Structure and Fluorescence Properties of Three 1D/2D/3D Zn/Cocomplexes Based on Flexible Tetracarboxylic Acid
LIU Lu JIN Ping-Ning YANG Jie SONG Li-Xing ZHAO Bo LI Jin-Ke HUANG Bin ZHANG Yu-Ping YANG Xiao-Xun*,( 4500)( 45000)( 455008)
Abstract:Three complexes,namely{[Zn(H2 L)(H2O)3]·H2O·0.5H4 L}n(1),{[Co(L)0.5(4,4′-bpy)]·0.5H2O}n(2)and{[Co(L)0.5(pbmb)(H2O)]·H2O}n(3)(H4 L=5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalic acid,4,4′-bpy=4,4′-bipyridine,pbmb=1,1′-(1,3-propane)bis-(2-methylbenzimidazole)),have been prepared by solvo-/hydrothermal reactions.Complex 1 belongs to a 1D chain structure.Complex 2 presents a 3D 3-fold interpenetrating framework with the Schl?fli symbol(64·7·8)(6·72).Complex 3 features a(3,4)-connected 2D network with the Schl?fli symbol(4·62)(42·62·82).Complex 1 exhibited better fluorescence properties.CCDC:1968748,1;1968750,2;1968751,3.
Keywords:Zn;Co;crystal structure;topology;fluorescence
Metal-organic frameworks,as a new kind of stable and tunable materials composing of organic bridging ligands and metal centers,have become very attractive candidates for gas storage,drug delivery,molecule separation,etc[1-5].In order to fabricate useful metal-organic frameworks materials,the researchist have made sorts of theoretical predictions and adopt various methods to control the topological structure and geometry configuration.The organic ligands as building blocks play a major role in the design of the materials.We know many multidentate aromatic carboxylic acid ligands have been widespread employed to fabricate metalorganic frameworks materials[6-9],because of their robustness and thermal stability[10].Based on our previous widespread research on metal-organic frameworks materials,we predict that the ether-linked flexible tetracarboxylic acids ligand 5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalic acid(H4L)would be a splendid precursor for the construction of the ideal structures.Firstly,the H4L ligand can afford various coordination fashions by deprotonated to the corresponding species(H3L-,H2L2-,HL3-,and L4-).Secondly,the existence of the —(CH2)6— spacers and the two methoxy groups will cause the carboxyl groups to joint to metal ions in different directions,resulting in the formation of more possibilities of topologies.On the side,N-heterocyclic spacers as secondary coligand will also be a good approach that could forward structural diversity.
Hence,by introducing two N-donor coligands into the Zn/Co/H4L synthesis system,three complexes,formulated as {[Zn(H2L)(H2O)3]·H2O·0.5H4L}n(1),{[Co(L)0.5(4,4′-bpy)]·0.5H2O}n(2)and{[Co(L)0.5(pbmb)(H2O)]·H2O}n(3)(4,4′-bpy=4,4′-bipyridine,pbmb=1,1′-(1,3-propane)bis-(2-methylbenzimidazole))were successfully acquired.The photoluminescent properties of complex 1 were also covered.
1 Experimental
1.1 Materials and methods
All reagents and solvents were commercially available except for pbmb,which was synthesized according to the literature[11].FT-IR spectra were recorded on a FTIR-7600 spectrophotometer with KBr pellets in 400~4 000 cm-1region.Elemental analyses(C,H and N)were carried out on a FLASH EA 1112 elemental analyzer.The fluorescence spectra for the samples were measured by a F7000 fluorescence spectrophotometer.The excitation slit and emission slit was 2.0 nm.
1.2 Synthesis of{[Zn(H 2 L)(H 2O)3]·H 2O·0.5H 4 L}n(1)
A mixture of Zn(NO3)2·6H2O(0.029 7 g,0.1 mmol),H4L(0.022 3 g,0.05 mmol),CH3CN(2 mL)and distilled H2O(6 mL)was placed in a 25 mL Teflonlined stainless-steel container.The mixture was sealed and heated at 130℃for three days.After the final mixture was gradually cooled to ambient temperature by a rate of 5℃·h-1,the colourless crystals of 1 were acquired with a yield of 50%(based on Zn).Anal.Calcd.for C33H35O19Zn(%):C,49.48;H,4.40.Found(%):C,49.45;H,4.42.IR(KBr,cm-1):3 444(w),2 946(w),1 619(m),1 554(s),1 459(m),1 390(s),1 322(m),1 261(m),1 135(m),1 041(s),995(w),935(w),779(s),725(m).
1.3 Synthesis of{[Co(L)0.5(4,4′-bpy)]·0.5H2O}n(2)
A mixture of Co(NO3)2·6H2O(0.058 2 g,0.2 mmol),H4L(0.044 6 g,0.1 mmol),4,4′-bpy(0.031 2 g,0.2 mmol)and distilled H2O(15 mL)was heated at 120℃for two days in a 25 mL Teflon-lined stainlesssteel vessel.The purple crystals of 2 were obtained with a yield of 25%(based on Co).Anal.Calcd.for C21H18CoN2O5.5(%):C,56.64;H,4.07;N,6.29.Found(%):C,56.60;H,4.05;N,6.31.IR(KBr,cm-1):3 338(m),3 072(vw),2 942(s),2 873(m),2 356(w),1 729(s),1 668(s),1 608(s),1 558(s),1 417(s),1 386(s),1 330(s),1 280(s),1 216(s),1 182(s),1 120(s),1 041(s),889(s),815(vs),771(vs),736(s),709(vs),669(vs),630(vs),592(s),568(s),512(vs).
1.4 Synthesis of{[Co(L)0.5(pbm b)(H2O)]·H2O}n(3)
A mixture of Co(OAc)2·4H2O(0.049 8 g,0.2 mmol),H4L(0.044 6 g,0.1 mmol),pbmb(0.060 8 g,0.2 mmol),NaOH(0.016 0 g,0.4 mmol)and distilled H2O(8 mL)was heated at 120℃for three days in a 25 mL Teflon-lined stainless-steel vessel.After the final mixture was gradually cooled to ambient temperature by a rate of 5 ℃ ·h-1,the purple crystals of 3 were obtained with a yield of 41%(based on Co).Anal.Calcd.for C30H33CoN4O7(%):C,58.06;H,5.36;N,9.02.Found(%):C,58.09;H,5.33;N,9.04.IR(KBr,cm-1):3 542(w),3 451(w),3 413(w),2 946(w),1 668(w),1 558(vs),1 459(vs),1 386(vs),1 288(w),1 143(w),1 033(vs),892(w),736(s).
1.5 Crystal structural determination
The crystal data of 1~3 were collected on a Rigaku Saturn 724 CCD diffractomer(MoKα,λ=0.071 073 nm)at temperature of 293(2)K.Absorption corrections were applied by using multi-scan program.The data were modified for Lorentz and polarization effects.The structures were solved by direct methods and refined with a full-matrix least-squares technique based onF2with the SHELX-97 crystallographic software package[12]or OLEX2[13].SQUEEZE was used in the crystal structure analysis of complex 2.Crystallographic crystal data and structure processing parameters for 1~3 are summarized in Table 1.Selected bond lengths and bond angles of 1~3 are listed in Table 2.
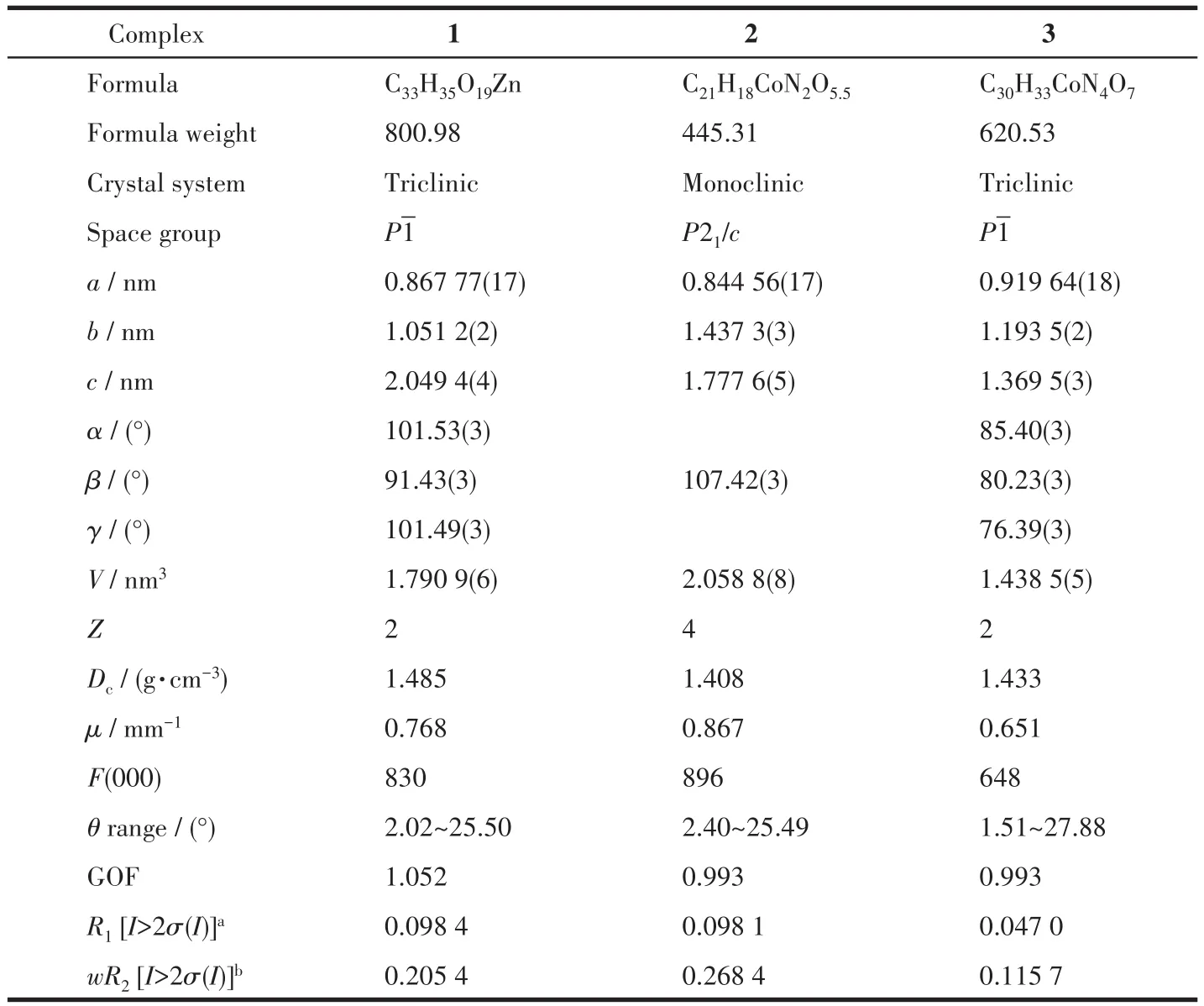
Table 1 Crystallographic data and structure refinement details for complex 1~3
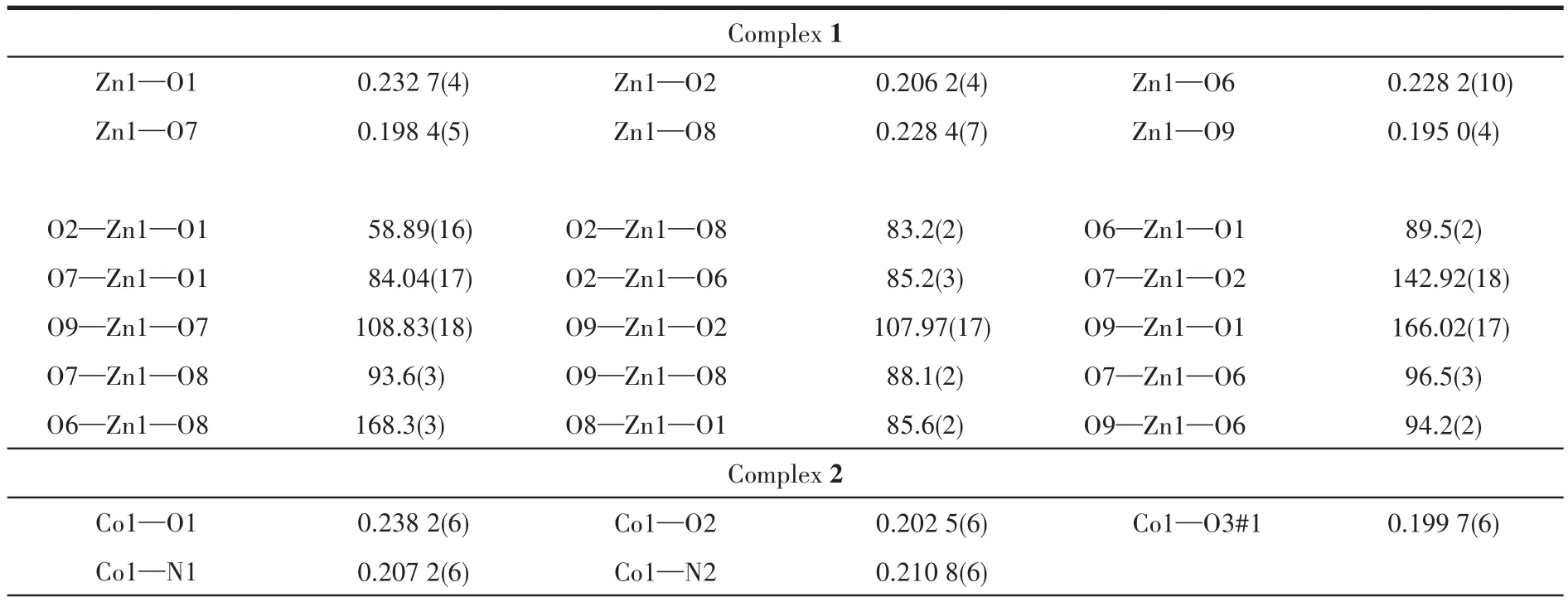
Table 2 Selected bond lengths(nm)and bond angles(°)for 1~3
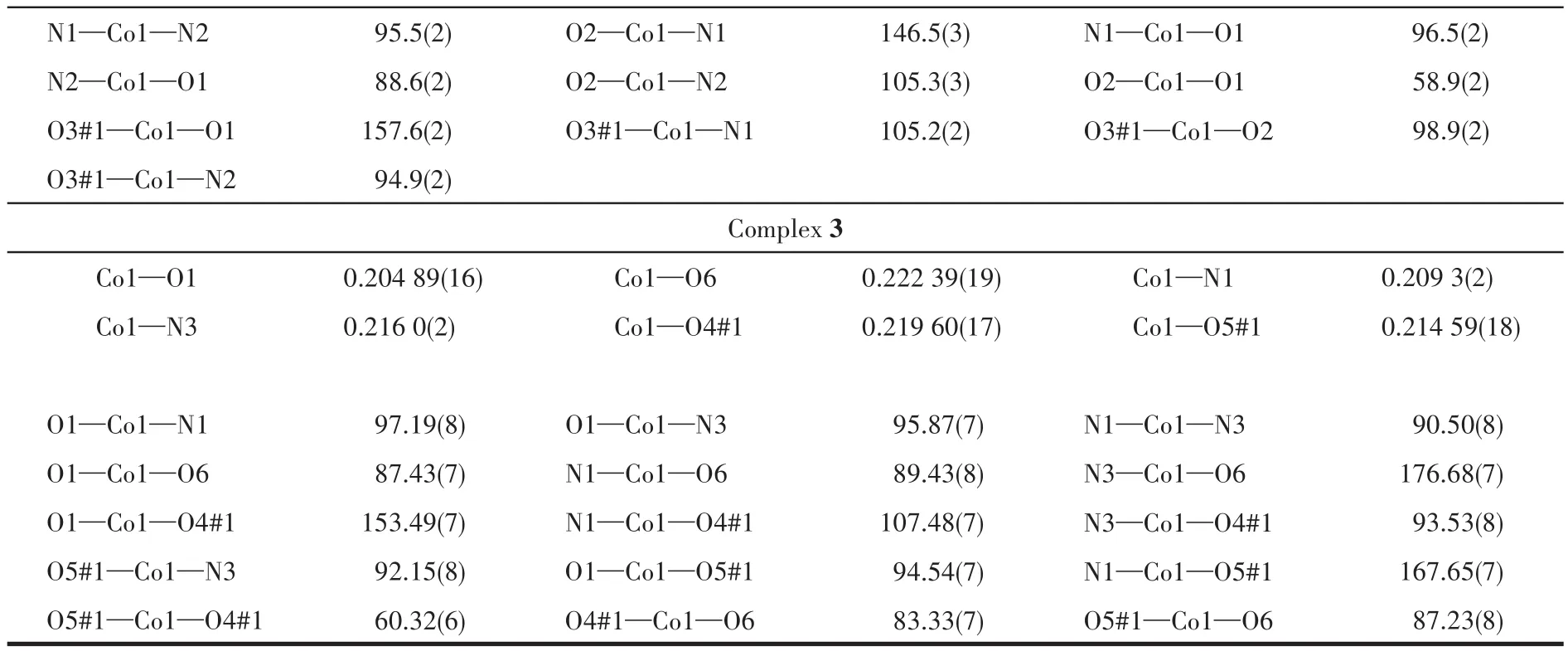
Continued Table 2
CCDC:1968748,1;1968750,2;1968751,3.
2 Results and discussion
2.1 Crystal structure of{[Zn(H2 L)(H2O)3]·H2O·0.5H4 L}n(1)
Crystallographic analysis reveals that 1 crystallizes in the triclinic system withP1 space group.As depicted in Fig.1a,the asymmetric unit comprises one crystallographically independent Znion,a H2L2-ligand,three coordinated water molecules,one lattice water molecule and half a dissociative H4L ligand.Znion is six-coordinated with coordination geometry of octahedron,which is completed by two oxygen atoms(O1 and O2)originating from one bidentate chelated carboxylate,one oxygen atom(O9)afforded by one monodentate carboxylate,and three oxygen atoms(O6,O7 and O8)from three coordinated water molecules.The Zn—O bond distances vary from 0.195 0(4)to 0.232 7(4)nm.The H4L in 1 are incompletely deprotonated(H2L2-),exhibiting two different coordination modes,and there are only two carboxylate groups participating in the coordination with Znions,as shown in Scheme 1.So,there are two different kinds of H2L2-ligands(namely,H2L2--Ⅰ and H2L2--Ⅱ).Two carboxylate groups of the first type(H2L2--Ⅰ)both adopts monodentate bridging modes(Mode Ⅰ of Scheme 1),and there exist five torsion angles:169.601°,-76.517°,-180.00°,76.517°and 169.601°.The two carboxylate groups of the second type(H2L2--Ⅱ)both adopt bidentate chelating modes(Mode Ⅱ of Scheme 1),and there exist five torsion angles of-60.766°,170.892°,180.000°,170.892°and 60.766°in H2L2--Ⅱ.On the basis of the connection mode,each Znion is joined by H2L2-anions into a 1D chain(Fig.1b).
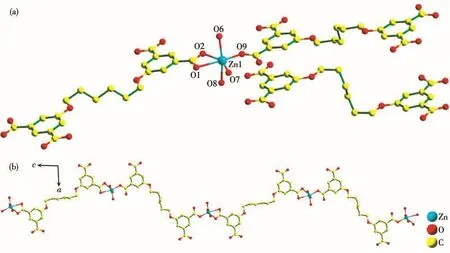
Fig.1 (a)Coordination environment of Znion in 1 with hydrogen atoms omitted for clarity;(b)View of 1D infinite chain formed by H2 L2-and Znions
2.2 Crystal structure of{[Co(L)0.5(4,4′-bpy)]·0.5H2O}n(2)
When 4,4′-bpy was introduced into the reaction system,complex 2 was obtained.Single crystal X-ray diffraction analysis declares that 2 crystallizes in the monoclinic system withP21/cspace group.As portrayed in Fig.2a,the structure of 2 contains one crystallographically unique Coion,half a L4-ligand,one 4,4′-bpy ligand and half a water molecule.Each fivecoordinated Co1 center is coordinated by three carboxylate oxygen atoms(O1,O2 and O3A)from two different L4-ligands(Co1—O1 0.238 2(6)nm,Co1—O2 0.202 5(6)nm,Co1—O3A 0.199 7(6)nm)and two nitrogen donors(N1 and N2)from two distinct 4,4′-bpy ligands(Co1—N1 0.207 2(6)nm,Co1—N2 0.210 8(6)nm).The bond angles around Co1 range from 58.9(2)°to 157.6(2)°.There is one kind of L4-ligand in 2,and there exist five torsion angles:176.737°,-179.920°,180.000°,179.920°and-176.737°.Two carboxylate groups of L4-ligand adopt chelating bidentate modes,and the other two are in bridging monodentate modes(Mode Ⅲ of Scheme 1).By this way,each Coion is joined by L4-anions into a 2D net running alongbandcaxes(Fig.2b).The 1D infinite wave chain(Co/4,4′-bpy chain)along the crystallographica-axis is formed by the 4,4′-bpy spacers and Coions with a Co…Co distance of 1.109 54 nm(Fig.2c).The Co/L4-nets are pillared by 1D chains of Co/4,4′-bpy via the Co—N connections to produce a 3D framework(Fig.2d).Topologically,the Co cation can be clarified as a 4-connected node,which is connected to four equivalent nodes through two 4,4′-bpy ligands and two L4-anions.Each L4-anion links four Coions,so the L4-is taken as a 4-connected node.Furthermore,the 4,4′-bpy is simplified as linear linkers.So,2 represents a 3D topological net(Fig.2e)and the Schl?fli symbol for 2 is(64·7·8)(6·72).Careful inspection of the structure of complex 2 suggests that it is a 3D 3-fold interpenetrating framework,as shown in Fig.2f.
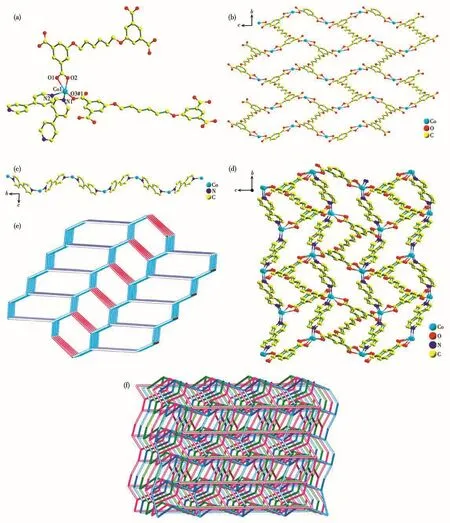
Fig.2 (a)Coordination environment around the Co centers in 2;(b)2D sheet structure constructed from Cocenters and parts of L4-anions;(c)1D infinite chain constructed by 4,4′-bpy and Co( ions along a axis;(d)Schematic view of 3D architecture of 2;(e)Single 3D topology network;(f)Schematic representation of 3-fold interpenetrated topology nets for 2
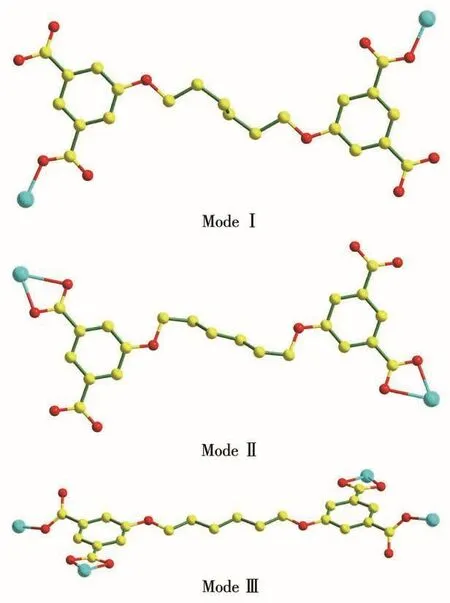
Scheme 1 Schematic view of versatile coordination modes of H2 L2-/L4-ligand
2.3 Crystal structure of{[Co(L)0.5(pbm b)(H2O)]·H2O}n(3)
The X-ray structural determination manifests that complex 3 is a 2D network.The asymmetric unit of 3 possesses one crystallographically independent Coion,half a L4-ligand,one pbmb ligand,one coordinated water molecule and one lattice water molecule.In accordance with Fig.3a,Co1 ion is in a six-coordinated mode,in which Co1 is equatorially bonded to three carboxylic oxygen atoms(O1,O4A and O5A)from two different L4-ligands(Co1—O1 0.204 89(16),Co1—O4A 0.219 60(17),Co1—O5A 0.214 59(18)nm)and one N atom from one pbmb ligand(Co1—N1 0.209 3(2)nm).The two axial sites on the metal are occupied by one N atom from one pbmb ligand(Co1—N3 0.216 0(2)nm)and one carboxyl O from one coordinated water molecule(Co1—O6 0.222 39(19)nm).The bond angles around Coions range from 60.32(6)°to 176.68(7)°.
There are also one kind of L4-ligand in 3,and there exist five torsion angles:177.774°,-176.531°,180.000°,176.531°and-177.774°.Two carboxylate groups of L4-ligand adopts chelating bidentate modes,and the other two are in bridging monodentate modes(Mode Ⅲ of Scheme 1).By the coordination mode,the L4-anion acts as a bridging ligand to bind the Coions forming a 1D chains alongbdirection(Fig.3b).The pbmb adopts asymmetricaltrans-conformation with two differenttorsion angle of 90.508°and 102.50°.Co1 atom and symmetry-related Co atom are connected by two pbmb ligands forming a[Co(pbmb)]2unit with a 20-membered metallorings.The bridged Co…Co distance alongμ-pbmb is 0.997 38 nm.Two neighboring 1D Co/L4-chains are connected together by sharing the metal ion from[Co(pbmb)]2unit,leading to the formation of the 2D layer of 3 alongaandcaxes(Fig.3c).Better insight into the 2D sheet can be fulfilled by topology analysis.Each Coion links two L4-and one[Co(pbmb)]2unit.As a consequence,the Coion is regarded as a 3-connector.While each L4-anion links four Coions,hence,the L4-can be known as a 4-connected linker.At last,the whole structure of 3 can be rationalized as a 2D topology network with the Schl?fli symbol(4·62)(42·62·82)(Fig.3d).
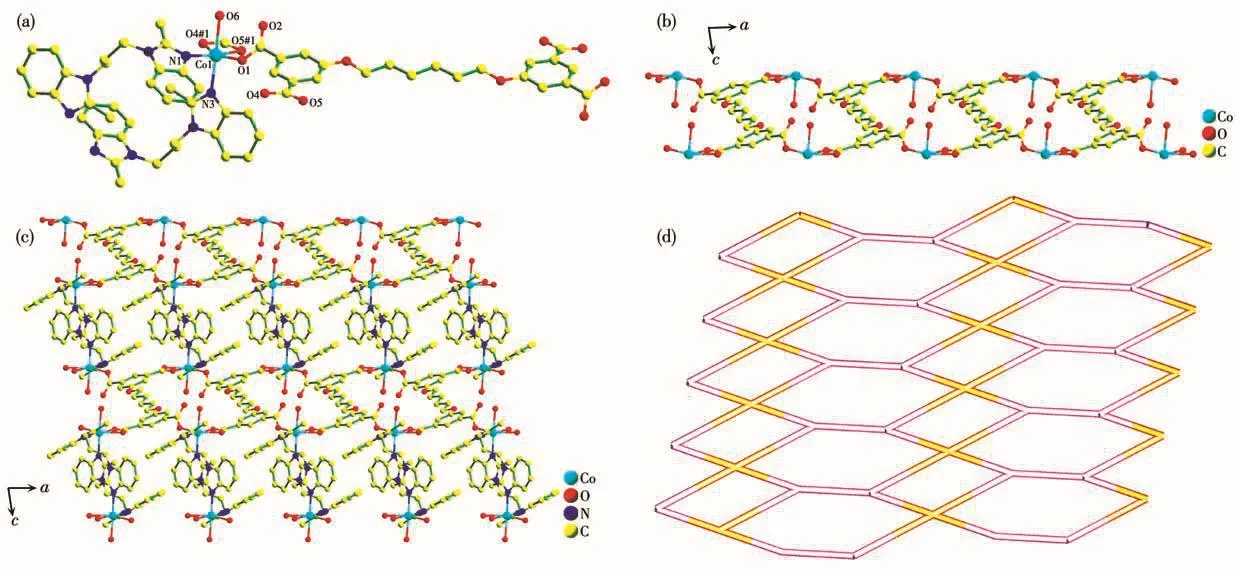
Fig.3 (a)Coordination environment of Co ion in 3 with hydrogen atoms omitted for clarity;(b)1D Co/L4-chains;(c)2D layer structure of 3;(d)Schematic view of 2D topological network for 3 with point symbol of(4·62)(42·62·82)
2.4 Effects of coordination modes of H2 L2-/L4-anion and N-donor ligands on the frameworks of complexes 1~3
On the basis of the descriptions above,we find that the H2L2-/L4-anion can adopt different coordination modes,linking to two(complexes 1~2)or four(complex 3)metal ions.
In 1~2,each H2L2-/L4-anion all connects two Zn/Coions corresponding to Mode Ⅰ and Ⅱ,severally(Scheme 1).Different from 1~2,each L4-anion in 3 links with four Coions corresponding to Mode Ⅲ (Scheme 1).The structural diversities of 1~3 can be attributed to the disparate coordination modes of carboxylate groups and the distinction of distortion degree of flexible chain in the H4L molecule.For 1,the carboxylate group of H2L2-anion bind with the central metals in a(κ1)-(κ1)-μ2fashion and(κ2)-(κ2)-μ2fashion.This kind of coordination fashion in 1 leads to the formation of 1D Zn/H2L2-chain.For 2 and 3,the carboxylate group of L4-anion coordinate with the central metals in a(κ1)-(κ2)-(κ1)-(κ2)-μ4fashion.This type of coordination fashion in 2 gives rise to the presence of 2D Co/L4-net;while that in 3 brings about the generation of 1D Co/L4-double chain.
The N-donor ligands have a profound influence on the resultant structures of the complexes.The 4,4′-bpy in 2 bridge Coions to yield a 1D chain of Co/4,4′-bpy.The Co/4,4′-bpy chain serves as pillars,linking the neighbouring Co/L4-nets into a 3-fold interpenetrated 3D framework with a vertex symbol of(4·62)(42·62·82).In 3,asymmetricaltrans-conformational pbmb ligand coordinates with Co1 atom and symmetry-related Co atom giving rise to a[Co(pbmb)]2unit with a 20-membered metallorings.The combination of the 1D Co/L4-chain and[Co(pbmb)]2unit leads to a 2D net with point symbol of(66)2(64·82).
2.5 Thermal analyses
The thermal stability of complexes 2~3 were also estimated(Fig.4).For 2,the TGA shows that the first weight loss of 2.14% in a range of 117~163℃ is relat-ed to the loss of half a lattice water molecule(Calcd.2.02%).The overall framework of 2 began to decompose from 224℃,corresponding to the losses of L4-and 4,4′-bpy,and the CoO residue of 16.54%(Calcd.16.82%)was observed at 494℃.For 3,the TGA shows that the first weight loss of 6.28% in a range of 114~151℃is related to the loss of one coordinated water molecule and one lattice water molecule (Calcd.5.80%).The overall framework of 3 began to decompose from 292℃,corresponding to the losses of L4-and pbmb,and the CoO residue of 12.03%(Calcd.12.07%)was observed at 472℃.
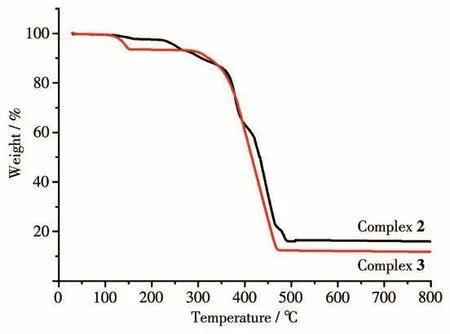
Fig.4 TG curves of complexes 2 and 3
2.6 Photoluminescence properties
Photoluminescence properties of the complexes withd10metal centers have aroused great interest owing to their latent applications[14-18].Hence,the photoluminescent spectra of the free H4L ligand and complex 1 were investigated at room temperature(Fig.5).The spectrum of free ligand H4L displayed an emission band at 429 nm(λex=330 nm).The spectrum of complex 1 exhibited the emission maxima at 339 nm(λex=277 nm).As compared to the H4L ligand,a hypsochromic shift(90 nm)was observed.The emission spectrum of 1 was parallel to that of H4L ligand,which also be mainly attributed to the intraligand emission of H4L[19].
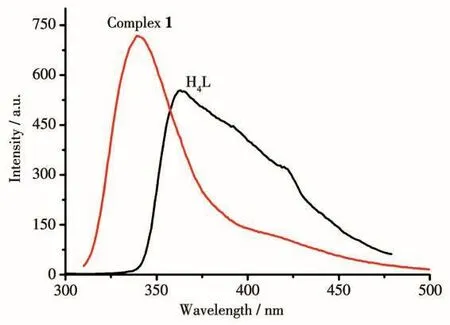
Fig.5 Photoluminescent spectra of free H4L ligand and complex 1
3 Conclusions
 無(wú)機(jī)化學(xué)學(xué)報(bào)2021年5期
無(wú)機(jī)化學(xué)學(xué)報(bào)2021年5期
- 無(wú)機(jī)化學(xué)學(xué)報(bào)的其它文章
- Synthesis and Characterization of Metal-Organic Framework Based on 2,6-Bis(4-carboxybenzylidene)cyclohexanone
- Two Metal-Organic Frameworks Built from 2,2′-Dimethyl-4,4′-biphenyldicarboxylic Acid
- Three Photochromic Co-crystals Based on Viologen Moiety
- Effect of Mass Ratio of Ni and Co in Initial Solution on Oxygen Evolution Reaction Performance of Ni-Co-S-O/NF Catalyst in Alkaline Water Electrolysis
- Two Nitronyl Nitroxide Biradical-Bridged Lanthanide One-Dimensional Chains:Crystal Structure,Magnetic Properties and Luminescent Behavior
- Synthesis and Visible-Light-Driven Photocatalytic Properties of Floating BiFeO3/Expanding Perlite Photocatalysts
