Solvent-Induced Inversion of Pickering Emulsions for In Situ Recycling of Enzyme Biocatalysts
Houbing Zou , Rammile Ettelaie , Shuai Yan , Nan Xue , Hengquan Yang ,*
1 School of Chemistry and Chemical Engineering, Shanxi University, Taiyuan 030006, P.R.China.
2 Food Colloids Group, School of Food Science and Nutrition, University of Leeds, Leeds LS2 9JT, United Kingdom.
Abstract: Separation and recycling of catalysts are crucial for realizing the objectives of sustainable and green chemistry but remain a great challenge, especially for enzyme biocatalysts.In this work, we report a new solvent-induced reversible inversion of Pickering emulsions stabilized by Janus mesosilica nanosheets (JMSNs), which is then utilized as a strategy for the in situ separation and recycling of enzymes.The interfacial active solid particle JMSNs is carefully characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen sorption experiments, Fourier transform infrared (FT-IR) spectroscopy, and thermogravimetric analysis (TGA).The JMSNs are demonstrated to show order-oriented mesochannels with a large specific surface area, and the hydrophobic octylgroup is selectively modified on one side of the nanosheets.Furthermore, the inversion is found to be a fast process that is strongly dependent on the interfacial activity of the solid emulsifier JMSNs.Such a phase inversion is also a general process that can be realized in various oil/water phasic systems, including ethyl acetate-water, octane-water, and cyclohexane-water systems.By carefully analyzing the capacity of JMSNs with different surface wettabilities for phase inversion, a triphase contact angle (θ) close to 90° and a critical oil-water ratio of 1 : 2 are identified as the key factors to achieve solvent-induced phase inversion via a catastrophic phase inversion mechanism.Importantly, this reversible phase inversion is suitable for the separation and recycling of enzyme biocatalysts that are sensitive to changes in the reaction medium.Specifically,during the reaction, the organic substrates are dissolved in the oil droplets and the water-soluble catalysts are dispersed in the water phase, while a majority of the product is released into the upper oil phase and the enzyme catalyst is confined inside the water droplets in the bottom layer after phase inversion.The perpendicular mesochannels of JMSNs provide a highly accessible reaction interface, and their excellent interfacial activity allows for more than 10 rounds of consecutive phase inversions by simply adjusting the ratio of oil to water in the system.Using the enzymatic hydrolysis kinetic resolution of racemic acetate as an example, our Pickering emulsion system shows not only a 3-fold enhanced activity but also excellent recyclability.Because no sensitive chemical reagents are used in this phase inversion process, the intrinsic activities of the catalysts can be preserved even after seven cycles.The current study provides an alternative strategy for the separation and recycling of enzymes, in addition to revealing a new innovative application for Janus-type nanoparticles.
Key Words: Janus nanosheet; Emulsion inversion; Mesoporous silica; Catalyst recycling; Enzyme catalysis;Kinetic resolution reaction

1 Introduction
The ability to recycle catalysts is one of the important criteria for their practical applications1-5.For example enzyme catalysts have high catalytic activity and selectivity even under mild reaction conditions6-8, but suffer from the technological challenge of separation and recycling, as they often are dissolved in the reaction media.Over the past decade, a biphasic aqueousorganic system has been developed as a gentle and general way to enable theinsituseparation and recycling of homogeneous catalysts or enzymes9-11.In the biphasic system, catalyst is dissolved in the aqueous phase whereas the reactants and products are both dissolved in the oil phase, allowing efficient isolation of the organic products from the catalystviaa simple liquid transfer.Despite facile separation and recycling of catalysts, barriers to mass transfer and the limited oil-water interfacial area dramatically suppresses the reaction rate.In this context, to simultaneously realize both separation-recycling of catalysts while maintaining a high degree of reactivity is crucial in developing efficient oil-water biphasic systems.
Recently, Pickering emulsions stabilized by solid particles have been widely viewed as an excellent biphasic system and have received ever-increasing attention as such12-22.Sufficient micromixing of immiscible phases can provide a large oil-water interfacial area and a short molecular diffusion pathway so as to greatly improve catalysis efficiency of these biphasic reactions23,24.More interestingly, tuning the liquid-liquid interfacial properties of the Pickering emulsions makes it possible to achieve emulsion type inversion, from oil-in-water to water-in-oil25-27and vice versa.Taking advantage of these unique features allows one to develop a potentially promising technique, based on Pickering emulsion systems, to address the aforementioned issue of reactivityvsseparation-recycling.For example, our group reported a series of pH-triggered reversible inversion of Pickering emulsions stabilized by different solid particles since 2013 and then achievedinsituseparation and recycling of nanocatalysts using this method28-30.In a similar type of work, CO2as well as light-induced inversion of Pickering-emulsions were also reported by other groups31-36.However, these processes all involved a wettability inversion of the surface of the solid emulsifier, achieved by the addition of chemical auxiliaries (for example HCl or NaOH) or by changing the temperature of the biphasic systems.This drawback makes them unsuitable for the separation and recycling of enzymes that are sensitive to changes in the reaction medium.Therefore, a new phase inversion method, not involving such modifications,is highly desirable forinsituseparation and recycling of the catalysts, especially for enzyme biocatalysts.
Herein, we develop and report on a hitherto unexplored solvent-induced Pickering emulsion inversion method, to provide an effective strategy forinsituseparation and recycling of enzymatic biocatalysts.As described in Scheme 1, the organic substrates are dissolved in the oil droplets and the water-soluble catalysts are dispersed in the water phase surrounding the oil droplets, during the reaction.The large oil-water interfacial area,available in the emulsion system, allows the reaction to proceed efficiently even under stirring-free conditions.At the end of reaction, a desired amount of oil phase is added into the emulsion system in order to trigger the phase inversion.This leads to the formation of a water-in-oil emulsion, where the vast majority of product is released into the upper oil phase.The catalyst is confined in the water droplets at the bottom layer.The upper layer of product-containing oil phase can now be removed by a simple decantation.In the next reaction cycle, fresh reactants with a small amount of oil phase are introduced.The water-inoil emulsion is inversed back to an oil-in-water emulsion again.Interestingly, the mesochannels of JMSNs, perpendicular to their surfaces, provide a highly accessible reaction interface, while their proper interfacial activity ensures that the phase inversion can be consecutively performed (in the reported case here more than ten times) without the need for any sensitive chemical reagents.As a result, our Pickering emulsion system exhibits the advantage of both a 3-fold enhanced activity and also an excellent level of recyclability, for the studied example of enzyme-catalyzed hydrolysis kinetic resolution of racemic acetate.
2 Experimental
2.1 Materials and methods
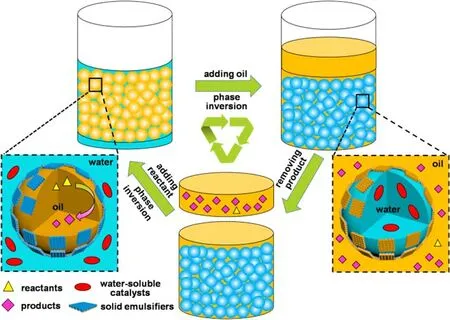
Scheme 1 Schematic illustration for the strategy of solvent-induced Pickering emulsions inversion for in situ separation and recycling of water-soluble catalysts.
Tetraethylorthosilicate (TEOS, 98%), aqueous ammonia(NH3·H2O, 28%), cetyltrimethylammonium bromide (CTAB,99.0%), styrene (> 99%), Gold(III) chloride trihydrate(HAuCl4·3H2O, 99.7%) and 2,2-azobis (2-methylpropionitrile)(AIBN) were purchased from Sinopharm Chemical Reagent.OrganosilanesN-octyltrimethoxysilane (OTMS, 96%) was obtained from Meryer.Poly(vinyl pyrrolidone) (PVP, MW =40000, 96%) was obtained from Beijing Solarbio Science &Technology Co., Ltd.Nile red (95%) was obtained from Sigma-Aldrich.Native Lipase B fromCandidaAntarctica(CALB,99%) was purchased from Novozymes.All esters were purchased from Admas Reagent Co., Ltd.All chemicals were used as received without any further purification.
2.2 Synthesis of Janus mesoporous silica nanosheets
Janus mesoporous silica nanosheet was prepared according to our previous report37.Typically, 0.96 g of polystyrene (PS)microspheres was dispersed in a mixture of ethanol (64 mL),water (58 mL) and NH3·H2O (0.4 mL, 28% (w, mass fraction)by ultrasonication.After addition of CTAB (0.48 g), the mixture was stirred for 30 min and TEOS (0.3 mL) was then quickly added.The obtained solution was thereafter stirred at 25 °C for 6 h.After that, temperature was increased to 80 °C and the mixture was kept undisturbed overnight.The resulting solid material PS@mSiO2was collected by centrifugation and washed with deionized water.Surface modification was then performed to graft octylgroup on the external surface of PS@mSiO2microspheres.1.0 g of PS@mSiO2was dispersed in the mixture of isopropanol, octyltrimethoxysilane and triethylamine by ultrasonication.After refluxing at 100 °C for 6 h, the material was isolated by centrifugation and washed four times with ethanol.The surfactant CTAB and PS microspheres were subsequently removed by stirring as-made sample (1.0 g) in ethanol (100 mL) containing 1.0 mL of 36% (w) HCl at 60 °C for 6 h, followed by stirring in THF (75 mL) at room temperature for 12 h.The obtained Janus hollow microspheres were recovered by centrifugation and washed with THF/EtOH for several times.In order to completely remove CTAB and PS, this solvent-extraction procedure was conducted twice.Finally, the Janus mesosilica nanosheets were obtained by a simple grinding.Generally, 20 min was essential to crushing the hollow microspheres.The density of the octyl group on the surfaces was tunedviaadjusting the amount of the organosilaneN-octyltrimethoxysilane.
2.3 Solvent-induced reversible inversion of Pickering emulsions
Typically, 1.0 mL of oil (toluene or octane, cyclohexane, ethyl acetate) was first added into a vial containing 10 mg of Janus mesoporous silica nanosheets.Then 2.0 mL of deionized water was added into the vial and the oil-water ratio was kept fixed at 1 : 2.After vigorously shaking/stirring, solid particle-stabilized oil-in-water (O/W) emulsions were observed to be in the upper layer.The oil to water ratio was then adjusted from 1 : 2 to 2 : 1,by introducing another 3 mL of oil into the system.After vigorously shaking or stirring, the O/W emulsions were inversed to water-in-oil (W/O) emulsions that settled in the bottom layer.Once the oil-water ratio was restored back to 1 : 2, after removing the introduced oil followed by vigorous shaking,pristine O/W emulsions were re-obtained in the upper layer of the reaction system.Adding oil into the system initiated the next cycle and this reversible inversion could be conducted for at least ten times.
2.4 Catalytic tests
For the CALB enzymatic hydrolysis kinetic resolution of racemic esters, 2 mL of PBS (phosphate buffer: 0.1 mol·L-1Na2HPO4-0.1mol·L-1NaH2PO4, pH = 7.0) solution containing CALB was mixed with 1 mL of octane containing 5 mg of Janus mesoporous silica nanosheets and 0.25 mmol of 1-phenylethyl acetate to obtain an O/W Pickering emulsions following vigorous shaking.The reaction was conducted at 35 °C for 3 h without stirring.At the end of reaction, 3 mL of toluene was added into the reaction system and once again vigorously shaken in order to invert the emulsions from O/W to W/O.The upper resolved organic layer was transferred through a decantation.This process was conducted twice to extract the products and residual reactants from the emulsions completely.For the next reaction cycle, fresh solvent and substrates were added into the residual emulsion phase.Pristine O/W emulsions were formed once the system was vigorously shaken.The other procedures,for the reaction and catalyst recycling, were the same as those for the first reaction cycle.The collected organic phase was analyzed by a gas chromatograph (Agilent 7890A), equipped with a flame ionization detector (FID).The product was further confirmed with GC-MS.
2.5 Materials characterization
Scanning electron microscope (SEM) images were taken on a JEOL JSM-6700F field-emission electron microscope.Transmission electron microscope (TEM) images were obtained from an FEI Tecnai G2 F20s-twin D573 field emission transmission electron microscope at an accelerating voltage of 200 kV.Results for N2adsorption-desorption isotherms were obtained at -196 °C on a Micromeritics ASAP 2010 sorption analyzer.Samples were degassed at 120 °C, for a minimum period of 12 h, prior to their analysis.Brunauer-Emmett-Teller(BET) surface areas were calculated from the linear part of the BET plot.Pore size distribution was estimated from the adsorption branch of the isotherm by the BJH method.The total pore volume was estimated from the adsorbed amount atP/P0=0.995.The FT-IR spectra were acquired using a Bruker IFS 66 V/SFTIR spectrometer in the range 400-4000 cm-1.The thermogravimetric analysis (TGA) was obtained in a flow of nitrogen from 30 to 800 °C (10 °C·min-1) using a Netzsch STA 449F3 thermogravimetric analyzer.The analysis of inductively coupled plasma mass spectrometry (ICP) was carried out on a NexION 350 ICP-MS instrument.Pickering emulsions were observed with an optical microscope (XSP-8CA, Shanghai)equipped with 4 × or 10 × magnification lens.
3 Results and discussion
3.1 Structure characterization of Janus mesoporous silica nanosheets (JMSN)
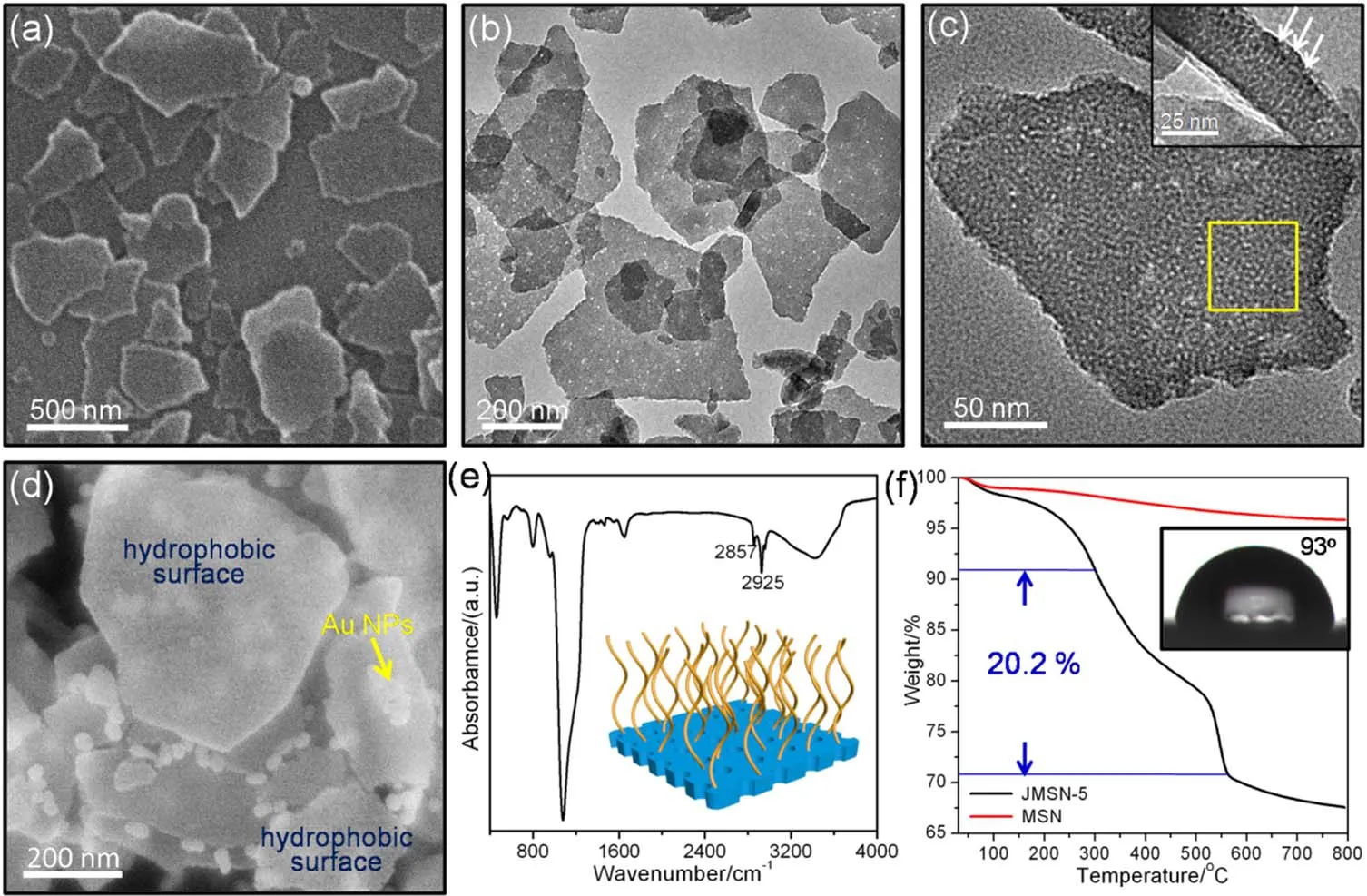
Fig.1 SEM image (a) and TEM images (b, c and the inset) of the sample JMSN-5.Sample JMSN-5 with PVP-capped Au nanoparticles selectively labeled on the hydrophilic surfaces (d).FT-IR spectrum (e) and TG curve (f) of the sample JMSN-5.The inset in e is the model of the sample JMSN-5.The inset in f is the water contact angle.
Janus mesoporous silica nanosheets with tunable interfacial activities (JMSN-x,xdenoted as the density of octyl group on the surface) were prepared through grinding of the hollow microspheres with hydrophobic external surfaces (grafted with octyl group) and hydrophilic internal surfaces (silanol group)37,38.The scanning electron microscopy (SEM) image in Fig.1a shows that the sample JMSN-5 has a typical morphology of twodimensional nanosheet.The particles have a broad size distribution from 200 to 800 nm.The transmission electron microscopy (TEM) image further confirms that the sample consists of irregular nanosheets (Fig.1b).From the highmagnification TEM image (Fig.1c), one can clearly observe both a uniform mesoporous structure and a defect structure,originating from the rigorous milling process.A hexagonal pattern, as marked by the yellow box, is also observed to be similar to the [110] direction of conventional ordered mesoporous material MCM-41, suggesting an ordered and perpendicular mesopore array.The cross-sectional TEM image further confirms that these mesochannels are indeed perpendicular to the nanosheet surfaces, with a nanosheet thickness of approximately 30 nm (Fig.1c, inset).The nitrogen adsorption-desorption isotherm of the sample JMSN-5 presents a typical type IV curve with a hysteresis loop in theP/P0range of 0.2-0.4 (Fig.S1a, see Supporting Information (SI)).Moreover, the corresponding Barrett-Joyner-Halenda (BJH)pore size distribution curve shows two pore diameters centered at 2.8 and 4.1 nm (Fig.S1b (SI)), which can be attributed to the ordered mesoporous structure and the disordered defect structure, respectively.These findings are in good agreement with the TEM results.The surface area and pore volume are measured to be as high as 927 m2·g-1and 0.68 m3·g-1,respectively.
The Fourier transform infrared (FT-IR) spectrum of the sample JMSN-5 clearly exhibits bands of C―H stretching vibration in the range of 2850-2980 cm-1(Fig.1e), indicating that the hydrophobic octyl groups have been successfully grafted on their surfaces.In the thermogravimetry (TG) curve, there is a distinct weight loss from 300 to 500 °C (Fig.1f) while a negligible weight loss (1% (w)) presents in the sample mesosilica nanosheet (MSN), revealing that the octyl group ratio can to be as high as 20.2% (w).The surface wettability is also examined and it is found that the JMSN-5 has a water contact angle of 93°.In order to directly observe the Janus structure of the nanosheets, a labeling experiment is performed using hydrophilic Au nanoparticles37,38.From the SEM image in Fig.1d, it can clearly be seen that almost all of the Au nanoparticles are selectively coated on one side of the nanosheets, while their other surface remains absolutely bare.This result indicates that nanosheets have one clean hydrophobic surface on one side,while the Au-absorbed surface is hydrophilic, providing an asymmetric surface structure.Taken together, these results demonstrate that we have successfully prepared Janus type nanosheets, with perpendicularly orientated mesochannels,having two distinctly different surfaces.
3.2 Solvent-induced inversion of Pickering emulsions stabilized by JMSN

Fig.2 Solvent-induced phase inversion of Pickering emulsions using toluene as the oil phase and JMSN-5 as the solid emulsifier.(a–c) Appearance of oil-in-water and water-in-oil Pickering emulsions stabilized by JMSN-5 under different toluene-water volume ratios.(d–e) Corresponding optic microscopy images of the Pickering emulsions in the a1–a3.(g–i) Fluorescence confocal microscopy images of the Pickering emulsions with the oil phase dyed by oil-soluble Nile red.The scale bar is 300 μm.
Janus nanoparticles have been widely demonstrated to possess excellent interfacial activity due to their distinct physical and chemical properties on two sides37-40.Here we first examine the emulsifying capacity of JMSN-5 using toluene/water with a volume ratio of 1:2 as a typical oil/water biphasic emulsion system.As show in the Fig.2a1, oil-in-water emulsions are observed in the upper layer after vigorously shaking (or stirring)with 0.5% (w) of JMSN-5 used as the solid emulsifier.The optical micrograph shows that the oil-in-water droplets are irregular spheres or ellipsoids with an average size of 110 μm(Fig.2d).The formation of these non-spherical droplets is typical of Pickering type emulsions and is most likely due to the unique interfacial jamming effect of irregular Janus nanosheets on the surface of droplets41,42.Regardless of the irregular nonspherical morphology, these oil-in-water droplets are extremely stable.After standing at room temperature for six months, or at 80 °C for 4 h, there are no distinct variations in the droplet morphology (Fig.S2 (SI)).Interestingly, such an extremely stable oil-in-water emulsion nonetheless easily inverts to a water-in-oil emulsion, once the oil-water ratio is adjusted from 1 : 2 to 2 : 1 and the system is vigorously shaken.The formation of inverted emulsion droplets is observed in the bottom layer, as seen in Fig.2a2.The optical micrographs indicate that most of water-in-oil droplets are also ellipsoid (Fig.2e).Furthermore, if the introduced toluene is removed from the system to restore the oil-water ratio back to the initial 1 : 2,irregular nonspherical oil-in-water droplets are once again reobtained.These reside in the upper layer, and are formed after vigorous shaking (Fig.2a3, 2f).The oil-in-water and water-in-oil emulsions are further confirmed through the fluorescence dyeing of oil phase with Nile red (Fig.2g, h, i).This proedure clearly reveals that by regulating the oil to water volume ratio one can efficiently induce the inversion of the Pickering emulsions, as stabilized by our JMSN-5.
This solvent-induced inversion behavior of Pickering emulsions is reversible.As shown in the Fig.2b, c and Fig.S3(SI), oil-in-water and water-in-oil emulsions can still be obtained from each other by inversion process in a consecutive manner for as many times as ten.Furthermore, this is simply achievedviaadding and removing more or less oil.This good ability to repeat the inversion cycle can be mainly ascribed to the excellent and stable interfacial activity of the material JMSN-5.Moreover,this inversion process can be realized in various oil/water phasic systems, including ethyl acetate-water, octane-water and cyclohexane-water (Fig.S4 (SI)).These results indicate that our Janus materials allows for a highly flexible solvent-induced emulsion inversion process.
In order to obtain further insight into the mechanism of inversion, we prepared JMSN-xwith different surface wettabilitiesviavarying the density of octyl group on the surface of nanosheets and then examined their capacity to invert the Pickering emulsions.As shown in the SEM images (Fig.S5a, b(SI)), both JMSN-0.5 and JMSN-30 share a similar morphology typical of irregular nanosheets.Two samples also show the C―H stretching vibration in the range of 2850-2980 cm-1(Fig.S5c (SI)) in their FT-IR spectra, but with different intensities.Furthermore, the thermogravimetric (TG) tests reveal that the weight ratios of surface octyl group for the samples JMSN-0.5 and JMSN-30 are 13.9% (w) and 22.1% (w) (Fig.S5d (SI)),respectively.Different surface densities of octyl group would lead to different hydrophilic/hydrophobic balance.As evidenced by the water contact angle measurements (Fig.S5e, f (SI)),where JMSN-0.5 has a relatively more hydrophilic surface wettability (80°), while JMSN-30 possesses a more hydrophobic surface wettability (110°).When we examine the emulsifying abilities of JMSN-0.5 and JMSN-30 using a typical toluenewater biphasic system with a volume ratio of 1 : 1, it is found that well-defined oil-in-water emulsions are obtained for JMSN-0.5 (Fig.S6 (SI)) while corresponding water-in-oil emulsions are observed for JMSN-30 (Fig.S7 (SI)).Furthermore, in a wide range of oil to water ratios from 2 : 1 to 1 : 2, oil-in-water and water-in-oil emulsions always result, without any occurrence of phase inversion, for both JMSN-0.5 and JMSN-30,respectively.This result indicates that the more hydrophilic JMSN-0.5 and the more hydrophobic JMSN-30 prefers to stabilize oil-in-water and water-in-oil emulsions,respectively.Any phase inversions cannot be realized for these two types of particulate emulsifiers.According to Binks and co-workers43-45, the desorption of a solid particle from the oil-water interface is determined by the triphase contact angleθbetween the particle, the oil phase and the water phase at the interface (Eq.(1)).

whereΔEis the desorption energy,ris the particle radius,γowrefers to the interfacial tension between the oil and the water,θis the three-phase contact angle between the nanoparticle, the oil phase, and the water phase at the interface.
When the contact angleθis 90°, the desorption energy is maximum, meanings that it is most difficult for the particle to dispalce from the oil-water interface into the bulk phase.On the other hand, it has been well documented that if theθis less than 90°, water phase will wet the particle more preferentially than the oil phase, thus leading to the formation of oil-in-water emulsions.In contrast, water-in-oil emulsions are generated whenθis larger than 90°.Whenθis extremely close to 90°,theoretically both oil-in-water emulsions and water-in-oil emulsions can be obtained and the other conditions such as oilwater ratio will determine the emulsion type.To sum up, this solvent-induced inversion of Pickering emulsions is highly dependent on the interfacial activity of JMSN and a triphase contact angle of 90° is most favorable for to induce phase inversion.
Currently, transitional phase inversion (TPI) and catastrophic phase inversion (CPI) are two typical mechanisms for solvent induced inversion of Pickering emulsions stabilized by solid particles46-50.The TPI mechanism usually involves a transformation of surface wettability in the solid emulsifiers49.In contrast, tuning the oil to water volume ratio in systems can invert the emulsion typeviaa CPI mechanism50.Here, we adjust the system composition of Pickering emulsions stabilized by JMSN-5 to check the process of phase inversion.It is found that the emulsion type is dramatically inversed from oil-in-water to water-in-oil once the volume fraction of water (φw) is adjusted to around 0.33.When theφwis higher than 0.33, oil-in-water emulsion is always presented.On the contrary, water-in-oil emulsion is always formed ifφw≤ 0.33.The emulsion inversion point (EIP) in our case is consistent with that of the typical catastrophic inversion of oil-in-water emulsions stabilized by hydrophilic silica particles (0.3)50.This result suggests that our solvent-induced inversion of Pickering emulsions stabilized by JMSN-5 is indeed likely to involve CPI mechanism.
It has been documented that the emulsion system will undergo limited coalescence51when the solid emulsifiers can adsorb rapidly to the oil-water interface and are almost completely depleted from the bulk in the early stages of the coalescence.In this case, the theoretical value for the size of dropletsDi(i = w or o, to indicate water or oil droplets) will need to satisfy the following two equations:

whereNiis the number densities of the droplets,npis number densities of the particles,rpis the radius of the particles,φiis the volume fraction of the ith phase (oil or water) andαis the critical degree of particle coverage needed to stabilise the droplets,respectively.Combining the above two equations to eliminateNi,we have

Since the particles with a contact angel close to 90° are capable of stabilizing both oil-in-water and water-in-oil emulsions, we suspect that initially both oil and water droplets are formed and are present in the system during the homogenization process.In a system undergoing limited coalescence, both these water and oil droplets will coalesce and coarsen with time until the criteria (Eq.(2) and Eq.(3)) are satisfied by at least one set of droplets.The set of droplets with a smaller size, as given by Eq.(4), are the ones that will meet the above criteria (Eq.(2) and Eq.(3)) first, as the droplets coalesce and grow.This is the set of droplets that we propose will eventually be the ones achieved, when the system is fully stable.Thus, in cases whereφw>φo(i.e.low volume fraction of oil) the oil droplets (i.e.oil-in-water emulsions) with a smaller size than water droplets, otherwise possible if a water-in-oil emulsion was formed instead (Eq.(4)), will be obtained.The opposite is true ifφw<φowhere water-in-oil droplets can be formed at a smaller size (Eq.(4)).In principle, these consideration only apply if all else is taken as equal.However, because the viscosities of the oil and water are quite different, resulting in different dropletbreakup and initial size distribution for the two groups of droplets, immediately post homogenization, the water droplets meet the requirement for arrest of limited coalescence only whenφois significantly larger thanφwand not just marginally above 0.5.Therefore, the oil-in-water emulsions rapidly invert into water-in-oil emulsions at aφw≤ 0.33 (i.e.φo≥ 0.67, and not our otherwise predicted 0.5 when the two immiscible fluids are perfectly symmetrical).
In addition, it is worth mentioning that our solvent-induced phase inversion has two advantages compared with previous strategies.On the one hand, the employed solid particle JMSN-5 possesses an excellent interfacial activity, ensuring that this phase inversion process can consecutively be performed.On the other, the perpendicular mesochannels of JMSN-5 can provide a highly efficient passageway for both the oil-soluble and the water-soluble reactants37.These unique features make this type of solvent-induced phase inversion an efficient strategy forin situseparation and recycling of enzyme biocatalysts.
3.3 In situ separation and recycling of biocatalyst

Fig.3 (a) Schematic illustration for the strategy of solvent-induced Pickering emulsions inversion for in situ separating and recycling of enzyme biocatalyst.(b) Photograph of the Pickering emulsions stabilized by JMSN-5 for the CALB catalyzed hydrolysis kinetic resolution of racemic acetate.(c) Kinetic profile, (d) activity comparison and (e) recyclability tests for two different catalytic systems.(f) The ee value of 1-phenethyl alcohol vs reaction cycle in the Pickering emulsion reaction system.
The solvent-induced phase inversion is applied toinsituseparating and recycling enzyme biocatalysts.As displayed in Fig.3a, the CALB-catalyzed hydrolysis kinetic resolution of racemic acetate, a very important enzymatic reaction for producing chiral alcohols and acetates52,53, is used as a model reaction to assess the feasibility of our strategy.The oil-in-water emulsions make it possible for the reaction to proceed efficiently without need for stirring (Fig.3b).Fig.3c shows the kinetic plots of the hydrolysis kinetic resolution of racemic 1-phenylethyl acetate in different catalytic systems.The enantiomeric excess value of 1-phenylethyl acetate (ees) can reach values as high as 90% within 1.0 h in our Pickering emulsion reaction system.However, using the conventional enzyme-immobilized CALB/DSN (the enzyme CALB was immobilized in typical mesoporous silica nanoparticles with a pore size of 30 nm) as the catalyst (Fig.S8 (SI)), an eesvalue of only 37.1% is obtained in 1.0 h, even with a stirring rate of 800 r·min-1.To achieve eesof≥ 90% requires 2.5 h.The specific activity of CALB in these systems is estimated according to the eeswithin the first 1.0 h.It can be clearly seen that the specific activity of our Pickering emulsion system is 3 times higher than that of CALB/DSN (Fig.3d).The much higher activity should be attributed to the fact that Pickering emulsions stabilized by JMSN cannot only provide an extremely fast mass transfer, but also perfectly retains the intrinsic activity of free enzyme molecules26,27.Furthermore, we evaluate the recyclability of two systems.As shown in the Fig.3e, our Pickering emulsion reaction system still gives a conversion of ≥ 45% (the theoretical conversion is 50%) within a prolonged reaction time (8 h) after seven consecutive cycles.Interestingly, the yield of hydrolysis product 1-phenethyl alcohol is as high as 45.1%, which is close to the equilibrium conversion.This is due to the fact that successive extraction can release the vast majority of products from the water-in-oil emulsions.The ee value is always ≥ 99% (Fig.3f).In sharp contrast, the CALB/DSN only retains 47% of the initial activity after three cycles, under the same conditions.This dramatic activity loss is mainly due to the leaching of enzyme from the support and the negative impact of the polar surface of support, which is often observed in the enzyme-immobilized catalysts54.Because any sensitive reagents are not involved in our separating and recycling process, the enzyme CALB can retain their intrinsic activity.On the other hand, the enzyme catalyst is well confined within the water droplets and away from the upper organic layer,when the products are being removed from the latter after the reaction.This significantly improves any leaching of enzyme molecules from the emulsion system.Moreover, the excellent interfacial activity of JMSN-5 can offer a large and stable oilwater interfacial area.These merits thus endow our proposed Pickering emulsion reaction system a remarkable level of recyclability.
4 Conclusions
In summary, we have developed a new solvent-induced Pickering emulsions inversionviatuning the interfacial activity of JMSN.This emulsion phase inversion is a fast process and is applicable to various oil-water biphasic systems.Moreover, the permanent interfacial activity of JMSN ensures that the inversion process can be repeated more than ten times without the aid of any sensitive chemical reagents.In addition, the perpendicular mesochannels of JMSN can provide a highly accessible reaction interface for significantly improved mass transfer.These unique features make the inversion process a highly efficient technology forinsituseparation and recycling of enzyme biocatalysts.As the CALB-catalyzed hydrolysis kinetic resolution of racemic acetate demonstrates here, our Pickering emulsion system offers both a significantly enhanced activity, as well as an excellent level of recyclability.The present study not only provides an alternative strategy for separation and recycling of enzymatic catalysts but also indicates a novel application for Janus type nanoparticles.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化學(xué)學(xué)報的其它文章
- 基于兩親性喹喔啉的超分子凝膠:手性信號反轉(zhuǎn)以及多重響應(yīng)手性光學(xué)開關(guān)
- Structure-Property Relationship of Light-Responsive Wormlike Micelles Using Methoxycinnamate Derivatives as Light-Switchable Molecules
- 環(huán)糊精與表面活性劑主客體作用誘導(dǎo)的金納米棒可控自組裝
- Wormlike Micelle to Gel Transition Induced by Brij 30 in Ionic Liquid-Type Surfactant Aqueous Solution
- 含有酰胺基或酯基的可降解陽離子Gemini表面活性劑在水溶液中的聚集行為
- 基于多酯頭基的“油-二氧化碳兩親分子”設(shè)計及其助混規(guī)律

