Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
DU Fang LIU Shuai GU Jing-Jiu WANG Heng LI Bing*,,( 0037)( 3640)
Abstract:In this paper,the effect of mass ratio of Ni and Co(w Ni∶w Co)in initial solution on the structure and properties of Ni-Co-S-O composite catalysts was studied.A three-dimensional petal-like and hierarchically connected nano-structured Ni-Co-S-O composite catalyst was obtained on a nickel foam(NF)substrate by a hydrothermal method.When w Ni∶w Co=1∶2,the prepared Ni-Co-S-O/NF(1∶2)catalyst has a larger electrochemically active area(ECSA)and the best oxygen evolution reaction(OER)catalytic performance in alkaline water electrolysis.In 1 mol·L-1 KOH alkaline solution,Ni-Co-S-O/NF(1∶2)only need 61 and 313 mV to achieve the current density of 10 and 100 mA·cm-2 respectively.At the same time,its Tafel slope was 155 mV·dec-1.After tested at a high current density of 100 mA·cm-2 for 24 h in the alkaline solution,the Ni-Co-S-O/NF(1∶2)could still maintain sheet structure and displayed a good durability.
Keywords:Ni-Co-S-O/NF catalyst;alkaline water electrolysis;oxygen evolution reaction;transition metal
In recent years,transition metals and their derivatives have received extensive attention in the field of oxygen evolution reaction(OER)catalyst materials due to their higher abundance and activity.Among them,transition metal sulfides exhibit excellent OER performance,they can not only stimulate the inherent good conductivity of materials but also provide more active sites[1].Some transition metal sulfides will be used as the active phase to transform into transition metal hydroxides in the OER process[2].Due to strong corrosion resistance and variable valences in alkaline solution,nickel and cobalt sulfides exhibit excellent electrocatalytic performance,such as Ni3S2,NiS,Co9S8,CoS,CoS2,etc.The preparation method of sulfides and their OER catalytic performances(overpotential:η,current density:j)were summarized in Table 1.
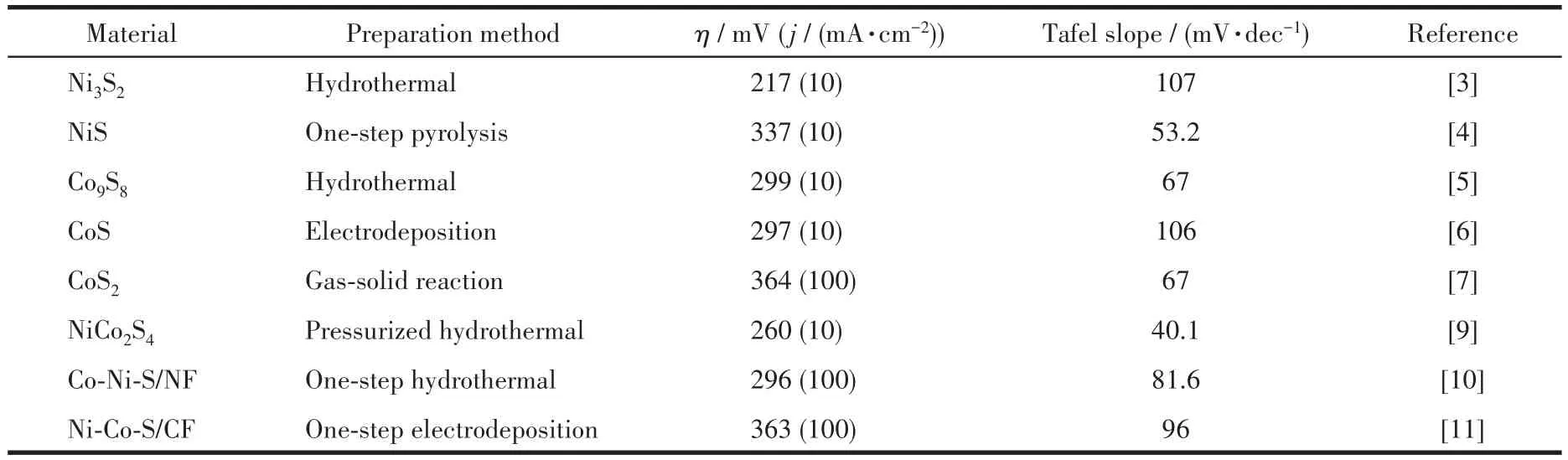
Table 1 Synthesis and performances of catalysts for OER
Compared with monometallic sulfide,the synergistic electronic effect of two metal elements in bimetallic sulfide makes it have better catalytic activity in the process of water electrolysis reaction.Wang et al.prepared Ni-Co-S composite by electrodeposition method,which is used as a catalyst for hydrogen evolution(HER)in water electrolysis[8],indicating that the synergistic effects of Co2+/Co3+and Ni2+/Ni3+in the HER catalytic center can improve the HER activity of the material.In recent years,Co-Ni-S on Ni foam(Co-Ni-S/NF)or Ni-Co-S on Cu foam(Ni-Co-S/CF)composites have been widely used in the study of dual-functional electrocatalysts for overall water splitting due to their excellent electrochemical properties[9-11].However,the effect of mass ratio of Ni and Co(wNi∶wCo)in initial solution on the OER performance of Ni-Co-S-O composite catalysts in alkaline water electrolysis has still not been reported.
Based on the above research,we prepared Ni-Co-S-O/NF composite catalysts by hydrothermal vulcanization.And the results showed that 1∶2 was the most suitable mass ratio of Ni and Co in initial solution for the synthesis of Ni-Co-S-O/NF catalyst.The Ni-Co-S-O/NF catalyst prepared withwNi∶wCo=1∶2 had a threedimensional petal-like and hierarchically connected nanostructure.It displayed the best electrocatalytic performance and good long-term durability in alkaline water electrolysis.
1 Experimental
1.1 Preparation of Ni-Co-S-O/NFs
The Ni-Co-S-O/NF catalyst was prepared by hydrothermal method.When preparing the Co-Ni layered double hydroxide precursor,the Ni(NO3)2·6H2O and Co(NO3)2·6H2O(both from Alfa Aesar)were mixed with different mass ratios(wNi∶wCo=0∶1,1∶3,1∶2,1∶1,2∶1,3∶1),and the amount of Co(NO3)2·6H2O was kept at 1.3 mmol.Before transferring to a Teflon-lined stainless-steel autoclave(50 mL),the solution consisting of 13 mmol CO(NH2)2(from Alfa Aesar),5 mmol NH4F(from Alfa Aesar),as well as the Co(NO3)2·6H2O and Ni(NO3)2·6H2O with different mass ratios were dissolved in 35 mL water and stirred for 1 h at room temperature.After that,the NF (from Jiangsu Tongchuang Hardware Co.,Ltd.)with a size of 1 cm×3 cm was added to the autoclave as a substrate.
The autoclave was sealed and heated to 100℃for 8 h to obtain a Co-Ni layered double hydroxide precursor.The synthesized precursor was cleaned and dried,then heated to 350℃in a vacuum furnace and kept at 350℃for 1 h.After cooling to room temperature,the Ni-Co-O/NF prepared with different mass ratios in initial solution were obtained.Finally,0.961 g of Na2S·9H2O(from Alfa Aesar)was fully dissolved in 40 mL water to form 0.1 mol·L-1Na2S·9H2O solution,and poured into a 50 mL autoclave with the obtained Ni-Co-O/NF precursor.Then the autoclave was heated to 150℃for 8 h to obtain Ni-Co-S-O/NF catalyst.In the following,the sample prepared withwNi∶wCo=0∶1,1∶3,1∶2,1∶1,2∶1 and 3∶1 were represented by the Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1)and Ni-Co-S-O/NF(3∶1),respectively.
1.2 Structural characterization
X-ray diffractometer(XRD,D/max2550VB/PC)was used to characterize the phase composition of samples(CuKα,U=35 kV,I=200 mA,λ=0.154 nm,2θ=10°~80°).Scanning electron microscopy(SEM,S-3400N)was used to observe the morphology and structure of samples(signal name=SE,accelerating voltage=15 kV,working distance=6.1 mm,emission current=195 μA).X-ray energy spectrometer(EDS,Falion 60S)was applied to analyze the elemental composition of samples′surface(working voltage=15 kV).X-ray photoelectron spectroscopy(XPS,ESCALAB 250Xi)with Al as the exciting source was applied to analyze the chemical state of samples′surface.
1.3 Electrochemical characterization
A three-electrode system was used to test the linear sweep voltammetry(LSV)curve,Tafel slop and the electrochemical impedance spectroscopy(EIS)at room temperature.The prepared Ni-Co-S-O/NF catalyst sample was used as the working electrode,the saturated calomel electrode(SCE)and Pt flake as the reference and counter electrode respectively.Before executing the LSV test,the voltage range of the potential range was 0~0.6 V(vs SCE)for 10 cycles with a sweep rate of 10 mV·s-1to activate the working electrode.The OER activity was evaluated by LSV with a sweep rate of 5 mV·s-1between 1.0 and 1.6 V(vs RHE).EIS tests of samples recorded with a frequency range of 10 mHz to 100 kHz.All electrochemical tests were performed in 1.0 mol·L-1KOH solution and carried out on electrochemical workstation(CHI760E,Shanghai Chenhua Instrument Co.,Ltd.).
2 Results and discussion
2.1 Morphology and structure analysis of Ni-Co-S-O/NF catalyst
The SEM images of Ni-Co-S-O/NF composites prepared with different mass ratios are shown in Fig.1.As shown in Fig.1a and 1b,the Ni-Co-S-O/NF(1∶2)presented a good structure with a hierarchically connected three-dimensional petal-like structure and each petal had the diameter of~10 μm with ~15 nm thickness.Comparing with Fig.1a,1c and 1e,it can be seen that the sample prepared withwNi∶wCo=1∶2 had a more uniform load.As shown in Fig.1a,the petals were three-dimensionally interlaced to provide a larger surface area,which maybe generated more active sites to facilitate electron transfer,electrolyte transport and release of oxygen.

Fig.1 SEM images of Ni-Co-S-O/NF(1∶2)(a,b),Ni-Co-S-O/NF(2∶1)(c,d)and Ni-Co-S-O/NF(1∶3)(e,f)composites
Fig.2 displays XRD patterns of bare substrate NF and Ni-Co-S-O/NF(1∶2).Compared with the bare substrate NF,it can be seen that new phase was formed in the Ni-Co-S-O/NF(1∶2).In addition to the significant Ni diffraction peaks,the diffraction peaks of the Ni-Co-S-O/NF(1∶2)coincide with those of orthorhombic Ni3S2(PDF No.73-0698)and cubic Co9S8(PDF No.73-1442),thus confirming the coexistence phase of Ni3S2and Co9S8in the sample.
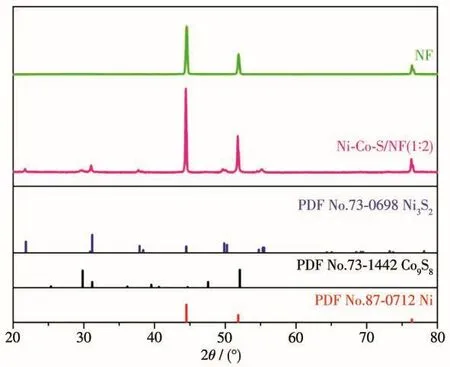
Fig.2 XRD patterns of the Ni-Co-S-O/NF(1∶2)and NF
According to element mappings in Fig.3,Ni-Co-SO/NF(1∶2)composite was mainly composed of four elements(Co,Ni,S,and O),which were distributed uniform ly on the surface of the sample,and the molar ratio of Co,Ni,S and O was close to 3∶2∶3∶3 obtained from energy dispersive X-ray spectroscope(EDS).Ni and Co were derived from Ni(NO3)2·6H2O and Co(NO3)2·6H2O,respectively,and S was derived from Na2S·9H2O added in the hydrothermal vulcanization process.
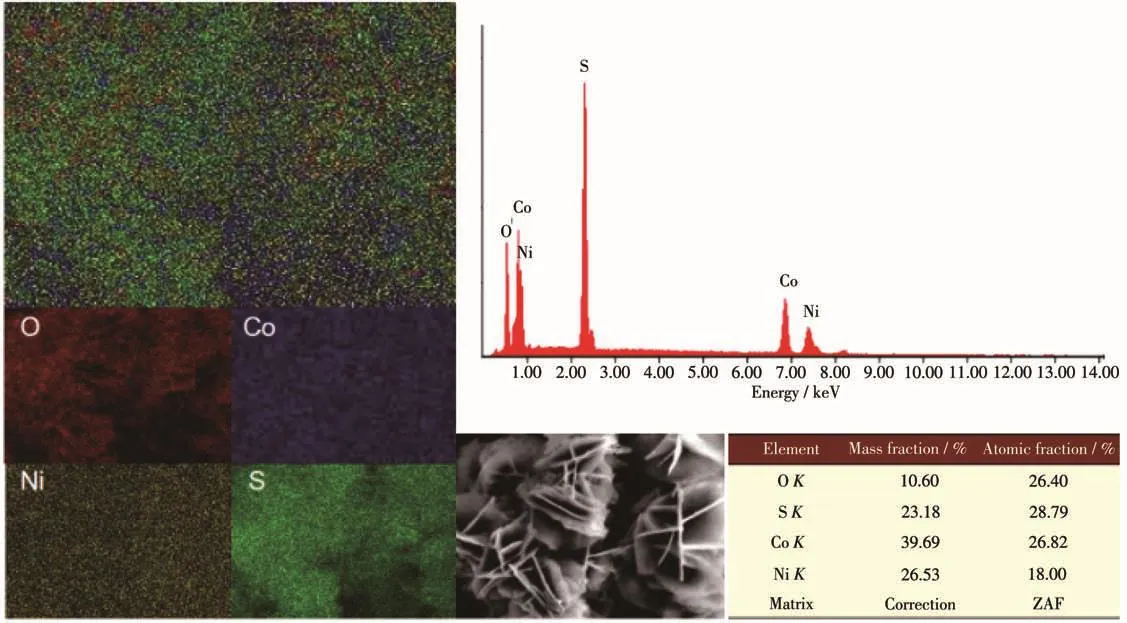
Fig.3 Element mappings and EDS spectrum of Ni-Co-S-O/NF(1∶2)composite
The XPS survey spectra for the prepared Ni-Co-SO/NF(1∶2)composite are shown in Fig.4.According to the full spectrum data in Fig.4a,the peaks of Ni2p,Co2p,S2p,O1s,and C1shave been marked.As revealed in Fig.4b,the binding energies(BEs)of 856.1 and 873.7 eV are assigned to Ni2p3/2and Ni2p1/2,along with two satellite peaks at 862.5 and 880 eV,demonstrate the coexistence of Ni2+and Ni3+[12-13].As shown in Fig.4c,two prominent peaks at 781.6 and 797.1 eV are assigned to Co2p3/2and Co2p1/2respectively,along with two satellite peaks at 784.2 and 805 eV.The spin-orbit splitting value of Co2p3/2and Co2p1/2was 15.5 eV,which can be ascribed to the coexistence of Co3+and Co2+[14].As shown in Fig.4d,the BEs at 161.4 eV for S2p3/2and 163.8 eV for S2p1/2are spin-orbit characteristics of S2-.The peak at a binding energy of 163.8 eV is also a typical of metal-sulfur bond(M—S)in Ni-Co-SO/NF(1∶2)material,while the component peak present at 168.6 eV is suggested the sulfur oxides oxidized by air at the surface[15-16].In Fig.4e,the BEs of O1sXPS spectra at 530.9 and 531.6 eV correspond to O2-and OH-,respectively[17].The peak at BE of 532.3 eV represents the oxygen combined with the metal[18-19].The XPS spectrum of C1sis shown in Fig.4f,the peak at about 284.9 eV corresponds to the carbon element adsorbed from the air,and the peak at 287.9 eV corresponds to the carboxyl group in urea[20-21].
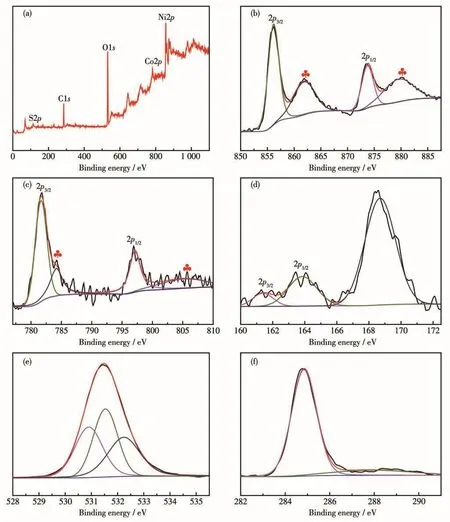
Fig.4 XPS spectra of Ni-Co-S-O/NF(1∶2):(a)survey;(b)Ni2p;(c)Co2p;(d)S2p;(e)O1s;(f)C1s
2.2 Electrochemical performance
The LSV test was used to study the OER performance in alkaline solution.Fig.5 shows the LSV curves of Ni-Co-S-O/NF samples prepared with different mass ratios and bare NF substrates in 1 mol·L-1KOH alkaline solution.As shown in Table 2,the overpotential(η10)required to achieve a 10 mA·cm-2current density of Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1)and Ni-Co-S-O/NF(3∶1)was 145,88,61,68,76 and 114 mV,respectively.It was obvious that the overpotential(η10)of Ni-Co-S-O/NF composites were much lower than bare NF(370 mV).Compared with previously reported single metal sulfides,the catalytic performance of Ni-Co-S-O/NF(1∶2)has been significantly improved.This may be due to the synergistic electronic effect of the two metal elements,the octahedral and tetrahedral sites of the spinel crystal structure have Co and Ni metal cations with various valence states,respectively,with the large S2-anions to form a closely packed arrays,resulting in more octahedral active sites of Co3+have efficient OER activity at a very low overpotential[22],which makes the Ni-Co-S-O/NF composites exhibit better catalytic activity in the OER process.
As shown in Fig.5,we can find that the Ni-Co-SO/NF samples prepared withwNi∶wCo=0∶1,1∶3 and 1∶2 did not show the oxidation peak,which maybe because of high Co contents in the samples.The Ni-Co-S-O/NF samples prepared withwNi∶wCo=1∶1,2∶1 and 3∶1 showed the obvious oxidation peak at 1.40~1.50 V(vs RHE),that maybe because of the high Ni contents in the samples,and Ni2+is oxidized to Ni3+[23].Obviously,the samples with higher Co content had shown a distinct lower OER overpotentials(η100)to reach 100 mA·cm-2than those with higher Ni contents,indicating that the catalytic activity of Co element may be higher than that of Ni element.By comparison of the overpotentials in Table 2,the Ni-Co-S-O/NF(1∶2)only need the lowest overpotentials of 61 and 313 mV to achieve the current density of 10 and 100 mA·cm-2,respectively,indicating that 1∶2 was the most suitable initial solution mass ratio of Ni and Co for the synthesis of Ni-Co-S-O/NF catalyst.
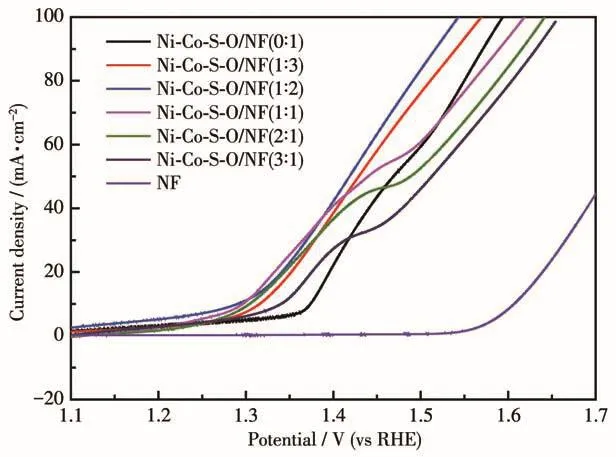
Fig.5 LSV curves of Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1),Ni-Co-S-O/NF(3∶1)catalysts and bare NF substrates in 1 mol·L-1 KOH
The Tafel slope test can be used to study the kinetic performance of the OER in the water electrolysis.The smaller the Tafel slope is,the faster the current density increases and the smaller the overpotential changes,indicating the better the electrocatalytic performance is.The Tafel slopes of Ni-Co-S-O/NF samples prepared with different mass ratios and bare NF substrates are shown in Fig.6a.As can be seen from the Fig.6a,the samples prepared withwNi∶wCo=0∶1,1∶3,1∶2,1∶1,2∶1 and 3∶1 corresponded to the Tafel slopes of 184,237,155,499,463 and 369 mV·dec-1respectively.By comparison,the Tafel slope of the synthesized Ni-Co-S-O/NF sample was the smallest whenwNi∶wCo=1∶2.EIS was also often used to characterize the kinetics of materials.In Fig.6b,the Nyquist semicircular diameter of Ni-Co-S-O/NF(1∶2)was smallest among the six samples,indicating that the petal-like nanostructure of Ni-Co-S-O/NF(1∶2)had a fast faradaic process and smaller reaction resistance.which was consistent with the previous LSV results and again proved that 1∶2 was the best mass ratio of Ni and Co in initial solution for Ni-Co-S-O/NF to achieve a good electrocatalytic performance.All of the electrochemical test data is shown in Table 2.

Table 2 Overpotential and Tafel slope of the samples
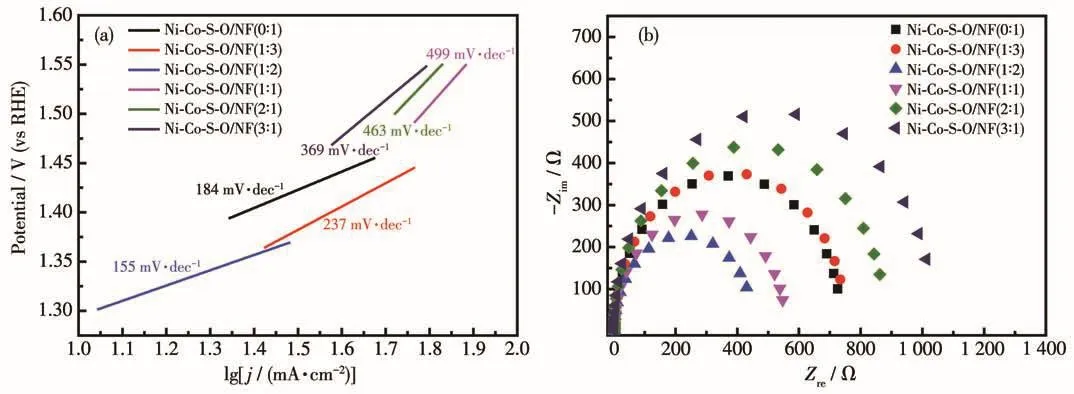
Fig.6 (a)Tafel slope of Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1)and Ni-Co-S-O/NF(3∶1)catalysts in 1 mol·L-1 KOH;(b)Nyquist plots of Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1)and Ni-Co-S-O/NF(3∶1)catalysts
The electrochemical active surface areas(ECSAs)of the Ni-Co-S-O/NF catalysts were determined through their electrical double-layer capacitance tests in 1 mol·L-1KOH.The cyclic voltammetry(CV)curves of all samples were recorded at a window from-0.205 to-0.095 V(vs SCE)with different scan rates(from 10 to 100 mV·s-1).Fig.7a is the CV curves of Ni-Co-S-O/NF(1∶2).As shown in Fig.7b,the obtained double layer capacitance(Cdl)for Ni-Co-S-O/NF(0∶1),Ni-Co-S-O/NF(1∶3),Ni-Co-S-O/NF(1∶2),Ni-Co-S-O/NF(1∶1),Ni-Co-S-O/NF(2∶1)and Ni-Co-S-O/NF(3∶1)were 20,25,46,10,18 and 8 mF·cm-2,respectively.The Ni-Co-SO/NF(1∶2)showed the highestCdl,indicating the largest ECSA and the corresponding highest surface roughness[24].This result was consistent with the SEM images in Fig.1.The three-dimensional petal-like nanostructure of the Ni-Co-S-O/NF(1∶2)provided a higher specific surface area and had more active sites.In addition,the high surface roughness of Ni-Co-S-O/NF(1∶2)made it highly hydrophilic in the electrolyte solution,which is conducive to the full progress of the reaction.
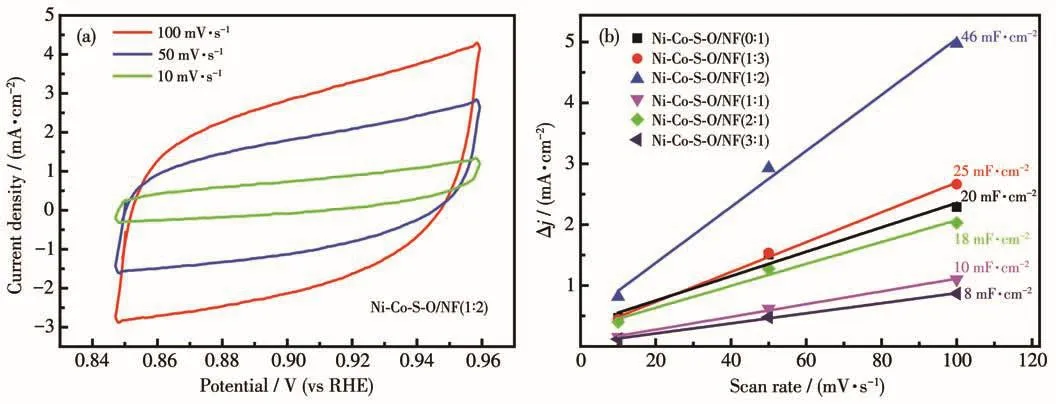
Fig.7 (a)CV curves for Ni-Co-S-O/NF(1∶2);(b)Capacitive current densities of the catalysts at 0.90 V vs RHE with a scan rate of 10,50 and 100 mV·s-1 in 1 mol·L-1 KOH
Fig.8a is the potential-time curve of Ni-Co-S-O/NF(1∶2)catalyst material at a current density of 100 mA·cm-2.It can be seen that there was almost no change in the potential under the high current density of 100 mA·cm-2after 24 h.As shown in Fig.8b,the sample after 24 h stability test can still maintain a sheet structure.The good stability under such high current density may be related to the high temperature and the high pressure conditions of the hydrothermal process,it made the Co-Ni and the substrate more tightly bonded to improve the catalytic stability of the composite material.
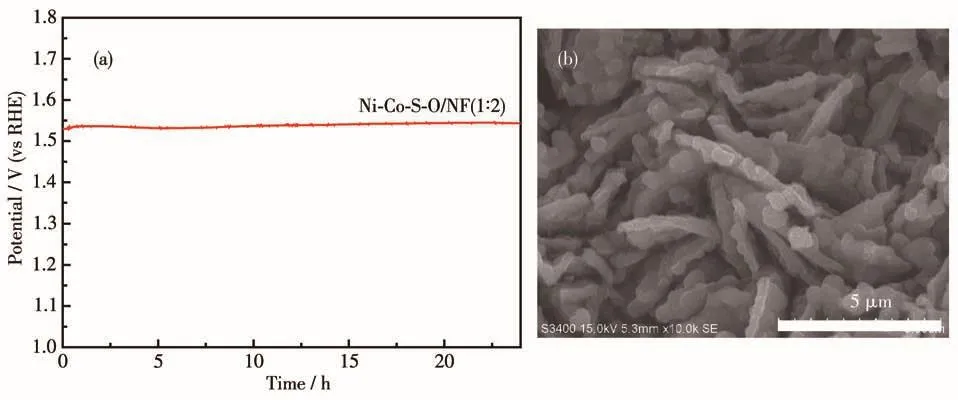
Fig.8 (a)Potential-time curve and(b)SEM image of Ni-Co-S-O/NF(1∶2)catalyst after 24 h durability test
3 Conclusions
In this paper,we used nickel foam(NF)as the substrate to prepare Ni-Co-S-O/NF composite catalysts with different mass ratio of Ni and Co in initial solution by hydrothermal method.WhenwNi∶wCo=1∶2,the prepared Ni-Co-S-O/NF catalyst material presented hierarchically connected three-dimensional petal-like nanostructures with the petal diameter was ~10 μm and the thickness was~15 nm.The hierarchically connected three-dimensional petal-like nanostructures made the Ni-Co-S-O/NF(1∶2)have the highestCdl,ECSA and surface roughness,which promoted the catalyst to be highly hydrophilic in the electrolyte solution and conducive to the full progress of the OER.Ni-Co-S-O/NF(1∶2)required the lowest overpotential 61 and 313 mV(vs RHE)respectively to reach the current density of 10 and 100 mA·cm-2and its Tafel slope was 155 mV·dec-1,showing good electrocatalytic performance.After tested at a high current density of 100 mA·cm-2for 24 h,Ni-Co-S-O/NF(1∶2)can still maintain a sheet structure,showing a good long-term durability.Therefore,1∶2 was the most suitable mass ratio of Ni and Co in initial solution for the synthesis of Ni-Co-S-O/NF catalyst.The development of this work will help broaden the application of low-cost,high-activity transition metal sulfide catalysts in the field of alkaline water electrolysis.
 無機(jī)化學(xué)學(xué)報(bào)2021年5期
無機(jī)化學(xué)學(xué)報(bào)2021年5期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Three Photochromic Co-crystals Based on Viologen Moiety
- Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
- Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
- Synthesis and Visible-Light-Driven Photocatalytic Properties of Floating BiFeO3/Expanding Perlite Photocatalysts
