Applying Both Chemical Liquefaction and Enzymatic Catalysis Can Increase Production of Agaro-Oligosaccharides from Agarose
JIANG Chengcheng LIU Zhen LIU Jie SUNJianan XU JiachaoLI Laihao3and MAO Xiangzhao 2
Applying Both Chemical Liquefaction and Enzymatic Catalysis Can Increase Production of Agaro-Oligosaccharides from Agarose
JIANG Chengcheng1), LIU Zhen1),*, LIU Jie1), SUNJianan1), XU Jiachao1),LI Laihao3),and MAO Xiangzhao1),2),*
1),,266003,2),266237,3),,,,510300,
Red algae represents an important marine bioresource. One high-value utilization of red algae is the production of agaro-oligosaccharides which have many positive biological effects. However, the lack of an efficient production route seriously limits the application of agaro-oligosaccharides. In this study, we established a green route that combines chemical liquefaction and enzymatic catalysis for the efficient production of agaro-oligosaccharides, and we used the production of neoagarotetraose (NA4) as an example. Agarose (150gL?1) liquefaction by citric acid was controlled with respect to two targets: a 100% liquefaction rate and a high average degree of polymerization (>4) of the liquesced agaro-oligosaccharides, which were then catalyzed by β-agarase into an oligosac- charides mixture with a high concentration of NA4 (30.8gL?1) in a 1-L reaction volume. After purification, we obtained 25.5g of NA4 with a purity of 92%. This work establishes an easy route for the efficient production of pure agaro-oligosaccharides from agarose.
neoagarotetraose; agarose;agarase; marine polysaccharide; expression
1 Introduction
Red algae is an important marine bioresource for the production of functional sugars and biofuels (Kim., 2013; Wei., 2013; Yun., 2016; Wu., 2017). The dominant component of red algae is the polysaccha- ride agarose, which consists of the units D-galactose (D- Gal) and 3,6-anhydro-L-galactose (L-AHG) with alternate α-1,3- and β-1,4-linkages (Chen., 2015). Agarose can be degraded by an acid or enzyme into agaro-oligosac- charides that contain agaro-oligosaccharides (AOSs) with D-Gal as the non-reducing end and neoagarooligosaccha- rides (NAOSs) with L-AHG as the non-reducing end (Yun., 2015). Both NAOSs and AOSs are valuable func- tional oligosaccharides that feature many positive biolo- gical effects, such as anti-aging (Ma., 2019a), pro- tection against alcoholic liver injury (Jin., 2017) and the prevention of gut dysbiosis (Higashimura., 2016). Therefore, the production of agaro-oligosaccharides con- stitutes a high-value utilization of red algae. Moreover, a NAOS or AOS with a specific polymerization degree (PD) will exhibit specific biological effect. For example, neoagarotetraose (NA4) can protect mice against damage from intense exercise-induced fatigue (Zhang., 2017b), agar- opentaose can protect SH-SY5Y cells against 6-hydro- xydopamine-induced neurotoxicity (Ye., 2019), and NAOSs and AOSs exhibit different skin-whitening effects (Kim., 2017b). NA4 is a well-known NAOS with many biological functions, including anti-fatigue, the mo- dulation of intestinal microbiota, antiin?ammatory proper- ties, and anti-obesity and anti-diabetic effects (Chen., 2016; Hong., 2017; Wang., 2017; Zhang., 2017a, 2017b). For further clinical application, it is necessary to produce an agaro-oligosaccharide with specific PDs rather than a mixture of NAOSs and AOSs with different PDs.
Specific NAOSs or AOSs with certain PDs can be pro- duced from agarose by agarolytic enzymes such as agar- otetraose-producing α-agarase (Liu., 2019) and NA4- producing β-agarase (Liang., 2017). Both heterologously expressed agarases (Li., 2019; Ma., 2019b) and engineered microorganism cells containing agarases (Gao., 2019) can be used as catalysts for the production of agaro-oligosaccharides. However, because of the gelling properties of agarose, once its concentration is high- er than 1%,., the typical concentration used in agarose gel electrophoresis, the agarose solution will transform into a gel or become very sticky, which limits the enzymatic production of NAOS or AOS to a low level. In contrast, the agarose concentration can become very high (307gL?1) during acid hydrolysis (Kim., 2018), but the degradation products of agarose by acid hydrolysis comprise a complex mixture with consecutively distributed PDs (Lee., 2012; Xu., 2018). Therefore, an efficient method is needed to produce large amounts of pure agaro-oligosaccharides.
In this study, we established an easy method for efficiently producing pure agaro-oligosaccharide with a high yield. Using the production of NA4 as an example, we employed a chemical-biological route to produce high volumes of pure NA4. This route has potential for use in the large-scale production of either high-purity NAOSs or AOSs in practical applications.
2 Materials and Methods
2.1 Materials
For the NA4 preparation, we purchased agarose from Sigma (Vetec? Reagent Grade, USA), and usedBL21(DE3) for the expression of AgWH50B with plasmid pET21a (+)-. The yeast extract and tryptone were purchased from Oxoid (Basingstoke, Eng- land). The citric acid monohydrate was purchased from Chi- nese Medicine Ltd. (China).
2.2 Chemical Liquefaction
Agarose was dissolved in 2.5%, 5%, or 7.5% (w/v) citric acid solutions with a final concentration of 150gL?1. The citric acid hydrolysis was performed for 4h at 90℃ using a sterilizer. After cooling to room temperature, the insoluble material of the liquefied agar was collected by centrifugation at 12000rmin?1for 30min at 4℃. We determined the dry weight of the insoluble material after heating at 105℃ for 12h in a drying oven. Then, we calculated the liquefaction rate using formula below:
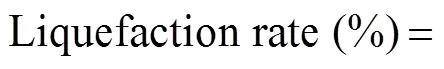
Next, after 0.75h, 1h, 2h, 3h, and 4h, the dissolvedsamples were neutralized to a pH of 7 with 20% (w/v) sodium hydroxide. The concentrations of sugar were de- termined using the 3,5-dinitrosalicylic acid (DNS) me- thod (Miller, 1959). During the analysis, 300μL of DNS was mixed with 200μL sample, which was then boiled immediately for 5min followed by cooling with cold water. The samples were subsequently diluted with 1.5mL of water, and the absorbance peak was determined to be 540nm. We used 200μL buffer as a control and all measurements were performed in triplicate.
2.3 Preparation of β-agarase and the Enzymatic Catalysis
The engineered strainBL21(DE3)-pET21a (+)-was cultured for activation using 5mL of Luria-Bertani medium (1% peptone, 0.5% yeast extract, and 1%NaCl) at 37℃, and was shaken overnight at 220rmin?1. Then, to produce the target enzyme, 10mL of the above seed solution was inoculated in 1L of ZYP-5052 medium (1% tryptone, 0.5% yeast extract, 0.2% MgSO4, 1.25%glycerin, 0.125% glucose, and 10% α-lactose as inducer), and was shaken with 220rmin?1at 20℃ for 48h. Theprecipitates were collected after centrifugation at 8500×for 15min at 4℃, resuspended in binding buffer (20mmolL?1PBS buffer, pH 7.0), and subsequently disrupted by sonication. Crude extracts were obtained by centrifugation at 8500×for 20min at 4℃, then freeze-dried to obtain crude enzyme powder. Using the DNS method (Miller, 1959), the crude enzyme activity level of Ag- WH50B was dedected to be 0.195Umg?1. Next, 200μL of 0.3% (w/v) agarose was digested with 1mg of crude enzyme at 40℃ for 15min, and then was boiled for 2min to terminate the reaction. Then, 300μL DNS was added to the reaction system and boiled for 5min to color, and then cooled it to room temperature, after which the absorbance peak was immediately determined to be 540nm. In this study, one unit of enzymatic activity (U) is defined as the amount of enzyme that produces 1μmol of reducing sugar per min by hydrolyzing agarose under the assay conditions. Before the last reaction in the NA4 preparation, we optimized the amount of AgWH50B crude enzyme, using 25, 50, or 75gL?1of crude enzyme powder for the optimization with a 10-mL reaction volume at 40℃ for 12h. We then boiled it for 10min and obtain the supernatant by centrifugation at 10000×for 15min at 4℃. To determine the concentration of NA4, we used high-perfor- mance liquid chromatography (HPLC) with a Sugar-Pak I column (Waters, U.S.A.) under the following conditions: a mobile phase of EDTA calcium disodium (50mgL?1), column temperature of 75℃, flow velocity of 0.5mLmin?1, and detection by a refractive index detector (Liang., 2017).
In the enzymatic catalysis step, we hydrolyzed the neutralized oligosaccharide solution obtained during the liquefaction step using 25gL?1of AgWH50B in a 1-L reaction mixture at 40℃ for 12h. After the reaction mixture was boiledfor 10min, it was centrifuged at 8500×g for 20min to obtain a supernatant containing a mixture of NA4 and other oligosaccharides. The supernatant was then freeze-dried it to obtain agaro-oligosaccharide powder for the next step of purification.
2.4 Purification of NA4
The agaro-oligosaccharide powder was then resuspend- ed in hyperpure water, and separated using a Bio-Gel P2 chromatography column, eluting the hyperpure water at a flow rate of 2mLmin?1. Aliquots were sampled every 2.5min and analyzed by thin-layer chromatography (TLC), and the plates were eluted in a developing solvent composed of n-butanol/acetic acid/water (2:1:1, v:v:v). The agaro-oligosaccharide spots were visualized by soaking the TLC plate in an ethanol solution containing 10% (v/v) H2SO4, and then heating it at 100℃ for 5min. Based on the TLC results, we collected and identified aliquots containing NA4 using the HPLC method described in Section 2.3.
3 Results and Discussion
3.1 Chemical Liquefaction of Agarose
Fig.1 shows an overview of the preparation routes used in this study. First, agarose was liquesced into soluble AOSs by citric acid, which is a food additive. Next, β- agarase AgWH50B (details regarding AgWH50B can be obtained from the study by Liang. (2017)) was used to hydrolyze the AOSs into NA4 and other agaro-oligo- saccharides with low PDs. In Step I, the PDs of the AOSs after liquefaction must be low enough to ensure their com- plete solubility and easy catalysis by β-agarase in Step II. Meanwhile, AOSs with PDs higher than 4 were hoped to be the products as they are the substrate for β-agarase AgWH50B to produce NA4 in Step II.
We treated 150gL?1of agarose using citric acid with con- centrations ranging from 2.5% to 7.5% at 90℃. As shown in Fig.2A, when the citric acid concentration was 2.5% and the treatment time was 0.75h, the agarose liquefaction rate was 92%, whereas in all other conditions, the liq- uefaction rate for each sample was 100%. This means that 0.75h was not long enough to achieve complete liquefaction. As shown in Fig.2B, with increasing the citric acid concentration, the molar concentration of the reducingsugars (MCRS) increased, and a higher MCRS was attained by increasing the treatment time. The initial agarose concentration (150gL?1) was the same in all the samples, so the higher PD,., the higher the relative molecular mass, the lower was the MCRS. If the average PD is 4 after liquefaction, the MCRS would be 0.22molL?1, so the actual MCRS must be lower than 0.22molL?1. Therefore, the liquefaction conditions must be controlled to achieve two goals: a 100% liquefaction rate and an MCRS lower than 0.22molL?1. In other words, in Fig.2C, the desired sample-condition data point should be just on the MCRS coordinate axis (vertical line) and lower than the liquefaction rate coordinate axis (horizontal line). There are only two conditions that qualify: 2.5% citric acid for 1h and 5% citric acid for 0.75h. The MCRS value in the condition (2.5%, 1h) was 0.18±0.01molL?1, which is lower than that (0.20±0.01molL?1) in the condition (5%, 0.75h), which indicates that the average PD in the condition (2.5%, 1h) is higher than that in the condition (5%, 0.75h). Therefore, the optimal condition in Step I was 2.5% citric acid at 90℃ for 1h. The HPLC results in Fig.2D showed that no NA4 was produced after chemical liquefaction. However, we observed one unknown oligosaccharide (US1, US2) with a PD higher than NA4, and three other unknown oligosaccharides (US3, US4) with PDs lower than NA4.

Fig.2 Chemical liquefaction of agarose. (A) Liquefaction rate of agarose after chemical liquefaction. (B) Molar concentration of reduced sugars (MCRS) after chemical liquefaction. (C) Scheme for screening the optimal liquefaction conditions. (D) Oligosaccharides produced during the chemical liquefaction step. US, unknown saccharide; PD, degree of polymerization; MCRS, molar concentration of reducing sugars. Allmeasurements were performed in triplicate. Error bars indicate the standarddeviation of measurement.
In previous studies, acetic acid and HCl were used in the preparation of oligosaccharides from agarose (Kaz- ?owski., 2008; Kim., 2012). In this study, for the agarose liquefaction, citric acidused as a food additive was employed (Henry., 1985). The citric acid treatment seems to be an extra step compared to the one-step enzyme hydrolysis method (Kim., 2017a). However, without citric acid treatment, prior to hydrolysis by agarase, the agarose must be melted at high temperature and then cooled to the appropriate temperature for enzymatic hydrolysis. The time and workload associated with this pretreatment are comparable to that (90℃, 1h) of our che- mical liquefaction step. Moreover, citric acid can be used to pretreat agarose in high concentrations, which enables large-scale enzymatic preparation.
3.2 NA4 Production by β-agarase
Fig.3 shows that with increases in the enzyme concentration, the titer of NA4 decreased, whereas the titers of US3, US4, and US5 increased. The highest titer of NA4 (30.8gL?1) was produced using 25gL?1of β-agarase AgWH50B. Fig.3A shows that US1, whose PD is higher than that of NA4, was completely degraded after hydro- lysis by AgWH50B. However, the titer of US3, another by-product whose PD is slightly lower than NA4, significant- ly increased. Therefore, it is important that US3 is removed in the purification step. Fig.4 shows the time course of NA4 formation in Step II. At 12h, the NA4 titer reached 30.8±0.52gL?1with a productivity of 2.57gL?1h?1in 1L of reaction broth.

Fig.3 Optimization of enzymatic catalysis. (A) Oligosaccharides produced using different amounts of enzyme. (B) NA4 produced using different amounts of enzyme. US, unknown saccharide. All measurements were performed in triplicate.Error bars indicate the standard deviation of measurement.

Fig.4 Time course of NA4 formation during enzymatic catalysis by 25gL?1 of β-agarase AgWH50B. US, unknown saccharide. All measurements were performed in triplicate.Error bars indicate the standard deviation of measurement.
In most of the previous studies on agarose degradation, oligosaccharides were produced at laboratory scales to study their function or activity.So a much lower initial agarose concentration was enough, such as 5.5gL?1(Kaz- ?owski et al., 2015) or 2gL?1(Li et al., 2007). An exception was an agaro-oligosaccharides production at the medium scale, in which the researchers used 289g of agarose as a substrate in 3-L buffer for hydrolysis by agarase, which means the initial agarose concentration was 96.3gL?1(Pan et al., 2010). In this study, combiningboth che- mical liquefaction and enzymatic catalysis in the preparation of NA4 enabled the use of a larger concentration of substrates (up to 150gL?1) and consequently drastically reduced the amount of water, which facilitates the subsequent purification steps.
3.3 Purification of NA4
In total, we collected 100 aliquots after purification by Bio-Gel P2 chromatography (Li., 2007; Jang., 2009; Lin., 2019). The TLC results shown in Fig.5A reveal that purified NA4 was present in aliquots 30 to 50, the details of which are shown on another TLC plate (Fig.5B). These results indicate that aliquots 38–42 were well purified, but aliquots 30–37 and 43–50 contained a few other oligosaccharides and required further purifica- tion. The Bio-Gel P2 column results for the different ali- quots after the second purification showed in Fig.5C sug- gested that the purified NA4 were in aliquots 20–28. Ali- quots 38–42 from the first purification and 20–28 from the second purification were examined by HPLC, which revealed that purified NA4 had been successfully ac- quired. After vacuum distillation and freeze-drying, 25.5±0.31g of NA4 powder was isolated, the purity of which, based on the HPLC results, was calculated to be 92%.
β-Agarase has been used previously to produce NAOSs, whereby a mixture of 6.4gL?1NA4 and 3.8gL?1neoa- garobiose (NA2) were produced from 10gL?1of agarose (Seo., 2014). Using 35g of agar as the substrate, 53mg of purified NA4 was also obtained after enzyme hydrolysis followed by purification (Jang., 2009). By producing NAOSs with a PD range of 2–22, 13.18g of NA4 was prepared from 289g of agarose with a concen- tration of 2.46%, which is the highest concentration of purified NA4 that have been reported (Pan., 2010). Using the engineeredBL21(DE3) containing a β- agarase, 0.45gL?1of NA2 was also produced from 2gL?1of agarose (Gao., 2019). As shown in Table 1, the 25.5g of NA4 obtained in this study is the highest pro- duction of any kind of agaro-oligosaccharide ever pro- duced by any method to date. Considering the purity and yield of the products, the advantage of our green chemical-biological route is obvious. In the future, we expect that by combining controlled chemical liquefaction with one or more other agarolytic enzymes such as α-agarase (Liu., 2019), α-neoagarobiose hydrolase (Jiang., 2020), and/or β-galactosidase (Yang., 2018), AOSs or NAOSs with different PDs can be efficiently produced. Furthermore, our method is easy to scale up to produce agaro-oligosaccharides at both pilot-project and industrial levels to yield high-purity NAOS or AOS for practical ap- plication.
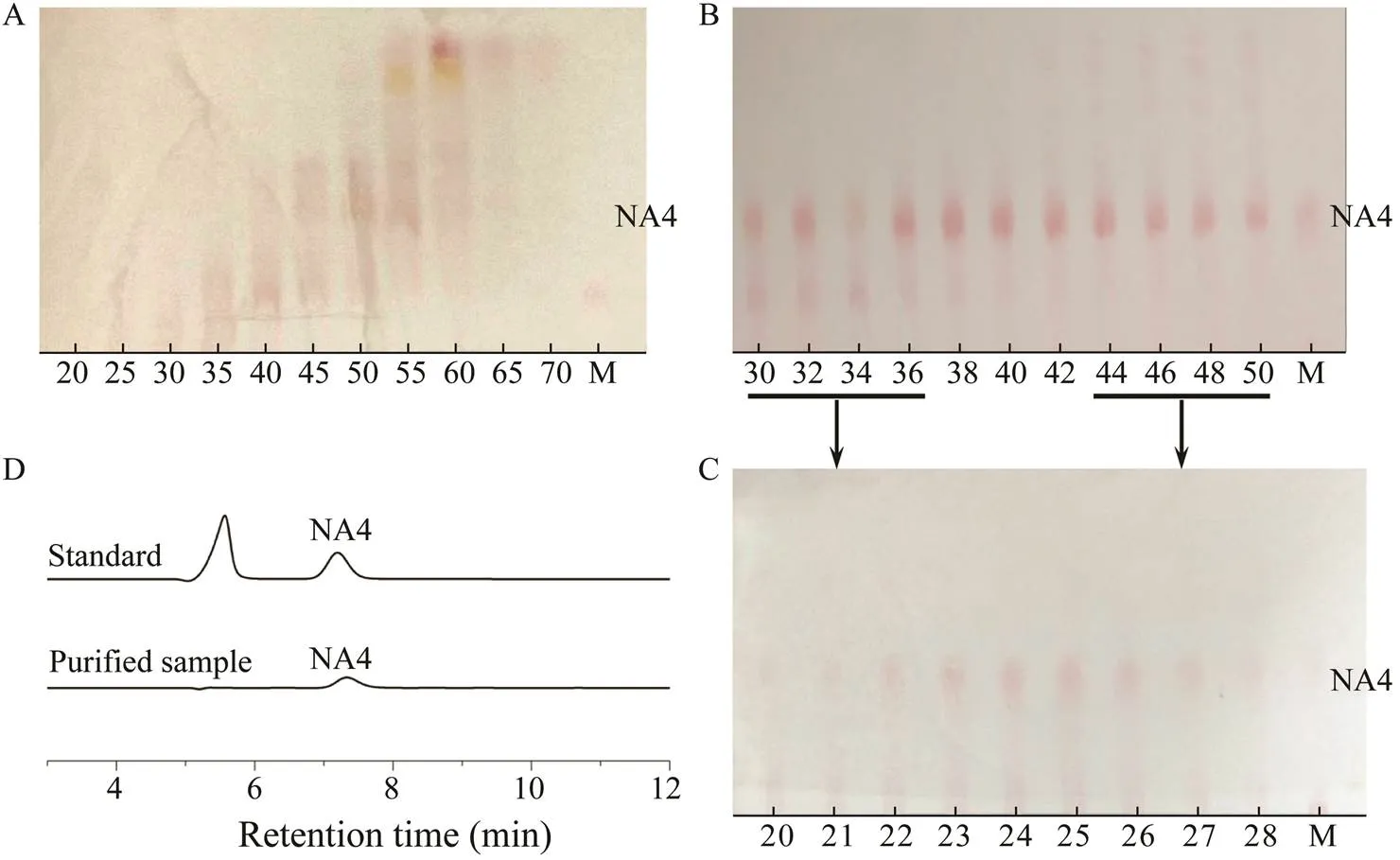
Fig.5 Purification of NA4 by gel chromatography. (A), (B) TLC results for different aliquots from the first purification. (C) TLC results for the different aliquots from the second purification. (D) HPLC results for purified NA4.

Table 1 The production of agaro-oligosaccharides (PD≤8) by chemical and/or biological methods in previous researches
Note:?The purity and yield were not calculated if there was no purification step.
4 Conclusions
In this work, we established a route that combines controlled chemical liquefaction with enzymatic catalysis in the preparation of purified NA4. Using 2.5% citric acid, we liquesced 150gL?1of agarose dissolved in 1L of water at 90℃ for 1h to obtain an agaro-oligosaccharide solution with an MCRS value of 0.18±0.01molL?1. Enzymatic catalysis was then performed using β-agarase AgWH50B to produce NA4, followed by the use of gel chromatography to purify NA4. Ultimately, we obtained 25.5±0.31g NA4 with a purity of 92%, which is the highest production of NA4 to date. Our proposed method is suitable for producing many pure agaro-oligosaccharides and enables their large-scale production.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2018YFC0311200), the Fundamen- tal Research Funds for the Central Universities (No. 201941002), and the Taishan Scholars Project (No. tsqn201812020).
Chen, H., Yan, X., Peng, Z., and Jing, L., 2016. Antioxidant activity and hepatoprotective potential of agaro-oligosaccha- ridesand.,5: 31-31, DOI: 10.1186/1475-2891-5-31.
Chen, H., Zhou, D., Luo, G., Zhang, S., and Chen,J., 2015. Ma- croalgae for biofuels production, progress and perspectives.,47: 427-437, DOI: 10.1016/j.rser.2015.03.086.
Gao, B., Li, L., Wu, H., Zhu, D., Jin, M., Qu, W., and Zeng, R., 2019. A novel strategy for efficient agaro-oligosaccharide pro- duction based on the enzymatic degradation of crude agarose inWPAGA1.,10: 1231-1242, DOI: 10.3389/fmicb.2019.01231.
Henry, R.W., Pickard, D.W., and Hughes, P.E., 1985. Citric acid and fumaric acid as food additives for early-weaned piglets., 40: 505-509, DOI: 10.1017/S0003356100040204.
Higashimura, Y., Naito, Y., Takagi, T., Uchiyama, K., Mizushi- ma, K., Ushiroda, C., Ohnogi, H., Kudo, Y., Yasui, M., Inui, S., Hisada, T., Honda, A., Matsuzaki, Y., and Yoshikawa, T., 2016. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice.,310: G367-G375, DOI: 10.1152/ajpgi.00324.2015.
Hong, S. J., Lee, J. H., Kim, E. J., Yang, H. J., Park, J. S., and Hong, S. K., 2017. Anti-obesity and anti-diabetic effect of neo-agarooligosaccharides on high-fat diet-induced obesity in mice.,15: 31-41, DOI: 10.3390/md15040090.
Jang, M.K., Lee, D.G., Kim, N.Y., Yu, K.H., Jang, H.J., Lee, S.W., Jang, H.J., Lee, Y.J., and Lee, S.H., 2009. Purification and characterization of neoagarotetraose from hydrolyzed agar.,19: 1197-1200, DOI: 10.4014/jmb.0906.06045.
Jiang, C., Liu, Z., Sun, J., and Mao, X., 2020. Characterization of a novel-neoagarobiose hydrolase capable of preparation of mediumand long-chain agarooligosaccharides., 7: 470-480, DOI: 10.3389/fbioe.2019.00470.
Jin, M., Liu, H., Hou, Y., Chan, Z., Di, W., Li, L., and Zeng, R., 2017. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from.,100: 186-195, DOI: 10.1016/j.foodres.2017.08.032.
Kang, O. L., Ghani, M., Hassn, O., Rahmati, S., and Ramli, N., 2014. Novel agaro-oligosaccharide production through enzy- matichydrolysis: Physicochemical properties and antioxidant activities.,42: 296-304, DOI: 10.1016/j. foodhyd.2014.04.031.
Kaz?owski, B., Pan, C. L., and Ko, Y. T., 2008. Separation and quantification of neoagaro- and agaro-oligosaccharide products generated from agarose digestion by beta-agarase and HCl in liquid chromatography systems.,343: 2443-2450, DOI: 10.1016/j.carres.2008.06.019.
Kaz?owski, B., Pan, C.L., and Ko, Y.T., 2015. Monitoring and preparation of neoagaro- and agaro-oligosaccharide products by high performance anion exchange chromatography systems.,122: 351-358, DOI: 10.1016/j.carbpol.2014.09.003.
Kim, D.H., Yun, E.J., Lee, S.H., and Kim, K.H., 2018. Novel two-step process utilizing a single enzyme for the production of high-titer 3,6-anhydro-L-galactose from agarose derived from red macroalgae., 66(46): 12249-12256, DOI: 10.1021/acs.jafc.8b04144.
Kim, H.T., Lee, S., and Kim, K.H.,2012. The complete en- zymatic saccharification of agarose and its application to simultaneous saccharification and fermentation of agarose for ethanol production.,107: 301-306, DOI: 10.1016/j.biortech.2011.11.120.
Kim, H.T., Yun, E.J., Wang, D., Chung, J.H., Choi, I.G., and Kim, K.H.,2013. High temperature and low acid pretreatment and agarase treatment of agarose for the production of sugar and ethanol from red seaweed biomass.,136: 582-587, DOI: 10.1016/j.biortech.2013.03.038.
Kim, J.H., Yun, E.J., Seo, N., Yu, S., Dong, H.K., Cho, K.M., An, H.J., Kim, J.H., Choi, I.G., and Kim, K.H., 2017a. En- zymatic liquefaction of agarose above the sol-gel transition temperature using a thermostable endo-type β-agarase, Aga16B.,101: 1111-1120, DOI: 10.1007/s00253-016-7831-y.
Kim, J. H., Yun, E. J., Yu, S., Kim, K. H., and Kang, N. J., 2017b. Different levels of skin whitening activity among 3,6-anhy- dro-L-galactose, agarooligosaccharides, and neoagarooligosa- ccharides.,15: 321-330, DOI: 10.3390/md15 100321.
Lee, D. G., Myong, J. J., and Lee, S. H., 2012. Cloning, expression, and characterization of a glycoside hydrolase family 118 β-agarase fromsp. JA-1.,22: 1692-1697, DOI: 10.4014/jmb.1209.09033.
Li, J., Han, F., Lu, X., Fu, X., Ma, C., Chu, Y., and Yu, W., 2007. A simple method of preparing diverse neoagaro-oligosaccha- rides with beta-agarase.,342: 1030-1033, DOI: 10.1016/j.carres.2007.02.008.
Li, L., Qu, W., Jin, M., Di, W., and Zeng, R., 2019. Extracellular expression of agarase rAgaM1 inand its abi- lity for neoagaro-oligosaccharide production.,59(4): 359-367: DOI: 10.1002/jobm.201800442.
Liang, Y., Ma, X., Zhang, L., Li, F., Liu, Z., and Mao, X., 2017. Biochemical characterization and substrate degradation mode of a novel exo-type β-agarase fromWH0801.,65: 7282-7988, DOI: 10.1021/acs.jafc.7b01533.
Lin, F., Ye, J., Huang, Y., Yang, Y., and Xiao, M., 2019. Simple preparation of diverse neoagaro-oligosaccharides.,7: 267-280, DOI:10.3390/pr7050267.
Liu, J., Liu, Z., Jiang, C., and Mao, X., 2019. Biochemical cha- racterization and substrate degradation mode of a novel α- agarase from.,67(37): 10373-10379, DOI: 10.1021/acs.jafc.9b03073.
Ma, C., Yang, K., Wang, Y., and Dai, X., 2019a. Anti-aging ef- fect of agar oligosaccharide on maleand its preliminary mechanism.,17: 632-645, DOI: 10.3390/md17110632.
Ma, J., Yan, Q., Yi, P., Yang, S., Liu, H., and Jiang, Z., 2019b. Biochemical characterization of a truncated β-agarase fromsp. suitable for efficient production of neoaga- rotetraose.,87: 119-127, DOI: 10.1016/j.procbio.2019.08.021.
Miller, G. L., 1959. Use of dinitrosalicylic acid reagent for de- termination of reducing sugar.,31:426-428, DOI: 10.1021/ac60147a030.
Pan, C., Kazlowski, B., Ko, Y., Kong, Z., Wu, S., and Wang, F., 2010. Manufacturing method of separating and purifying neo- agarooligosaccharides having degrees of polymerization from 2 to 22. US patent,0323407A1.
Seo, Y.B., Lu, Y., Chi, W.J., Park, H.R., Jeong, K.J., Hong, S.K., and Chang, Y.K., 2014. Heterologous expression of a newly screened β-agarase fromsp. GNUM1 inand its application for agarose degradation.,49: 430-436, DOI: 10.1016/j.procbio.2013.12.014.
Wang, W., Liu, P., Hao, C., Wu, L., Wan, W., and Mao, X., 2017. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappa B pathways.,7: 44252-44262, DOI: 10.1038/srep44252.
Wei, N., Quarterman, J., and Jin, Y.S., 2013.Marine macroalgae,an untapped resource for producing fuels and chemicals.,31: 70-77, DOI: 10.1016/j.tibtech.2012.10. 009.
Wu, Y. R., Zhang, M., Zhong, M., and Hu, Z., 2017. Synergistic enzymatic saccharification and fermentation of agar for bio- hydrogen production.,241: 369-373, DOI: 10.1016/j.biortech.2017.05.117.
Xu, X. Q., Su, B. M., Xie, J. S., Li, R. K., Yang, J., Lin, J., and Ye, X. Y., 2018. Preparation of bioactive neoagaroligosaccharides through hydrolysis ofagar, a comparative study.,240: 330-337, DOI: 10. 1016/j.foodchem.2017.07.036.
Yang, X., Liu, Z., Jiang, C., Sun, J., Xue, C., and Mao, X., 2018. A novel agaro-oligosaccharide-lytic β-galactosidase fromWH0801., 102(12): 5165-5172, DOI: 10.1007/s00253-018-8999-0.
Ye, Q., Wang, W., Hao, C., and Mao, X., 2019. Agaropentaose protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity through modulating NF-κB and p38MAPK sig- naling pathways.,57: 222-232, DOI: 10.1016/j.jff.2019.04.017.
Yun, E.J., Choi, I.G., and Kim, K.H., 2015. Red macroalgae as a sustainable resource for bio-based products.,33: 247-249, DOI: 10.1016/j.tibtech.2015.02.006.
Yun, E.J., Kim, H.T., Cho, K.M., Yu, S., Kim, S., Choi, I.G., and Kim, K.H., 2016. Pretreatment and saccharification of red macroalgae to produce fermentable sugars.,199: 311-318, DOI: 10.1016/j.biortech.2015.08.001.
Zhang, N., Hou, E., Song, J., Li, J., Tang, Q., and Mao, X., 2017a. Neoagarotetraose-modulated gut microbiota and alleviated gut inflammation in antibiotic treatment mice.,28: 1-16, DOI: 10.1080/09540105.2017.1346063.
Zhang, N., Mao, X., Li, R. W., Hou, E., Wang, Y., Xue, C., and Tang,Q., 2017b. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut mi- crobial composition and function.,61: 1-12, DOI: 10.1002/mnfr.201600585.
. E-mail: liuzhenyq@ouc.edu.cn
E-mail: xzhmao@ouc.edu.cn
January 6, 2020;
April 5, 2020;
June 2, 2020
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年6期
Journal of Ocean University of China2020年6期
- Journal of Ocean University of China的其它文章
- Numerical Simulation and Analysis of Electromagnetic Fields Induced by a Moving Ship Based on a Three-Layer Geoelectric Model
- In situ Assemblies of Bacteria and Nutrient Dynamics in Response to an Ecosystem Engineer, Marine Clam Scapharca subcrenata, in the Sediment of an Aquaculture Bioremediation System
- Harvesting Microalgae Biomass Using Sulfonated Polyethersulfone (SPES)/PES Porous Membranes in Forward Osmosis Processes
- Simulation of the Internal Wave of a Subsurface Vehicle in a Two-Layer Stratified Fluid
- Effects of Different Weaning Protocols on Survival,Growth and Nutritional Composition of Pharaoh Cuttlefish (Sepia pharaonis) Juvenile
- Microalgal Cultivation in Secondary Effluents: Enhancement of Algal Biomass, Nutrient Removal, and Lipid Productivity
