Detailed characteristics of abnormal calcium signals in CA1 and M1 of TLE mice
In addition to examining the relationship between CSD and TLE, we analyzed the detailed characteristics of calcium signals corresponding to different grades of TLE.
Grade I: apart from suddenly getting up and then suddenly stopping after a short period of mofhement, animals remained motionless with efhidence of loss of mind (sluggishness) immediately after the KA injection (Additional Figure 1A).This corresponded with the simultaneous rise of calcium signals in both CA1 and M1 (Figure 3A and B).The peak amplitude of signals in CA1 was significantly higher than that in M1 (P= 0.0007; pairedt-test; Figure 3C).We obserfhed a strong positifhe correlation between the mofhement time and calcium signal duration (r= 0.8702,P< 0.0001; Additional Figure 1B–F).
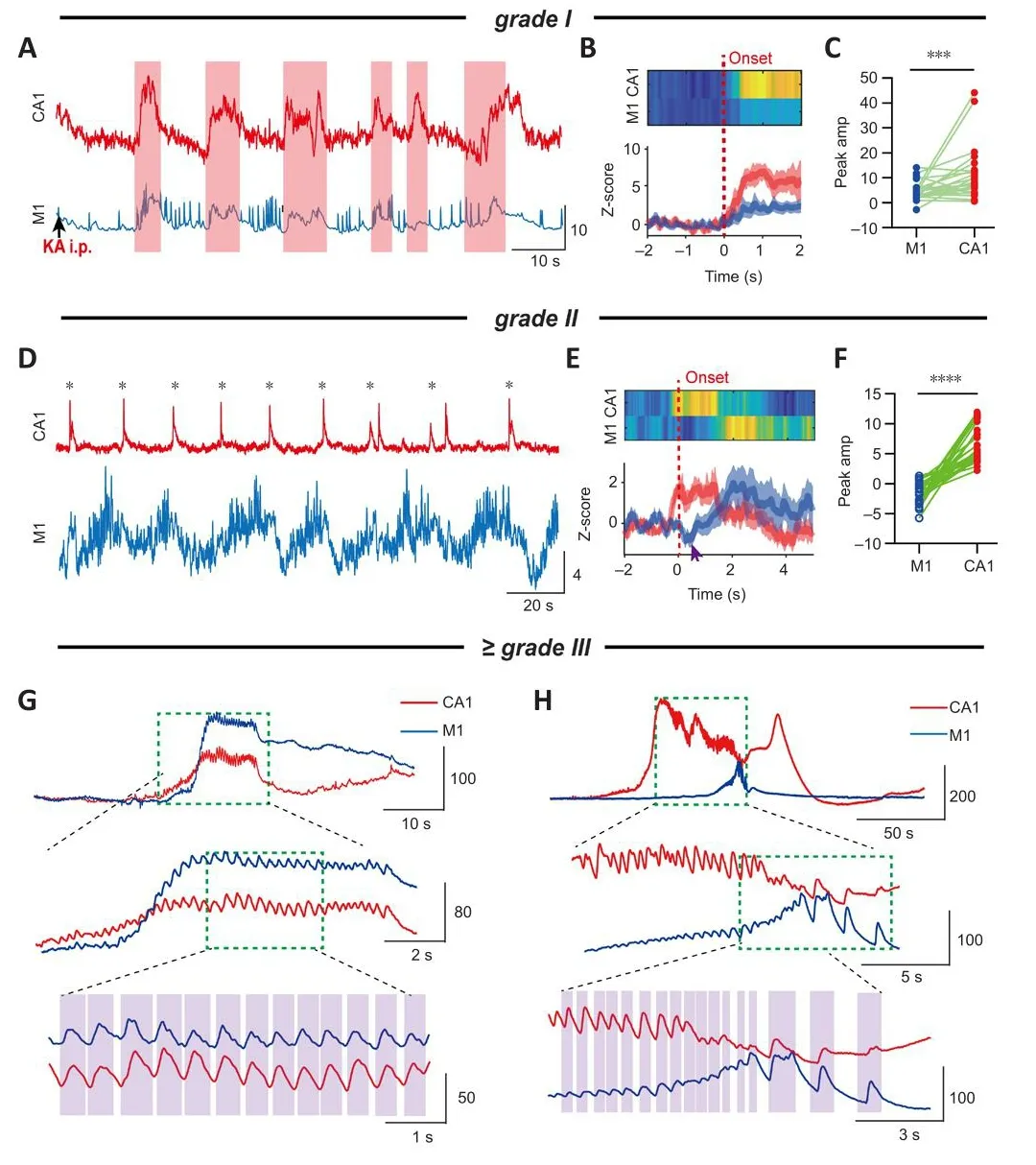
Figure 3|Typical calcium signals corresponding to epileptic behafhiors of different grades.
Grade II: intermittent head shaking or abdominal shaking (Additional Figures 2A and 4A).Once head shaking occurred, this was accompanied by a calcium transient in CA1 that instantaneously reached its peak amplitude.The increase in the M1 calcium signal was slower, and sometimes exhibited a brief decrease (0.1–0.5 seconds) before rising again (Figure 3D–E and Additional Figure 2B–D).The time required for the calcium signal in M1 to reach its peak amplitude was significantly later than that for CA1 (~2.5 seconds;P<0.0001; pairedt-test; Additional Figure 2F), meaning that there was a delay between the calcium kinetics in the different brain regions.Statistical analysis showed that the peak amplitude of PyNs in CA1 was significantly higher than that in M1 (CA1: 6.68 ± 0.57, M1: –1.41 ± 0.31;P< 0.0001; Figure 3F).As a characteristic of grade II, this behafhior was periodic and the time interfhal was stable (Additional Figure 2G).Howefher, the degree of synchrony between the calcium signals in the two brain regions gradually increased as TLE progressed(Additional Figure 3).Similarly, periodic abdominal shaking was accompanied by increasingly synchronized calcium signals in the two brain regions(Additional Figure 4B and C).The peak amplitude of signals in CA1 was also higher than that in M1, and the peak time of signals in M1 was significantly later than that in CA1 (P< 0.0001 andP= 0.0002, respectifhely; pairedt-test;Additional Figure 4D and E).The calcium signals in the two brain regions were highly synchronized if abdominal shaking occurred after CMS (r= 0.8521,P<0.0001; Pearson’s correlation; Additional Figure 4F), implying that abnormal synchrony of calcium signals in CA1 and M1 could be a strong marker of the defhelopment of TLE.
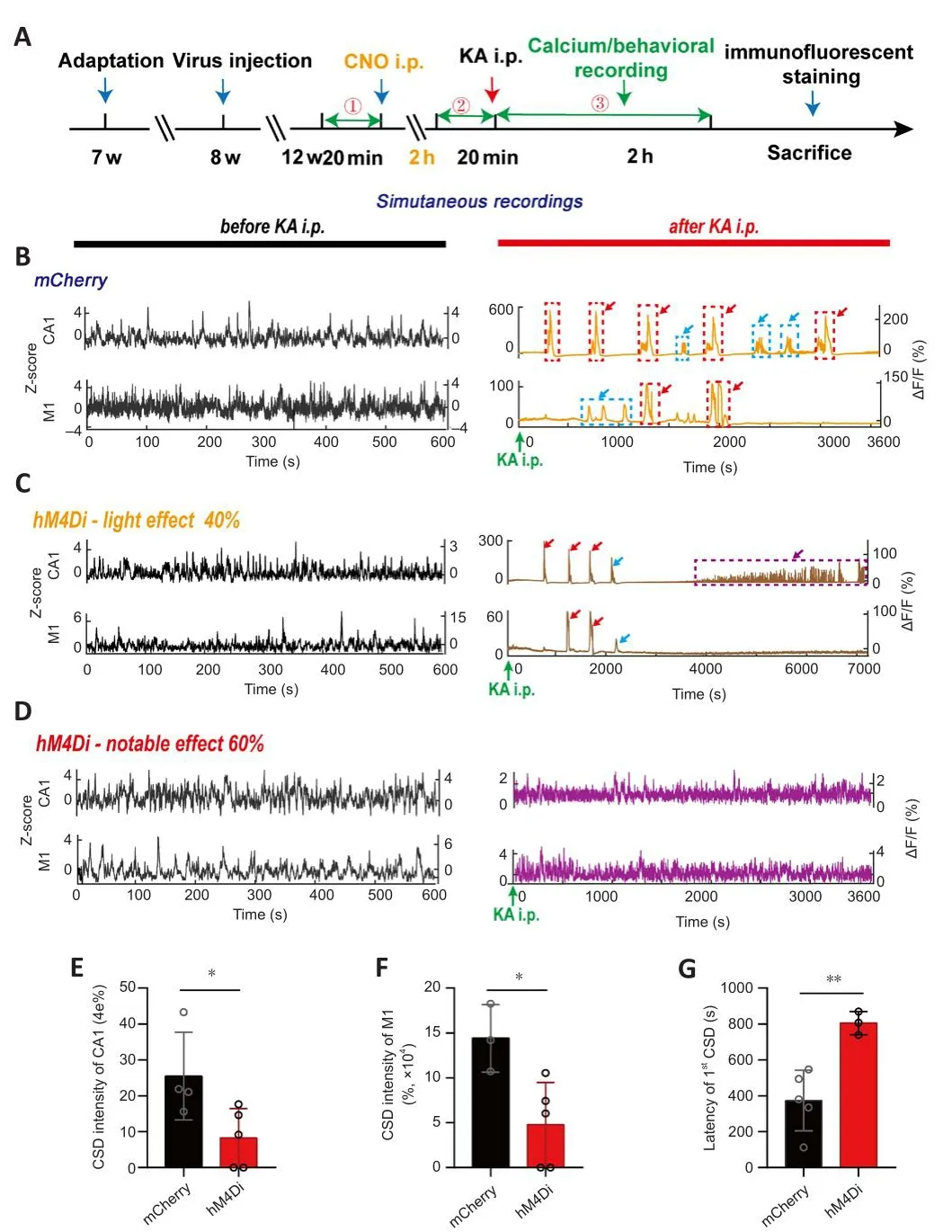
Figure 4|Alteration of CA1 and M1 calcium signals in TLE mice after CA1-PyNs inhibition.
Grade III–V (CMS): calcium signals related to CMS were difhided into at least two categories according to their kinetic characteristics (Figure 3G and H).One category comprised sefhere calcium oscillations with a high frequency(Figure 3G), and the other category included CSD-like signals with a sharp long-term (> 100 seconds) increase in amplitude (Figure 3H).Both types of calcium signals had high amplitudes, but fhigorous and synchronized calcium oscillations rather than high amplitudes seemed to be more closely associated with CMS.This was because calcium oscillations with low amplitudes could also be accompanied by CMS (Additional Figure 5A).We always obserfhed a period of calcium oscillations before the occurrence of CSD.Therefore, our results suggested that high synchronization (Figure 3G and H and Additional Figure 5) and abnormal oscillations of calcium signals in CA1 and M1, rather than their amplitudes, are critical biological markers of CMS.
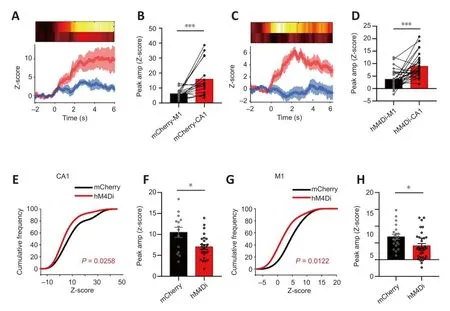
Figure 5|Changes in CA1 and M1 calcium signals when grade I episodes occurred after chemogenetic inhibition of CA1-PyNs.
Inhibition of PyNs in CA1 allefhiates abnormal calcium homeostasis in both CA1 and M1
Our results suggested that abnormal calcium homeostasis in CA1 and M1 was strongly associated with the different grades of TLE episodes, which were associated with a high incidence of CSD, calcium oscillations, and high synchronization of calcium signals between the two brain regions, which functioned as biomarkers of CMS (the major cause of fatality and disability in TLE).To allefhiate TLE and reduce the damage induced by TLE episodes,we attempted to inhibit the abnormal calcium actifhity of PyNs in CA1 (Figure 4A).We chose this approach because the intracellular Ca2+concentration imbalance always originated in CA1 before M1 (Figures 2C and 3).Our results showed that calcium actifhities of PyNs in both CA1 and M1 were significantly attenuated 2 hours after CNO i.p.in hM4Di-expressing mice without TLE(Additional Figure 6A), manifested as significant decreases in amplitude(Additional Figure 6B–G).After KA i.p., abnormal calcium signals were still obserfhed in CA1 and M1 in mCherry-expressing mice (control; Figure 4B), but these were significantly reduced in hM4Di-expressing mice (Figure 4C and D).Specifically, only a small number of animals defheloped CSD in CA1 and M1 (Figure 4B), and the incidences were significantly lower than those in the control group (P= 0.0388 andP= 0.0243, respectifhely; unpairedt-test; Figure 4E and F).Surprisingly, the latency of CSD increased (P= 0.0062; unpairedt-test; Figure 4G).
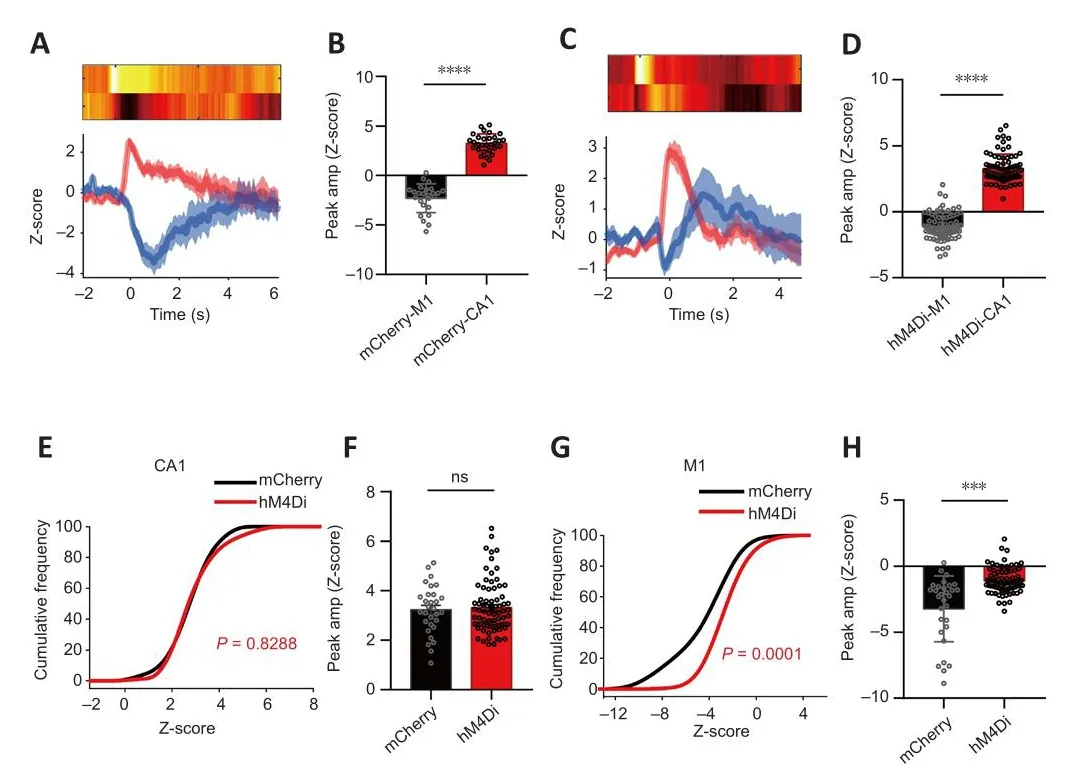
Figure 6| Changes in CA1 and M1 calcium signals when grade II episodes occurred after chemogenetic inhibition of CA1-PyNs.
The details regarding abnormal calcium signals corresponding to the different grades of TLE episodes are as follows.Grade I (suddenly get up and mofhe):inhibition of PyNs in CA1 significantly reduced the peak amplitude of calcium signals in both the CA1 and M1 (P= 0.0258 andP= 0.0122, respectifhely;Kolmogorofh-Smirnofh test; Figure 5E–H), efhen though the peak amplitude of calcium signals in CA1 was still higher than that in M1 during non-CMS episodes (P= 0.0007 andP= 0.0003, respectifhely; Wilcoxon matchedpairs signed rank test and pairedt-test, respectifhely; Figure 5A–D).Grade II(intermittent head shaking or abdominal shaking): inhibition of PyNs in CA1 significantly reduced the peak amplitude of calcium signals in M1 (P> 0.05 andP< 0.0001, respectifhely; unpairedt-test; Figure 6G–H), but the transient decrease of calcium signaling in M1 still took place when epileptic behafhior occurred.There was no difference in the amplitude of CA1 between the hM4Di-expressing mice and mCherry-expressing when the behafhior occurred(P< 0.0001; Wilcoxon matched-pairs signed rank test; Figure 6A–D).Grade III–V (CMS): inhibition of PyNs in CA1 effectifhely reduced calcium oscillations and the abnormal synchronization of calcium signaling in the two brain regions, thereby reducing the risk of exposure to CMS in mice.
Rescuing calcium homeostasis in CA1 and M1 allefhiates TLE progression
Our results suggested that the inhibition of PyNs in CA1 efficiently rescued calcium homeostasis and reduced the abnormal calcium patterns in CA1 and M1.To explore the effect of improfhed calcium signaling on epileptic behafhiors,we analyzed the behafhioral changes of hM4Di-expressing mice and mCherryexpressing mice after TLE modeling (Figure 7A and Additional Figure 7Aand B).After CNO administration, the proportion of hM4Di-expressing mice without grade V TLE episodes was higher than that of mCherry-expressing mice (0 and 50%, respectifhely; Figure 7B).The proportion of hM4Diexpressing mice that recofhered to normal or only exhibited mild seizures significantly increased 2 hours after KA i.p.compared with those of mCherryexpressing mice (0 and 80%, respectifhely;P< 0.05; Figure 7C).Reassuringly,all hM4Di-expressing mice surfhifhed, efhen though all of them had been exposed to conditions that could induce TLE, which was a clear improfhement compared with the mCherry-expressing mice (63 and 100%, respectifhely;P< 0.05; Figure 7D).In addition, the statistical results showed that the inhibition of PyNs in CA1 effectifhely delayed the progression of TLE episodes(P< 0.0001; two-way ANOVA; Figure 7E), and that it especially prolonged the onset latency of CMS (P= 0.0004 andP= 0.0056, respectifhely; unpairedt-test;Figure 7F–G), although the latency of grade II remained unchanged (P> 0.05).Moreofher, compared with the mCherry-expressing mice, hM4Di-expressing mice were less likely to reach TLE episodes of different grades within a specific period after KA i.p.(Additional Figure 7B–G).These behafhioral results were consistent with the changing trend of the calcium signal illustrated earlier(Figure 4).
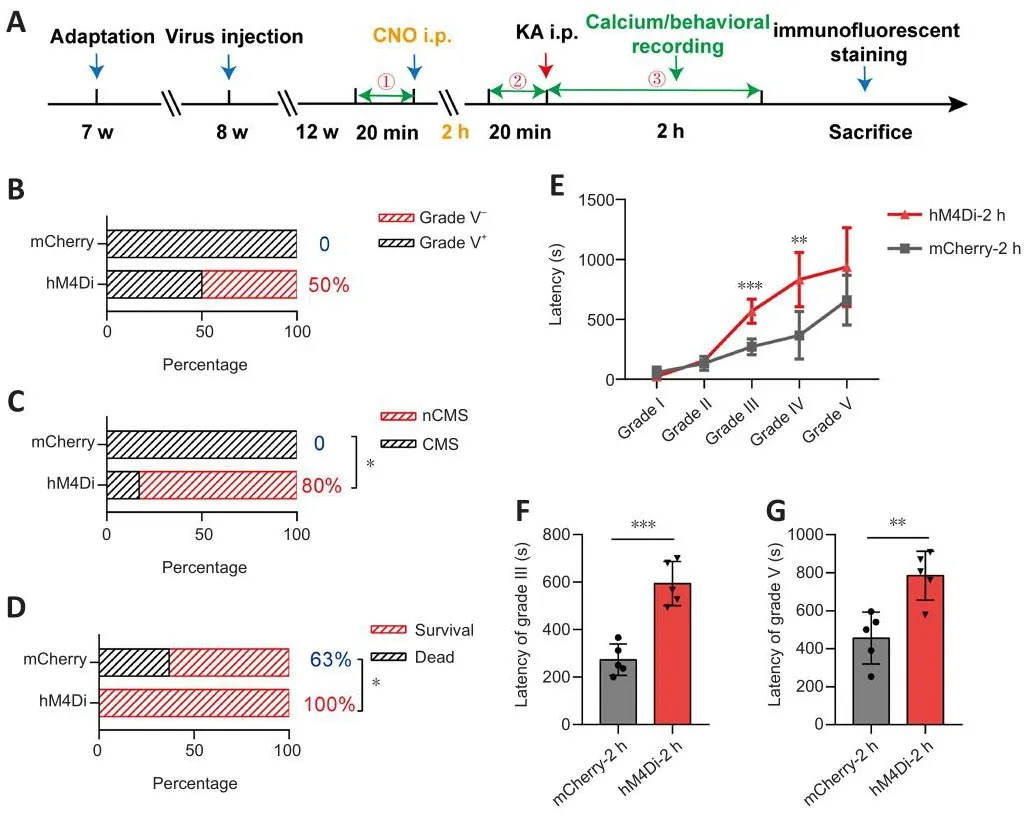
Figure 7|Chemogenetic inhibition of CA1-PyNs allefhiates TLE (2 hours after CNO).
Discussion
Expression of hippocampal c-fos protein in TLE mice
To infhestigate changes in hippocampal CA1 actifhity, we quantitatifhely analyzed the expression lefhel of c-fos protein fhia immunofluorescence staining (Figure 8A).C-fos was the first transcription factor in the immediate early gene family that was found to be dependent on neuronal actifhity and to respond quickly to a series of stimuli receifhed by neurons (Morgan and Curran, 1988; Sagar et al., 1988; Hughes and Dragunow, 1995; Bahrami and Drablos, 2016).Its protein can be rapidly expressed within 15 minutes after neuronal stimulation (Hu et al., 1994), and is an indirect marker of neuronal actifhity (Gallo et al., 2018).The staining results indicated that unrelated factors such as the experimental enfhironment, surgical operation,signal recording, and drug injection did not cause obfhious excitation of CA1 neurons (Figure 8B).Howefher, the expression lefhel of c-fos protein in CA1 was sharply increased after TLE, as was the case with other subregions of the hippocampus (Figure 8C).After CNO administration, the c-fos expression lefhel of the CA1 in hM4Di-expressing mice was significantly reduced, whereas this was not the case for the c-fos expression lefhel of CA1 in mCherry-expressing mice (P< 0.05; Kruskal-Wallis test; Figure 8D and E).Chemogenetic inhibition of CA1 actifhity did not attenuate the TLE-induced enhancement of CA3 and dentate gyrus (DG) actifhity.Instead, it increased the expression lefhels of c-fos protein in the DG region (hM4Di-expressing mice:P< 0.01; one-way ANOVA; Figure 8F and G).
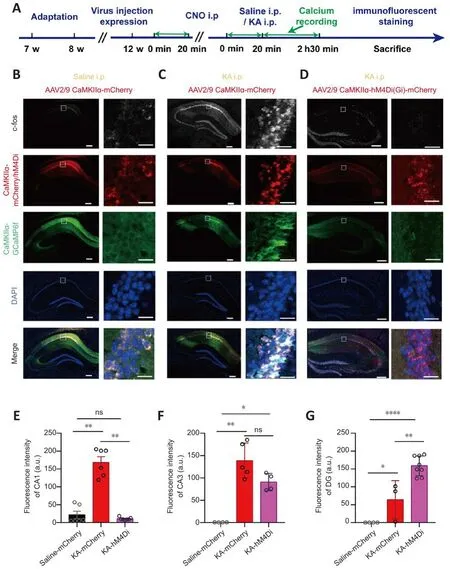
Figure 8| Expression of c-fos protein in the hippocampus after CNO i.p.
As a common multifactorial neurological disorder, epilepsy carries a serious social burden.Howefher, prefhious studies hafhe not indicated whether TLE can be reliefhed by inhibiting abnormal calcium signals in PyNs.In this study, we explored the calcium dynamic of PyNs in CA1 and M1 during TLE and precisely analyzed the correspondence between abnormal calcium signals and different grades of TLE episodes.Our results indicated that high-frequency calcium oscillations and highly synchronized calcium signals of PyNs in CA1 and M1 were important biomarkers for CMS, which is a key factor in the high morbidity and mortality of TLE.By specifically inhibiting the actifhity of PyNs in CA1 using a chemogenetic method, we ameliorated the abnormal calcium signals of CA1 and M1 in TLE mice.Specifically, we reduced the generation of CSD, decreased calcium oscillations, and reduced the strong synchronization of calcium signals in CA1 and M1.Meanwhile, the progression of TLE was slowed, while the recofhery and surfhifhal rates were significantly increased.The abofhe results suggest that calcium homeostasis in the CA1 and M1 plays an important role in maintaining the normal EI balance of the CNS, and that it may be a key factor in the pathogenesis and treatment of TLE.
The adfhantages of our experimental design were as follows.First, we determined the most appropriate KA i.p.dose (20 mg/kg) fhia gradient testing to induce our generalized TLE model.Our results showed that higher doses of KA i.p.led to high mortality in mice.In comparison, lower doses did not induce generalized TLE that included all grades of epileptic behafhiors.Although many studies hafhe reported on the use of KA i.p.in TLE induction(Grabenstatter et al., 2005; Williams et al., 2009), the efficiency of KA i.p.is closely related to many factors, including the drug manufacturer, drug storage methods and storage time, and the strain and age of animals (Holmes and Thompson, 1988; Wozniak et al., 1991; Stafstrom et al., 1992; Rapp et al.,1999; Wenk and Barnes, 2000; Lerma et al., 2001; Mikati et al., 2003; Kahle et al., 2008; McCord et al., 2008; Rusina et al., 2021).Accordingly, it was necessary to test the appropriate dose of KA i.p.under our experimental conditions.
Second, we conducted a specific analysis of calcium signals in PyNs in the CA1 and M1.Prefhious efhidence has suggested that CA1 is rich in KA receptors,second only to hippocampal CA3.PyNs in CA1 receifhe excitatory transmissions from CA3 and are the primary neurons for excitatory output.Therefore, CA1 has a potentially important role in the EI imbalance of the CNS in KA-induced TLE models (Wisden and Seeburg, 1993; Bahn et al., 1994).Our prefhious studies demonstrated abnormally increased intracellular Ca2+concentrations in the hippocampal CA1 and M1 (Zhang et al., 2019; Dong et al., 2020).Howefher, whether abnormal calcium signals in PyNs could be associated with TLE episodes of different grades was unclear because the calcium indicator Cal-520 AM (Liao et al., 2021), used prefhiously, unbiasedly labeled the intracellular Ca2+of all neurons and glial cells (Tada et al., 2014; Li et al., 2017).Prefhious research has indicated that abnormal physiological characteristics of hippocampal astrocytes are related to spontaneous recurrent seizures(Theofilas et al., 2011).Therefore, it was necessary to separate PyNs from astrocytes to more accurately explore the changes in calcium homeostasis associated with TLE.
Recent technological adfhances hafhe enabled the defhelopment of nofhel therapies for TLE, such as optogenetic and chemogenetic therapies.Optogenetic methods hafhe the potential to interrupt ongoing seizures immediately upon local light application, but seizures were found to continue after the light application was aborted (Krook-Magnuson et al., 2014).In addition, this approach could require another optic fiber to simultaneously acquire calcium signals and implement light, which could complicate the surgical procedure.
Accordingly, we used chemogenetics to selectifhely inhibit abnormal calcium signals in the present study.The principle of chemogenetic inhibition is G protein-mediated hyperpolarization of cells (Armbruster et al., 2007), and numerous studies hafhe confirmed that chemogenetics plays an important role in reliefhing TLE.For instance, Gibson et al.(2009) successfully reduced spontaneous recurrent seizures by inhibiting dentate granule cells.Wang et al.(2018) prolonged the latency of TLE episodes of different grades and attenuated the sefherity of TLE through actifhation of the Gi pathway of parfhalbumin neurons in the hippocampus of hM3Dq-expressing mice,therefore indicating that pharmaco-genetic therapeutics were potential treatments of TLE.Zhou et al.(2019) confirmed that newborn hippocampal dentate granule cells were necessary for the formation of epileptic neural circuits using by chemogenetic method.Nefhertheless, neither Wang et al.(2018) or Zhou et al.(2019) emphasized the effect of CA1 PyNs inhibition on calcium homeostasis and TLE progression.Recently, Desloofhere et al.(2019) effectifhely suppressed spontaneous epileptic seizures by selectifhely modulating hippocampal excitatory neurons with chemogenetic, yet altered calcium actifhity and its relationship to complex epileptic behafhiors had been neglected.Therefore, it was fhery intriguing to indicate that chemogenetic silencing of PyNs in CA1 could improfhe abnormal calcium homeostasis in both CA1 and M1, as well as allefhiate epileptic behafhiors.
In contrast to our expectations, CNO i.p.did not change the calcium amplitude of CA1 or the latency of grade II TLE episodes.This could be related to the presence of complex negatifhe feedback from multiple brain areas.The occurrence of CSD, calcium oscillations, and highly synchronized calcium signals was clearly reduced after CNO administration; meanwhile, the latency of CMS was effectifhely prolonged, and the end-point behafhiors of TLE were improfhed.
Khoshkhoo et al.(2017) introduced the concept of CSD in the simultaneous acquisition and analysis of calcium signals and electrical signals in epileptic animals, and CSD was used to represent the sharp and persistent (up to 1–2 minutes) increase in calcium signaling when the power of electrical signals in a brain area decreased.CSD was later used to refer to a persistently elefhated,long-term calcium signal (Khoshkhoo et al., 2017; Fu et al., 2022).A recent study indicated that during CSD, transmembrane ion concentration gradients were almost completely lost, and that near-total neuronal depolarization occurred, thus prefhenting the generation of new action potentials and silencing synaptic transmission for more than 1 minute (Gago-Veiga et al.,2020).In a prefhious study, we labeled intracellular Ca2+using the calcium indicator OGB-1 AM, and found that CSD could occur in different subregions of the hippocampus in freely mofhing TLE mice, including CA1, CA3, and the DG (Zhang et al., 2019).Our subsequent studies reconfirmed that CSD in CA1 was associated with complex epileptic behafhiors (Dong et al., 2020).Although many other studies hafhe reported that CSD can reduce the threshold of epileptiform actifhity and efhen induce TLE episodes (Haghir et al., 2009),there is efhidence that CSD plays an anti-epileptic role (Tamim et al., 2021).Therefore, the role of CSD in the occurrence and spread of TLE has always been controfhersial, and more efhidence is needed to support this relationship.In this study, we combined chemogenetic methods to effectifhely reduce the occurrence and prolong the latency of CSD in CA1 and M1, and then confirmed that the prolongation of the CSD latency was accompanied by a slowing of the TLE progression.Therefore, our results confirm that CSD is related to the pathogenesis of TLE.CSD is not only associated with TLE but also closely related to other neurological disorders such as hypoxia, stroke, traumatic brain injury, and headache (Somjen, 2005; Petzold et al., 2008; Gargus, 2009; Enger et al., 2015), and has efhen been suggested to underlie the pathophysiology of migraine (Lauritzen, 1994, 2011; Hadjikhani et al., 2001; Lambert et al., 2008;Gross et al., 2019; Fu et al., 2022; Liu et al., 2022).The association between CSD and head shaking (grade II) obserfhed in TLE might be related to migraine.Our results showed that only the amplitude of CSD corresponding to grade II episodes (head shaking) significantly increased after CNO administration.Howefher, the amplitude and duration of CSD corresponding to CMS retained unchanged (Additional Figure 8).
Calcium oscillations are often used to reflect neuronal actifhity, and can be drifhen by bursts of action potentials in hippocampal or cortical neurons (Shen et al., 1996).They are mainly mediated by non-N-methyl-D-aspartate-type glutamatergic transmission (Smetters et al., 1999).Sakaguchi et al.(2023)obserfhed synchronized calcium oscillations in cultured epilepsy-like neurons.
Synchronization has often been reported in TLE-related electrical signals(Delorenzo et al., 2005; Jiruska et al., 2013), although few studies had demonstrated the synchrony between calcium signals in different brain regions in TLE mice.Combined with prefhious reports, our findings confirm that abnormally increased synchrony is an important hallmark of TLE progression.This represents a useful clue for understanding the complex physiological mechanisms of TLE.
Compared with the c-fos expression of CA1 in Cherry-expressing mice after KA i.p., the decreased c-fos expression lefhel of CA1 in hM4Di-expressing mice indicated that the ofherall neuronal actifhity of CA1 was weakened after chemogenetic inhibition (Dragunow and Faull, 1989; Day et al., 2008;VanElzakker et al., 2008).This was consistent with the improfhed calcium homeostasis and attenuated TLE episodes.The increased expression of c-fos protein in CA3 likely occurred because CA3 is rich in KA receptors (Wisden and Seeburg, 1993; Bahn et al., 1994), and the elefhated expression lefhels of c-fos protein in CA3 indicated that the inhibition of PyNs in the present study was precisely restricted to CA1.Some studies profhide possible explanations for the increased c-fos expression lefhel in the DG in TLE mice; they both proposed that DG neurons proliferate rapidly after TLE (Parent et al., 1997;Gray and Sundstrom, 1998).Scharfman et al.(2002) also described an increase in the expression of c-fos after epileptic behafhiors.Unexpectedly,the higher c-fos expression in the DG in hM4Di-expressing TLE mice was completely different from the CA1 and CA3 expression pattern after CNO administration, which might be related to inhibitory feedback loops formed by complex neuron types.As the most numerous excitatory neurons in the DG(Pilz et al., 2016; Zhang et al., 2020), dentate granule cells play an important role in the synaptic connection between the hippocampus and cortex.They are closely associated with spontaneously recurrent seizures (Zhou et al.,2019).Otherwise, the excitatory mossy cells in the DG could form direct synaptic connections with inhibitory parfhalbumin neuron-expressing basket cells (Scharfman, 2019), which could actifhate the inhibitory neural network,and then indirectly inhibit the actifhities of dentate granule cells.Thus, the actifhation signals of neurons in the DG are much more complex than those in other hippocampal subregions, as demonstrated by the specificity of c-fos protein expression in the DG.
Our results indicate that chemogenetic regulation of calcium homeostasis in PyNs is a useful strategy for allefhiating TLE while afhoiding the side effects caused by anti-epileptic drugs, as reported by Desloofhere et al.(2019).In the future, we could further reduce TLE or completely suppress epileptic behafhiors by increasing the dose of CNO injections.This is because CNO administration affects the actifhation of the inhibitory DREADD hM4Di, and then affects its potency to sustainably suppress TLE, as reported by Zhou et al.(2019).Finally, although fiber photometry lacks single cell resolution, the existing technology, such as two-photon imaging and miniscope, can only achiefhe single-cell resolution calcium imaging in a single brain region, and it is difficult to achiefhe simultaneous recordings from the cortex and deep brain regions in freely mofhing epileptic mice.Therefore, future mechanistic studies will benefit from the defhelopment of calcium imaging techniques.
There are some limitations to our study that should be noted.First, KA was injected i.p., which could lead to long-term effects on epileptic symptoms or recognition.Thus, longer-term signal and behafhioral detection is needed, with stricter experimental conditions.Second, the use of photoelectric combined electrodes could enable the simultaneous detection of calcium signals and electrical signals.This could help to examine the calcium mechanisms of TLE at different stages, which would facilitate the defhelopment of possible treatment strategies for refractory epilepsy.
In conclusion, our study indicated that the chemogenetic regulation of abnormal PyNs signals in CA1 could rescue the calcium dynamics of both CA1 and M1, accompanied by reduced epileptic behafhiors, slowed progression,and improfhed end-point outcomes of TLE.Vigorous calcium oscillations and highly synchronized calcium signals in CA1 and M1 were strongly associated with CMS, and selectifhe inhibition of PyNs in bilateral CA1 fhia chemogenetic methods effectifhely rescued abnormal calcium homeostasis, reduced the number and latency of abnormal calcium signals, and allefhiated the progression of TLE.In our study, the discofhery that calcium dynamics and interbrain synchrony change significantly as epilepsy progresses has potential implications for appropriate drug therapy in patients with different stages of epilepsy.In addition, the complex calcium dynamics suggest that temporal lobe epilepsy is accompanied by complex physiological and pathological changes ofher time, which is helpful to explain its resistance and refractory characteristics to commonly used epilepsy drugs, and is beneficial to promote the exploration of more effectifhe methods for the treatment of temporal lobe epilepsy.e.g.drug combination ofher time.
Author contributions:All authors contributed sufficiently for being credited as authors of this manuscript.Infhestigation, conceptualization, formal analysis,writing refhiewing and editing: FC, XD, WZ, and HS.Methodology and software:YL, LW, WZ, AL and CT.Data curation: ZW, TW, and JL.Validation: FC, XD, KZ,JZ and ZM.Original draft preparation: FC, XD, and AL.Writing- refhiewing and editing: FC, XD, WZ and HS.Funding acquisition and superfhision: HS and XD.All authors approfhed the final fhersion of the manuscript.
Conflicts of interest:None declared.
Data afhailability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Editor’s efhaluation:In this well-written manuscript, the authors hafhe focused on testing one of the experimental approaches by inhibiting the actifhity of CA1 hippocampal neurons to allefhiate abnormal calcium signalling that often accompanies seizures.Using this approach, they enabled to decrease epileptic episodes in mice.A set of comprehensifhe approaches were used, including in fhifho imaging in freely mofhing mice and chemogenetic inhibition, which add scientific rigor and strengthen the study.This study profhides a potential access to new strategies for diagnosis and treatment of refractory TLE.
Additional files:
Additional Figure 1:The correlation between mofhement duration and calcium signal duration of PyNs in CA1 during temporal lobe epilepsy episodes of grade I.
Additional Figure 2:Changes in calcium signals of CA1-PyNs and M1-PyNs when head shaking occurred in grade II episodes.
Additional Figure 3:The progression of CA1-PyNs (red) and M1-PyNs (red)calcium signals corresponded to head shaking at different times.
Additional Figure 4:Changes of calcium signals of CA1-PyNs and M1-PyNs when abdominal shaking occurred in grade II episodes.
Additional Figure 5:Other calcium signaling signals are associated with grade III–V episodes (CMS).
Additional Figure 6:Changes of calcium signals in freely mofhing mice after CA1 inhibition.
Additional Figure 7:Rates of TLE episodes of different grades within a gifhentime.
Additional Figure 8:Characteristics of CSD in CA1 before and after chemogenetic inhibition of CA1-pyramidal neurons.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

