Wormlike Micelle to Gel Transition Induced by Brij 30 in Ionic Liquid-Type Surfactant Aqueous Solution
Yimin Hu, Jie Han , Rong Guo
School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, Jiangsu Province, P.R.China.
Abstract: Wormlike micelles and low-molecularweight hydrogels are composed of threedimensional networks that endow them with viscoelasticity, but their viscoelastic properties are markedly different.The viscosity of wormlike micelles is attributed to a transient network, while that of gels is due to a stable network.Under certain conditions, wormlike micelles can undergo transition to gels with an increase in the density of the network.In our previous study, we found that the wormlike micelle formed by the ionic liquid-type surfactant 1-hexadecyl-3-octyl imidazolium bromide ([C16imC8]Br) without any additive has high viscoelasticity.The inclusion of a nonionic surfactant polyoxyethylene lauryl ether (Brij 30) is expected to enhance the viscoelasticity of [C16imC8]Br wormlike micelles via electrostatic shielding and strong hydrophobic interactions, which may be the driving factor for the wormlike micelle-to-gel structural transition.The morphology and viscoelasticity of [C16imC8]Br wormlike micelles with Brij 30 were studied as a function of concentration by rheological measurements and freeze-fracture transmission electron microscopy.The thermal stability and gel-sol transition temperature of the Brij 30/[C16imC8]Br gels were studied using rheology.The interaction between Brij 30 and [C16imC8]Br was studied by zeta potential measurements and nuclear magnetic resonance (NMR) spectroscopy.Upon the inclusion of Brij 30 into the [C16imC8]Br wormlike micelles,the viscoelasticity of the Brij 30/[C16imC8]Br samples first increased and then decreased with an increase in the Brij 30 concentration, at different initial concentrations of [C16imC8]Br.At a certain Brij 30 concentration, the Brij 30/[C16imC8]Br samples rheologically behaved as a gel.The maximum viscoelasticity of the [C16imC8]Br (4.06% (w))/Brij 30 gel was observed at a Brij 30/[C16imC8]Br molar ratio of 4.55.The viscoelasticity of the Brij 30/[C16imC8]Br gels was positively correlated with the activation energy of the gels.The gel-sol transition temperature of the Brij 30/[C16imC8]Br gels also increased first and then decreased with an increase in the Brij 30 concentration.The highest gel-sol transition temperature of the Brij 30/[C16imC8]Br (4.06% (w)) gel was observed at a Brij 30/[C16imC8]Br molar ratio of 2.93.The Brij 30 concentration had a notable impact on the viscoelasticity, thermal stability, and gel-sol transition temperature of the Brij 30/[C16imC8]Br gels.The zeta potential and 1H NMR measurements revealed that the neutral Brij 30 molecules are inserted into the palisade layer of the [C16imC8]Br wormlike micelles via hydrophobic interactions.This decreased the electrostatic repulsion between the [C16imC8]Br headgroups, which in turn induced the rapid growth of wormlike micelles and the formation of a stiffer network structure.Finally, the wormlike micelles underwent a structural transition to gels.The obtained results would aid in better understanding the relationship between wormlike micelles and gels, and may be of potential value for industrial and technological applications.
Key Words: Wormlike micelle; Gel; Ionic liquid-typed surfactant; Brij 30; Activation energy; Transition

1 Introduction
The molecular self-assembly of surfactants based on noncovalent interactions in solution has been a very important research field in colloidal and interfacial chemistry1,2.These self-assemblies include micelles, vesicles, emulsions, wormlike micelles, gels,etal.Wormlike micelles are flexible, long,cylindrical micelles with contour lengths of a few micrometres,which significantly increases the viscoelasticity of the solution.3In the past two decades, wormlike micelles have been used as thickeners for personal and home care products4,5, dragreducing agents6, fracturing fluid in oil fields7and as templates for materials synthesis8,9.Supramolecular gels have also attracted widespread attention due to their unique supramolecular structures and potential applications.Most supramolecular gels consist of fibers that noncovalently entangle with one another to form a 3D network10,11.The intermolecular forces include hydrogen bonding, electrostatic interaction,hydrophobic interaction andπ-πstacking.They can be used for controlled release of medicine12, regenerative medicine13,biomimetic14, catalyst carrier15, soft template for the synthesis of nanomaterials16,etal.The gelation process can be achieved by external stimuli, such as pH, temperature, ionic strength,light, electric field or magnetic field.By changing different external conditions, the transition of sol-gel17,18, gel-sol-gel19,gel-liquid crystal20,21and gel-vesicle22,etal.can be realized.However, the transition of wormlike micelle-gel is still rare.The wormlike micelles and gels are all viscoelastic fluids which are consist of 3D network.The wormlike micelles are characterized by viscosity and the gels by elasticity.In certain situation, the wormlike micelles can transit to gels.For example, the wormlike micelles formed by bromo-1-hexadecyl-3-methylimidazole and sodium salicylate can transit into gel by lowering the temperature23.The erucyl bis-(hydroxyethyl) methylammonium chloride (EHAC) and erucyl trimethylammonium chloride(ETAC) wormlike micelles can transit into gel in the presence of either sodium salicylate (NaSal) or sodium chloride (NaCl)24.The wormlike micelles of ionic liquid-typed surfactant 1-hexadecyl-3-nonyl imidazolium bromide can transit to gels by increasing surfactant concentration, which promotes the growth of micelles and the three-dimensional networks, as we reported25.However, similar surfactants with shorter alkyl chain (the carbon atom number is less than 9) form wormlike micelles in aqueous solution.Surfactants with longer alkyl chain(the carbon atom number is larger than 9) form gels in aqueous solution26.Can the wormlike micelles formed by surfactants with shorter alkyl chain length transit to gels with additives?
The nonionic surfactant polyoxyethylene (4) laurel ether (Brij 30) is very efficient in inducing the one-dimensional micellar growth in mixed surfactant systems27-29.The viscoelasticity of the cetyltrimethylammonium bromide, gemini surfactants solution can be highly increased with the addition of Brij 30,which induces the increase of the micellar length30,31.The ionic liquid-typed surfactant [C16imC8]Br aqueous solution forms strongly viscoelastic wormlike micelles without any additives26.So we try to add Brij 30 to the [C16imC8]Br wormlike micelles to examine whether it can transit to gels by increasing the micellar length and inducing the growth of 3D networks.The results indicate that the presence of appropriate amount of Brij 30 induces the transition of [C16imC8]Br wormlike micelles to Brij 30/[C16imC8]Br gels.On the other hand, the role of fundamental parameters in controlling the viscoelasticity of gels is still known little32-34.So there is still needed to investigate the complex relationship between the macroscopic properties of gels and the molecular dynamics.The obtained results in this work are expected to be helpful for better understanding of the relationship between wormlike micelles and gels and have potential value for food, cosmetics industry, tertiary oil recovery and other fields.
2 Materials and methods
2.1 Materials
The [C16imC8]Br (99.0%,w, mass fraction) was synthesized and characterized according to the procedures described in our previous work26.Polyoxyethylene 4 laurel ether (Brij 30,C20H42O5, 99.0% (w) as shown in Fig.S1 (see Supporting Information (SI)).) was purchased from Sigma ( > 99.5% (w),America).Water was Millipore water (resistivity > 18.2 MΩ·cm,America).
2.2 Mixed solution preparation
The mixed solution was prepared by mixing the [C16imC8]Br surfactant, Brij 30 and water directly in a test tube at various weight ratios and different total surfactant concentrations.After sealing, the sample was vortex mixed and equilibrated at a high temperature for 1 h to ensure complete solubility and uniformity.The resulting mixture was vortex mixed and equilibrated at 25.0 ±0.1 °C in a thermostatted bath.
2.3 Rheological measurements
2.4 Freeze-fractured transmission electron microscopy (FF-TEM)
For the preparation of replica, a small amount of sample was placed on a 0.1 mm thick copper disk.Then, the copper sandwich with the sample was plunged into liquid N2to freeze.Fracturing and replication were carried out on a Balzers BAF-400D(Balzers) equipment.Replicas were examined with JEM2100FS TEM (200 kV, Japan).
2.5 Zeta potential measurements
Zeta potential measurements were conducted on a MalvernZetasizer NanoZS (England) system with irradiation from a 632.8 nm He-Ne laser.The [C16imC8]Br aqueous solution was loaded in folded capillary cells and measured using a mixed mode method combining fast field reversal and slow field reversal, which eliminated electroosmotic effects.
2.6 Hydrogen nuclear magnetic resonance (1H NMR)
1H NMR experiments were carried out with a Bruker AV-600 NMR (American) spectrometer with a1H frequency of 600.1 MHz.Sixty-four times of accumulations were acquired generally.D2O (99.5% (w)) was used as solvent, in which sodium 3-(trimethylsilyl)-1-propanesulfonate was added as standard substance.Samples were prepared in the water bath(25.0 ± 0.1 °C) and equilibrated for 2 h.The samples were then transferred to the NMR tube and equilibrated after 24 h before the1H NMR spectra examination.
3 Results and discussion
3.1 Morphology observation of [C16imC8]Br wormlike micelles and Brij 30/[C16imC8]Br gels
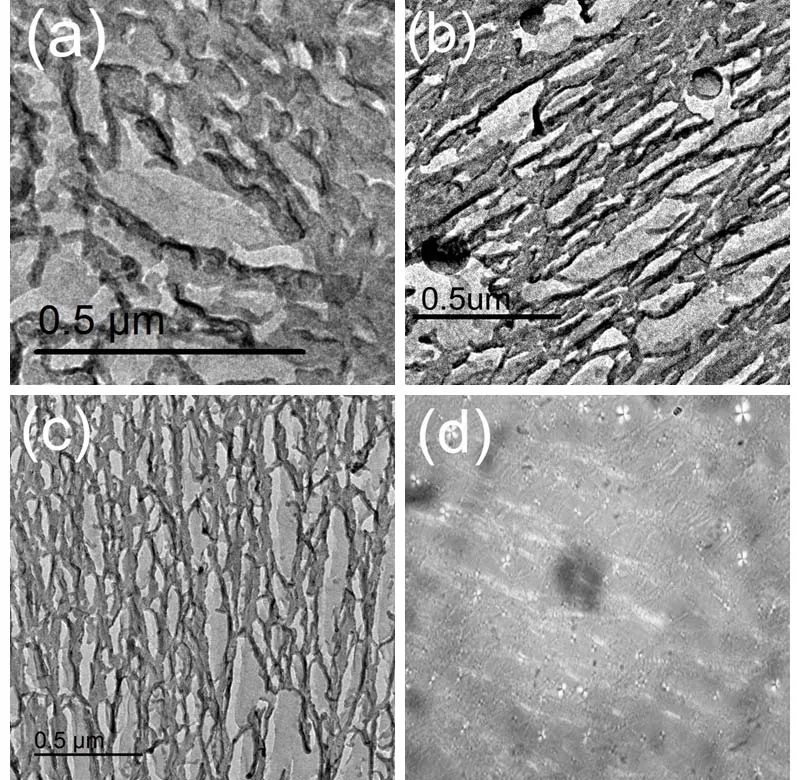
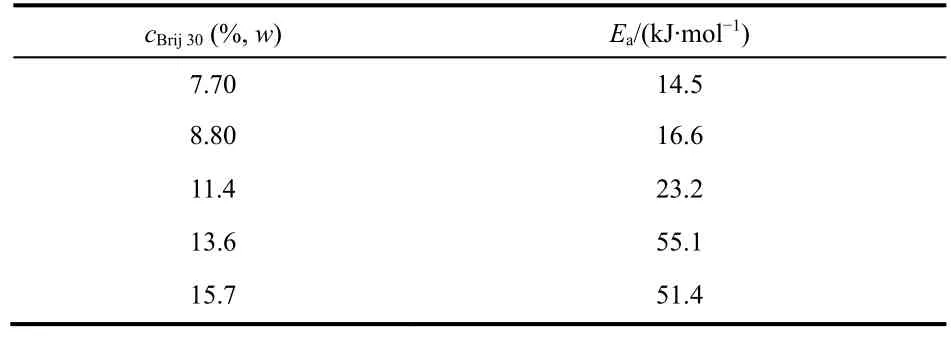
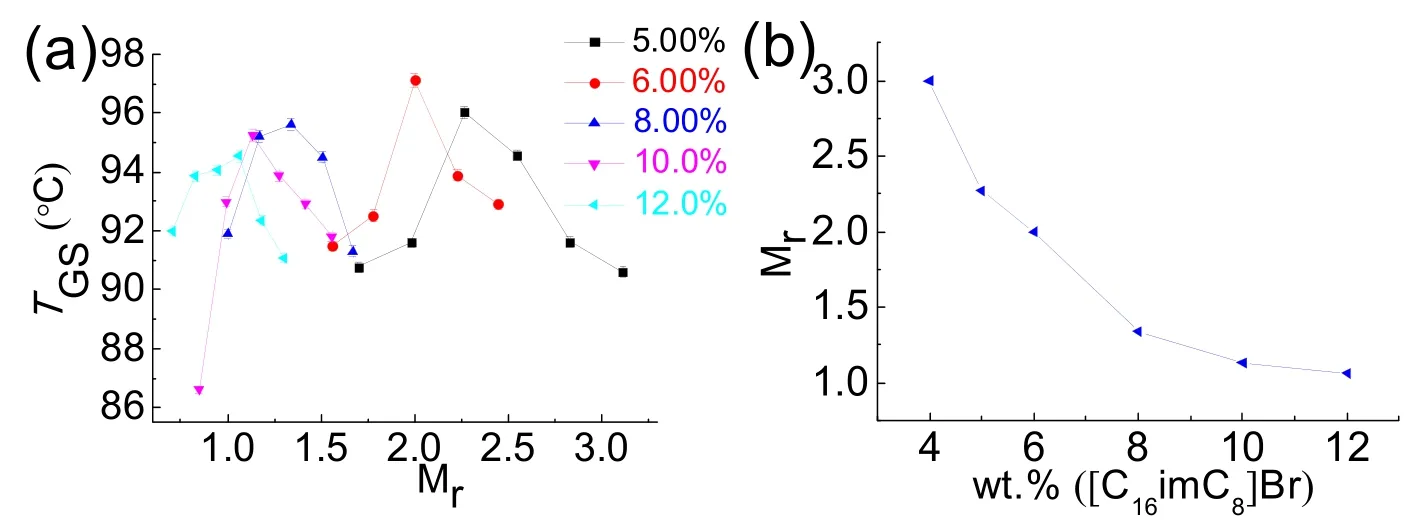
The wormlike micelle and gel phase are traditionally determined by rheological method.The [C16imC8]Br aqueous solution can form wormlike micelles at 3.50%-11.0% (w)without any additive26.The elastic modulus (G’) cross over the viscous modulus (G”) at the critical frequency (ωc) in the frequency spectrum of [C16imC8]Br (4.06% (w)) aqueous solution (Fig.S2 (see SI)).The sample behaves as solid matter at high-ωregion (G’ >G”) and as liquid at low-ωregion (G’ The digital photographs of Brij 30/[C16imC8]Br (4.06% (w))mixed solution at different Brij 30 concentrations at 25 °C are shown in Fig.1.When the concentration of Brij 30 is less than 1.25% (w) (Mr = 0.418; Mr represents the molar ratio of Brij 30 to [C16imC8]Br.), the mixed Brij 30/[C16imC8]Br (4.06% (w))solution is still wormlike micelles.When the Brij 30 concentration is 1.25% (w) the solution turns turbid and a two-phase appears.The gels are completely formed when the Brij 30 concentration reaches 6.50% (w) (Mr = 2.17).When the test tube is inverted, the gels will not flow downward.The lamellar liquid crystal phase is formed when the Brij 30 concentration reaches as high as 30.0% (w).The samples will flow downward when the test tube is inverted. Fig.1 The digital photographs of the Brij 30/[C16imC8]Br (4.06%(w))aqueous solutions at different Brij 30 concentrations. In order to gain an insight into the microscopic aggregation morphology, freeze-fractured transmission electron microscopy(FF-TEM) images were performed.Fig.2a-c show FF-TEM images of the [C16imC8]Br (4.06% (w)) wormlike micelles and the Brij 30/[C16imC8]Br (4.06% (w)) gels.Wormlike micelles were found in 4.06% (w) [C16imC8]Br sample, but the degree of entangle between micelles was low and the network structure was loose (Fig.2a).With the increase of Brij 30 concentration,three-dimensional networks are composed of elongated flexible micelles with several micrometers in contour length (Fig.2b, c)can be clearly observed.When Brij 30 concentration is 30.0%(w), the sample exhibits clear maltese crosses under polarizing microscope (Fig.2d), which are the classical optical patterns of lamellar liquid crystal phase35,36. The [C16imC8]Br (4.06% (w)) aqueous solution shows the microstructures of wormlike micelles (Fig.S2 and Fig.2a).When the Brij 30 concentration is less than 1.25% (w), the Brij 30/[C16imC8]Br (4.06% (w)) aqueous solutions also behave as wormlike micelles (Fig.3a).With the increase of Brij 30 concentration, theG’ increases gradually which is due to the increase of micellar length.The insertion of the electrical neutrality of Brij 30 into the palisade of the [C16imC8]Br wormlike micelles will shield the electrostatic repulsion between the [C16imC8]Br surfactants which induces the increase of the micellar length.When the concentration of Brij 30 is 1.25% (w),the sample begins to turn opaque and theG’ decreases because of the branch of wormlike micelles.When the concentration of Brij 30 reaches 5.85% (w) (Mr = 1.95), theG’ increases because the Brij 30/[C16imC8]Br complex micelles grow (Fig.3a). When the King heard this, he told Cola-Mattheo to bring the snake to the palace, and said that he was prepared to receive the creature as his son-in-law Fig.2 The FF-TEM images of (a) [C16imC8]Br (4.06% (w)),(b) Brij 30 (8.80% (w))/[C16imC8]Br (4.06% (w)), and(c) Brij 30 (12.0% (w))/[C16imC8]Br (4.06% (w)) aqueous solutions.(d) The polarization micrograph of Brij 30 (30.0% (w))/[C16imC8]Br(4.06% (w)) aqueous solution. Interestingly, the dynamic oscillation curve of the sample shows no crossover frequency within the range of accessible frequencies when Brij 30 concentration is 6.50% (w) (Mr = 2.17)(Fig.3b).This rheology is reminiscent of a cross-linked gel in whichG’ exceedsG” over the entire range of frequencies37.With the further increase of Brij 30 concentration theG’ of the gels increases first and then decreases (Fig.3b), which indicates the viscoelasticity of the gels increases initially and then decreases.TheG’ reaches the maximum which is attributed to the formation of fine gel network.The decrease of theG’ is due to the fuse of the micelles, which is caused by the decrease of the curvature of the micelles.With the increase of Brij 30 concentration, the increase of the hydrophobicity will cause the decrease of the curvature of micelle interface.The stress sweep measurement and creep and recovery measurement of the gels also show the similar trend with the increase of Brij 30 concentration (Fig.S3 (see SI)).When the concentration of Brij 30 is 30.0% (w), the rheological behavior of the sample is the typical for the lamellar liquid crystal (LLC) phase because the curvature of the micelles is nearly zero.The variation ofG’ andG” of gels with surfactant concentration or temperature is usually consistent.For the convenience of comparison, the variation ofG’ with Brij 30 concentration is given in Fig.3c.With the increase of Brij 30 concentration, theG’ of the gels reaches the maximum when the Brij 30 concentration is 13.6%(w) (Mr = 4.55) and then decreases. Fig.3 (a, b) The G’ (filled symbols) and G” (unfilled symbols)versus frequency for Brij 30/[C16imC8]Br (4.06% (w)) systems at different Brij 30 concentrations.(c) The variation of G’ of different samples with the Brij 30 concentration. Fig.4 (a) Zeta potential of Brij 30/[C16imC8]Br (4.06% (w)) aqueous solution at different Brij 30 concentrations.(b) Zeta potential of Brij 30 (8.80% (w))/[C16imC8]Br (4.06% (w)) gels at different temperatures. Generally, the maximum viscoelasticity of the gel composed of ionic and nonionic surfactants is determined by the balance between repulsion and attraction of the micelles in the gel.The relative strength of repulsive force or attraction between micelles can be characterized by zeta potential.The stability of micelle is very high when the zeta potential is above 61 mV.The micellar stability is preferable when the zeta potential is between ±40 and±60 mV and normal when the zeta potential is between ±30 and±40 mV.However, the micelle will become unstable when the zeta potential is between ±10 and ±30 mV.When the zeta potential is between 0 and ±5 mV the micelles will rapidly coagulate38. As is shown in Fig.4a, the zeta potential of [C16imC8]Br(4.06% (w)) aqueous solution is 56.7 mV.The repulsive force among the wormlike micelles is large.The stability of the wormlike micelles is good.When the concentration of Brij 30 was 1.00% (w) (Mr = 0.33), the zeta potential decreases to 41.2 mV which indicates the increase of the attraction force between micelles.The wormlike micelles grow along one-dimensional direction which slightly increases the viscoelasticity of the solution (Fig.3c).Upon the addition of Brij 30 the zeta potential decreases greatly, which is due to insertion of the electrical neutrality of Brij 30 into the palisade of the [C16imC8]Br wormlike micelles.The electrical repulsion force between the[C16imC8]Br head groups is weakened, and the surface charge density of the micelle decreases39-41.On the other hand, the shield of electrostatic repulsion between the hydrophilic headgroups can decrease the effective cross-sectional area of hydrophilic ionic head group, which results in the decrease of the aggregates interfacial curvature.This will induce the onedimensional micellar growth and even the fusion between wormlike micelles, leading to the collapse of the structure.The wormlike micelles aggregate into the gel with a stable threedimensional network structure when the zeta potential decreases to 14.1 mV at the Brij 30 concentration of 6.00% (w) (Mr =2.01).With the further increase of Brij 30 concentration, the zeta potential decreases to less than 5 mV.The network structure of gel is growing with the strengthened hydrophobicity of Brij 30.Accordingly, the viscoelasticity of the gel increases and reaches the maximum at the Brij 30 concentration of 13.6% (w) (Mr =4.55).TheG’ value is related to the perfection of gel network,i.e.the proportion of coarse and fine strands.TheG’ reaches the maximum which is attributed to the formation of fine gel network42.With the further increase of Brij 30 concentration, the curvature of micelle interface decreases with the increase of hydrophobicity which induces the fuse of the micelles43and the decrease of the network structure intensity and viscoelasticity of gel.When the concentration of Brij 30 increases to 30.0% (w),the curvature of micelle interface decreases to nearly zero which causes the collapse of the network structure.In the end the gel transits to lamellar liquid crystal and the viscoelasticity of the solution decreases rapidly. As shown in Fig.5a, theG’ of the Brij 30/[C16imC8]Br (4.06%(w)) gels increases first and then decreases with the increase of Brij 30 content at different temperatures.For a given Brij 30/[C16imC8]Br (4.06% (w)) gel, theG’ of all samples decreased monotonously with the increase of temperature.For gels with different Brij 30 concentrations, at low temperature range (25-55 °C), the maximum elastic modulusG’maxis relatively high at the Brij 30 concentration of 13.6% (w) (Mr = 4.55).However, it decreases abruptly to 8.80% (w) (Mr = 2.93) at high temperature range (65-85 °C) (Fig.5b).With the increase of Brij 30 concentration, the slope ofG’ gradually increases first and then decreases with the increase of temperature (Fig.5c), indicating that the gels with higher Brij 30 content were affected more by the temperature. As shown in Fig.5a, theG’ of the Brij 30/[C16imC8]Br (4.06%(w)) gels increases first and then decreases with the increase of Brij 30 content at different temperatures.For a given Brij 30/[C16imC8]Br (4.06% (w)) gel, theG’ of all samples decreased monotonously with the increase of temperature.For gels with different Brij 30 concentrations, at low temperature range (25-55 °C), the maximum elastic modulusG’maxis relatively high at the Brij 30 concentration of 13.6% (w) (Mr = 4.55).However, it decreases abruptly to 8.80% (w) (Mr = 2.93) at high temperature range (65-85 °C) (Fig.5b).With the increase of Brij 30 concentration, the slope ofG’ gradually increases first and then decreases with the increase of temperature (Fig.5c), indicating that the gels with higher Brij 30 content were affected more by the temperature. Fig.5 (a) The G’ versus Brij 30 concentration of Brij 30/[C16imC8]Br (4.06% (w)) gels at different temperatures.(b) Brij 30 concentration corresponding to the maximum elastic modulus G’max of different Brij 30/[C16imC8]Br (4.06% (w)) gels at different temperatures.(c) The G’ versus temperature of Brij 30/[C16imC8]Br (4.06% (w)) gels.(d) The G’ versus 1/T of different Brij 30/[C16imC8]Br (4.06% (w)) gels. In the case of mixed systems with a nonionic surfactant, the temperature has a pronounced effect, particularly when the surfactant contains polyoxyethylene head groups because of the sensitive dehydration of oxyethylene unit with temperature44.With the increase of temperature the dehydration of Brij 30 and the breakage of hydrogen bond will cause the disintegration of the Brij 30/[C16imC8]Br mixed micelles, resulting in the decrease of theG’.With the increase of Brij 30 concentration the viscoelasticity decreases more because temperature has more negative effect on the stability of micelles.Therefore, the higher the temperature and the Brij 30 concentration, the more obvious the decrease of viscoelasticity.So which parameter determines the macroscopic properties of the gel? Generally, the influence of temperature on chemical reaction or process is determined by the activation energy (Ea) of the system45.The activation energy of the Brij 30/[C16imC8]Br (4.06% (w)) gels can be obtained from an Arrhenius plot of these quantities: hereTis the absolute temperature (K),Ris the gas constant(8.314 J·mol-1·K-1), andAis a pre-exponential factor.Semilogarithmic plots ofG’vs1000/T(Fig.5d) fall on straight lines with identical slopes at different concentrations.The calculated value of activation energy is shown in Table 1. As shown in Table 1, the activation energy of Brij 30/[C16imC8]Br (4.06% (w)) gels increases first and then decreases with the increase of Brij 30 concentration.The energy required for the gel formation increases firstly with the increase of Brij 30 concentration.Accordingly, the stability andG’ of gelsincrease.When Brij 30 concentration is 13.6% (w) (Mr = 4.55),the activation energy of the gel reaches the maximum, and the stability of the gel is the best.With the further increase of Brij 30 concentration, the activation energy of the gel decreases which indicates the decrease of the stability of the gel.Generally,the reaction or system with low activation energy is less affected by temperature.On the contrary, the reaction or system with high activation energy is more affected by temperature46.The activation energy andG’ of Brij 30/[C16imC8]Br (4.06% (w))gels with low Brij 30 concentration is low.With the increasing of temperature, the decline extent of theG’ is small.However,the activation energy of Brij 30/[C16imC8]Br (4.06% (w)) gels with high concentration of Brij 30 is high.TheG’ decreases more with increasing temperature. Table 1 The activation energy of different Brij 30/[C16imC8]Br(4.06% (w)) gels. Fig.6 (a) The G’ (filled symbols) and G” (unfilled symbols) versus temperature for Brij 30/[C16imC8]Br (4.06% (w)) systems at different Brij 30 concentrations.(b) The TGS of the Brij 30/[C16imC8]Br (4.06% (w)) gels versus Mr.The added data are listed in Fig.S4. The gel-sol transition temperature (TGS) of gels indicates the temperature at which the system changes from gel to sol.The greater the value ofTGS, the higher the thermal stability of the gels.Generally, theG’ andG” of the gels decrease gradually with the increasing temperature in rheological measurement.At certain temperature theG’ andG” are equal.The corresponding temperature is defined as theTGS.As can be seen from Fig.6a and Fig.S4 (see SI), for the Brij 30/[C16imC8]Br (4.06% (w))gels theG’ decreases with the increase of temperature which is caused by the break of the gel networks.TheG’ decreases with increasing temperature is mainly due to the break of the hydrogen bond in the [C16imC8]Br molecule and the progressively dehydration of the EO chain of Brij 30 molecules47.On the other hand, the zeta potential decreases with the increase of temperature because of the decrease of micellar surface charge density (Fig.4b).The fusion of micelles makes the disintegration of the gel structure and leads to the decrease of the viscoelasticity of the gel.TheTGSof the Brij 30/[C16imC8]Br (4.06% (w)) gels was higher than 85 °C which indicates they have excellent thermal stability (Fig.6a). The curve ofTGSof [C16imC8]Br/Brij 30 gelsversusthe molar ratio of Brij 30/[C16imC8]Br (Mr) is shown in Fig.6b.TheTGSof the gels increases first to the maximum (Mr = 2.93) and then decreases gradually with the increase of Mr when the original[C16imC8]Br concentration is fixed at 4.06% (w).TheTGSof the gel is related to the stability of the network structure at different temperatures.The high viscoelastisity of the gel will reduce the diffusion rate of the gelators in the cavities formed by the micellar fibers.The network structure of the gel is stable and not easily destroyed.Accordingly, theTGSof the gel is high.Conversely, if the viscoelasticity of the gel is low, theTGSof the gel is low.As discussed above, the viscoelasticity of different gels increases first and then decreases with the increase of Brij 30 concentration.TheG’ reaches the maximum at Mr = 4.55.On the other hand, theG’ of gel with more Brij 30 decreases more with the increasing temperature because of the break of the hydrogen bond and the dehydration of the EO chain of Brij 30.So theTGSreaches the maximum at Mr = 2.93. In order to further inspect the variation tendency ofTGSwith Mr, the original concentration of [C16imC8]Br is fixed at 5.00%,6.00%, 8.00%, 10.0% and 12.0% (w), respectively.As shown in Fig.7, theTGSof the [C16imC8]Br/Brij 30 gels increases first with the increase of Mr, that is the increase of Brij 30 concentration, to the maximum and then decreases at different original [C16imC8]Br concentrations (Fig.7a). This trend is very similar to that in Fig.6.However, the Mr corresponding to theTGS,maxdecreases with the increase of the initial concentration of [C16imC8]Br (Fig.7b).When the[C16imC8]Br concentration is low, the amount of Brij 30 required for the most stable network structure increases accordingly because the wormlike micelles are less in the system.The increase of Brij 30 can promote the growth of micelles and entanglement between micelles.So the Mr corresponding to theTGS,maxis larger.With the increase of the [C16imC8]Br concentration the initial concentration of wormlike micelles is larger in the system.The amount of Brij 30 required for the most stable network structure is smaller.Accordingly, the Mr corresponding to theTGS,maxis smaller. Fig.7 (a) The TGS of Brij 30/[C16imC8]Br gels versus Mr at different [C16imC8]Br concentrations.(b) The Mr versus [C16imC8]Br concentration given the maximum TGS. Fig.8 (a, b) 1H NMR spectra of Brij 30/[C16imC8]Br (4.06%(w)) samples at different concentrations of Brij 30 in D2O for (a) H1, H2 and(b) H4 of [C16imC8]Br.(c) The relative chemical shifts of H1, H2 and H4 at different concentrations of Brij 30 of Brij 30/[C16imC8]Br (4.06% (w))samples.(d, e) 1H NMR spectra of Brij 30 (8.80%(w))/[C16imC8]Br (4.06% (w)) gels at different temperatures in D2O for (d) H1, H2 and (e) H4 of[C16imC8]Br.(f) The relative chemical shifts of H1, H2 and H4 of Brij 30 (8.80% (w))/[C16imC8]Br (4.06% (w)) gels at different temperatures. It can provide very useful information about the molecular conformation, the molecular interaction and the formation process of the micelles by the determination of the chemical shift of1H NMR48.Generally, the chemical shift of protons of surfactants moves downfield during aggregation in the absence of any other specific interaction49.So the1H NMR was further conducted to examine the interaction between [C16imC8]Br and Brij 30.The proton assignments of the [C16imC8]Br molecule is shown in Scheme 1, and the1H chemical shifts of [C16imC8]Br of the Brij 30/[C16imC8]Br gels are given in Fig.8. With the increasing concentration of Brij 30, the chemical shifts of the protons of H1 (0.8 ppm), H2 (1.25 ppm) (Fig.8a)and H4 (7.65 ppm) (Fig.8b) of [C16imC8]Br undergo significant downfield shifts.When the concentration of Brij 30 reaches 15.7% (w) (Mr = 5.25), the relative chemical shifts of H1, H2 and H4 all showed rapid increase (Fig.8c).In the Brij 30 and[C16imC8]Br complex micelles, the alkyl chain of the Brij 30 is close to that of [C16imC8]Br with increasing concentration of the Brij 30, which lead to the decrease of electronic cloud density of the H1, H2 and H4.So the1H peaks of the H1, H2 and H4 shift gradually to downfield50.In addition, the overlapping conformation of Brij 30 and [C16imC8]Br hydrophobic alkyl chains changes to cross-conformation with the increase of Brij 30 concentration which decreases the energy of the system.The H1 and H2 signal on the hydrophobic chain will move to downfield35.Obviously, when the concentration of Brij 30 reaches 15.7% (w) (Mr = 5.25), the surfactant molecules in the gel accumulated very closely and the conformation of hydrophobic alkyl chains changed from overlapping to crossing,which resulted in the fusion between micelles and the decrease of the structure strength of the gel network.This trend is same as the variation of the viscoelasticity of the gel with the concentration of Brij 30 in Fig.3c. Fig.8d, e shows the effect of temperature on the chemical shifts of [C16imC8]Br molecules in Brij 30 (8.80%(w))/[C16imC8]Br (4.06% (w)) gels.With the increasing temperature, the chemical shifts of the protons of H1 (0.8 ppm),H2 (1.25 ppm) (Fig.8d) and H4 (7.65 ppm) (Fig.8e) of the alkyl chain undergo significant downfield shifts (Fig.8f), which indicates the agglutination or even fusion of the micelles.This is mainly due to the dehydration of Brij 30 and the decrease of the intermolecular force because of the severe thermal movement of surfactant molecules in the gel during heating.Accordingly, the stability and viscoelasticity of the gel network structure are reduced. As viscoelastic aggregates, wormlike micelles and gels have been widely studied.However, the research on the transition of wormlike micelles to gels is still rare.Normally, the wormlike micelles-gels transitions are induced by lowering temperature23or adding salts24,49.In this paper, the transition from the[C16imC8]Br wormlike micelles to gels is realized by adding nonionic surfactant Brij 30.The zeta potential and the1H NMR measurements reveal that the neutral Brij 30 molecules insert into the palisade layer of the [C16imC8]Br wormlike micelles based on the hydrophobic action.It decreases the electrostatic repulsion between the [C16imC8]Br headgroups which induces the rapid growth of wormlike micelles and the formation of stiffer network structure.In the end the wormlike micelles transit to gels. The viscoelasticity and thermostability of the gels can be tuned by the Brij 30 concentration.Normally the viscoelasticity and thermostability of gels increases monotonously with concentration50-52.However, with the increase of Brij 30 concentration, the viscoelasticity and thermostability of the Brij 30/[C16imC8]Br gels increase first and then decrease.The viscoelasticity of the Brij 30/[C16imC8]Br gels is positively correlated with the activation energy of the gels.In the future,the mechanism and rules of the transition from wormlike micelles to gels will be further studied.It is of great scientific significance and industrial and technological application value to understand the relationship between wormlike micelles and gels.The relevant research results may be applied to the food,cosmetics industry, tertiary oil recovery and other fields. Acknowledgment:We would like to acknowledge the technical support received at the Testing Center of Yangzhou University. Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.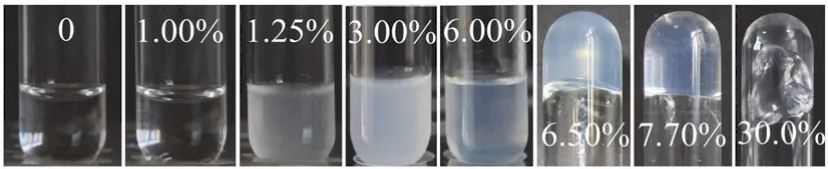
3.2 Rheological behavior of [C16imC8]Br wormlike micelles and Brij 30/[C16imC8]Br gels


3.3 Activation energy Ea of Brij 30/[C16imC8]Br gels


3.4 The gel-sol transition temperature of the Brij 30/[C16imC8]Br (4.06% (w)) gels

3.5 The interactions between the Brij 30 and[C16imC8]Br studied by 1H NMR

4 Conclusions
- 物理化學學報的其它文章
- 基于兩親性喹喔啉的超分子凝膠:手性信號反轉(zhuǎn)以及多重響應手性光學開關(guān)
- Solvent-Induced Inversion of Pickering Emulsions for In Situ Recycling of Enzyme Biocatalysts
- Structure-Property Relationship of Light-Responsive Wormlike Micelles Using Methoxycinnamate Derivatives as Light-Switchable Molecules
- 環(huán)糊精與表面活性劑主客體作用誘導的金納米棒可控自組裝
- 含有酰胺基或酯基的可降解陽離子Gemini表面活性劑在水溶液中的聚集行為
- 基于多酯頭基的“油-二氧化碳兩親分子”設(shè)計及其助混規(guī)律

