Synthesis,Structure,Magnetic and Photocatalytic Properties of Nickel(Ⅱ)Coordination Polymer Based on 1-(3,5-Dicarboxybenzyl)-1H-pyrazole-3,5-dicarboxylic Acid Ligand
WANG Lao-Bang WANG Ji-Jiang YUE Er-Lin TANG Long WANG Xiao HOU Xiang-Yang ZHANG Yu-Qi
(Yan′an City Key Laboratory of New Energy & New Functional Materials,Shaanxi Key Laboratory of Chemical Reaction Engineering,College of Chemistry and Chemical Engineering,Yan′an University,Yan′an,Shannxi 716000,China)
Abstract:A new coordination polymer{[Ni2(L)(bib)1.5(H2O)4]·3H2O}n(1)(H4L=1-(3,5-dicarboxybenzyl)-1H-pyrazole-3,5-dicarboxylic acid,bib=1,4-bis(1-imidazolyl)benzene)was synthesized by hydrothermal method and characterized by single-crystal X-ray diffraction,elemental analyses and thermogravimetric analysis.Complex 1 features a 3D network structure.The variable temperature magnetic susceptibility measurements reveal that there are antiferromagnetic interactions between the Ni(Ⅱ)ions in complex 1.In addition,photocatalytic experiments indicated that complex 1 exhibited high photocatalytic activity up to 82.7% for the degradation of methylene blue pollutant.CCDC:2030837.
Keywords:coordination polymer;Ni(Ⅱ);1-(3,5-dicarboxybenzyl)-3,5-pyrazole dicarboxylic acid;magnetic property;photocatalytic properties
Coordination polymers(CPs)[1-2]as an appealing class of high ordered and crystalline porous functional materials have developed rapidly in the past two decades due to their novel and diverse structures and unique properties,and significant progress have been made in gas storage and separation[3],luminescent[4],sensors[5],drug carriers[6],heterogeneous catalysis[7],magnetism[8]and other fields[9-10].Great efforts have been made to construct CPs by a rational selection of metal ions and bridging/chelating organic ligands[11-12].Among these ligands,pyrazole carboxylic acids are one of the intriguing ones in constructing the CPs with interesting topologies and functional properties owing to their diversity of the coordination modes,high structural stability,good flexibility and strong coordination ability[13-14].For example,Li et al.[13]reported three Co(Ⅱ)-CPs based on the combination of flexible 1,2-,1,3-,1,4-bis(1,2,4-triazol-1-ylmethyl)benzene and rigid 5-nitroisophthalate.Three new coordination polymers possessing two types of structures through employing a 1-(3,5-dicarboxybenzyl)-3,5-pyrazole dicarboxylic acid(H4L)ligand and two various N-donor ligands under similar conditions were described by Hu and Liu[14].
On the other hand,the influence of metal ions is also key factor for the preparation of CPs besides the impacts of the main ligand and ancillary ligand[15-16].Among these metal ions,the Ni(Ⅱ)ions were extensively used to construct CPs due to its nontoxic,abundant earth′s crust element,relatively large radius,relatively large electronegativity and a high ligand-field stabilization energy(LFSE)[17].Therefore,it is possible that they can generate CPs base on Ni(Ⅱ)ions with rich topological structures and various properties.
Recently,we are interested in the construction of CPs from the carboxyl derivatives,such as terphenyl-2,2′,4,4′-tetracarboxylic acid[18],3,3′,5,5′-benzene-1,3-biy-ltetrabenzoic acid[19-20],2,2′-oxybis(benzoic acid)[21-22]and 5-(3′,4′-dicarboxylphenoxy)isophthalic acid[23].They can coordinate with oxygen atoms from carboxyl groups and some auxiliary ligands with nitrogen atoms.As an extension of our investigation of CPs based on the carboxyl acid ligands,we combine H4L as the main ligand and 1,4-bis(1-imidazolyl)benzene)(bib)as the auxiliary ligand as well as Ni(Ⅱ)ion for the construction of CPs.Herein,we report the synthesis and structure characterization of a new Ni(Ⅱ)-CPs,namely{[Ni2(L)(bib)1.5(H2O)4]·3H2O}n(1).Furthermore,the thermogravimetric,magnetic and photocatalytic properties of complex 1 have been also investigated in detail.
1 Experimental
1.1 Materials and methods
All synthetic reagents and solvents employed were commercially available and used directly without further purification.The C,H and N elemental analyses were conducted with a PerkinElmer PE-2400 elemental analyzer.The FT-IR spectrum(400~4 000 cm-1)was recorded on a Nicolet 170SX FT-IR spectrophotometer.Thermal gravimetric analysis(TGA)was performed with a NETZSCH STA 449F3 thermal gravimetric analyzer in flowing nitrogen at a heating rate of 10 ℃·min-1.In a range of 2~300 K,the magnetic susceptibility data were investigated by using Quantum Design MPMS SQUID VSM instrument.The ultravioletvisible spectra were measured using a UV-2700 spectrophotometer.Powder X-ray diffraction(PXRD)patterns were recorded with a Bruker D8ADVANCE diffractometer operating at 40 kV and 40 mA using Cu Kα radiation(λ=0.154 18 nm)at a scanning rate of 2(°)·min-1from 5°to 50°.
1.2 Synthesis of{[Ni2(L)(bib)1.5(H2O)4]·3H2O}n(1)
A mixture of Ni(NO3)2·6H2O (29.1 mg,0.10 mmol),H4L(16.7 mg,0.05 mmol),bib(10.5 mg,0.05 mmol),NaOH(8.00 mg,0.20 mmol)and 13 mL H2O was stirred for 30 min and then placed in a 25 mL Teflon-lined stainless-steel vessel and heated at 160℃for 4 d.Green block crystals of 1 were obtained.Yield:34%(based on bib).Anal.Calcd.for C32H35N8O15Ni2(%):C,43.23;H,3.97;N,12.60.Found(%):C,43.45;H,3.86;N,12.82.IR(KBr,cm-1):3 414(w),2 374(w),2 343(w),1 605(s),1 531(s),1 352(w),1 313(s),1 063(w),835(s),761(w),720(w),655(w).
1.3 X-ray crystallographic studies
Diffraction intensities for the complex 1 was collected at 293(2)K on a Buker SMART 1000 CCD diffractometer employing graphite-monochromated Mo Kα radiation(λ=0.071 073 nm).A semiempirical absorption correction was applied using SADABS program[24].By using SHELXS 2014 and SHELXL 2014 programs,the structure was solved by direct methods and refined by full-matrix least-squares on F2[25-26].All non-hydrogen atoms were refined anisotropically and all of the hydrogens were geometrically placed in calculated positions.The detailed crystallographic data are presented in Table 1.Selected bond lengths and bond angles are listed in Table 2.
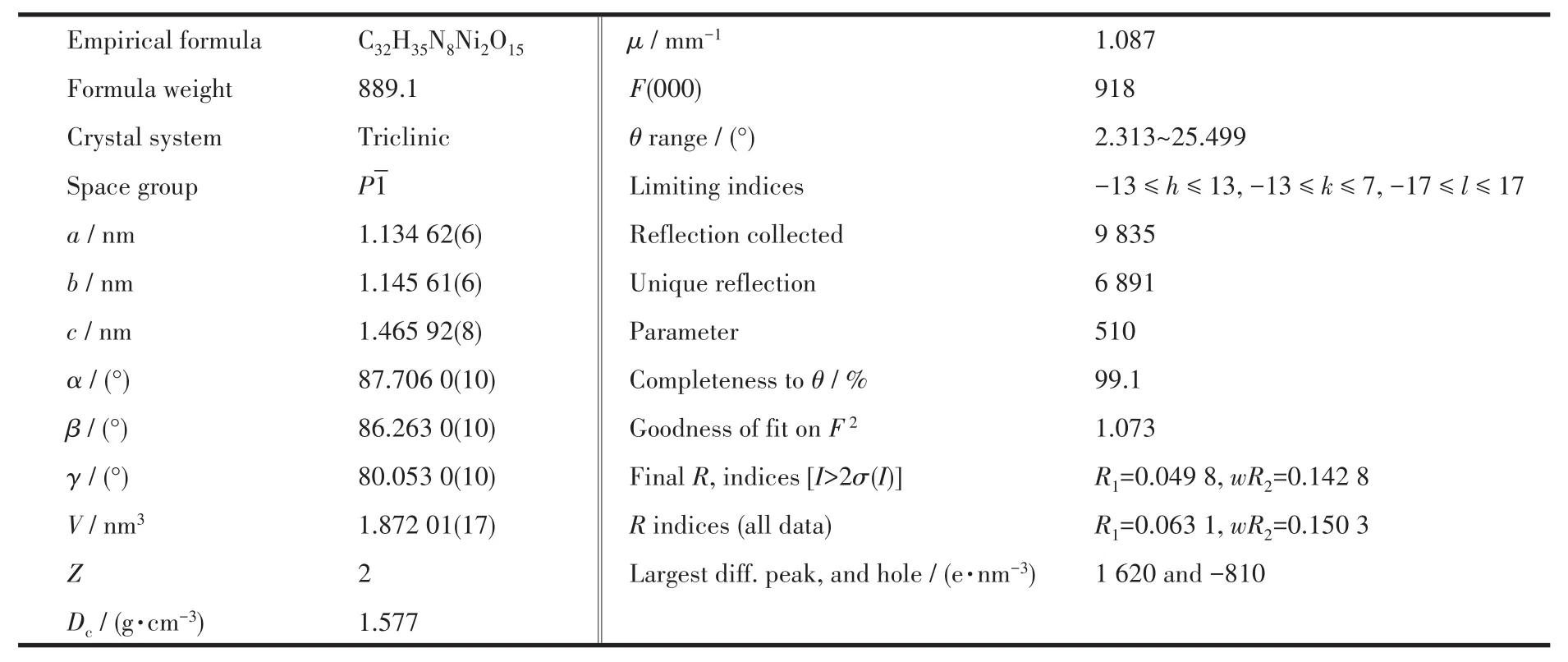
Table 1 Crystal data and structure refinement for complex 1
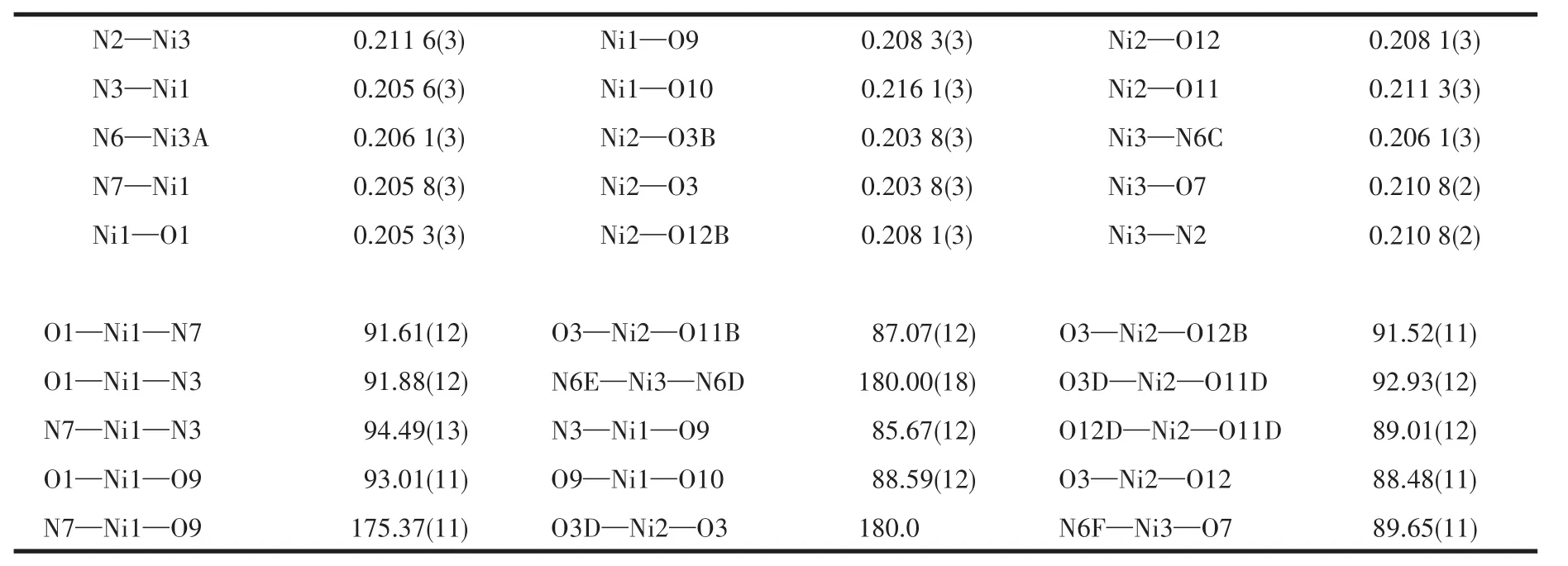
Table 2 Selected bond lengths(nm)and bond angles(°)for complex 1
CCDC:2030837.
2 Results and discussion
2.1 Description of the crystal structure
The result of X-ray diffraction analysis indicates that complex 1 crystallizes in the triclinic system,Pspace group.The asymmetric unit of complex 1 comprises two Ni(Ⅱ)ions(Ni2 and Ni3 are located in special positions),one L4-ligand,one and a half bib ligands,four coordinated water molecules as well as three free water molecules(O14,O15,O16,and O17 are placed in special positions).As shown in Fig.1,Ni1 center coordinates two oxygen atoms from two L4-ligands,two nitrogen atoms from two bib ligands and two oxygen atoms from two water molecules,displaying a slightly distorted octahedron coordination geometry.Ni2 coordinates two oxygen atoms from two L4-ligands and two oxygen atoms from two water molecules.Ni3 coordinates two oxygen atoms from two individual L4-ligands,four nitrogen atoms from two bib ligands and two L4-ligands.All the Ni—O(0.203 9(3)~0.216 2(4)nm)and Ni—N(0.205 4(4)~0.212 5(4)nm)bond lengths fall into the normal ranges(Table 2).
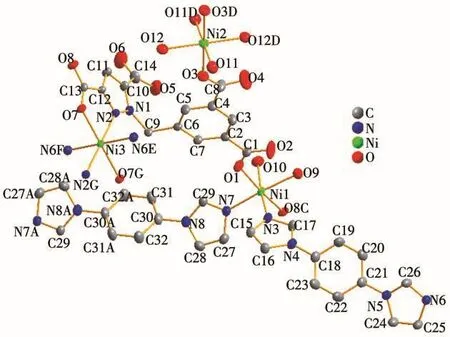
Fig.1 Coordination environment of Ni(Ⅱ)in 1
The coordination mode of H4L connecting four Ni(Ⅱ)ions in complex 1 is shown in Fig.2.Fig.3 indicates that in the ab plane,the carboxylic acid groups of H4L are connected with Ni(Ⅱ)ions to form a similar“step-like”structure.The horizontal direction of the step is the side containing the benzene ring of H4L,and the vertical direction is the side containing the imidazole ring.Therefore,the 2D structure of 1 is formed.The "step-like" structure is connected by N atoms at both ends of bib to form a 3D structure in ab plane,as shown in Fig.4.
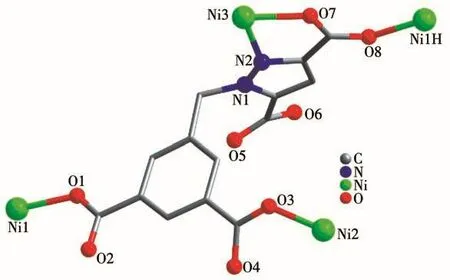
Fig.2 Coordination mode of H4L ligand in 1
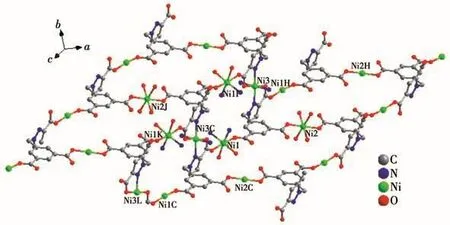
Fig.3 Two-dimensional framework of 1
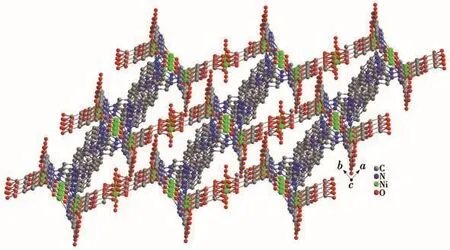
Fig.4 Three-dimensional framework of 1
2.2 Thermal gravimetric analysis
To determine the thermal stability of complex 1,TGA experiment was conducted under a nitrogen atmosphere with a heating rate of 10℃·min-1(Fig.5).As depicted in Fig.5,the first weight loss of 14.29% for complex 1 was in a range from 25 to 195℃corresponding to the removal of four coordinated H2O and three free water(Calcd.14.17%).The 3D supramolecular framework started to decompose upon further heating up to about 370℃,and the remaining weight of 16.52% might be NiO(Calcd.16.80%).A DSC(differential scanning calorimetry)curve can provide the enthalpy change and starting temperature associated with physical or chemical changes.The DSC curve in Fig.5 indicated that there were two obviously endothermic peaks corresponding to the loss of coordination water as well as free water and the collapse of the complex framework.
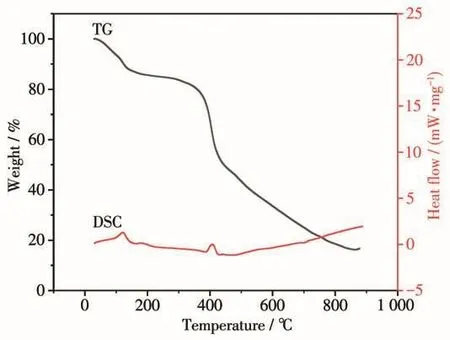
Fig.5 TGA and DSC curves for 1
2.3 Magnetic properties
Under a static field of 1 kOe,the variabletemperature magnetic susceptibility measurement of complex 1 was investigated in a range of 2 to 300 K[27](Fig.6).Over 30 K,the relationship between reciprocal susceptibility and T obeyed the Curie-Weiss Law(χM=C/(T- θ))with C=5.778 cm3·K·mol-1and θ=-3.8 K,demonstrating the antiferromagnetic interaction between Ni(Ⅱ)ions.
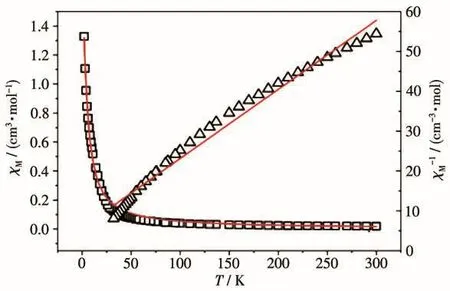
Fig.6 Plots of χMand 1/χMvs T for complex 1
2.4 Photocatalytic activity
2.4.1 Absorption spectrum and optical gap
The solid state UV-Vis diffuse-reflectance spectra of complex 1 and H4L ligand were examined at room temperature(Fig.7),respectively.It can be found that the absorption peak of complex 1 was wider and stronger than that of H4L ligand in the visible light region(greater than 400 nm).Complex 1 exhibited the main absorption peak at 280 nm in the ultraviolet region and at near 652 nm in the visible region.In addition,we drew the graph according to the Kubelka-Munk equation[28-29]and the line extrapolated from the linear portion of the adsorption edge in a plot of Kubelka-Munk function F versus energy E[30].Complex 1 behaved as a semiconductor with a band gap of 2.85 eV(Fig.8).This suggests that it could be used as a photocatalyst using ultraviolet radiation.
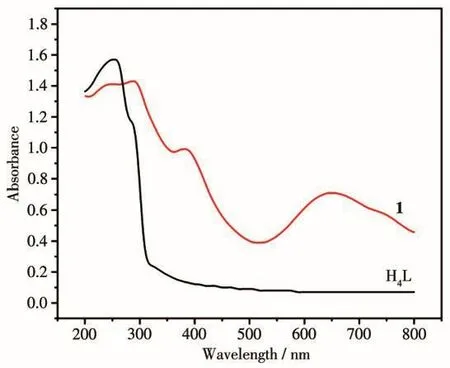
Fig.7 UV-Vis diffuse-reflectance spectra of H4L and 1
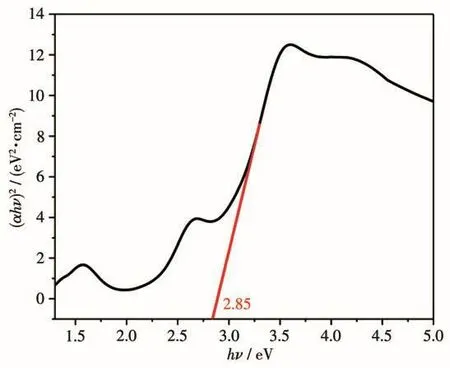
Fig.8 Kubelka-Munk transformed diffuse reflectance spectra of 1
2.4.2 Photocatalytic degradation of the organic pollutant
In this work,a 500 W xenon lamp(with a visible light filter above 420 nm)was used to provide the visible light source.Methylene blue(MB,25 mL,10 mg·L-1)was selected as the representative to evaluate the photocatalytic activity of complex 1 by studying degradation of organic pollutants.A comparative experiment without any catalyst was also investigated under the same conditions.In the process of photocatalytic degradation of pollutants,when H2O2(30%)was present,the·OH concentration increased,indicating H2O2(30%)played an important role in degradation process.So,we chose MB as the target product of the photocatalytic degradation activity experiment of complex 1,and chose to add H2O2to improve the photocatalytic degradation efficiency.By measuring the absorption intensity at λ=664 nm,we could estimate the photocatalytic activity.The results showed that the degradation rate of MB solution increased by 20% in the system with 80μL of H2O2(Fig.9).Then,we tested the degradation effect of complex 1 on MB in the presence of H2O2.Under the same experimental conditions,the control experiment without H2O2was carried out.The efficiencies of MB degradation for complex 1 are shown in Fig.10.It can be seen from Fig.10 that when H2O2was present,the degradation of MB solution by complex 1 was very obvious.Fig.11 shows the degradation effi-ciency curves of MB solution.It can be seen that 82.7% MB was photodegraded in the presence of complex 1 and H2O2after 180 min.In the control experiment,after the same time of illumination,the degradation efficiency in the absence of 1 was less than 20%.These results indicate that complex 1 may have potential applications in photocatalytic materials.
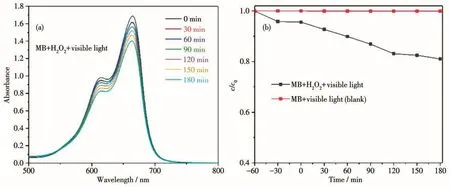
Fig.9 (a)Time-varying UV-Vis spectra of MB solution degraded by visible light in the presence of H2O2;(b)c/c0of MB solution versus time
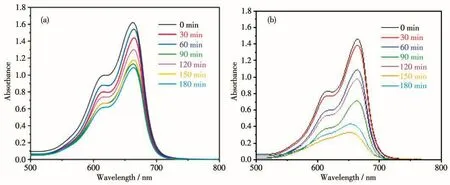
Fig.10 Time-varying UV-Vis spectra of MB solution degraded by complex 1 under visible light in the absence of H2O2(a)and presence of H2O2(b)
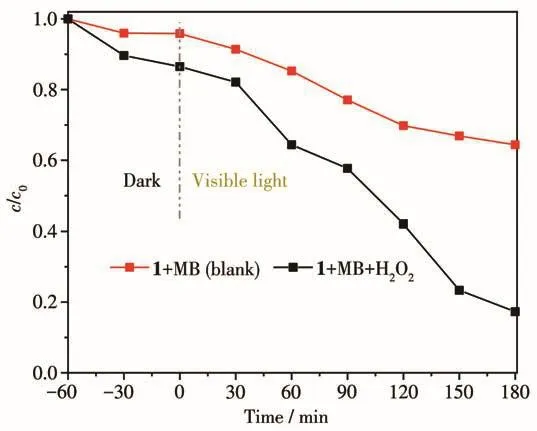
Fig.11 Degradation efficiency of MB in solution under different conditions
To confirm the recyclability of complex 1 after degradation,the PXRD pattern of complex 1 was measured.The PXRD experimental and simulated patterns are presented in Fig.12.Complex 1 performed excel-lent recyclability because the PXRD pattern of complex 1 after degradation was in accordance with the simulated one.
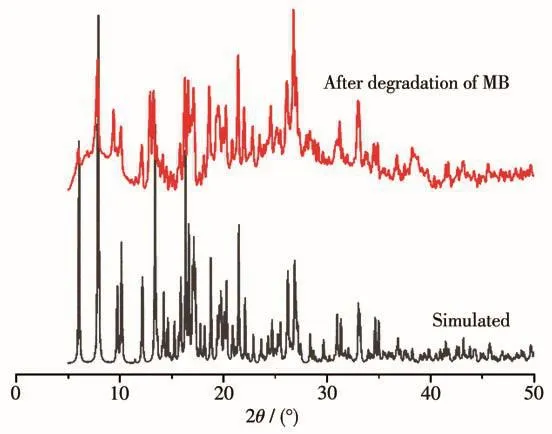
Fig.12 PXRD patterns of 1
3 Conclusions
In summary,a novel Ni(Ⅱ)coordination polymer based on a main pyrazole carboxylic acid ligand(H4L)and an auxiliary N-donor ligand(bib)was successfully synthesized by hydrothermal method.The investigation of crystallographic structure of complex 1 indicates that it features a 3D network framework.Magnetic studies reveal that there is an antiferromagnetic interaction between Ni(Ⅱ)ions in complex 1.Additionally,the investigation of the photocatalytic degradation of methylene blue suggests that complex 1 may be an excellent candidate for the degradation of MB.
 無機(jī)化學(xué)學(xué)報(bào)2021年4期
無機(jī)化學(xué)學(xué)報(bào)2021年4期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- Lamellar-Smectic Polyoxometalate Liquid Crystal Encapsulated by Branched Bola-Form Amphiphiles
- Synthesis,Structure and Catalytic Properties of Mn(Ⅱ)Coordination Polymer through in Situ Ligand Reaction
- Flexible Bis(imidazole)-Based Ligands Oriented Co(Ⅱ)-Glutarate Metal-Organic Frameworks:Syntheses,Structures and Properties
- 以四乙基氫氧化銨為模板并通過轉(zhuǎn)晶法合成高硅鋁比的SSZ-13沸石分子篩
- Fe物種改性海膽狀Nb2O5光催化降解類吩噻嗪染料
- Cu3P納米板陣列@泡沫銅三維骨架的原位構(gòu)筑及其作為高性能鋰金屬負(fù)極
