Surface active agents stabilize nanodroplets and enhance haze formation*
Yunqing Ma(馬韻箐), Changsheng Chen(陳昌盛), and Xianren Zhang(張現(xiàn)仁)
State Key Laboratory of Organic-inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
Keywords: nanodroplet,stability,aerosol,haze,nucleation
1. Introduction
Haze, also known as atmospheric aerosols that represent microscopic particles suspended in the atmosphere, becomes a major environmental problem now. It is not only a type of serious air pollution that affects human health, but represents one of the largest uncertainties in our prediction of climate trends.[1–3]For the formation of haze droplets, two rate limiting processes appear sequentially, including nucleation of aerosols and subsequent formation of haze droplets from pre-existing aerosol particles. For the first step, various species, including inorganic and organic compounds and water, are involved to account for the nucleation and growth of aerosol particles. Those organic and inorganic compounds are either emitted directly by plants, industry, and automobiles or formed by the spontaneous clustering of gaseous precursors.[4–7]
The second rate limiting step is the hygroscopic nucleation[8]of haze particles from the pre-existing aerosols.At water vapor subsaturation(relative humidity,RH <100%),newly formed aerosols would undergo hygroscopic growth.Instead,at water vapor supersaturation(RH >100%)aerosols may act as the cloud condensation nuclei (CCN) and induce the formation of haze droplets through absorbing and evaporating water.[9,10]Numerous studies suggested that inorganic components,such as sulfate acid,play an important role in the nucleation and growth processes.But for organic components,their role in the haze/cloud formation remains unclear.[2,11–14]
It has been recognized that less- and non-hygroscopic organic compounds can even dominate atmospheric aerosol mass concentrations and cloud condensation nuclei (CCN)activity.[11,12]This is interpreted by the so-called surface tension effect that organic molecules can effectively reduce the surface tension of water, allowing for more efficient formation of haze particles. Recent experiments confirmed that surface active organic molecules affect significantly the properties of CCN.[12]In particular, under supersaturated conditions,the formed cloud droplets based on particles composed of dicarboxylic acids or secondary organic aerosol and ammonium sulfate showed a larger diameter than that predicted by considering alone the lowering of water activity, pointing to the essential role of lowering of the surface tension.[13,14]Based on their observations,[14]the authors proposed an alternative mechanism for cloud droplet formation in mixed organic/inorganic aerosol: most of the organic material exists as an interfacial compressed film,which reduces the surface tension, allowing larger droplets to form before activation. Despite this,the role of organics in the haze/cloud formation remains unclear: there is no evidence in the atmosphere that organic species actually nucleate new particles.[2]Therefore,understanding CCN activity of aerosols containing surface active materials is one of the central topics in atmospheric science.
Inspired by aforementioned works,[12–14]in this paper we revisit the role of organic species played in haze and cloud formation,from the point of view of thermodynamics. Based on a simple theoretical analysis,we report that the adsorption of amphiphilic organics not only lowers the surface tension, but also unexpectedly stabilizes nanodroplets of specific size at water vapor supersaturation(RH >100%).We then study how the nanodroplet stability is jointly determined by the aerosol composition and RH. The existence of stable nanodroplets leads us to propose a new mechanism for haze droplet formation from the existing aerosols. In this mechanism, we stress the essential role of stable nanodroplets in the formation of haze particles: the existence of stable nanodroplets induces a non-classical two-step nucleation pathway.
2. Theory analysis
Haze particles are mainly composed of inorganic and organic compounds and solvent water.[4–7]Therefore,in our calculations each tiny aerosol particle, which consists of water,solvable inorganic salt, and partially solvable/insolvable organic acid, is assumed to be spherical and placed into an environment featured with a given RH (see Fig.1). The system is characterized at a fixed relative humidity(equivalently at a fixed water vapor pressure P)and temperature T. In the process of droplet formation,the evolution of the aerosol particle is controlled by the Gibbs free energy cost for forming the droplet. The differential form of the corresponding Gibbs free energy cost can be written as
where μiand nirepresent respectively the chemical potential and molar amount of the i component,σ is the surface tension,and A is the area of the droplet interface. Equation(1)denotes that for forming a haze particle, both the droplet volume and the interface contribute differently to ΔG.
Fig.1. Schematic diagram of a nanodroplet in thermodynamic equilibrium with an infinite vapor phase with a given relative humidity S. The droplet contains initially inorganic and organic substance and then leads to hygroscopic growth into a stable nanodroplet. We assumed that the organic substances are surface active and adsorbed on the interface of the droplet.
For simplification,we neglect the negligible partial pressure of the solute in the gas phase as well as the exchange of the solute particles between the gas and liquid droplet phase.For most atmospheric situations,the partial pressure of water vapor overtakes that of solute by several orders of magnitude.Therefore,it is the condensation of water vapor that dominates the droplet growth. With the above approximations, the water vapor in the air would continuously condensate into liquid droplet
Here, to separate the effects of organic molecules on lowering the surface tension from that on lowering of water activity,we assume that all organic molecules are surface-active and insoluble, and thus they keep staying at the droplet interface when the droplet size changes (setting fsurf=0 in Eq. (4)).If one wants to precisely determine the surface partitioning of organic molecules onto a microscopic droplet interface,the Gibbs adsorption equation can be applied. In this work, the surface tension lowering due to the surface enrichment of organic compounds is determined via a simple mixing rule,
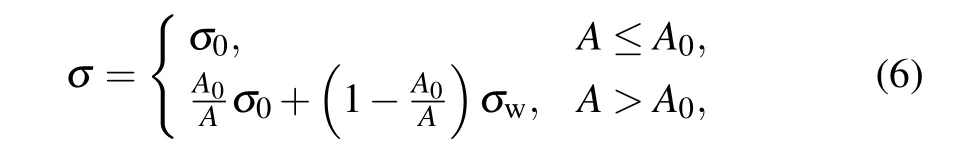
where σwand σ0are the interfacial tensions of pure water and pure organic substance, respectively, A0is the surface area of the adsorption sites that are occupied by organic molecules, and A is the total surface area of the droplet. According to Eq.(6),when A=A0,the organic substances completely cover the droplet, and the surface tension is that of the pure organic substance. When the droplet grows continuously,A >A0,the organic substances cannot completely occupy the adsorption site on the surface of the droplet. As a result,the interfacial tension is obtained by the simple mixing rule (Eq. (6)). Here, the organic substance is assumed to be lipid,with σ0=21 mN/m,and the interfacial tension of water is set to 72 mN/m.
Inserting Eqs.(2),(3),and(5)into Eq.(1)yields
for which either d2ΔG/dD2>0 or d2ΔG/dD2<0,depending on the composition of the droplets and relative humidity.
Note that in Eqs.(8)and(9),σ(D)is a function of droplet size D (see Eq. (6)). It is this variation of surface tension σ(D) with droplet size that induces the nanodroplet stability(see Fig. 2). What is important is that amphiphilic organics alone can stabilize nanodroplets at a water vapor supersaturation. This behavior is not captured by the current theory.
Fig.2. The Gibbs free energy cost for droplet formation as a function of the droplet diameter D and Sc. In this figure, the nanodroplet contains only organic substances (norg =6.4×10-22) and the amount of inorganic substances is set to zero,namely,nino=0. The inset denotes how to determine the size of stable nanodroplet,D* and the nucleation barrier for the formation of a haze particle from a pre-existing stable nanodroplet,ΔG*.
It is noticed that by ignoring the influence of the solute on the surface tension (σ = σw= const.) and letting dΔG/dD = 0, equation (8) is reduced to the conventional K¨ohler equation[16,17]that is extensively used to describe the growth of aerosols with relative humidity and activation of cloud droplets,
However,equation(10)leads to d2ΔG/dD2<0 in the absence of inorganics, indicating that the droplet is in fact thermodynamically unstable if the variation of surface tension with droplet size is not taken into account. For a single-component droplet,σ=σwand aw=1,equation(8)reduces to the Kelvin equation

2.1. Metastability of nanodroplets stabilized by amphiphilic organic molecules
By analyzing Eq. (8), we are able to investigate the effect of surface tension lowering on the free energy cost for nanodroplet formation. Note that in this section,the effect of water activity is absent by setting nino=0.The determined ΔG is shown in Fig.2 as a function of droplet diameter D.The figure indicates that for a nanodroplet containing a fixed amount of amphiphilic molecules,three different situations appear according to Eq. (8). The first scenario, which occurs at large RH, e.g., Sc>4.6% (see the solid line in Fig. 2), is characterized by a monotonic decrease of ΔG. In this case, the nanodroplets become unstable and will grow spontaneously from the initial size. On the other hand, at rather weak RH of Sc<3.0% (e.g., Sc=2.0% in Fig. 2), ΔG monotonously increases with the droplet size, indicating that the state with nanodroplets again becomes unstable. At moderate water supersaturation (3.0%<Sc<4.6%), however, the free energy profile ceases to decrease or increases monotonically. Instead,it displays two equilibrium states. One is a maximum that represents an unstable state although the nanodroplet is in equilibrium with the solvent, whereas the other corresponds to a local free energy minimum,which indicates the metastability of the nanodroplet(see the inset of Fig.2). Therefore,figure 2 clearly demonstrates that at the certain range of water supersaturation, a nanodroplet covered by amphiphilic molecules can be thermodynamically stable (in fact metastable) in an open system.
Beside the stable nanodroplet,the other equilibrium state is featured with the maximal of free energy cost (the inset of Fig.2),indicating that this type of nanodroplet is in an unstable state. In this case the nanodroplet in equilibrium with the surroundings would either shrink or grow by a small thermodynamic perturbation(see the inset of Fig.2). It corresponds to the critical nucleus for haze formation,i.e.,when a nucleus is larger than the critical one,it can grow spontaneously with the decrease of the system Gibbs free energy. Otherwise, it prefers to shrink to the stable nanodroplet with the minimal of free energy cost. As shown in the inset of Fig. 2, the free energy difference between the minimal and maximal of free energy costs, ΔG*, is identified as the free energy barrier for haze nucleation.
Figure 3(b) shows the size of stable nanodroplets as a function of the amount of adsorbed amphiphilic molecules norg. It is found from the figure that as the amount of organics norgincreases, the size of the stable nanodroplet increases monotonously, which in turn decreases the energy barrier for haze formation. This agrees with the experimental observation[14]that under supersaturated conditions, organic material reduces surface tension and thus allows larger droplets to form before activation. The size of stable nanodroplets and hence the free energy barrier for haze formation are also affected by RH (Fig.3(a)).
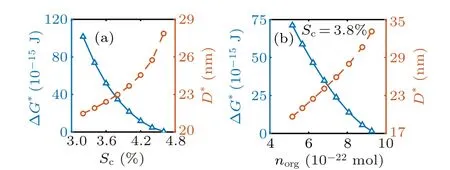
Fig. 3. The size of stable nanodroplets and the nucleation barrier for haze formation from the nanodroplets. In this figure, nino = 0.(a) The effect of water vapor supersaturation Sc under condition of norg =6.4×10-22 mol. (b) The effect of the amount of adsorbed organic substance at Sc=3.8%.
In general, strong interface affinity of organic components causes their surface enrichment and,as expected,results in additional decrease of the surface tension. What is important is that the adsorption of amphiphilic organic molecules onto a nanodroplet surface may lead to its stability even in an open environment. From a mechanical point of view, the given adsorption of surface active molecules would generate a size-dependent surface tension. It is the size-dependent surface tension that behaves as a restoring force when the nanodroplets are thermodynamically perturbed away from their equilibrium state: Under the condition of constant adsorption of organic molecules, the initial increase of the droplet size induces simultaneously the increase of the surface tension(Eq. (6)), which in turn prevents the nanodroplet from continuously growing. Similarly, when the nanodroplet initially shrinks, the resulting decrease of the surface tension would counterbalance the shrinking, resulting in a stable droplet of fixed size.
2.2. Nanodroplet stability as a function of norg and nino and relative humidity
Above we demonstrate that the surface enrichment of organics alone could lead to nanodroplet stability. Then,in what follows we show that when both the organic and inorganic components are present, they cooperatively stabilize the nanodroplet.
By analyzing Eq.(8),we are able to illustrate how the stability of bulk nanodroplets depends on RH and on the amounts of organic and inorganic compounds. Figure 4(a)shows how the amount of inorganic compounds, which are dissolved in nanodroplet, affects the droplet stability at a supersaturation condition of Sc=4%. First,the occurrence of minimal of free energy cost indeed indicates that the nanodroplets initially stabilized alone by organics are also stable if the inorganics is added. Figure 4(a)demonstrates that increasing the amount of inorganic compounds would enhance the nanodroplet stability and enlarge the droplet size.Figure 4(b)shows the relationship between the diameter for stable nanodroplets and norgat two distinct values of nino(6.4×10-22mol and 15.1×10-22mol)and RH (water vapor supersaturation of Sc=3%and subsaturation of Sc=-5%). In general,the amount of surface active species has a strong effect on the size of stable nanodroplets,both in supersaturated and at subsaturated water vapor surroundings. Differently,the effect of inorganic species depends strongly on RH. At a subsaturated environment, both the organic and inorganic compounds play an important role. Under conditions of water vapor supersaturation,however,ninoshows rather weak effect on the size of the stable droplet,in contrast to that of norg. This can be interpreted by the slow rate of decay for the effect of surface adsorption of organics(~1/D2),while the effect of solvable inorganics decays at a higher rate of ~1/D3. This observation also implies that the haze droplet activation should be more sensitive to organic compounds than to inorganic ones,and how stable nanodroplets affect haze formation is in detail discussed below.
Fig.4. The stability of nanodroplets as a function of droplet composition and relative humidity. (a)The effect of adsorbed inorganics on the free energy cost for nanodroplet formation. The symbols of solid circles denote the corresponding size of stable nanodroplets.In this figure,norg =6.4×10-22 mol and Sc =4%. (b) The diameter of the stable nanodroplets as a function of norg,nino,and Sc.
2.3. The existence of stable nanodroplets changes the pathway for haze formation
In what follows we will discuss how the existence of stable nanodroplets would change the pathway for haze formation. As discussed above, the surface tension effect due to surface enrichment of organics and the effect of water activity decreasing due to the dissolution of inorganics cooperatively stabilize nanodroplets. The occurrence of stable nanodroplets would have a profound effect on the formation of haze droplets from pre-existing aerosols: it in fact changes the nucleation pathway, in which, as shown in the inset of Fig. 2, the stable nanodroplets would behave as intermediate states for nucleating haze droplets. With continuous adsorption of water from the surrounding, the aerosol volume increases gradually until the state with stable nanodroplet is reached (the inset of Fig.2). Then the nucleation would proceed via the crossing of a free-energy barrier of the height ΔG*,after which the droplet would grow spontaneously. Hence, haze droplets tend to be nucleated from a pre-existing aerosol via an energetic favorable,two-step pathway.
More importantly, the metastable nanodroplets appeared during haze activation would show a long-term lifetime. During this period of time, the nanodroplets can serve as a condensation sink for inorganic and organic air pollutants, and their amount of adsorption would increase with time in the case with severe air pollution(Fig.5). This may be a slow but long-term process so that substantial amount of inorganic and organic compounds can be adsorbed even at low and fixed vapor supsaturation. The effect of increasing organic/inorganic content on haze nucleation can be inferred from Fig.4(b): Increasing their amount would reduce the energy barrier of nucleation because of the corresponding increase in the size of the stable nanodroplets (Fig. 3(b)). In this way, the presence of stable nanodroplets would facilitate the nucleation of haze particles through a continuous organics/inorganics adsorption within the lifetime of the stable nanodroplet (Fig. 5). Therefore,this two-step pathway would substantially reduce the energy barrier for nucleation of haze droplets from pre-existing aerosols,and thus enhance haze formation. This can partially interpret why less- and non-hygroscopic organic compounds can substantially enhance or even dominate CCN activity or cloud formation.[2,11–14]
Fig. 5. Schematic diagram for haze generation with stable nanodroplets involved. The sources of inorganic and organic aerosols are mainly traffic exhaust emissions, the combustion of coal and biomass, and the secondary inorganic enrichment and secondary organic enrichment. In the air, inorganic, organic, and water will first form an aerosol particle. Under a certain relative humidity (RH <100%), the aerosol undergoes hygroscopic growth,and the surface tension lowering effect due to surface enrichment of organic components and water activity decreasing effect due to increasing inorganic concentration cooperatively stabilize the nanodroplet. The stable droplet acts as a condensation sink for inorganic and organic air pollutants, and therefore while keeping stable the droplet would gradually increase its size with continuous adsorption. When the air humidity increases (RH >100%), the nanodroplet changes the hygroscopic growth process into a growth-nucleation process. In this process the nanodroplet can serve as a condensation sink for the continuous adsorption of inorganic and organic air pollutants, which gradually reduces the nucleation barrier for haze formation and eventually leads to sudden nucleation of a haze droplet with water condensation.
A model for haze formation different from the conventional hygroscopic growth pathway is then in detail discussed in Fig. 5. During the formation of haze/cloud particles, the system does not go from aerosols directly to the most stable conformation of haze or cloud particles, but prefers to reach an intermediate stage featured with stable nanodroplets.The appearance of stable nanodroplets changes the haze formation into a two-step process, which is quite different from the conventional hygroscopic growth pathway. This nonclassical pathway would start from the hygroscopic growth until the pre-existing aerosol reaches a stable state. The stable nanodroplet would evolve through a slow process for the adsorption of air pollutants, followed by a nucleation process when the reduced nucleation barrier can be crossed through absorbing and evaporating water(Figs.2 and 5). This pathway hence enhances haze formation. During this nucleation process, as shown in Fig. 4(b), the organic components play at least the same important role as the organic components.
3. Conclusions
Although many organic molecules commonly found in the atmosphere are known to be surface-active in macroscopic aqueous solutions, the effect of their surface partitioning on the mechanisms underlying haze nucleation remains unclear.In this study, based on a simple thermodynamic analysis, we report an unexpected stability of nanodroplets induced by the surface tension effect of organic compounds,i.e.,the adsorbed organic components alone can stabilize the nanodroplets. Further analysis indicates that when both the organic and inorganic components are present,they cooperatively stabilize the nanodroplets.
The existence of stable nanodroplets would profoundly change the nucleation and growth scenarios for haze formation. During the formation of haze droplets from pre-existing aerosols,the system does not proceed from the classical hygroscopic growth pathway. Instead,a two-step nucleation mechanism takes effect. As the first step, the system prefers to reach an intermediate stage(with the presence of a stable nanodroplet). Then, the stable nanodroplet can serve as a condensation sink for the continuous adsorption of inorganic and organic air pollutants,and this slow adsorption process would lead to gradually lowering of the nucleation barrier for haze formation. Subsequently, a nucleation process may occur if the barrier becomes low sufficiently or if the vapor supersaturation increases. During the nucleation process, the organic components would play at least the same important role as the organic components. After the droplet size exceeds that of the critical nucleus, the droplet would grow rapidly and spontaneously through condensation of water vapor.
- Chinese Physics B的其它文章
- Numerical simulation on ionic wind in circular channels*
- Interaction properties of solitons for a couple of nonlinear evolution equations
- Enhancement of multiatom non-classical correlations and quantum state transfer in atom–cavity–fiber system*
- Protein–protein docking with interface residue restraints*
- Effect of interaction between loop bases and ions on stability of G-quadruplex DNA*
- Retrieval of multiple scattering contrast from x-ray analyzer-based imaging*