Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update
Sasidharan Rajesh, Tom George, Cyriac Abby Philips, Rizwan Ahamed, Sandeep Kumbar, Narain Mohan,Meera Mohanan, Philip Augustine
Abstract More than five decades after it was originally conceptualized as rescue therapy for patients with intractable variceal bleeding, the transjugular intrahepatic portosystemic shunt (TIPS) procedure continues to remain a focus of intense clinical and biomedical research. By the impressive reduction in portal pressure achieved by this intervention, coupled with its minimally invasive nature, TIPS has gained increasing acceptance in the treatment of complications of portal hypertension. The early years of TIPS were plagued by poor long-term patency of the stents and increased incidence of hepatic encephalopathy. Moreover, the diversion of portal flow after placement of TIPS often resulted in derangement of hepatic functions, which was occasionally severe. While the incidence of shunt dysfunction has markedly reduced with the advent of covered stents, hepatic encephalopathy and instances of early liver failure continue to remain a significant issue after TIPS. It has emerged over the years that careful selection of patients and diligent post-procedural care is of paramount importance to optimize the outcome after TIPS. The past twenty years have seen multiple studies redefining the role of TIPS in the management of variceal bleeding and refractory ascites while exploring its application in other complications of cirrhosis like hepatic hydrothorax, portal hypertensive gastropathy, ectopic varices, hepatorenal and hepatopulmonary syndromes, non-tumoral portal vein thrombosis and chylous ascites. It has also been utilized to good effect before extrahepatic abdominal surgery to reduce perioperative morbidity and mortality. The current article aims to review the updated literature on the status of TIPS in the management of patients with liver cirrhosis.
Key Words: Early transjugular portosystemic shunt; Preemptive transjugular intrahepatic portosystemic shunt; Portal hypertension; Esophageal varices; Gastric varices; Refractory ascites
INTRODUCTION
Portal hypertension (PH) is the primary vascular consequence of cirrhosis and responsible for the majority of its potentially life-threatening complications. Transjugular intrahepatic portosystemic shunt (TIPS), involving the creation of a sideto-side shunt between the portal and hepatic vein, was envisaged as a salvage therapy for patients with acute variceal hemorrhage (VH) not responding to standard medical care[1]. With the discovery of self-expandable metal stents, TIPS started gaining wider acceptance not only for managing episodes of acute VH but also in other complications of PH like refractory ascites (RA) and hepatic hydrothorax (HH)[2]. The diversion of portal flow, so effectively achieved by TIPS, also resulted in hepatic hypoperfusion resulting in hepatic encephalopathy (HE) and deterioration in liver functions. Moreover, the uncovered self-expandable metal stents used in the initial years of TIPS were notorious for early thrombosis due to leakage of bile into the stent within the hepatic parenchymal tract and pseudointimal hyperplasia at the hepatic venous end of the stent. This resulted in frequent shunt dysfunctions necessitating multiple reinterventions. With the advent of expanded-polytetrafluoroethylene (e-PTFE) covered stents for TIPS in 2004, the incidence of shunt dysfunction reduced markedly. Additional studies showed that the use of covered stents for TIPS may not increase episodes of de novo or worsening HE, although this issue is still debatable[3]. Liver failure after TIPS continues to remain an area of concern. Appropriate patient selection for TIPS plays a major role in clinical outcomes. Significant modifications in patient selection criteria for TIPS have occurred in the recent past. The role of TIPS in management of other complications of cirrhosis and PH such as HH, portal hypertensive gastropathy (PHG), ectopic varices, hepatorenal syndrome (HRS) and hepatopulmonary syndrome (HPS), non-tumoral portal vein thrombosis (PVT) and chylous ascites has also been explored. With the introduction of the novel controlledexpansion stent, options for modulation of the portosystemic gradient (PSPG) after placement of TIPS stent has evolved. Recent studies have demonstrated a reduction in the incidence of HE, stent dysfunction, readmission for sepsis, and ascites with the use of these stents[4,5]. In this review, we present an exhaustive update of current literature on the role of TIPS in the management of PH in patients with cirrhosis with emphasis on emerging indications of TIPS, evolving patient selection criteria, and technical aspects of the procedure.
ESTABLISHED AND EMERGING INDICATIONS FOR TIPS
Acute esophageal VH
VH is one of the most severe and life-threatening complications in cirrhosis patients and constitutes the second most frequent decompensating event after ascites[6]. About 10%-15% of patients experience treatment failure, warranting repeated endoscopic interventions, with up to 80% mortality[6,7]. The overall mortality at 6 wk with each episode of VH also remains high at around 15%-25%, despite improvements in therapy[8,9].
Rescue TIPS and the role of early/preemptive TIPS:TIPS is highly effective in reducing the portal pressure, control of bleed, and prevention of early rebleeding. Due to the increased risk of HE and the absence of survival benefit with the use of uncovered stents, TIPS was traditionally recommended as rescue therapy for uncontrolled bleeding. The prognosis of patients undergoing rescue (or salvage) TIPS is dismal, with 35%-55% mortality due to failure to control bleeding or early rebleeding. The time-delay associated with the decision on performing TIPS also contributes to poor outcome[7,10,11]. A recent large observational study showed a 6-wk mortality of 36% in patients undergoing rescue TIPS[11]. The model for end-stage liver disease (MELD) and Child–Turcotte-Pugh (CTP) scores were predictive of short- and long-term mortality, respectively, and pre-TIPS intensive care unit stay was independently associated with TIPS failure and mortality at 6 wk and 12 mo. Rescue TIPS was found futile in patients with CTP score > 13. With the advent of e-PTFE–covered stents, the incidence of TIPS dysfunction and recurrence of complications related to PH reduced drastically. Additionally, it was found that covered TIPS did not significantly increase the frequency and severity of episodes of de-novo HE[3]. Hepatic venous pressure gradient (HVPG), the surrogate marker of portal pressure, is an objective and reproducible measurement. Moitinhoet al[12]found that measurement of HVPG in patients with cirrhosis admitted with acute VH provided useful prognostic information, and those with HVPG > 20 mmHg required closer surveillance. Monescillo and colleagues showed that early portal decompression by TIPS placement in those with HVPG > 20 mmHg significantly reduced the risk of treatment failure, prevented recurrent VH, and improved short and long-term survival despite having higher baseline bilirubin levels[13]. HVPG was found more accurate than the CTP score for 6 wk survival prediction. This study, however, used endoscopic sclerotherapy in the medical treatment group, and bare stents were used in the early-TIPS group, both of which are not the current standard of care.
To address these issues, a multicentre randomized controlled trial (RCT) was conducted in which patient selection was based on clinical and endoscopic criteria[3]. In this study, early treatment with covered TIPS (within 72 h, and preferably within 24 h) in high-risk patients-defined as CTP score 10-13 points and CTP class B with active bleeding at endoscopy-resulted in significant bleed control and reduction in mortality, without an increase in the risk of HE. Additionally, the study found lower rates of ascites formation, HRS, and reduced hospital stay. A retrospective post-RCT surveillance study by the same group found only a trend to improvement in survival when compared with standard medical therapy[14]. The Baveno VI consensus endorsed these findings and recommended that "an early TIPS within 72 h (ideally < 24 h) should be considered in patients at high risk of treatment failure after initial pharmacological and endoscopic therapy"[15].
Further, a meta-analysis confirmed the survival benefit offered by early TIPS in high-risk patients[16]. The original trial by Garcia-Paganet al[3]was not powered to conduct appropriate subgroup analyses to identify benefits on survival between CTP B and C groups. Studies conducted later showed that clinical outcomes among CTP B patients on standard medical treatment were significantly better than that of Child-Pugh C patients without added benefits with early-TIPS[17]. The re-calibrated MELD score as an alternative to the CTP score was shown to have better prognostic value in patients with acute VH on standard care[18]. CTP C patients with a baseline creatinine ≥ 1 mg/dL (Child C-C1 criteria) were found to have high-risk of death after VH[17]. A recent multicentre study showed that the mortality risk among CTP B compared to CTP class C patients with active bleeding at endoscopy, on the standard of care was lower[17]. The study also identified MELD score ≥ 19 as a high risk for death with standard care alone. This implied that the grouping of Child-Pugh B and Child-Pugh C as high-risk for mortality on standard therapy of acute VH was inaccurate. Subsequently, observational studies showed that the early use of TIPS was justified in those with MELD ≥ 19 or Child-Pugh class C[19]. For patients with MELD 12–18 or Child-Pugh B patients, survival benefit could not be uniformly demonstrated.
An RCT from a single center in China reported improved control of bleeding and rebleeding and better transplant-free survival (TFS) at 6 wk and one year with early TIPS[20]. The benefit was seen in all groups regardless of active bleeding or stage of liver disease. There was no difference in the incidence of HE. Besides, the actuarial probability of remaining free from new or worsening ascites was higher in the early TIPS group than in the control group at one year. A slight increase of median bilirubin levels and the international normalized ratio at 1 and 3 mo was observed in the early-TIPS group, which improved after 6 mo. Similarly, median MELD scores were significantly higher at 1 and 3 mo in the TIPS group disappearing after 6 mo. Notably, all patients with Child-Pugh class B and class C disease were included irrespective of active bleeding, and 75% had a chronic hepatitis-B infection. Therefore, antiviral therapy could have influenced the outcome. Another recent RCT from the United Kingdom reported that early-TIPS reduced rebleeding without survival benefit and higher incidence of HE in those undergoing early TIPS[21]. However, out of the 29 patients enrolled in the TIPS-arm of this study, only 13 underwent TIPS stent placement within 72 h of index bleeding, making it underpowered to derive any conclusions. Despite the contradictory results shown by these two recent RCTs, there is enough evidence now (Table 1) to recommend early TIPS in patients with Child-Pugh class C disease and MELD > 19; however, the upper limit of MELD requires confirmation. Even though the question of survival benefit in patients with Child-Pugh class B and MELD score of 12-18 remains open to debate, the reduction in rebleeding and ascites, without increasing the risk or severity of HE could also justify the use of early TIPS in this subgroup of patients. In keeping with this, the British society of interventional radiology and British association of the study of the liver recommends that "in patients who have Child’s C disease (C 10-13) or MELD ≥ 19, and bleeding from esophageal varices (EV) or GOV1 and GOV2 gastric varices (GV) and are hemodynamically stable, early or pre-emptive TIPS should be considered within 72 h of a variceal bleed where local resources allow"[22]. Despite these recommendations, the rate of implementation of early TIPS in a real-world situation is dismal, with only 6%-13% of eligible candidates undergoing the procedure according to two recent large multicentre observational studies[23,24].
Secondary prophylaxis of variceal bleeding:Patients who survive an episode of acute VH are at high risk of rebleeding and death. The 1-year rate of recurrent VH is approximately 60% in patients without treatment, with a mortality rate approaching 30%[25]. It is recommended that endoscopic band ligation (EBL), in combination with non-selective beta-blockers (NSBB), be the first line of therapy for the prevention of recurrent VH with reservation of TIPS only for non-responders[15,22]. In this regard, two RCTs compared covered stents with EBL[26,27]. TIPS significantly reduced VH without any remarkable effect on overall survival. In one study, 8 mm stents were used, leading to a comparatively lower rate of HE[26]. However, in the other trial using 10 mm stents, although early HE (within one year) was significantly more frequent in the TIPS group (35%vs14%), during long-term follow-up, this difference disappeared[27]. In the previous study, there was no difference in rebleeding or mortality rates beyond 6 wk. Two RCTs conducted later comparing TIPS with EBL plus NSBB in patients with PVT found no increase in the rates of HE in the two groups[28,29]. The absence of survival benefit offered by TIPS in this clinical setting when compared to early TIPS can be explained by the fact that liver failure and infection were the most common cause of death of patients in studies for secondary prevention of variceal bleeding. Contrarily, in the studies on the role of early TIPS, the most common cause of death was early rebleeding, which can be effectively controlled by TIPS, thus conferring survival benefit. A recent study, published in abstract form, demonstrated that TIPS performed at first symptomatic portal hypertension related decompensation (VH or ascites requiring paracentesis) event termed ‘a(chǎn)nticipant TIPS‘ improved overall survival even on sub-group analysis for VH. On further grouping, based on CTP and MELD scores, a higher proportion of patients survived after anticipant-TIPS for allcauses at 1 year. Compared to standard treatment, those undergoing anticipant TIPS had significantly lesser sepsis events and hospitalization and recurrence of varices at one year, even though overall and grouped survival outcomes and were similar[30]. TIPS is not indicated for the prevention of varices (pre-primary prophylaxis) orprevention of the first episode of bleeding from varices (primary prophylaxis), since the risks associated with TIPS, clearly outweighs the potential benefits in this group. Based on the current evidence, it may be appropriate to stratify the patients with cirrhosis with index VH into a “high-risk” and “l(fā)ow-risk” group based on their CTP score and endoscopic findings (Figure 1).

Table 1 Summary of the randomised controlled trials on early (preemptive) transjugular intrahepatic portosystemic shunt
Gastric VH
GV are seen in 5%-33% of patients with cirrhosis and PH[31]. Although they bleed less often than EV-accounting for only 10%-30% episodes of VH–the bleeding is often severe with higher transfusion requirements[31]. GV is frequently associated with large gastrorenal shunts (GRS) and have a “downhill” drainage as opposed to “uphill” drainage of EVviaazygos-hemiazygos venous system[32]. GVs exist as “l(fā)ow pressure, high volume” channels, and can bleed at lower portal pressures than EVs[33,22]. Between 10%-16% of GV can bleed at PSPG < 12 mmHg[33]. Thus, the management of GV hemorrhage (GVH) requires a different therapeutic approach compared to EV.
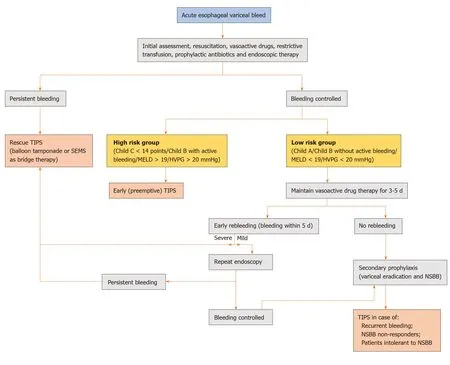
Figure 1 Proposed algorithm for the management of index acute esophageal variceal hemorrhage. Mild rebleeding is defined as clinical symptoms of bleeding only while severe rebleeding is bleeding associated with hemodynamic compromise or requirement of blood transfusion. TIPS: Transjugular intrahepatic portosystemic shunt; SEMS: Self-expandable metal stents; MELD: Model for end-stage liver disease; HVPG: Hepatic venous pressure gradient; NSBB: Non-selective beta-blockers.
Recently, endosonographic coiling and glue have shown promising results in the management of GV but may not suffice for those associated with large portosystemic shunts[34]. Significantly more failure to control bleeding, early rebleeding, and recurrent bleeding were notable in GOV2 and IGV1 related bleeds, with mortality rates reaching up to 20%[35]. For cases unresponsive to pharmacological and endoscopic management, percutaneous endovascular therapy is indicated. Although TIPS can establish initial hemostasis in up to 90% of cases of acute GVH, it has not proven to be as efficacious as in EV hemorrhage[22,33,35]. Indeed, multiple studies have shown that GV can persist and rebleed (incidence of 25%-30%) after successful TIPS placement[35]. Other explanations proposed for the suboptimal efficacy of TIPS in controlling GVH are the “proximity”, “throughput”, and “recruitment” theories[35-37]. The 'proximity theory' suggests that since GV (supplied more commonly by posterior and short gastric veins) are anatomically farther away from the TIPS shunt, they are less likely to be decompressed as compared to EV (supplied predominantly by left gastric vein). The “throughput theory” states that large GRS associated with GV can compete with the TIPS stent and may lead to early TIPS dysfunction. The “recruitment theory” describes the development of new feeders after the proximal embolization of a GV complex. These factors have led to the development of obliterative therapies, like balloon-occluded retrograde transvenous obliteration (B-RTO), in the management of GV. B-RTO and its other variants, like coil-assisted retrograde transvenous obliteration, plug-assisted retrograde transvenous obliteration, and balloon-assisted antegrade occlusion, have emerged as a popular method for treatment of GV. The goal of these therapies is to trap sclerosant within the gastric variceal complex by controlling both inflow and the outflow using balloon, coils, or plug. Since its introduction, multiple studies and metaanalyses have reported technical and clinical success rates over 95% for B-RTO[38-40]. Also, GV rebleed rates of patients who had undergone a successful B-RTO procedure range between 0-20%[38-40]. Notably, compared to TIPS, these shunt occlusion therapies divert blood towards the liver and have shown to preserve or improve the liver functions in the first 6-9 mo[40,41].
Additionally, B-RTO is a proven therapy for patients with severe recurrent shuntrelated HE, unresponsive to medical therapy[40,42]. Thus, patients with spontaneous portosystemic shunts, who are at high risk of developing HE after TIPS can safely undergo B-RTO. However, occlusion of GRS can also aggravate the PH. Long term follow-up of patients undergoing B-RTO revealed development or aggravation of esophageal and duodenal varices, ascites, hydrothorax, and PHG[40]. Prospective studies and meta-analysis comparing TIPS and B-RTO in the management of GV have found that B-RTO is at least as efficacious as TIPS in controlling the acute bleeding with a trend towards a lower incidence of rebleeding[43-46]. Of note, B-RTO was associated with lower post procedure HE and mortality at one year. More recently, a combination of TIPS and B-RTO (Figure 2) has been utilized for the management of GV[32]. Since the obliteration of GRS can lead to worsening of PH, simultaneous or staged placement of TIPS could ameliorate the associated symptoms. The combined procedure can also reduce the risk of development of HE since GRS are often larger in diameter and have higher flow rates compared to TIPS.
Moreover, occluding a competing GRS (shunt steal phenomenon) may decrease the risk of TIPS dysfunction in the long run. Typically, TIPS is performed first, and a splenoportal venogram is obtained. The GRS is cannulated retrogradely, and suitable sized coils or vascular plug deployed. The inflow vein is then occluded with a balloon and sclerosant injected into the variceal complex to achieve complete obliteration. Conversely, doing B-RTO first may make TIPS less technically challenging in cases where the portal vein is severely attenuated due to the siphoning of blood away from the liver by the large GRS. These tiny portal veins can be difficult to target during TIPS. Following B-RTO, due to the diversion of blood towards the liver, portal vein caliber may improve, making it easier to access. A proposed algorithm for the management of GVH is shown in Figure 3.
Ectopic varices
The term ectopic varices are used to describe portosystemic collaterals located at sites other than the gastroesophageal region. Stomal varices are the most common, followed by small bowel (predominantly duodenum), colon, rectum, and peritoneum. Rare sites include the biliary tree, umbilicus, and pelvic organs[47]. Bleeding from ectopic varices represents an uncommon but challenging clinical problem. The prevalence ranges between 1%-5% of all variceal bleeds with a higher prevalence (up to 40%) seen in patients with extrahepatic PH and after surgery[47-49]. Postoperative adhesions and the creation of an enterostomy can facilitate the formation of portosystemic collaterals. Depending on the location of varices, clinical presentation, and available local expertise, the management of bleeding ectopic varices can differ. After initial resuscitation and pharmacological treatment, the treatment options include endoscopic management, percutaneous variceal embolization, TIPS, or surgical therapies[50]. Endoscopic therapy is often not feasible or successful due to an inaccessible location. Percutaneous variceal embolization using balloon-occluded sclerotherapy, coils, glue, gel foam, thrombin, or a combination of these is an effective short-term therapy for bleeding ectopic varices. However, it fails to decompress the portal venous system resulting in high 1-year rebleeding rates. Surgical treatment options such as local suturing, segmental bowel resection, devascularisation procedures, or stomal revision are associated with a high risk of recurrence[50]. TIPS has shown excellent results in achieving initial hemostasis and reducing the incidence of recurrent bleeds. However, the available evidence is limited to case reports, small case series, and related reviews[50-54]. A recent multicentre cohort study showed that TIPS was particularly effective in patients with less severe liver disease and those with stomal varices[50]. Contrarily, the rebleeding risk in patients with duodenal varices was surprisingly high. Rebleeding in 75% of patients was associated with TIPS stent dysfunction. Multiple studies have shown that patients with ectopic varices can rebleed after TIPS despite the achievement of hemodynamic target and stent patency[51,52]. Notably, the overall risk of rebleeding after TIPS in patients with ectopic varices was significantly higher than in patients with gastroesophageal variceal bleeding (23%vs0-6%, respectively, at 1 year)[50]. This discrepancy can be explained by the anatomical differences between varices at these two sites. It has been suggested that unlike gastroesophageal varices, ectopic varices are true veins and are likely to have larger diameters resulting in greater wall tension resulting in higher rates of bleeding[55,56].
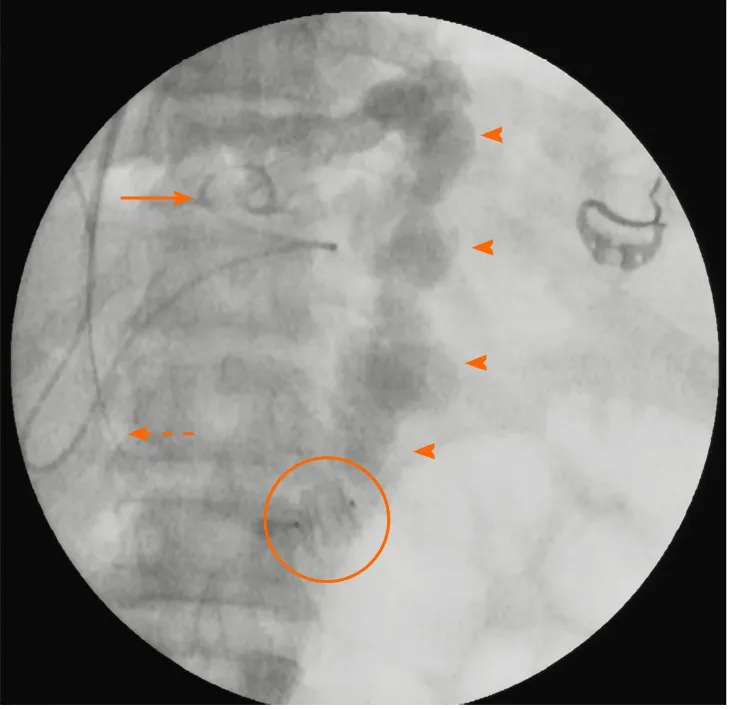
Figure 2 Fluoroscopic spot image demonstrating the “combined approach” to management of a patient with intractable gastric variceal bleeding due to IGV1 and severely attenuated portal vein. A type-II amplatzer vascular plug (encircled) has been deployed within the gastrorenal shunt retrogradely through the jugular route with vascular access sheath (dashed arrow) in situ. Subsequently, a catheter (solid arrow) was used to inject the sclerosant mixture into the shunt (arrowheads) antegrade through the transjugular intrahepatic route. The transjugular intrahepatic portosystemic shunt stent was then placed in the usual way within the intrahepatic tract after ensuring stasis of sclerosant mixture within the shunt and detachment of the vascular plug.
Based on this, few authors recommend concomitant embolization of variceal complex during TIPS (Figure 4). In the series by Vangeliet al[51], rebleeding was more commonly seen in those who had TIPS alone compared to those who had concomitant variceal embolization. Furthermore, they also found that the rebleeding in the majority of patients responded to subsequent variceal embolization. However, multiple other studies have reported contradictory results[52-54]. Technically, variceal embolization in this setting can be difficult since ectopic varices are frequently multiple, tortuous, and complex, and access to them may be challenging. Even when accessible, complete obliteration of ectopic varices may not be possible because of the presence of other communications with the systemic or mesenteric venous system.
Moreover, variceal embolization has the risk of inherent complications, such as propagative thrombus or paradoxical systemic embolization. Although a recent metaanalysis showed a trend favoring variceal embolization along with TIPS for ectopic variceal bleeding, the evidence is insufficient to recommend the same routinely[57]. However, when the target PSPG could not be achieved after TIPS stent placement and in whom the ectopic varices continue to be opacified on completion splenoportogram, concomitant variceal embolization may be appropriate. Variceal embolization alone can be offered to patients in whom TIPS is contraindicated due to advanced cirrhosis or overt HE (Figure 5).
PHG
PHG is characterized by vascular ectasia, which appears as a mosaic-like pattern of gastric mucosa on endoscopy[58,59]. The reported prevalence of PHG ranges from 20%-98% in patients with known cirrhosis[59-62]. Studies have shown an increased prevalence in patients with high CTP scores, EV, or history of treatment for EV (sclerotherapy or ligation)[60,61]. PHG is thought to be a direct consequence of passive congestion induced by increased portal pressure because it does not develop in the absence of established PH. A direct correlation between portal pressure values and severity of PHG remains to be demonstrated[63,64]. The incidence of acute PHG related bleeding varies between 2%-12%[60,61]. NSBB, octreotide, and terlipressin are effective in the initial treatment of PHG with reported rates of hemostasis between 93%-100%[65,66]. Endoscopic argon plasma coagulation, sclerotherapy, and coagulation therapy with the heater probe may be considered with focal bleeding. Antioxidants like vitamin E, thalidomide, and prednisolone have also been used to treat acute PHG bleeding, with anecdotal success in case reports[67,68]. After the resolution of the episode of acute bleeding, propranolol should be initiated as secondary prophylaxis. The published evidence for TIPS in the management of PHG is limited to a few case reports[69-71]. Current evidence suggests that TIPS reduces the severity of PHG, ameliorates mucosal lesions, and could be considered in patients with transfusion-dependent PHG when pharmacological measures and endoscopic interventions fail. It is important to differentiate PHG from gastric antral vascular ectasia (GAVE) as the latter can be seen in patients with and without PH or cirrhosis. GAVE has a characteristic endoscopic appearance but can coexist with PHG[72,73]. TIPS does not have a role in the management of bleeding solely from GAVE.

Figure 3 Proposed algorithm for the management of acute gastric variceal hemorrhage. TIPS: Transjugular intrahepatic portosystemic shunt; NSBB: Non-selective beta-blockers; HE: Hepatic encephalopathy; HPS: Hepatopulmonary syndrome; B-RTO: Balloon-occluded retrograde transvenous obliteration; CARTO: Coil-assisted retrograde transvenous obliteration; PARTO: Plug-assisted retrograde transvenous obliteration.
ASCITES

Figure 4 Ectopic umbilical variceal bleeding controlled using TIPS procedure and adjuvant percutaneous intravariceal glue injection. A: The patient with cirrhosis and portal hypertension presented with spontaneous blood soakage of clothes associated with painless spurting of blood from umbilical region; B: Contrast imaging of abdomen revealed large umbilical varix with extracutaneous component; C: The umbilical varix supply was from the splenic vein; D and E: Fluroscopy guided transjugular intrahepatic portosystemc shunt placement, shunt embolization with multiple coils followed by percutaneous glue injection for variceal obliteration was performed; F: Complete resolution of the variceal complex was noted clinically post transjugular intrahepatic portosystemc shunt procedure.
Ascites is the most common complication of PH in cirrhosis, with approximately 60% of compensated cirrhosis patients developing the condition within ten years of diagnosis[74]. The 5-year survival is approximately 30% in patients with decompensated cirrhosis and ascites[75]. Moreover, ascites is a direct cause of further complications, such as spontaneous bacterial peritonitis, hyponatremia, and HRS. For patients developing grade 3 ascites, large-volume paracentesis (LVP) with intravenous albumin (8 g for every L of fluid removed above 5 L) supplementation is the treatment of choice[76]. However, despite optimal medical therapy, 5%-10% of these patients develop RA, which is associated with an extremely poor prognosis and median survival of 6 mo[74,76,77]. Liver transplantation, the only definitive treatment of RA, is limited by donor resources and high costs in developing countries. Repeated LVP with albumin infusion is currently recommended as the first-line therapy for RA[76]. Current guidelines recommend consideration of TIPS placement if more than three sessions of LVP have to be performed per month for symptomatic relief or procedure intolerance[78].
Although the efficacy of TIPS in controlling ascites has been well validated by several RCT's (between 1996-2004) and subsequent meta-analysis (2005-2006), the increased incidence of HE and controversial results on survival benefit resulted in LVP to be continually recommended as the first-line therapy for RA, ahead of TIPS[79-86]. However, these RCTs were primarily evaluating the efficacy of ascites control rather than survival. Moreover, the early meta-analysis did not analyze survival as a timedependent variable, and the confounding effect of liver transplantation on survival in patients with advanced cirrhosis was not considered[84-86]. A meta-analysis conducted later using individual patient data of these RCTs confirmed that TIPS significantly improved TFS and reduced the recurrence of tense ascites[87]. Another RCT conducted later employed even stricter inclusion criteria (Child-Pugh score of < 11, serum bilirubin < 3 mg/dL, and creatinine < 1.9 mg/dL) and found that TIPS was significantly superior to paracentesis in the control of ascites in cirrhotic patients with RA with response rates of up to 60% at one year[88]. More importantly, survival was significantly higher in the TIPS group attesting to the fact that careful patient selection is a pre-requisite for better outcomes after TIPS in patients with RA. This finding was confirmed in a recent updated meta-analysis[89]. However, the probability of posttreatment HE was increased by TIPS in all the studies with a significantly higher average number of episodes per patient. Nevertheless, all these RCTs have used baremetal stents for TIPS, and there was a high incidence of shunt dysfunction requiring stent revision. Thus, the conclusions drawn cannot be applied to the current clinical scenario where covered stents for TIPS are the norm.
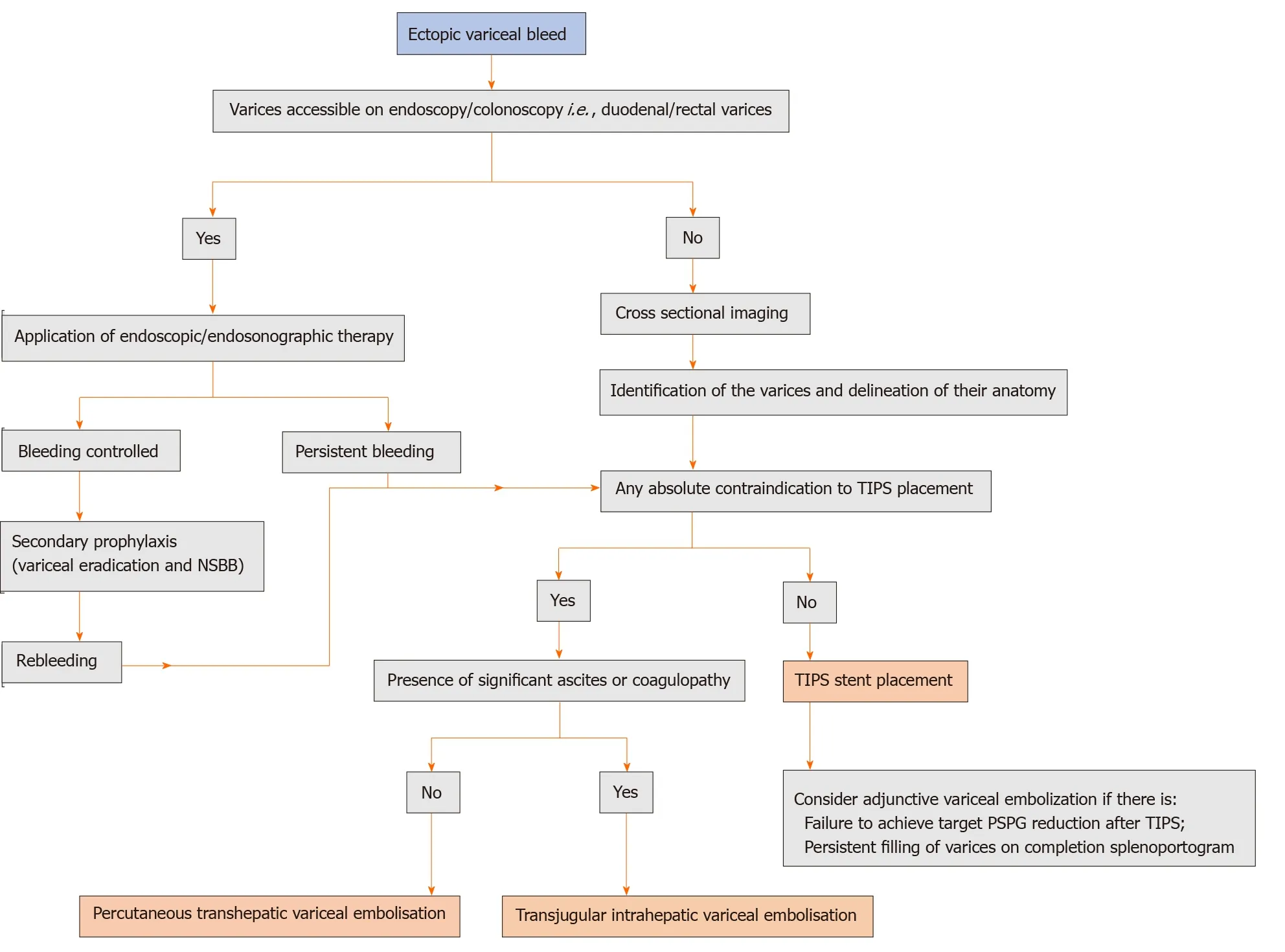
Figure 5 Proposed algorithm for the management of ectopic variceal bleeding. NSBB: Non-selective beta-blockers; TIPS: Transjugular intrahepatic portosystemic shunt; PSPG: Portosystemic gradient.
Multiple retrospective studies since then have reported survival benefit after covered TIPS in this clinical setting[90,91]. Interestingly, the most recent RCT comparing TIPS (using covered stents) with LVP in patients with ascites found that covered TIPS improved survival and did not increase the risk of HE[92]. Another retrospective study conducted later, which included patients with RA similarly showed that the risk of denovo HE was not increased in the TIPS group[93]. Notably, this study employed smaller 8 mm diameter TIPS stents and found that while ascites control was similarly effective between TIPS responders and non-responders (as defined by a decrease in portal pressure to < 12 mmHg after TIPS implantation), HE occurred more often in patients with hemodynamic TIPS response, implying that a less aggressive PSPG reduction might be sufficiently effective for ascites control, while concomitantly decreasing the risk of post-TIPS HE. However, a randomized study comparing 8 mmvs10 mm covered TIPS for RA had to be stopped midway after early results revealed worse ascites control with 8 mm stents[94]. Another recent retrospective study reported higher post-TIPS PSPG and greater need for LVP with 8 mm stents, with similar rates of encephalopathy[95]. Therefore, the optimal diameter of covered TIPS stents for this indication remains unclear. Some studies have suggested that TIPS should not be undertaken in patients with a high (≥ 18) MELD score[96,97]. However, the role of MELD in patient selection remains unclear. In the meta-analysis by Salernoet al[87], it was shown that compared with paracentesis, the benefit of TIPS on TFS could be seen across all MELD scores. More recently, two retrospective studies found no evidence that TIPS creation confers worse survival in patients with higher MELD scores compared with serial LVP[98,99]. A higher MELD score predicted poor survival, but survival was equally poor among patients whose RA was treated with serial LVP compared to TIPS. Another retrospective review showed that early death after elective TIPS was highest in patients with MELD greater than 24[100]. Gabaet al[101]compared various scores, including MELD and CTP score in the prediction of outcome after TIPS, and found that CTP score had the best overall capability at predicting mortality when TIPS is used for ascites. Bureauet al[102]have proposed the use of simple laboratory parameters (bilirubin < 50 μmol/L and platelets > 75 × 109/L) to predict 1-year survival following TIPS for RA, which form the basis of European Association for the Study of Liver Disease guidelines.
There has been a renewed interest in the role of TIPS in patients with recurrent ascites (three recurrences of symptomatic ascites within a year). Studies, including the initial RCT's comparing TIPS with LVP, have grouped patients having recurrent ascites with those having RA. However, subgroup analyses performed on the pooled data of these RCTs showed that TIPS significantly improved TFS regardless of whether recurrent ascites patients were included or not in the trials[89]. A recent single-center retrospective study of 128 patients showed that placement of TIPS in patients with lower LVP frequency and creatinine levels is associated with superior ascites control[103]. Similar findings were reported by a prospective RCT comparing TIPS to LVP in patients with recurrent ascites and a limited LVP frequency, which demonstrated benefits in ascites control and survival in TIPS-treated patients but no difference in HE between the two groups[92]. This was reiterated in the recent study on very early TIPS performed in patients with cirrhosis and first symptomatic ascites development[30]. Thus, currently available data (Table 2) suggest that TIPS should be considered early in patients with difficult-to-treat ascites (not necessarily fulfilling the criteria of RA) having a stable underlying liver disease with relatively preserved renal function. However, a recent observational study on outcomes and mortality of patients with cirrhosis with recurrent ascites found that mortality does not differ significantly between patients with recurrent ascites and patients with ascites responsive to medical treatment and that recurrent ascites is not necessarily a sign of worsening of the liver disease, implying that these patients should not be prioritized for TIPS or liver transplant[104]. Further large multicentre prospective RCTs are needed to assess the role of “early TIPS” in ascites.
HH
HH is the accumulation of a significant amount of transudative fluid, usually over 500 mL, in the pleural cavity of patients with decompensated liver cirrhosis without coexisting primary cardiopulmonary or pleural diseases[105]. It is a relatively uncommon complication of end-stage liver disease, seen in approximately 5%-10% of patients and constitutes 2%-3% of all cases of pleural effusions[105,106]. It has a dismal prognosis with a median survival of 8-12 mo[107]. Approximately 20%-25% of patients with HH have persistent symptomatic rapidly refilling HH despite adequate dietary sodium restriction and maximum tolerated diuretic dose[107]. Early liver transplantation is the only curative treatment for these patients, but it is not always available because of recipient condition and limited donor availability. Therapeutic thoracentesis can be offered as an alternative for symptomatic relief. However, therapeutic thoracentesis is not recommended as a long-term treatment due to the risk of re-expansion pulmonary edema, pneumothorax, bleeding, and infection. TIPS effectively reduce the portal pressure, thereby providing symptomatic relief in close to 2/3rds of patients[108,109]. However, since HH is relatively uncommon, controlled studies assessing the role of TIPS for this condition are lacking. Recently, Ditahet al[110]and colleagues conducted a systematic review and meta-analysis of 6 retrospective studies involving a total of 198 patients suffering from HH. The analysis of pooled data showed that TIPS was successful in relieving the symptoms in 73% of cases, with complete response seen in 56% of patients. The occurrence of HE and overall mortality was found to fall within the observed range, as seen with TIPS performed for other established indications. In the absence of controlled studies comparing TIPS with standard medical treatment, the benefit of TIPS on TFS in HH cannot be commented upon. In a recent retrospective single-center analysis, despite the selection of patients with lower mean CTP (9.9 ± 1.6) and MELD score (18.7 ± 5.4), the 6-mo mortality after TIPS for HH was close to 36%[111]. The independent predictors of mortality were MELD > 25, spontaneous bacterial peritonitis, and septic shock. The study found no difference in 6 mo mortality and complication rates when TIPS was compared to other treatment groups (standard medical therapy, thoracentesis, and catheter drainage) based on propensity matching analysis. Early TIPS in selected patients may be effective as a bridge to liver transplantation.
Chylous ascites and chylothorax
Of all cases of cirrhosis-related ascites, only 0.5%-1% is chylous[112]. The underlying mechanism is believed to be excessive hepatic and gastrointestinal lymph flow andpressure secondary to PH, which may lead to spontaneous rupture of serosal lymphatic channels[113]. Triglyceride level > 110 mg/dL or the presence of
chylomicrons in pleural or ascitic fluid is used to confirm the diagnosis[113-116]. High protein, low-fat diet (supplemented with medium-chain triglycerides) with sodium restriction and diuretics form the first-line of management[113]. Octreotide, a somatostatin analog, has been used successfully in some patients but requires longterm therapy to achieve and maintain consistent symptom control[117]. Given the rarity of the disease, the evidence on the role of TIPS in this condition is limited to seven case reports and one series of 4 patients[118-126]. Four patients received covered stents, while five patients received bare stents. In two patients, the stent type was not described[118,123]. TIPS was uniformly successful in providing symptomatic relief in all these cases without any major procedure-related complications, except self-limiting HE in three patients. On mean patient follow-up of 13.9 mo (range 0.6-35), one patient had TIPS dysfunction with recurrence of chylous ascites twice (at 43 d and 70 d post procedure), but the ascites improved after TIPS revision on both occasions. Based on available published literature and the fact that prospective controlled trials with adequate sample size are likely to remain unavailable shortly, TIPS can be considered an effective and safe method for treating chylothorax and chylous ascites in patients with cirrhosis.

Table 2 Summary of the randomised controlled trials on transjugular intrahepatic portosystemic shunt in patients with ascites
Portal vein thrombus
Non-tumoral PVT is the most common thrombotic event in patients with cirrhosis, with an annual incidence of up to 12%[127,128]. Asymptomatic presentation is common with incidental diagnosis during routine surveillance or pretransplant workup. PVT has a significant but variable influence on the outcome of patients with cirrhosis. Multiple studies have found that in the natural history of cirrhosis and PVT, 40%-70% of patients will have a progression of thrombus leading to complete occlusion of portal vein or extension to other splanchnic vessels[129-131]. While anticoagulation is considered to be the mainstay of therapy in PVT in the absence of cirrhosis, optimal management of PVT in cirrhosis has not been addressed adequately in clinical guidelines. In a prospective study on the role of anticoagulation and TIPS in 56 patients from Europe, only 36% of patients on anticoagulation showed complete recanalization, while 27% of patients showed partial recanalization[130]. The presence of ascites and splenic vein thrombosis were independently associated with the failure of anticoagulation therapy. Previously considered a contraindication for TIPS, multiple case reports, and few case series have described the successful placement of TIPS in patients with cirrhosis with PVT with acute VH, and RA[132-135]. Besides, recently two RCTs comparing TIPS with EBL plus propranolol in patients with PVT showed that TIPS was more effective than medical and endoscopic therapy without an increase in the risk of HE in the vast majority of patients leading to a recanalization rate of 95%[28,29]. Studies had also shown that even when persistent thrombus on completion splenoportogram was not stented (to preserve the long length of the unstented portal vein for liver transplant) and TIPS was not followed by anticoagulation or thrombolytic therapy, recanalization was frequently observed, implying that PVT in these patients is mainly due to hemodynamic factors[133]. With the advent of multiple imaging techniques for real-time visualization of the portal vein during TIPS, PVT is no longer considered as an absolute contraindication to TIPS placement. Also, portal vein thrombolysis and balloon angioplastyviarecently described percutaneous transhepatic and transsplenic routes allow better visualization of the portal vein before transjugular puncture, resulting in markedly improved outcomes[133,136]. However, the presence of portal cavernoma has been associated with high failure rates despite the use of three dimensional (3D) imaging and fusion technology during TIPS. Concomitant embolization of varices has also been found to increase the long-term patency rates of TIPS and prevent thrombosis. Based on the current evidence, TIPS can be utilized for cirrhosis patients in whom thrombosis persists or progresses, despite optimal anticoagulation therapy and in those who present with complications of PH, such as acute variceal bleeding or RA (Figure 6). Standard anticoagulation following the TIPS procedure for other indications is not recommended.
Pre-surgical/neoadjuvant TIPS

Figure 6 Contrast-enhanced computed tomography image. A: Coronal image showing bland occlusive thrombus involving the main portal vein, superior mesenteric vein (SMV) and splenic vein (encircled) with gross ascites (asterisk); B: Image taken 2 wk after transjugular intrahepatic portosystemic shunt (TIPS) shows the stent in situ (arrowhead) with its distal end in one of the major tributaries of SMV. The main trunk of SMV (solid arrow) and splenic vein could not be fully recanalized during TIPS. Trans-splenic access was not taken due to gross ascites; C and D: Corresponding axial images show marked enlargement of the gastroepiploic collaterals (solid orange arrows) arising from the patent portion of splenic vein at splenic hilum draining through the TIPS stent (black arrow in D) into the portal venous system. Note the significant regression of ascites on the follow up scans.
Extrahepatic surgery is associated with higher postoperative morbidity and mortality in patients with cirrhosis[137]. The reported mortality is between 10% to 30%, while the perioperative morbidity is about 30%. The outcome is mainly influenced by the severity of liver disease, type of surgery, and the degree of PH[138,139]. Pre-operative TIPS may reduce the portal pressure and decrease the risk of bleeding as well as help in managing pre-or-post operative ascites[140-147]. The optimal time between TIPS and the performance of surgery is controversial. Nonetheless, a delay of 1 mo from TIPS to surgery has been suggested to be the most appropriate for optimal portal decompression[142]. That being, the perceived benefit of TIPS must be weighed against the risk of the procedure itself and the associated time delay. All publications on the role of pre-operative TIPS are retrospective in the form of single clinical reports or case series with a fairly small number of patients[140-147]. Out of these, only two studies have had a control group, but both were retrospective comparative studies without randomization[143,146]. A systematic analysis of all the published data showed that there is marked heterogeneity with regards to patient selection based on the severity of the underlying liver disease, indication for TIPS, criteria for successful TIPS, and timelapse between TIPS placement and surgical procedure[148]. The study by Vinetet al[143]compared patients who underwent an elective abdominal surgery after preoperative TIPS placement (n= 18) with those who underwent surgery without TIPS (n= 17) during the same period. The authors found that the preoperative portal decompression with TIPS did not improve outcome after abdominal surgery in patients with cirrhosis. However, the TIPS group in this study had a higher mean CTP score compared to the control group. The other retrospective, multi-institutional, comparative study by Tabchouriet al[146]also did not find any significant differences between TIPS and control groups in terms of severe postoperative complications and mortality. Notably, they found deterioration of hepatocellular function after TIPS placement, which persisted postoperatively despite a mean interval of 51 d between TIPS placement and planned surgery. In this study, a subset of patients with less severe PHT (HVPG ≤ 13 mmHg) and less advanced liver dysfunction (MELD-sodium score ≤ 15) seemed to benefit from preoperative TIPS placement in terms of postsurgical complications in the absence of statistical significance. Contrarily, in the study by Kimet al[144], despite a preoperative mean MELD score of 15 among the patients (n= 6), the 1-year survival rate was 74%. A recent prospective study showed the value of HVPG in predicting outcomes in cirrhosis patients undergoing nonhepatic surgery, with no patient having HVPG < 10 mmHg or indocyanine green clearance > 0.63 developing decompensation[149]. On the other hand, HVPG > 16 mmHg was independently associated with higher mortality, and patients with HVPG > 20 mmHg were found to be at the highest risk. Interestingly, MELD and CTP scores were not independent predictors of post-surgical mortality. The findings of this study reiterate that the potential of pre-surgical TIPS in high-risk patients deserves further research to improve outcomes. Based on all the available published evidence, routine TIPS placement cannot be recommended before surgical procedures in all patients with cirrhosis and PH. Pre-operative TIPS is likely to benefit cirrhosis patients having preserved liver function but with features of severe PH who are undergoing curative oncosurgery. For patients who require emergency surgery, TIPS might still be beneficial by decreasing the risk of perioperative hemorrhage related to venous congestion and varices.
HRS
HRS is usually manifested in the advanced stage of cirrhosis with PH. International Club of Ascites has defined HRS as an increase in serum creatinine ≥ 0.3 mg/dL (≥ 26.5 mmol/L) within 48 h; or a percentage increase in serum creatinine ≥ 50% from the baseline that is known, or presumed, to have occurred within the previous seven days[150]. As per the recent International Club of Ascites classification, patients with cirrhosis and acute kidney injury (AKI) are subgrouped into HRS AKI and HRS non-AKI[150,151]. HRS non-AKI is further subdivided into HRS-acute kidney disease and HRS-chronic kidney disease. In the former, the calculated glomerular filtration rate (eGFR) is < 60 mL/min per 1.73 m2for < 3 mo in the absence of other (structural) causes along with percent increase in serum creatinine < 50% using the last available value of outpatient creatinine value within 3 mo as the baseline value. In the latter, the eGFR is < 60 mL/min per 1.73 m2for ≥ 3 mo in the absence of other (structural) causes. In patients not responding to medical management in the presence of ascites, TIPS is a useful procedure in the management of HRS.
The utility of TIPS in patients with HRS non-AKI has been discussed previously in the section on RA as most of these patients present with the need for repeated paracentesis. In a recent systematic review on TIPS in HRS, nine publications with 128 patients were analyzed. The pooled short-term and 1-year survival rates were 72% and 47% in HRS-AKI and 86% and 64% in HRS non-AKI. The pooled rate of HE after TIPS was 49%. The pooled rate of renal function improvement post-TIPS was 93% in HRSAKI and 83% in any type of HRS. Post-procedure, creatinine, blood urea nitrogen, serum sodium, sodium excretion, and urine volume significantly improved with a nonsignificant elevation in serum bilirubin[152]. The use of TIPS in patients with HRSAKI remains controversial since a majority of these patients are sick at presentation with sepsis or acute decompensation. A recent retrospective cohort study in HRS patients showed TIPS is a relatively safe, bridging therapeutic option in patients who underwent TIPS in comparison to patients who received dialysis[153]. Decreased recurrence of ascites and increased incidence of HE in the TIPS group was seen in a small randomized study where they compared patients with Type 2 HRS (HRS non-AKI) who underwent TIPS with another group of patients receiving paracentesis plus albumin[81]. TIPS may prevent permanent renal damage and the need for further liverkidney transplantation due to portosystemic shunting and resultant hemodynamic changes[154]. However, further RCTs showing the role of TIPS in HRS patients are required.
HPS
In HPS, patients with underlying chronic liver disease present with shortness of breath and hypoxemia, which occurs secondary to pulmonary vasodilation and intrapulmonary shunts[155]. Liver transplantation is considered as the most effective treatment in HPS[156]. PH is one of the key events that is considered to play a role in the pathogenesis of this syndrome, and hence reduction of portal pressure using TIPS may be considered as an alternative therapeutic procedure[157,158]. Few studies have compared the difference between a left branch of the portal vein (LPV-TIPS) and right branch of the portal vein (RPV-TIPS) for performing TIPS and have shown that the incidence of HE is lower in LPV-TIPS group[159,160]. Zhaoet al[155]in their study recommend LPV-TIPS over RPV-TIPS to improve the symptoms of hypoxemia and thereby improve the arterial oxygenation. Even though controlled studies assessing the role of TIPS in HPS are lacking, there is evidence of improvement in oxygenation after the procedure[161-163]. TIPS also has a role in patients awaiting liver transplantation[155,164,165]. Additional prospective studies are required to understand the pathogenesis of this syndrome and identify the effects of reducing portal pressure.
CONSIDERATIONS DURING SELECTION OF PATIENTS FOR TIPS
Age
Initial studies identified advanced age as an independent predictor of early mortality after TIPS, attributing it to age-related physiologic decline in hepatic functional reserve, which might not be picked up on routine laboratory tests[166,167]. However, the majority of patients in these studies received bare-metal stents for TIPS. Also, there was significant heterogeneity in terms of severity of underlying disease in the study group with different cut-offs for defining advanced age, precluding drawing of any robust conclusions. Nevertheless, a recent study using covered stents for TIPS did find a trend towards greater mortality and hospitalization in the elderly, without reaching statistical significance[168]. Another retrospective study in which the subjects were well matched for MELD score, indication for TIPS, and comorbidities showed that age is strongly and independently associated with 90-day post-TIPS mortality risk, particularly in those > 70 years[169]. Adlakhaet al[170], in a retrospective study of 100 patients, similarly showed that re-admission rates and incidence of severe HE requiring hospital admission were higher in elderly patients, even after accounting for MELD score. They also found that TIPS for secondary prophylaxis of variceal bleeding, RA, and HH had acceptable morbidity and mortality. However, there was high mortality when TIPS was placed for acute variceal bleed, even in patients with MELD score < 18. There was a trend towards increased 30 d mortality despite a low baseline MELD, particularly in patients aged 80 years and more, without reaching statistical significance. Current evidence suggests that older age (no absolute cut-off; generally accepted as > 65 years) is a relevant consideration in assessing mortality risk of TIPS. However, advanced age alone should not be an absolute contraindication for TIPS, especially for conditions in which TIPS has proven benefit in terms of symptomatic relief and survival, like acute variceal bleeding or RA. These patients should be followed for occurrence of HE after TIPS closely. Moreover, the need for frequent readmissions and the heightened risk of early mortality should be part of routine counseling before TIPS in this subset of patients.
HE
Multiple studies have shown that TIPS, by portosystemic shunting, increases the risk of HE[171,172]. The median cumulative 1-year incidence of overt HE after TIPS has been reported to be between 10% and 50%[172,173]. The incidence of persistent overt HE is around 8% and that of de-novo, covert HE around 35%[171]. However, even in the mildest form, HE significantly reduces health-related quality of life and reflects a poor outcome of TIPS, especially when the procedure was done as palliative therapy in an elective setting. One study showed that neither rifaximin nor lactulose prevented post-TIPS HE any better than the placebo[174]. Thus, careful case selection is the most effective way to reduce the incidence of HE after TIPS. Risk factors for HE post-TIPS include advanced age, the severity of the liver disease, sarcopenia, history of prior encephalopathy, and the presence of any pre-existing portosystemic shunt[173]. Diabetes has also recently been recognized as a risk factor for HE, which is particularly important in the current scenario where a significant proportion of patients who come for TIPS have NASH-related cirrhosis associated with diabetes[175]. Although age > 65 is not an absolute contraindication, it might increase the risk of encephalopathy and should be taken into account when deciding the eligibility, especially for elective TIPS. Similarly, although studies have suggested that sarcopenia and HE are causally related, an overall improvement in muscle mass and density after TIPS has also been reported in recent literature, which resulted in a reduction in episodes of overt HE and venous ammonia levels. Furthermore, the majority of patients who come for elective TIPS will have relatively well-preserved hepatic and renal functions without any documented history of overt HE. Diligent screening of these patients to identify signs of covert HE is crucial. Patients who have evidence of covert HE should ideally not undergo TIPS for an elective indication unless there is a large portosystemic shunt that can be embolized during TIPS. Stent characteristics and desired portal pressure gradient reduction has been implicated in post TIPS HE. Recent studies have shown reduced rates of HE with covered TIPS stents compared to bare-metal stents[3]. However, conclusive evidence is still lacking. Similarly, there is a lack of consensus on whether to aim to reduce PSPG by 20% or below 12 mmHg (discussed later). Too low a pressure because of large stent diameter has been shown to predispose to intractable HE in some studies.
Cardiopulmonary status
In advanced stages of cirrhosis, structural, and functional cardiac abnormalities occur. This cirrhosis associated cardiomyopathy (CCM) leads to impaired contractile responsiveness to stress, diastolic dysfunction, myocardial hypertrophy, and electrophysiological abnormalities in the absence of other known cardiac disease[176,177]. Cirrhosis associated cardiomyopathy has been suggested as a key factor in the development of RA, hyponatremia, and HRS. As many as 50% of end-stage patients undergoing liver transplantation show signs of cardiac dysfunction[177-179]. Shunting of portal blood into the systemic circulation after TIPS leads to a sudden increase in cardiac preload and output that can rapidly worsen the hyperdynamic circulatory state in patients with cirrhosis. Cardiac complications noted post-TIPS commonly include clinically evident heart failure in those with RA. Long-term cardiovascular changes, including cardiac volume overload and an increased rate of pulmonary hypertension, have also been reported[180]. Initial prospective studies reported that the presence of diastolic dysfunction before TIPS was associated with post-procedural mortality within one year[178,181]. However, these studies lacked an independent, blinded review of the echocardiography and relied solely on E/A (early maximal ventricular filling velocity/atrial maximal ventricular filling velocity) ratio < 1.0 to define diastolic dysfunction.
Recent studies have found no relationship between diastolic dysfunction and post-TIPS survival or cardiac failure despite pre-TIPS rates of diastolic dysfunction ranging from 30%-45%[180,182,183]. Another study found that symptomatic heart failure was rare after TIPS (seen in < 1% of patients) and that this condition can be managed successfully when it is recognized early[184]. However, a recent prospective study of 100 patients from France undergoing a complete cardiac evaluation before TIPS found that hospitalization for cardiac decompensation was observed in 20% of patients in the year after TIPS insertion[185]. The serum N-Terminal pro-B-type natriuretic peptide (NTproBNP) was found to be predictive of cardiac decompensation after TIPS, but not mortality. The authors recommended that combining BNP or NT-proBNP levels and echocardiographic parameters should help improve patient selection. Recently left ventricular global longitudinal strain has been utilized to identify cirrhotic patients with underlying cardiac dysfunction[186]. It was found that impaired cardiac contractility, reflected by higher left ventricular global longitudinal strain, predisposes to the development of acute-on-chronic liver failure and death in cirrhosis.
Current guidelines suggest a detailed cardiac history, physical examination, 12-lead electrocardiogram, echocardiography, and NT-proBNP in all patients undergoing elective TIPS placement with invasive cardiac assessment reserved for patients in whom the initial evaluation is abnormal[187]. Severe PAH-defined as mean pulmonary artery pressure (mPAP) > 45 mmHg-represents an absolute contraindication to TIPS. In patients with moderate PAH (mPAP between 35-45 mmHg) with elevated pulmonary capillary wedge pressure (> 15 mmHg), TIPS can be placed in emergencies for established indications (like variceal bleeding refractory to endoscopic and pharmacologic treatment)[187]. In patients with severe left ventricular dysfunction, elective TIPS is contraindicated. The cardiologic workup should also include contrast echocardiography aimed to demonstrate a patent foramen ovale, particularly in patients with PVT. Foramen ovale may serve as a conduit for paradoxical embolization, the occurrence of which has been reported following TIPS[188].
Nutritional status
Alterations in the nutritional status are one of the most frequent complications of cirrhosis that worsens with disease progression and negatively affects the outcome in these patients. The etiology is multifactorial and includes reduced caloric and protein intake, increased catabolism, malabsorption, reduced protein synthesis, and anabolic resistance[189]. Malnutrition in cirrhosis can lead to reduced muscle mass and strengthalso called sarcopenia-as well as the loss of subcutaneous and visceral fat mass called adipopenia[190]. Sarcopenia is the predominant nutritional consequence of cirrhosis, with a reported prevalence as high as 95%[191]. The risk of malnutrition is assumed to be high in Child-C patients and those with BMI < 18.5[192]. The Royal free hospitalnutritional prioritizing tool is a screening score that has been reported to correlate with clinical deterioration, the severity of the liver disease, and clinical complications[193]. CT image analysis at L3 vertebra (L3 skeletal muscle index; L3SMI) is widely recognized as a specific method to quantify the loss of muscle mass[194]. Bedside anthropometric methods like mid-arm muscle circumference, triceps skinfold, and mid-arm muscular area have also shown comparable predictive value to L3SMI with good intra and interobserver agreement[195,196].
TIPS has been shown to improve body composition and increase fat-free mass in cirrhotics in observational studies[189,197,198]. Resolution of ascites leading to better nutritional intake, improvement in splanchnic venous return, a reversal of proteinlosing enteropathy, prevention of further episodes of bleeding and paracentesis, and a possible reversal of hypermetabolism have been proposed as possible mechanisms by which TIPS improves the muscle mass[189]. A recent study showed that the creation of TIPS was strongly associated with an increase in cross-sectional area and attenuation of truncal musculature with maximal gains noted by 6 mo after TIPS[198]. Furthermore, TIPS related increase in muscle mass was independently associated with lower patient mortality. This study also identified a positive effect of TIPS on muscle attenuation, an indicator of myosteatosis that has been associated with sarcopenia and mortality in patients with cirrhosis. This survival advantage could prove crucial in patients awaiting a liver transplant. Multiple other studies have shown that reversal of sarcopenia and improvement of muscle attenuation after TIPS were independently associated with a reduction in mortality[197].
Similarly, the persistence of sarcopenia after TIPS is associated with a reduced response to TIPS and a higher risk of acute-on-chronic liver failure development and mortality[199]. A retrospective observational study found that the measurement of psoas muscle density improved overall survival predictability in patients with cirrhosis undergoing TIPS creation when used in conjunction with the MELD score[200]. Another retrospective study found that sarcopenic obesity is a risk factor for mortality after TIPS and contributes additional prognostic information beyond the MELD score[201]. However, sarcopenia has also been shown to increase the incidence of post TIPS HE[202,203]. This is because skeletal muscle is an important site for ammonia metabolism in cirrhosis. Also, hyperammonemia can impair muscle function and contribute to muscle loss, leading to a vicious cycle[192]. A prospective study of 46 patients from Italy showed that sarcopenia was independently associated with the development of post TIPS HE[202]. However, compared to the patients without sarcopenia, patients with muscle depletion in this study were older, had a higher MELD score, and more often had a previous episode of HE before TIPS.
Nevertheless, all the patients who developed HE in this study could be managed medically. Another retrospective study found a correlation between sarcopenia and development of HE within 6 mo of a TIPS procedure without reaching statistical significance[203]. More recently, a study published in abstract form showed that amelioration of muscle wasting after TIPS resulted in a decrease in the episodes of overt HE and venous ammonia levels, suggesting that sarcopenia and HE are causally related[204]. Contrarily, another study published in abstract form showed that in patients undergoing TIPS for RA, sarcopenia did not have any impact on mortality, HE, or ascites control and that sarcopenia should not be considered as a contraindication for TIPS[205]. Available evidence suggests that TIPS has a positive influence on muscle mass and overall body composition, and the addition of nutritional indices to the MELD score could enhance its predictive value. Although TIPS might increase the incidence of HE and acute-on-chronic liver failure in patients with sarcopenia, further studies are needed to identify patients who might be at risk of these complications.
UPDATE ON THE TECHNICAL ASPECTS OF TIPS
Optimal stent diameter
The 8-mm vs 10-mm debate:The availability of covered stents for TIPS has significantly reduced the incidence of stent dysfunction with attendant improvement in patient outcomes[206]. While covered stents have become the standard of care for TIPS world over, the question of optimal stent diameter for TIPS remains unanswered. The diameter of the stent determines the amount of portal blood shunted into the systemic circulation and the PSPG. Several studies have found a relationship between the degree of portosystemic shunting and post TIPS HE[172]. Similarly, a lower PSPG has also been identified as a risk factor for HE after TIPS[207,208]. Also, impairment of hepatic function often seen after TIPS could be reduced by decreasing the size of the stent to avoid significant portal flow diversion and maintain sufficient hepatic perfusion. According to Poiseuille's law, shunt flow is proportional to the fourth power of the stent radius. This underlines the impact of small variations of the stent diameter on shunt flow and, eventually, shunt-related complications. Thus, the use of a smaller diameter stent is desirable. However, placement of smaller diameter stent runs the risk of not achieving adequate portal pressure reduction defeating the purpose for which TIPS was done. The earliest RCT comparing 8-mm and 10-mm covered stents for TIPS had to be stoped early after the results in the first 45 patients showed significantly less efficient control of complications of PH in the patients receiving 8-mm stents[94]. Due to the premature closure of the study, the trial could not provide any evidence on the risk of development of HE. Contrarily, another randomized multicentre trial from Germany comparing covered 8-mm diameter TIPS with HVPG-guided medical therapy for prophylaxis of rebleeding from EV showed that TIPS prevented variceal rebleeding more effectively than drugs without any improvement in survival or quality of life[26]. Compared to other studies using covered TIPS stents, the two-year incidence of overt encephalopathy in the TIPS group in this study was low at 18%. However, the patients included in this study had rather compensated liver disease (Child A or B cirrhosis), and there was no head-to-head comparison between 8-mm and 10-mm stents. Notably, only 43% of patients in the TIPS group had a reduction of PSPG below 10 mmHg. TIPS revisions were required in 8% of the patients with PSPG < 10 mmHg and in 29% of patients with PSPG ≥ 10 mmHg. Nevertheless, a recent RCT from China of 127 patients found that 8 mm covered TIPS stents showed similar shunt function to 10-mm stents, with the halved risk of spontaneous overt HE and less hepatic function impairment[209]. Notably, the majority of patients in this study had hepatitis-B as the etiology of cirrhosis, which is different from the earlier study by Sauerbruchet al[26], in which more than 60% of patients had alcoholic cirrhosis. Although the stent used for TIPS was Fluency?and not Viatorr?, the same stent was used in both groups and might not have influenced the outcomes. Whether the trend towards beneficial effects of 8-mm stents could be extended to patients receiving TIPS for RA is unclear. A retrospective study of 171 patients in this regard showed that 10-mm covered stents for TIPS resulted in better control of ascites compared to an 8-mm stent without increasing the incidence of HE[95]. They found that the mean PSPG after TIPS was significantly higher in the 8-mm stent group than in the 10-mm stent group, and in the overall study cohort, the need for paracentesis was associated with a higher PSPG. Another recent analysis of 185 patients from the German TIPS registry showed that patients receiving 8-mm stents had prolonged survival compared to those receiving 10-mm stents[210]. However, in this study, 8-mm stents were used more frequently in patients with variceal bleeding, while 10-mm stents were placed more commonly in patients having RA. Since patients with RA are generally at a more advanced stage of liver cirrhosis than those with variceal bleeding, derivation of any robust conclusion on survival benefit is not possible from this study. Moreover, although patients in the two groups were matched for age, MELD score, and serum bilirubin concentration, they remained different concerning CTP score and creatinine concentration. Thus, the 10 mm group had more patients with Child C cirrhosis, and the mean creatinine concentration of patients in this group was higher. Other confounding factors affecting survival like sarcopenia were not available for analysis, and the incidence of HE in both groups was not compared. The incidence of rebleeding and recurrence of ascites was also not analyzed in this study. Thus, comparisons on the clinical efficacy of TIPS in both groups of patients cannot be drawn and properly matched patient cohort with adequate subgroup analysis followed by quality prospective studies remain an unmet need to clarify the current issue at hand. Notably, 8-mm stents resulted in less reduction of the PSPG (45%vs65%) compared to 10-mm stents, and patients with an 8-mm stent required significantly more revisions. Current evidence is inadequate to recommend routine use of smaller diameter stents in all patients. However, in patients who are at higher risk of development of HE or liver failure, especially when TIPS is used in the setting of acute variceal bleeding, there may be a role of 8-mm stents.
Target PSPG reduction and passive expansion of under dilated TIPS stents:It has been found that barring few exceptions, patients with de novo or worsening HE after TIPS had PSPG of < 12 mmHg, while those with rebleeding often had stent dysfunction with gradients of > 12 mmHg[207]. Thus, a cut-off of 12 mmHg for post-TIPS PSPG is useful to stratify patients into high or low-risk groups when it comes to HE or rebleeding[207]. It is recommended that a relative reduction of PSPG by 20%-50% may be more practical[211]. In contrast to the situation for variceal bleeding, the optimum target PSPG when placing TIPS for RA remains unclear. A threshold of 5 mmHg or 8 mmHg is not as useful in risk stratification as the cut-off of 12 mmHg[212]. Despite this conflicting evidence, quality improvement guidelines of the American Society of Interventional Radiology recommend that the PSPG after TIPS should not be less than 5 mmHg[213]. Many centers have anecdotally adopted a strategy of step-wise dilatation of 10-mm diameter covered TIPS stents by using balloon catheters of increasing diameter, starting with a 6 mm or 8 mm balloon. The extent of dilatation is considered acceptable when the target PSPG is reached. Further balloon dilatation is reserved for patients with insufficient clinical response. This approach is based on the assumption that TIPS stents which are made of nitinol do not have the necessary radial force to self-expand within a cirrhotic liver. However, it has been reported that under dilated stents passively auto-expand over a variable period[214,215]. Therefore, the practice of under dilating the stent may only have a temporary benefit and may not sufficiently decrease the risk of shunt-related complications. To overcome this limitation, modifications in the TIPS technique have been described by multiple authors, which essentially involve deploying a covered TIPS stent within a smaller balloon-expandable stent allowing calibration of PSPG to a predetermined value at the time of TIPS creation or at a later time, as and when needed. This technique is called as 'incrementally expandable' TIPS stents[216]. However, this requires the placement of an additional stent, adding to the cost and complexity of the procedure.
Controlled expansion stents:Recently, a new controlled expansion stent has been introduced into clinical practice by Gore and associates (Viatorr?controlled expansion endoprosthesis; VCX, Flagstaff, AZ, United States), which allows more accurate diameter control in the diameter range 8 to 10 mm during implantation. VCX is similar to the regular 10-mm Viatorr?e-PTFE stent graft with the added feature of an outer constraining balloon-expandable sleeve that allows adjustment of the stent diameter[217]. Thus, it allows the calibration of PSPG with a single device. In vivo studies have shown that VCX can assume and maintain the intended diameter on clinical follow-up[217]. VCX was associated with a good short term clinical success with a lower rate of HE and stent dysfunction[4,5]. Also, a reduced rate of readmission for sepsis and ascites was observed over a three-month follow-up[217]. However, further studies with longer follow up are needed to confirm this data.
Update on portal venous puncture technique
Cannulation of the portal vein is one of the most crucial and technically challenging steps during TIPS and often determines the duration of the procedure and total radiation dose[218,219]. The majority of potential intraoperative complications are also related to this part of the procedure, including arterial and biliary tract injury and hepatic capsular penetration. A “blind” fluoroscopic approach was originally described to access the portal vein during TIPS. Many centers have switched over to wedged carbon dioxide portovenography to facilitate the advancement of the needle towards the portal vein under two dimensional (2D) fluoroscopy[220]. However, it cannot be used in cases with occlusive portal vein thrombus. Arterial portography is another technique of navigation but requires intra-arterial injection of contrast, and visualization of portal vein may be suboptimal, particularly when the vein is small in caliber or shows hepatofugal flow[221]. Many studies have described computed tomography or ultrasound -guided percutaneous marking of portal vein using guidewires or metallic coils, which is not without risk in patients with advanced cirrhosis[222,223]. The use of transabdominal ultrasound-guided portal vein puncture (Figure 7) overcomes these problems and has been shown to reduce the radiation dose[224]. It is also useful in patients with portal vein thrombosis. Intravascular ultrasound guidance is a potentially exciting tool for portal vein access and has been shown to reduce the radiation dose, multiple needle passes, and volume of contrast used compared to the conventional technique[225,226]. However, intravascular ultrasound has a learning curve and requires additional expensive equipment. Recently, 3D conebeam computed tomography-guided portal vein cannulation using image fusion technology has been described[227,228]. It allows registration of pre-procedural 3D multimodality imaging data sets with 2D fluoroscopy for real-time instrument visualization and has been shown to reduce the number of liver puncture, complications, and failed attempts at TIPS stent placement. Apart from the difficulty in portal venous access during procedure and associated technical challenges, various other complications associated with the technical aspect of TIPS have been described. A comprehensive discussion on these technical aspects is beyond the scope of this review. Nonetheless, Table 3 shows a concise and clarified discussion of these pertinent challenges.
Adjunctive embolization of varices and portosystemic shunts
Persistence of varices after deployment of TIPS can potentially cause recurrent variceal bleeding, especially in cases where adequate reduction of PSPG could not be achieved. Few retrospective studies and one RCT have explored this aspect of the TIPS procedure[229-234]. Angiographic filling of varices despite the adequate reduction of PSPG, presence of gastric or ectopic varices, and suboptimal reduction of PSPG after TIPS have been identified as some of the clinical situations in which patients may benefit from concomitant embolization of varices[229]. Recently, a prospective RCT of 106 patients from China compared TIPS alone with TIPS and coronary vein embolization to assess the rates of rebleeding and stent dysfunction[232]. They found that the cumulative rates of recurrent variceal bleeding in the two groups were not significantly different, except at 6 mo, when the bleeding rate in the embolotherapy group was 2.5-fold lower than that in the TIPS group, without any survival advantage.Interestingly, the primary stent patency rates in the adjunctive embolization groupwere higher than the TIPS group at 6 mo. This was attributed to the increased antegrade flow in the TIPS shunt due to the embolization of the varices. However, the incidence of stent dysfunction in the TIPS group in this study at 6 mo (18%) was worse than the 1-year incidence of shunt dysfunction (12.8%) reported by another RCT from Europe in which majority of the patients underwent placement of covered TIPS stents for variceal bleeding. One reason for this discrepancy could be that Fluency?stents, instead of Viatorr?, were used for TIPS creation in the Chinese study, which tends to
have a higher rate of dysfunction. A meta-analysis of these studies suggested that the incidence of variceal rebleeding was significantly less in the group who underwent concomitant variceal embolization with TIPS[234].

Figure 7 Ultrasound image. A: Gray scale ultrasound image showing the stiff guidewire in the right hepatic vein (arrowhead) with the right portal vein branch in the same image (asterisk); B: The needle-catheter combination advancing towards the portal vein branch (arrow); C: Indentation of the needle (arrow); D: The needle is seen entering the portal vein (arrowhead).
It is generally accepted that liquid embolic agents should be used along with coils to achieve effective occlusion of the afferent veins as well as the variceal complex and prevent the persistent filling of varices. However, many studies have reported the use of coils alone for embolization[229]. Proximal embolization of the afferent vessels using only coils potentially allows persistent variceal perfusionviacollaterals and has poor outcomes. Embolization before TIPS insertion allows for better visualization of collateral vessels, which may decompress after shunt creation. Furthermore, a patent TIPS stent represents a potential channel for systemic non-target embolization of misplaced coils or liquid embolic material into the pulmonary circulation.
On the other hand, post-stent embolization allows the operator to determine the effect of PSPG reduction on the filling of varices. It is suggested that the use of extensive adjunctive variceal embolotherapy theoretically allows for the use of a smaller shunt diameter, which may lower rates of post-procedure encephalopathy[229]. However, the embolization of varices may lead to an increase in portal pressure necessitating placement of larger shunt for adequate decompression. Based on current evidence, adjunctive variceal embolization can be considered in patients in whom the target PSPG reduction could not be achieved after TIPS stent placement or when the persistent filling of variceal channels is noted on completion splenoportogram. It is important to note that the completion splenoportogram should be obtained from the catheter tip at the splenic vein near the splenic hilum to optimally assess the presence or absence of variceal filling after TIPS. Pre-existing large spontaneous portosystemic shunts can compete with the antegrade flow in the TIPS stent and theoretically lead to early stent dysfunction in addition to increasing the incidence of HE. While there are no dedicated studies to assess this aspect of TIPS, it is generally accepted that any large accessible portosystemic shunts should be embolized during TIPS.
Post-TIPS HE
The diagnosis and treatment of post-TIPS overt and covert HE is not different from that of HE occurring independently of the procedure. Embolization of any large preexisting spontaneous portosystemic shunts (if not already embolized during TIPS) is an important step in the management of post TIPS HE. Stent lumen reduction or occlusion is indicated in case of severe persistent overt HE. Unfortunately, complete shunt occlusion with the help of vascular plug, multiple coils, or detachable balloons has often resulted in life-threatening sequelae due to the sudden hemodynamic alterations. Even intentional reversible TIPS stent occlusion using latex balloons kept inflated for up to 48 h carries the risk of recurrent VH and death. To overcome these problems, multiple techniques of stent reduction have been described. Initially, constrained uncovered stents were utilized for shunt reduction. These were either customized or required use of a parallel balloon-expandable stent. However, this technique was limited by inaccurate regulation of blood flow even after the embolization of dead space surrounding the narrowed portion of the stent using coils. Nowadays, stent reduction is almost exclusively done using stent-grafts, which provide a more predictable outcome in terms of blood flow regulation. On-table customization of balloon-expandable stent-grafts into an hourglass configuration using sutures was initially described. Later, Szeet al[235]reported a technique of parallel placement of stent graft and balloon-expandable stent within the TIPS stent. The balloon-expandable stent is used to compress the stent-graft, which will determine the flow lumen.
Post TIPS liver failure
Despite its minimally invasive nature, TIPS inevitably cause stress on the liver due to the parenchymal injury and diversion of already compromised portal blood flow. Besides, depending on the location and configuration of the stent, one or more branches of the portal vein or hepatic artery might be obstructed or compressed, resulting in ischemia. Also, the covered portion of TIPS stent can occlude the drainage of one or more hepatic veins leading to venous congestion[236-238]. These can manifest as mild transient derangement of liver functions in the days after TIPS stent placement or liver failure. Studies have shown a two-to-three fold increase in liver enzymes and bilirubin after TIPS, independent of baseline[239,240]. These alterations usually get resolved within 2 wk because of a compensatory increase in hepatic arterial flow, also called as 'hepatic artery buffer response'[239]. However, marked derangement and delayed stabilization of liver functions might be an indicator of irreversible liver injury and liver failure. Bilirubin is an independent predictor of 30-d mortality after TIPS placement with a 40% increased risk of death for each 1 mg/dL increase above 3.0 mg/dL[241]. Bilirubin levels increased to at least triple the baseline value in approximately 50% of dying patientsvsonly 20% of surviving patients[240]. Similarly, patients with a MELD score of 18 or more have a significantly lower 3-mo survival rate than those with a MELD score of 17 or less[242]. It is recommended that patients with a CTP score > 10 or MELD score > 14 should not have their post-TIPS PSPG reduced to < 5 mmHg[243]. Thus, patients showing a persistent threefold increase in bilirubin after TIPS should be considered to be at risk of liver failure warranting aggressive management, including referral to a transplant center.
Follow-up imaging protocol and shunt revision
No rigorous studies have addressed the issue of optimal follow-up time intervals for doppler surveillance after TIPS, while majority of the centers have anecdotally adopted a strategy of doing doppler ultrasonography at 1, 2, 6 and 12 mo following stent placement and every 6-12 mo after that unless there is the recurrence of symptoms for which TIPS was done (ascites or hydrothorax)[244]. For patients in whom TIPS stent was placed for VH, a stringent doppler follow-up is required because they might not be immediately symptomatic even after shunt dysfunction. Evaluation of stent flow velocities is the primary tool for assessing shunt patency. Normal flow velocities within the stent fall within the range of 90-190 cm/s[245]. A main portal vein velocity below 30 cm/s is another useful parameter[246]. Another recent study found that a greater than 25% interval change in peak TIPS velocity was significantly more sensitive at detecting dysfunction in a covered TIPS stent[247]. If there is a suspicion of in-stent stenosis or occlusion on surveillance Doppler ultrasound, a TIPS venogram and pressure measurements should be carried out.
In-stent stenosis most commonly occurs at the hepatico-caval junction. Multiple studies have shown that angioplasty with the placement of covered stents gives better long term results compared to angioplasty alone[248]. Restenting is also useful in cases of stent shortening or portal venous stenosis. Shunt extension (placement of another stent in the portal venous or hepatic venous end) is used mainly to correct problems with angulation. In cases of chronic stent thrombosis, accessing the stent may be difficult from the transjugular route[249]. Percutaneous transhepatic and trans-splenic routes have been described for obtaining wire access in such cases[249,250]. The transhepatic route requires percutaneously puncturing the midportion of the stent and snaring the wireviathe internal jugular venous route[249]. For the trans-splenic approach, a small peripheral tributary of the splenic vein is punctured, and the caudal end of the stent is accessedviathe portal vein[250]. In cases where the primary stent is unsalvageable, placement of a parallel TIPS stent has been described to provide symptomatic relief[251].
CONCLUSION
Transjugular intrahepatic portosystemic shunt placement has been demonstrated to have benefit on the control and recurrence of PH events and transplant free survival in patients with cirrhosis. Nonetheless, various applicability of TIPS among specific subsets of patients with cirrhosis need further validation and thus form prospects for future studies in the form of observation, hypothesis generation and validation in controlled trials. Of these the most important include utility among Child Pugh class B patients with variceal bleeding, role in non-responders to primary prophylaxis with beta blocker therapy and benefits with early use in patients with recurrent ascites who do not fulfil criteria for refractoriness. Other pertinent areas for further research involve the role of TIPS in management of bleeding GV associated with large spontaneous shunts, in hepatopulmonary syndrome as a bridge to liver transplantation and biomarkers to predict post TIPS outcome such as stent dysfunction or death. Furthermore, an exciting area would also be the use of controlled-expansion stents for TIPS placement in those with advanced liver disease and recurrent or uncontrolled PH related complications.
 World Journal of Gastroenterology2020年37期
World Journal of Gastroenterology2020年37期
- World Journal of Gastroenterology的其它文章
- Artificial intelligence technologies for the detection of colorectal lesions: The future is now
- Abernethy syndrome in Slovenian children: Five case reports and review of literature
- Endoscopic retrograde cholangiopancreatography in the treatment of pancreaticopleural fistula in children
- Risk prediction rule for advanced neoplasia on screening colonoscopy for average-risk individuals
- Endoscopic ultrasound-fine needle biopsies of pancreatic lesions: Prospective study of histology quality using Franseen needle
- Helicobacter pylori infection with atrophic gastritis: An independent risk factor for colorectal adenomas
