Exogenous Peroxidase Mitigates Cadmium Toxicity, Enhances Rhizobial Population and Lowers Root Knot Formation in Rice Seedlings
Priyanka Singh, Chitra Pokharia, Kavita Shah
Research Paper
Exogenous Peroxidase Mitigates Cadmium Toxicity, Enhances Rhizobial Population and Lowers Root Knot Formation in Rice Seedlings
Priyanka Singh1, Chitra Pokharia2, Kavita Shah2
(Department of Biochemistry, Faculty of Science, Banaras Hindu University, Varanasi 221005, India; Environmental Biotechnology Laboratory, Department of Environment and Sustainable Development, Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi 221005, India)
Soil cadmium (Cd) causes toxicity and oxidative stress, alters biochemical processes and root knot formation in rice. Irrigation of exogenous peroxidase (POX) together with its co-substrate H2O2(POXRice+ H2O2), is likely to have protective effect upon the biochemical and nodular changes in rice grown in Cd-rich soil. Exposure to Cd concentration of 1.00 mg/L increased oxidative stress, loss of cell viability, electrolyte leakage and root knot formation, whereas it significantly lowered the chlorophyll level and rhizobium growth in rice. Irrigation of exogenous POXRice+ H2O2to Cd-stressed rice seedlings reversed the Cd-induced alterations in rice to levels similar in control (non-stressed) seedlings. Results provided strong evidence of exogenous POXRice+ H2O2-mediated reversal and restoration of physiological and biochemical processes as well as increased resistance of rice seedlings to root knot formation. Irrigation with POXRice+ H2O2appeared to contribute towards bringing normoxic conditions in the otherwise hypoxic soil environment by enhancing the O2in pot-experiments due to reduced Cd uptake, enhanced mineral homeostasis of essential elements viz. P, Fe, Mo, Mg and Mn for maintenance of root architecture damaged by lipid peroxidation and reduction in oxidative stress by reducing Cd-induced reactive oxygen species generation. Therefore, the mitigation of Cd-toxicity in rice through this novel approach appeared to be a promising mode to limit Cd-uptake, modulate protective and tolerance mechanisms for sustainable rice yield in Cd-contaminated rice-croplands and prevent nematode attack in rice, however, more detailed studies are needed prior to large scale applications.
cadmium; peroxidase; rice; rhizobium; root knot; reactive oxygen species; antioxidant enzyme
Agricultural efficiency depends significantly on the quality of soil (Kumar et al, 2014). Rapid urban development, industrial extension and other anthropogenic activities have led to a large load of toxic elements such as agro-chemicals, heavy metals to the agricultural soil. Most of these persistently and potentially deteriorate the quality of soil by interacting with various ions present therein (Singh and Shah, 2015). Among these, cadmium (Cd), a potentially toxic, non-redox, highly water-soluble heavy metal with no apparent biological function in organisms at higher levels, has become a major ecological concern (Shah et al, 2001). Nearly 9.9?45.0 t of Cd is discharged annually into the soil environment globally (Kamnev and van der Lelie, 2000). The regulatory limit of Cd in agricultural soil is 100 mg/kg (Salt et al, 1995), but this threshold is continuously exceeding owing to anthropogenic activities.The presence of ≥ 0.32 mmol/L Cd in soil is enough for hindering the growth, morphology and activity of microorganisms inhabiting the soil (DiToppi and Gabrielli, 1999; Lei et al, 2011). These microorganisms contribute significantly towards the soil-quality and hence agricultural productivity. Rhizobiumis an importantand beneficial microorganism, which is negatively influenced in high soil-Cd levels, resulting in significant reduction in rice yield (Bianucci et al, 2011).
Rice () provides 20% to 31% of the total calories required by the global population (Shah et al, 2001; Zeigler and Barclay, 2008). Accumulation of Cd in rice grains and its gradual transfer to the food chain results in serious health issues, e.g. kidney and bone damage in human (Shah and Nongkynrih, 2007; Nahakpam and Shah, 2011; Bolan et al, 2013; Naeem et al, 2015). Therefore, it is essential to reduce the concentration of Cd below the allowable level indicated by the Codex Alimentarius Commission of FAO/WHO (Codex, 2006). Suppressing the effect of Cd and corresponding yield loss in rice grown in Cd-contaminated soils has emerged as an urgent need to ensure food safety.
Owing to its solubility in water, Cd enters readily through plant roots and is transported to the aerial parts where it impedes vital cellular and physiological processes including photosynthesis and respiration (Hasanuzzaman et al, 2017). Chlorosis, necrosis, stunted growth, cell death and disturbance in mineral homeostasis are commonly noticed in Cd-stressed plants (Sandalio et al, 2001; Nazar et al, 2012).
Among several strategies evolved by the plants to counteract Cd-toxicity, the activation of antioxidant system which scavenges the different reactive oxygen species (ROS) and protects the plants is the most important. ROS includes superoxide anion (O2??), hydroxyl radical (OH?), alkoxyl (RO?), peroxyl (ROO?), hydrogen peroxide (H2O2) and singlet oxygen (1O2),resulting as a consequence of Cd-induced toxicity (Smeets et al, 2008; Shah and Nahakpam, 2012; Singh and Shah, 2015). It is taken care of by the antioxidant defence system of plants consisting of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidase (POX) and non-enzymatic low-molecular weight antioxidants such as ascorbic acid (AsA), reduced glutathione (GSH), flavonoids, phenolic compounds, carotenoids, alkaloids and proline (Shah et al, 2001; Hasanuzzaman et al, 2017).
H2O2is a relatively stable and versatile ROS as well as an early signalling molecule for activation of plant defense mechanisms under stress conditions (Quan et al, 2008; Moskova et al, 2009). H2O2acts not as substrate but as a co-substrate for POX which further catalyzes the oxidation of wide range of organic and inorganic substrates in its presence (Yoshida et al, 2003). There occurs a cross-talk between the signaling molecules and plant hormones that regulates plant metabolism both under normal and stress environments (Shah et al, 2013; Singh and Shah, 2015; Hasanuzzaman et al, 2017). The protective role of exogenously applied low concentrations of H2O2has been reported previously in plants exposed to different stress environments (Gechev et al, 2002; Moskova et al, 2009; Sohag et al, 2020), but its significance in Cd-stress mitigation is yet unknown.
POX is a major antioxidant enzyme involved in the H2O2scavenging (Cavalcanti et al, 2004) and catalyzingthe oxidative metabolism of xenobiotics as pesticides and agro-chemicals in plants that lead to soil deterioration and growth inhibition of soil micro-organisms. POX is the main component associated with tolerant waterlilies exposed to paper mill pollution (Roy et al, 1992) and provides protection against various biotic and abiotic stresses including defense against pathogen or insect attack (Popova et al, 2008). Endogenous POX is found to be involved in Cd accumulation in a H2O2- dependent oxidation manner (Lavid et al, 2001).
To our knowledge, the role of exogenously applied POX in Cd-stressed plants on growth and population of organisms including nematodes in Cd-contaminated soil is unknown. Earlier, we reported POX from rice (POXRice) to be catalytically stable, thermo-tolerant and owing to its redox capabilities may have potential in bioremediation and stress mitigation in Cd-contaminated croplands (Singh et al, 2012). It is likely that POXRice- enriched water supplemented with its co-substrate H2O2may promote Cd-stress mitigation paralleled with resistance to nematode infection in rice grown on Cd- contaminated soil. Therefore, the present work investedthe effects of simple aqueous soil irrigation of exogenous POXRice+ H2O2on soil rhizobia, morphological, biochemical and physiological parameters including nutrient homeostasis and resistance against pathogen attack in Cd-stressed rice plants as an economically viable and sustainable tool.
Results
Cd-induced decline in growth and population of rhizobium and its corresponding restoration upon irrigation with POXRice+H2O2
Fig. 1 shows the effects of varying concentrations of Cd on the growth and survival of free-living forms ofbefore and after treatment with exogenous POXRice+ H2O2. No significant change in rhizobial population was noted during 9 h of incubation in the control and Cd treatments, however, a 45%?50% time-dependent increase in rhizobial population was noted at relatively higher concentrations of Cd (i.e. 0.50?1.00 mg/L) in combination with POXRice+ H2O2in culture experiments (Fig. 1-A). In addition, with the increasing incubation time, the rhizobial populations appeared to suppress the toxic effects of Cd as evident from the absorbance. Streak plate observations of 107-fold diluted Cd-contaminatedsoil samples revealed the presence of a heavy secretion of exo-polysaccharides and 50%?60% reduction in bacterial colonies (Fig. 1-B), whereas the plate from Cd + POXRice+ H2O2soil samples revealed a marked reduction in exo-polysaccharides secretion along with restoration of normal colony morphology and population density (Fig. 1-B). The results suggested that the Cd-stress experienced by rhizobiumis synergistically mitigated by exogenous POXRice+ H2O2.
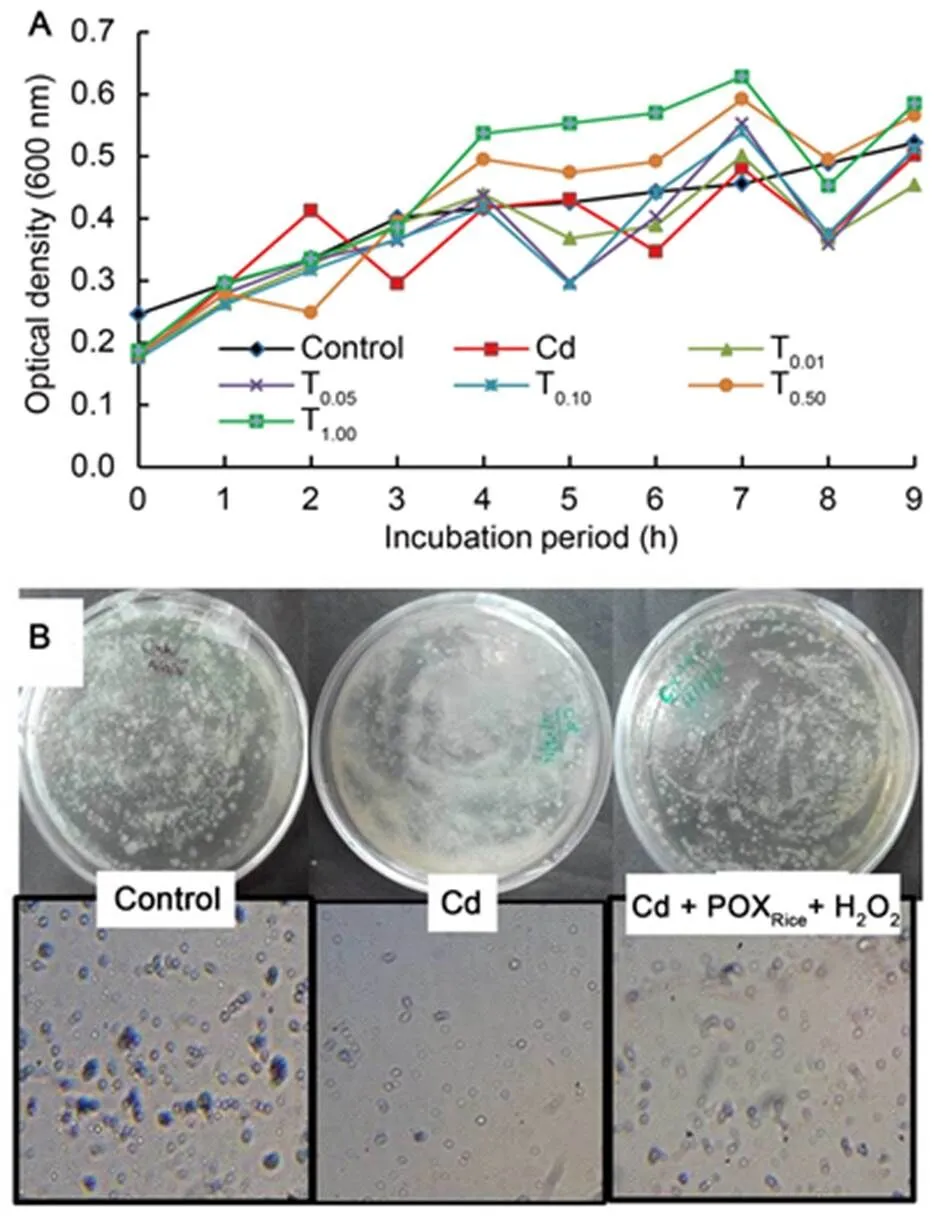
Fig. 1. Effects of exogenous peroxidase from rice (POXRice) +H2O2on growth and population of Cd-exposed
A, Growth inpopulation (600) with increasing incubation time pre-exposed to treatment (T) with 0.01, 0.05, 0.10, 0.50 and 1.00 mg/L Cd.
B,Rhizobia grown on yeast extract mannitol agarmedia in the absence (control) and presence of Cd and/or exogenous POXRice+ H2O2.
POXRice + H2O2 harmonized mineral nutrient balance in Cd-stressed rice
The effects of exogenously supplied POXRice+ H2O2on mineral homeostasis in plants exposed to Cd-stress were investigated by estimating the contents of Cd, P, Fe, K, Mo, Mg and Mn in 30-day-old rice plants (Table 1). In comparison with the control, the Cd- treated rice seedlings had elevated Cd levels, increased P content and decreased Fe, Mo, Mg and Mn levels. Addition of POXRice+ H2O2did not alter the levels of Cd in the plants, however, an increased uptake and accumulation of P, Fe, Mo, Mg and Mn were noted (Table 1). Almost 35%?40% decrease in essential mineral nutrients can be noted in Cd + POXRice-treated plants compared to the control. Results suggested a POXRice+ H2O2mediated harmonizing effect for most of the essential mineral nutrients under the Cd-stress in rice. Such Cd homeostasis explained a specific mechanism that restored the capability of plants to accumulate essential nutrients for normal metabolic functions, and at the same time avoid Cd toxicity by keeping its level below the toxicity threshold (Table 1).
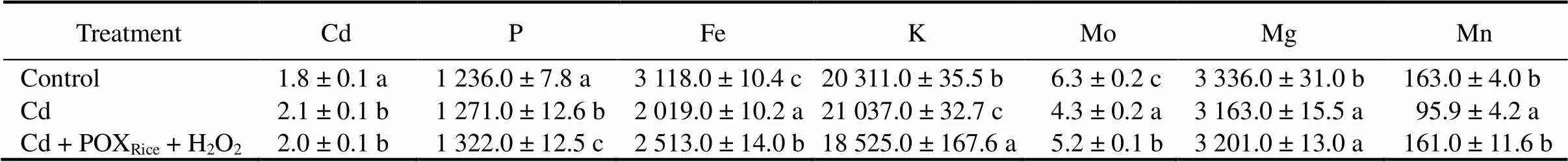
Table 1. Effects of Cd-stress on accumulation of Cd and essential mineral nutrients in rice with or without exogenous peroxidase from rice (POXRice) + H2O2 treatment to 30-day-old rice plants. mg/kg
Values are Mean ± SD (= 3).Different lowercase letters in the same column indicate significant difference among the treatments at the 0.05 level.
Synergistic effects of POXRice + H2O2 on growth and biomass of Cd-stressed rice
The effects of POXRiceand H2O2on the growth of rice plants under Cd-stress were investigated in terms of biomass (fresh and dry weights) and plant height (shoot and root lengths) (Table 2). Presence of Cd in soil resulted in an increase in plant height and higher biomass of rice seedlings during the first 15 d of growth followed by a regular suppression in shoot and root lengths and fresh weight at increasing days of growth, as compared to controls (Table 2). A gradual increase in dry weight with increasing growth days might be a result of the continuously increasing Cd- accumulation in roots of plants grown in Cd-rich soil. Exogenous application of POXRice+ H2O2to the Cd- stressed rice seedlings not only improved the plant height but also showed a significant increase in fresh and dry weights of rice seedlings compared to controls at the later growth stages. When compared for control and exogenous POXRice+ H2O2-treated plants, 40% and 110% increase in fresh weight and 202% and 151% increase in dry weight of plants were obtained at 75 and 90 d of rice growth, respectively.

Table 2. Effects of Cd-stress and simultaneous application of exogenous peroxidase from rice (POXRice) and H2O2 on growth parameters, electrolyte leakage (EL), cell viability and chlorophyll content (CC) in Cd-stressed rice plants.
Values are Mean ± SD (= 3). Different lowercase letters in the same line at each growth stage indicate significant difference among the treatments at the 0.05 level.
POXRice + H2O2 enhanced cell viability, reduced electrolyte leakage (EL) and restored chlorophyll levels in rice leaves exposed to Cd-stress
Cell viability when measured using Evans blue revealed cadmium stress caused more than 30% loss in rice plants at all days of growth (Table 2). Application of POXRice+ H2O2irrigation water to the Cd-treated plants resulted in 30?40 percent point increases in cell viability compared to rice samples from Cd-treatment alone and controls at increasing days of growth.
Plants treated with 1.00 mg/L Cd showed 68.9%, 60.6%, 30.0% and 31.0% increases in EL compared to control plants at 15, 30, 75 and 90 d of growth, respectively (Table 2). The control and Cd + POXRice+ H2O2-treated plants always had lower EL than Cd- stressed plants. POXRice+ H2O2, when applied to Cd- treated plants, significantly lowered EL (35.7%, 15.9%, 30.0% and 34.0% reduction at 15, 30, 75 and 90 d, respectively), suggesting a restoration of membrane damage during the growth period.
Exposure to Cd stress significantly reduced the chlorophyll content in rice leaves compared with those from control plants. Reductions of chlorophyll content by 41%, 32%, 28% and 27% were observed at 15, 30, 75 and 90 d of rice growth in leaves from Cd-treated plants, which were restored by 37.5%, 42.1%, 23.8% and 45.45% when supplemented with POXRiceand H2O2(Table 2).
POXRice + H2O2 alleviated Cd-induced oxidative stress in Cd-exposed rice plants
Formation of malondialdehyde (MDA) and increase in H2O2levels are the result of oxidative damage and are considered to be sensitive stress markers (Foyer et al, 1994). A gradual increase in MDA levels was observed with increasing days in Cd-stressed plants. MDA levels in 15, 30, 75 and 90 d Cd-stressed plants increased by almost 1.5?2.0 times in rice roots when compared to controls. Addition of exogenous POXRiceand H2O2to Cd-treated rice plants significantly restored the MDA contents in both shoots and roots to a baseline level at any stage of rice growth (Table 3).
Exposure of 1.00 mg/L Cd significantly increased H2O2concentrations by 1.14, 1.04 and 1.18 and 1.09 times in shoots and by 1.32, 1.26, 1.20 and 1.06 times in roots compared to controls at 15, 30, 75 and 90 d, respectively. H2O2concentrations were always higher in plants grown under Cd-stress alone (Table 3). Exogenous POXRice+ H2O2application in Cd-treated plants not only normalized the H2O2levels in shoots and roots but a lesser amount of H2O2was noted in exogenous POXRice+ H2O2-treated plants when compared to the controls.

Table 3. Effects of Cd-stress and simultaneous application of exogenous peroxidase from rice (POXRice) and H2O2 on malondialdehyde (MDA), H2O2 and oxidative (O2??) damage levels in shoots and roots of Cd-stressed rice plants.
Values are Mean ± SD (= 3).Different lowercase letters in the same line at each growth stage indicate significant difference among the treatments at the 0.05 level.
Increasing days of rice growth had increasing levels of O2??in shoots and roots of Cd-treated rice plants. Exogenous POXRice+ H2O2application followed the same pattern as for H2O2with significant reduction in Cd toxicity in shoots and roots, and the levels of O2??significantly decreased by 71%, 68%, 60% and 61% at 15, 30, 75 and 90 d of growth in shoots and 73%, 56%,57% and 59% in roots, respectively (Table 3). ExogenousPOXRice+ H2O2treatment to Cd-exposed plants loweredO2??levels compared to the controls.
The activity of CAT followed a generalized increasing trend throughout the growth period in shoots of rice plants grown under Cd-stress except for the first 15 d of growth. CAT activities increased by 44% to 83% during 30 to 90 d of growth (Fig. 2-A). Exogenous application of POXRice+ H2O2significantly lowered the CAT specific activity, which was sometimes even lower than that of the controls, suggesting removal of oxidative stress by exogenous POXRice+ H2O2in rice plants.
Exposure of 1.00 mg/LCd also affected the SOD activity in the same way as that of CAT activity. The highest level of SOD activity was found at 75 d, similar to CAT. Cd treatments seemed to exert a highly negative effect on plants in terms of SOD as its activities were 2.58, 1.71, 1.60 and 1.36 times higher compared to the controls at 15, 30, 75 and 90 d of growth, respectively. Application of exogenous POXRiceand H2O2decreased the SOD activity by 66.0%, 36.6%, 48.2% and 30.8% at 15, 30, 75 and 90 d of rice plants, respectively (Fig. 2-B).
The activities of POX increased by 13.3%, 19.0%, 21.3% and 34.0% in shootsof rice seedlings exposed to Cd-stress at 15, 30, 75 and 90 d of growth, respectively, against the controls. Results of Cd + POXRice+ H2O2treatments showed a significant decrease in POX levels in shoots compared to the Cd-treated plants (Fig. 2-C).

Fig. 2. Altered antioxidant enzyme activities.
Activities of catalase (CAT) (A), superoxide dismutase (SOD) (B) and guaiacol peroxidase (POX) (C) in 1.00 mg/L Cd-stressed rice shoots in presence/absence of exogenous peroxidase from rice (POXRice) + H2O2during 15?90 d of growth.
Values are Mean ± SD (= 3). Different lowercase letters above the bars indicate significant difference among the treatments at the 0.05 level.
POXRice + H2O2 suppressed nematode attack in Cd-exposed rice plants
The development of knots/galls in the roots of rice plants from control plants grown in agro-farm soil suggested nematode pre-infection and when cross- checked in root sections under microscope revealed presence of Meloidogyne spp. known to attack broad range of host crops.The effects of exogenously applied POXRice+ H2O2were evaluated for its ability to impart suppression for root-knot nematode attack in rice variety HUR-3022 grown in Cd-contaminated soil (Fig. 3). Our study showed a marked variation in susceptibility and sensitivity to M.infection among the rice plants examined from Cd and Cd + POXRice+ H2O2treatments. Out of nine plants (three from each set viz. control, Cd-treated and Cd + POXRice+ H2O2treatment) observed, the control and Cd + POXRice+ H2O2-treated plants had less than 10 galls per plant (which are typically hook-shaped at the root tip), and of these, Cd + POXRice+ H2O2-treated plants, on an average, exhibited the least number of galls (4 galls per plant), showing maximum suppression in nematode attack. Cd-treated plants were observed with significantly higher number of knots per plant (Fig. 3-B) in addition to growth reduction, stunting, wilting and chlorosis during the study period. The various stages of these obligate sedentary endo-parasite life cycles were observed in histopathological studies of moderate sized knots (Fig. 3-A). Furthermore, a complex network of nematode-feeding fungal mycelia was adhered to root knots in each experimental set (Fig. 3-C). A branched fungal hypha with typical non- constriction rings associated to nematode-killing and feeding upon observed in histopathological examination is shown in Fig. 3-D.
Discussion
Rice cultivation results in increased microbial biomass, respiration and bacterial and fungal abundance in paddy soils, collectively termed as rhizospheric effect (Zhang et al, 2016). Contamination of arable soil with Cd is a serious agricultural problem as it disturbs the plant rhizosphere structure, which forms an important soil ecological environment for plant-microbe interactions. High solubility of Cd in water allows it to enter the plant roots where its prolonged exposure disrupts the cellular homeostasis and enhances the production and accumulation of ROS, resulting in oxidative damage to lipids, proteins, DNA, intrinsic membrane properties and ultimately cell death and dramatic reduction in crop productivity in a concentration-dependent manner(Gill and Tuteja, 2010). Cd influx in plants inhibits the activities of enzymes involved in photosynthetic Calvin cycle (Sandalio et al, 2001), carbohydrate metabolism (DiToppi and Gabrielli, 1999) and phosphorus metabolism (Shah and Dubey, 1998).

Fig. 3. Altered nematode (Meloidogynespp.) infection in Cd-stressed rice plants.
A, Life-stages of M.as seen in transverse section of a moderate- sized root knot under a compound microscope.
B, Number of root-knot formation (inset graph) in control, Cd-exposure and its suppression in Cd + peroxidase from rice (POXRice) + H2O2. Yellow arrows show the locations of root knots. Values in the inset graph are Mean ± SD (= 3). Different lower- case letters above the bars indicate significant difference among the treatments at the 0.05 level.
C, Complex network of nematode- feeding fungal mycelia adhered to root knots.
D, Presence of non-constriction rings in fungal mycelia indicating an increased protection to the Cd- stressed rice plants.
To avoid oxidative damage, plants have a complex antioxidative defense mechanism comprising of non- enzymatic and enzymatic components. Among these, POX is widely accepted as stress mitigating enzyme. POX is associated with several important biosynthetic processes and bears inherent redox capability due to presence of four conserved disulfide bridges with two structural Ca2+that render it with high potential in altering the toxic form of Cd as well as quenching elevated levels of Cd-induced ROS (Schuller et al, 1996).
Fig. 4 illustrates the summary of the various facets of Cd-induced toxicity in rice plants and its effect upon rhizobiumin soil, together with the suppression of Cd-stress by irrigation with exogenous POXRice+ H2O2. Our results provided evidence that the applicationof exogenous POXRice+ H2O2to 15-day-old rice plantsin pot experiments effectively alleviated the Cd-induced reduction of soil rhizobium population and modulated physiological, biochemical and pathological mechanisms associated with Cd-stress tolerance. The redox activity of exogenous POXRice+ H2O2appeared to change the Cd from a readily water soluble to a less soluble form perhaps by metal chelation through thiol group, thereby reducing the biologically available form of Cd and resulting in its reduced mobility and toxicity as also evident from the biochemical tests.
Under natural conditions, normally 20.6% oxygen is present in soil. When there is a lack of O2, the transition states of hypoxia, anoxia and reoxygenation occur, which are characterized by different O2concentrations (Blokhina et al, 2003). Addition of O2to natural soil results in hyperoxygenation in soil.
H2O2and ROS form an integral part of hyperoxic- normoxic-hypoxic-anoxic-reoxygenic conditions in plants, however, the redox balance may alter under each of these conditions depending upon the partition between different pathways for generation of ROS, thereby regulating the O2concentration in the system.

Fig. 4. Schematic representation showing altered biochemical parameters and nematode infection in Cd-stressed rice in presence/absence of exogenous POXRice+ H2O2.
The relative stabilities of POX and CAT in soil and plant control the H2O2levels in almost all compartments of the plant cells. Lamb and Dixon (1997) reported that POX beside its main function in H2O2elimination can also catalyze O2??and H2O2formation by a complex reaction wherein NADH is oxidized using trace amounts of H2O2, first produced by the non-enzymatic breakdown of NADH. The NAD?radical then converts O2to O2??and some of them dismutate to H2O2and O2. This contributes in fine regulation of ROS concentration in plants. In this study, the rice plants were kept at field saturation conditions, therefore a hypoxic condition is likely to occur. Irrigation with POXRice+ H2O2would contribute towards bringing normoxic conditions in the soil environment by enhancing O2. A low O2concentration treatment of 5% is reported to confer tolerance to hypoxic conditions (Ellis et al, 1999).
Owing to its redox properties, exogenous POXRiceappears to be instrumental in stabilizing the dynamic O2conditions that are known to prevail in the rhizosphere under Cd stress in paddy soils (Xu et al, 2020). Occurrence of root knots in rice is due to poor aeration and hypoxic conditions in rhizopshere under Cd-stress. Li et al (2019) reported that the aeration increases Cd retention in rice roots and prevents its translocation to shoots along with improving nitrogen assimilation in rice. It is also likely that exogenous POXRice+ H2O2enhances aeration in the soil environment which in turn leads to improved retention of Cd in rice roots perhaps through iron plaque formation, thereby restricting root knot formation and alleviating the effect of Cd in rice (Li et al, 2019). Growth and population studies of pure cultures ofin presence of varying concentrations of Cd in toxic range and the restoration of normal rhizobiumcolony, morphology and density observed in streak-plate culture studies (Fig. 1-B) also suggested exogenous POXRice+ H2O2treatments not only alleviate Cd- induced reduction in rhizobial population but also successfully enhance the rhizobial population in presence of higher Cd-levels (Fig. 1-A). This could also be attributed to the redox activity of exogenous POXRice, which along with its co-substrate H2O2, either changed the Cd from a readily water soluble form to a less toxic, feebly soluble chemical form, thereby reducing the biologically available form of Cd for absorption by rice plants.
Results indicated that Cd-induced disturbance in mineral homeostasis hampered mineral-driven bio- chemical events in Cd-stressed rice plants. The exogenous POXRiceapplication considerably restricted the uptake of Cd probably by interacting with Cd itself, thereby reducing the quantities (bioavailability of the metal) and the activities as well as the ionic ratios of elements in soil solution (intensity), thereby minimizing antagonistic effects of Cd on essential mineral transportation in rice plants (Tables 1 and 2; Fig. 2). Cd homeostasis and mineral balance observed in presence of exogenous POXRice+ H2O2were in accordance with the findings of Ali et al (2014) insubjected to Cd-stress
Similar to findings of Mostofa et al (2015), a significant suppression was found in growth and biomass of Cd-stressed rice plants whereas treatment of exogenous POXRice+ H2O2acted synergistically in reversing the Cd-induced growth inhibition in plants in this study. It could be that exogenous POXRice+ H2O2either exerts a strong suppressive effect on Cd-induced reduction in growth and biomass of growing rice plants or has strong stimulating effects on the growth of rice plants (Table 2). It must be mentioned that Cd-stress results in the loss of cell viability and increasing EL in rice (Shah et al, 2013), however, in the presence of exogenous POXRice+ H2O2, a remarkable restoration of the physiological parameters were observed (Table2), suggesting maintenance of ROS levels and improved membrane integrity in rice irrigated with exogenous POXRice+ H2O2herein.
In our study, the rice plants treated with Cd exhibited a severe oxidative stress in aerial tissues as evident by increased levels of H2O2and overproduction of ROS like O2??and H2O2, which was significantly restored to the basal levels by the externally supplied ROS- scavenging exogenous POXRiceenzyme (Table 3). This was in accordance with the observation by Moskova et al (2009), where a lack of change in the endogenous H2O2content in pea plants after treatment with hydrogen peroxide alone were reported.
Once formed, ROS must be detoxified as efficiently as possible to minimize eventual damage to the plants. Though the expression for antioxidant enzymes is altered under stress conditions, its upregulation has a key role in combating the Cd-induced oxidative stress (Noctor and Foyer, 1998). In this study, Cd-stress induced significant increases in levels of CAT, SOD and POX. The possible mechanism might be that at low concentrations, Cd stimulates CAT, SOD and POXactivities to enhance the basal antioxidant capacity to overcome oxidative stress. Similar results are also obtained in different plant species, including rice under Cd-stress (Shah et al, 2001; Li et al, 2012; Sun et al, 2013; Bharwana et al, 2014). In the presence of externally supplied POXRice+ H2O2, the elevated levels of antioxidant enzymes appeared to revert back to the basal levels (Fig. 2 and Table 3), suggesting inclusive irrigation with exogenous POXRice+ H2O2contributes towards regulation of ROS and antioxidant levels in rice plants grown under Cd-stress.
Several reports indicate that prolonged exposure of plants to abiotic stress, such as heavy metals, drought and extreme temperatures, results in the weakening of plant defense and enhanced susceptibility to numerous biotic stresses such as attacked by parasitic root-knot nematodes (Amtmann et al, 2008; Mittler and Blumwald, 2010; Zhu et al, 2010). They penetrate into rice roots and complete their entire life cycle inside the host tissue, which is a conspicuous limiting factor for successful rice production in all rice ecosystems. To avoid huge losses (reduction of 2.6% in grain yield for every 1000 nematodes present around young seedlings), nematode management is of prime importance. Encouraging results for plant defense against nematode attack upon combined application of exogenous POXRice+ H2O2in Cd-stressed plants were observed in this study. We noticed a correlation between increased POXRice+ H2O2levels and plant’s sensitivity to nematode attack in Cd-stressed plants, as the plant’s resistance decreased upon increasing the concentration of Cd (Fig. 3), thereby intensifying nematode infection in presence of Cd in rice plants. On the contrary, the fewer number of root knots observed in exogenous POXRice+ H2O2-treated plants appeared to be associated with altered ROS, O2and antioxidative balance resulting from positive effect of exogenous POXRice+ H2O2in amplifying resistance of rice plants to nematode infection (Fig. 3).
Our results provided strong evidence that exogenous POXRice+ H2O2not only alleviated Cd-induced reduction in rhizobial growth and population, but also successfully led to increased growth of rhizobia under the elevated concentrations of Cd. Irrigation with exogenous POXRice+ H2O2also reversed and restored the Cd-induced alterations and damages in the rice plants. The exogenous POXRice+ H2O2-mediated reversalof oxidative stress and restoration of normal physiological and biochemical processes appeared to be mainly due to reduced Cd uptake, enhanced mineral homeostasis and reduction in oxidative stress. It is therefore recommended that mitigation of Cd-toxicity in rice can be brought about through inclusive irrigation with exogenous POXRice+ H2O2that serves as a promising tool in modulating protective and tolerance mechanisms for sustainable rice yield in Cd-contaminated rice- croplands, however, more detailed study need to be carried out in future.
Methods
Chemicals and reagents
Bovine serum albumin (BSA), guaiacol, H2O2, Folin-Ciocalteauphenol reagent (Loba-Chemie), cadmium nitrate [Cd(NO3)2?4H2O] and all other chemicals used were of analytical grade (Hi-media or E. Merck, USA). Filter membrane (GV 0.22 μm) was from Millipore. Milli-Q water was used in solution preparations for elemental analysis.
Stock solution of Cd (100 mg/L) was prepared by dissolving the required amount of Cd(NO3)2?4H2O in autoclaved double distilled water. The stock solution was sterilized by filtering through Millipore filter membrane and further 2, 2 × 5, 2 × 10 and 2 × 500 dilutions were made in autoclaved double distilled water for further experimentation.
POXRiceobtained from rice plants, purified and assayed for catalytic activity according to Singh et al (2012) were used and expressed as μmol H2O2reduced per mg protein per min.
Rice material and growth condition of rice seedlings
Rice variety HUR-3022, which is an early-maturing, high- yielding and dwarf variety with long slender grains and good cooking quality, has been used for different abiotic stress tolerance and mitigation studies and therefore used in the present study. Seeds were surface sterilized and imbibed in water for 24 h. Rice seedlings raised for 90 d in plastic pots containing 1.5 kg of soil were pre-conditioned and irrigated with 1.00 mg/LCd (cadmium nitrate) solution. Soil obtained from Agro-farm, Institute of Agricultural Sciences (25o15? N, 82o59? E), Banaras Hindu University, Varanasi, India, was sandy clay loam in texture with water holding capacity of 39.16%, pH of 7.05 and electrical conductivity of 0.145 dS/m. The organic carbon, available N, P2O5and K2O contents for the soil were 0.67%, 201 kg/hm2, 25 kg/hm2and 247 kg/hm2, respectively. The population of total bacteria, fungi and actinomycetes in the tested soil were 5.5 × 10-8, 3.5 × 10-8and 4.1 × 10-8CFU/g, respectively. The seedlings from control (no Cd) and Cd-only pots were irrigated with tap water alone, however, 15 mL of POXRiceand 3 mL of 2 mmol/L H2O2mixed in 500 mL water were added to the treatment pots (Cd + POXRice+ H2O2) at 0, 15, 30, 45, 60, 75 and 90 d of rice seedling growth. Pots were maintained at field saturation capacity throughout the study period and irrigation was done as and when required. Three plants were uprooted from each pot at 15, 30, 75 and 90 d of growth, washed and used for morphological, physiological and biochemical tests in triplicate.
H2O2is required in POX-catalyzed alleviation of Cd-induced toxicity in plants (Lavid et al, 2001). To check the effectiveness of the POXRiceand H2O2mixture in real conditions, the enzyme activity was assayed in irrigation water after 15 min in control and Cd treatments, using 9 mmol/L guaiacol as substrate (Singh et al, 2012).
Measurement of nutrient balance in rice seedlings
Whole rice seedlings (30-day-old) from the control, Cd-only and Cd + POXRice+ H2O2treated pots were weighed accurately (around 500 mg) and acid digested. After digestion, the vessels were cooled, solutions were diluted to 25 mL and thereafter analyzed for concentrations of essential elements viz. P, Fe, K, Mo, Mg and Mn using a microwave plasma-atomic emission spectrometer (Model 4210, Agilent, USA).
Determination of growth parameters, cell viability and electrolyte leakage (EL)in rice seedlings
Fresh and dry weights as well as root and shoot lengths were measured at 15, 30, 75 and 90 d of growth in control, Cd-only and irrigated with Cd + POXRice+ H2O2rice seedlings. The loss of cell viability was evaluated using the Evans blue staining method (Baker and Mock, 1994). EL was measured according to Rai et al (2012) using a conductivity meter (CM-180, ELICO, India). Ten leaf discs were placed in 25 mL water and conductivities were measured after 15 min of vacuum filtration and/or autoclaved at 121 oC for 30 min. EL values were calculated using the equation:(%) =/× 100, whereis conductivity at 15 min after vacuum filtration, andis conductivity at 30 min after autoclaving (Khare et al, 2010).
Estimation of oxidative damage in rice seedlings
The oxidative stress in plant samples was estimated as a measure of lipid peroxide, chlorophyll content, H2O2formation and superoxide anion in control, Cd-treated and Cd + POXRice+ H2O2-treated rice seedlings, respectively at 15, 30, 75 and 90 d of growth. Lipid peroxide from oxidatively modified proteins of root/shoot tissues was quantified in terms of MDA content (Shah et al, 2001). An extinction coefficient of 155 mmol/(L?cm) was used in calculation and MDA content was expressed in nmol/g fresh weight. The absorbance was read at 532 nm using a blank of 0.25% thiobarbituric acid in 10% tricholoroacetic acid.
Chlorophyll content was determined according to the method of Arnon with modifications (Rai et al, 2012). The absorbance was read at 645 and 663 nm using a UV-VIS spectrophotometer(SL-159, ELICO, India) and 80% acetone as a blank. Chlorophyll content was calculated and expressed in mg/g fresh weight.
The H2O2levels in roots and shoots of rice seedlings were measured using 0.1% titanium sulphate (Rai et al, 2012). The yellow colour intensity developed was measured at 410 nm. The amount of H2O2was calculated using an extinction coefficient of 0.2 μmol/(L?cm) and expressed as μmol/g fresh weight.
Superoxide anion (O2??) was measured in the plant samples according to Shah et al (2001). The 3 mL assay mixture contained 3 mmol/L epinephrine in phosphate buffer (pH 7.5), 0.3 mmol/L NADH and the extract from plant roots and shoots. The absorbance was recorded at 480 nm and NADH dependent adrenochrome formation was recorded for 7–8 min. The amount of O2??was calculated using the extinction coefficient of 4 mmol/(L?cm) and expressed as nmol/g fresh weight. All the experiments were carried out in sealed tube under N2atmosphere to minimize oxidation and generation of ROS.
Activities of CAT, SOD and POX were assayed as before (Shah et al, 2001) in shoots of rice seedlings grown in Cd-only or in presence of Cd + POXRice+ H2O2. All the enzyme preparation proteins were determined by the method of Lowry et al (1951) using BSA as a standard.
Determination of growth and population of R. leguminosarum
Exponential growth of rhizobial culture () was recorded using a UV-Visible spectrophotometer (Model-Systronic 2202, India) at 600nm with an initial absorbance of 0.25 at 0?9 h that served as a control. Rhizobial culture + Cd (1:1) was taken as a negative control whereas treatment cultures containing varied amounts of Cd (0.01, 0.05, 0.10, 0.50 or 1.00 mg/L) and 1.0 mL of rhizobial culture (initial=0.25) together with 100 μL of POXRice[pecific activity=13.49 μmol/(min?mg)] and 75μL of 2 mmol/L H2O2were mixed thoroughly. Absorbance was recorded at 600 nm for rhizobiumgrowth with time. One gram of rhizospheric soil was diluted 107-fold and streaked on yeast extract mannitol agar(YEMA) plates to obtain rhizobiumcolonies. The characteristic changes in bacterial population were visualized in the isolated colonies under a compound microscope.
Study on nematode attack in rice grown on Cd-rich soil irrigated with POXRice+ H2O2
Soil of agricultural fields usually contains infectious nematodes that infect the next crop grown in the same field. Therefore, roots of rice seedlings from control plants grown in agro-farm soil characterized above were checked for pre-infection of nematodes in the root sections under a microscope. The roots of rice seedlings from control, Cd-treated and Cd + POXRice+ H2O2irrigated pots were then observed for the development of nematode-induced root knots/galls. Plants were uprooted at 15, 30, 75 and 90 d of growth and washed with tap water. The number of root knots of 1 mmol/L or more was counted manually. For confirmation of nematode infection, a thin-section of root knot (1 mm) was cut, stained with 1% safranin stain and observed for presence of different stages of nematode life-cycle under a microscope.
Statistical analysis
Data were statistically analyzed using analysis of variance.
Ali B, Gill R A, Yang S, Gill M B, Ali S, Rafiq M T, Zhou W. 2014. Hydrogen sulfide alleviates cadmium-induced morphophysiological and ultrastructural changes in.,110: 197?207.
Amtmann A, Troufflard S, Armengaud P. 2008. The effect of potassium nutrition on pest and disease resistance in plants.,133: 682?691.
Baker C J, Mock N M. 1994. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue.,39: 7–12.
Bharwana S A, Ali S, Farooq M A, Ali B, Iqbal N, Abbas F, Ahmad M S A. 2014. Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton.,21: 717?731.
Bianucci E, Fabra A, Castro S. 2011. Cadmium accumulation and tolerance inspp. (peanut microsymbionts).,62: 96?100.
Blokhina O, Virolainen E, Fagerstedt K V. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review., 91: 179?194.
Bolan NS, Makino T, Kunhikrishnan A, Kirkham M, Kim PJ, Ishikawa S, Murakami M, Naidu R, Kirkham M B. 2013. Cadmium contamination and its risk management in rice ecosystems.,119: 183?273.
Cavalcanti F R, Oliveira J T, Martins-Miranda A S, Viégas R A, Silveira J A. 2004. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves., 163: 563?571.
Codex. 2006. Report of the 38th session of the Codex committee on food additives and contaminants. Hague, the Netherlands. 24?28 April, 2006.
DiToppi L S, Gabbrielli R. 1999. Response to cadmium in higher plants,41: 105?130.
Ellis M H, Dennis E S, Peacock W J. 1999. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance., 119: 57?64.
Foyer C H, Lelandais M, Kunert K J. 1994. Photooxidative stress in plants.,92: 696?717.
Gechev T S, Gadjev I, van Breusegem F, Inzé D, Dukiandjiev S, Toneva V, Minkov I. 2002. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes.,59: 708?714.
Gill S S, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.,48: 909?930.
Hasanuzzaman M, Nahar K, Gill S S, Alharby H F, Razafindrabe B H, Fujita M. 2017. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress inL.: An intrinsic study on antioxidant defense and glyoxalase systems.,8: 115.
Kamnev AA, van der Lelie D. 2000. Chemical and biological parameters as tools to evaluate and improve heavy metal phytoremediation.,20:239?258.
Khare N, Goyary D, Singh N K, Shah P, Rathore M, Anandhan S, Sharma D, Arif M, Ahmed Z. 2010. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance.,103: 267–277.
Kumar M, Shah K, Chand R. 2014. Role of melanin in the biology of spot blotch pathogen of barley and its management.: Hemantaranjan A. Advances in Plant Physiology. Vol.15. Jodhpur, India: Scientific Publishers: 49?76.
Lamb C, Dixon R A. 1997. The oxidative burst in plant disease resistance., 48: 251?275.
Lavid N, Schwartz A, Yarden O, Tel-Or E. 2001. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae)., 212: 323?331.
Lei M, Tie B, Williams P N, Zheng Y, Huang Y. 2011. Arsenic, cadmium, and lead pollution and uptake by rice (L.) grown in greenhouse.,11: 115?123.
Li H B, Zheng X W, Tao L X, Yang Y J, Gao L, Xiong J. 2019Aeration increases cadmium (Cd) retention by enhancing iron plaque formation and regulating pectin synthesis in the roots of rice () seedlings., 12:28.
Li L, Wang Y Q, Shen W B. 2012. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots.,25: 617?631.
Lowry O H, Rosenbrough R J, Farr A L, Randall R J. 1951. Protein measurement with Folin-phenol reagent.,193: 265–275.
Mittler R, Blumwald E. 2010. Genetic engineering for modern agriculture: Challenges and perspectives.,61: 443?462.
Moskova I, Todorova D, Alexieva V, Ivanov S, Sergiev I. 2009. Effect of exogenous hydrogen peroxide on enzymatic and nonenzymatic antioxidants in leaves of young pea plants treated with paraquat.,57: 193?202.
Mostofa M G, Rahman A, Ansary M M U, Watanabe A, Fujita M, Tran L S P. 2015. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice.,5: 14078.
Naeem A, Ghafoor A, Farooq M. 2015. Suppression of cadmium concentration in wheat grains by silicon is related to its application rate and cadmium accumulating abilities of cultivars.,95: 2467?2472.
Nahakpam S, Shah K. 2011. Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings.,63: 23?35.
Nazar R, Iqbal N, Masood A, Khan M I R, Syeed S, Khan NA. 2012. Cadmium toxicity in plants and role of mineral nutrients in its alleviation.,3: 1476?1489.
Noctor G, Foyer C H. 1998. Ascorbate and glutathione: Keeping active oxygen under control.,49:249?279.
Popova N V, Plotnikov A N, Ziganshin R K, Deyev I E, Petrenko AG. 2008. Analysis of proteins interacting with TRIP8b adapter.(),73: 644?651.
Quan L J, Zhang B, Shi WW, Li H Y. 2008. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network.,50: 2?18.
Rai A C, Singh M, Shah K. 2012. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants.,61: 108–114.
Roy S, Ihantola R, H?nninen O. 1992. Peroxidase activity in lake macrophytes and its relation to pollution tolerance., 32: 457–464.
Salt D E, Blaylock M, Kumar N P, Dushenkov V, Ensley B D, Chet I, Raskin I. 1995. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants.,13: 468?474.
Sandalio L M, Dalurzo H C, Gomez M, Romero-Puertas M C, Del Rio L A. 2001.Cadmium induced changes in the growth and oxidative metabolism of pea plants.,52: 2115?2126.
Schuller D J, Ban N, van Huystee R B, McPherson A, Poulos T L. 1996. The crystal structure of peanut peroxidase.,4: 311?321.
Shah K, Dubey RS. 1998. A18 kDa cadmium inducible protein complex: Its isolation and characterization from rice (L.) seedlings,152: 448?454.
Shah K, Nongkynrih J M. 2007. Metal hyperaccumulation and bioremediation.,51: 618?634.
Shah K, Nahakpam S. 2012. Heat exposure alters the expression of SOD, POD, APX and CAT isoenzymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars.,57: 106?113.
Shah K, Kumar R G, Verma S, Dubey R S. 2001. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings.,161: 1135?1144.
Shah K, Singh P, Nahakpam S. 2013. Effect of cadmium uptake and heat stress on root ultrastructure, membrane damage and antioxidative response in rice seedlings., 22: 103–112.
Singh I, Shah K. 2015. Evidences for suppression of cadmium induced oxidative stress in presence of sulphosalicylic acid in rice seedlings.,76: 99?110.
Singh P, Prakash R, Shah K. 2012. Effect of organic solvents on peroxidases from rice and horseradish: Prospects for enzyme-based applications., 97: 204?210.
Smeets K, Ruytinx J, Semane B, van Belleghem F, Remans T, van Sanden S, Vangronsveld J, Cuypers A. 2008. Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress.,63:1?8.
Sohag A A, Tahjib-Ul-Arif M, Brestic M, Afrin S, Sakil M A, Hossain M T, Hossain M A, Hossain M A. 2020. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice., 66: 7?13.
Sun J, Wang R G, Zhang X, Yu Y C, Zhao R, Li Z Y, Chen S L. 2013. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes incells.,65: 67?74.
Xu C M, Chen L P, Chen S, Chu G, Wang D Y, Zhang X F. 2020. Rhizosphere aeration improves nitrogen transformation in soil, and nitrogen absorption and accumulation in rice plants.,27(2):162?174.
Yoshida K, Kaothien P, Matsui T, Kawaoka A, Shinmyo A. 2003. Molecular biology and application of plant peroxidase genes., 60:665–670.
Zeigler R S, Barclay A. 2008. The relevance of rice.,1: 3–10.
Zhang J P, Zhou X H, Chen L, Cheng Z G, Chu J Y, Li Y M. 2016. Comparison of the abundance and community structure of ammonia oxidizing prokaryotes in rice rhizosphere under three different irrigation cultivation modes., 32: 85.
Zhu Y, Qian W Q, Hua J. 2010. Temperature modulates plant defenseresponses through NB-LRR proteins.,6: e1000844.
11 April 2020;
12 August 2020
Kavita Shah (kavitashah@bhu.ac.in)
Copyright ? 2021, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.01.006
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- High-Quality de novo Genome Assembly of Huajingxian 74, a Receptor Parent of Single Segment Substitution Lines
- Growth and Photosynthesis Responses of a Super Dwarf Rice Genotype to Shade and Nitrogen Supply
- Lodging Resistance Related to Root Traits for Mechanized Wet-Seeding of Two Super Rice Cultivars
- Effects of Early- and Late-Sowing on Starch Accumulation and Associated Enzyme Activities During Grain Filling Stage in Rice
- Osa-miR439 Negatively Regulates Rice Immunity Against Magnaporthe oryzae
- RAVL1 Activates IDD3 to Negatively Regulate Rice Resistance to Sheath Blight Disease

