Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species
Zi-Jian Zhang, Kun-Peng Wang, Jing-Gang Mo,Li Xiong, Yu Wen
Zi-Jian Zhang, Li Xiong, Yu Wen, Department of General Surgery, Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China
Kun-Peng Wang, Jing-Gang Mo, Department of General Surgery, Taizhou Central Hospital (Taizhou University Hospital), Taizhou 318000, Zhejiang Province, China
Abstract
Key words: Cancer stem cells; Photodynamic therapy; Reactive oxygen species; Photosensitizer; Mitochondrial; Endoplasmic reticulum
INTRODUCTION
Photodynamic therapy (PDT) is an effective and promising cancer treatment.By injecting a tumor-targeted photosensitizer (PS) into the patient’s body and directly irradiating the tissue with a laser, a significant tumor ablation effect can be achieved[1].The space- and time-selective uptake characteristics of the PS protect normal cells, while the laser radiation is directly pointed to the tumor[2].Thus, PDT is a multipletargeting method.Since the US Food and Drug Administration listed PDT as a new treatment method for the clinical treatment of cancer patients[3], alone or combined with surgery[4]and/or chemotherapy[5], PDT has been applied in large numbers worldwide.For PDT treatment, some Western countries have established relatively systematic treatment plans[6-8].The main mechanism of PDT depends on the reactive oxygen species (ROS) components generated by the photochemical reaction, which can oxidize a large number of intracellular active components (such as DNA and lipid compounds) in tumor cells[9,10].This chemical-dependent treatment is more sensitive than drug treatments and can minimize tumors in the short term.
It is currently recognized that ROS play decisive roles in the biological effects mediated by PDT because PDT is based on a natural cold photochemical reaction[11].Although PDT can be divided into two types of reactions, depending on the type of PS, the products can both be considered ROS.Essentially, the exogenous ROS induced by this cold photochemical reaction are the most important and first effector molecules of PDT, although they also depend on the intracellular oxygen levels most of the time.In addition to exogenous ROS, PDT-induced ROS can also cause intracellular metabolic changes, induce endoplasmic reticulum stress[12], and/or destroy mitochondrial potential[13]to increase endogenous ROS production.On the one hand, the photochemical reaction directly caused by PDT produces a short ROS duration (< 0.05 μs), with high reactivity and a limited diffusion distance (< 0.02 μm)[14].Therefore, the main target position of the photochemical reaction in the cell is often near the subcellular components where the PS is localized, which explains the heterogeneity of the effects of different PSs[14].On the other hand, the metabolic induction of mitochondrial ROS production is much more complicated; for example, it may involve the partial inactivation of respiratory complexes I, II, and III of the mitochondrial electron transport chain[15].In general, excessive ROS destroy the redox system in cells and cause oxidative damage to biomolecules, including DNA and other molecules[10,11].In previous studies, DNA was considered an important target of PDT because doublestrand DNA breaks are the most lethal form of damage to tumor cells[16].Recently, more studies have suggested that the activation of the mitochondrial permeability transition increases the levels of reactive nitrogen substances, such as nitric oxide[17,18].Of course, ROS-induced intracellular metabolism affects mitochondria to an even greater extent.The ROS-related effects on the endoplasmic reticulum, nucleus, and cell membrane are described in detail in a subsequent section.
There is growing evidence that ROS play roles in cell signaling.These signals are transmitted in tissues to coordinate various cellular processes.At physiological doses, ROS maintain cell nutrition and cytokine balance; however, in some specific cases, small changes in ROS level may have a profound impact on the fate of stem cells[19], directly induce cancer stem cell (CSC) differentiation, or induce CSC heterogeneity in tumors[20].Furthermore, ROS are related to the level of many biological processes, including but not limited to gene expression, protein translation, and protein or nucleic acid interactions[21].As genomics and proteomics advance, increasing pathway information on ROS, explaining the mechanisms by which they maintain and regulate cellular processes, is being mined.Especially in stem cells, changes in the oxidative state (also known as redox regulation) may indicate the regulatory elements for communication between key organelles such as endoplasmic reticulum-mitochondria and mitochondria-nucleus crosstalk[22,23].Redox-mediated mitochondrial-nuclear crosstalk can explain the coordination of cell metabolism and chromatin remodeling, gene expression, cell cycle progression, DNA repair, and cell differentiation.The endoplasmic reticulum-mitochondria crosstalk (as well as that of other organelles with mitochondria) can explain endoplasmic reticulum stress, mitochondrial autophagy, stemness induction, apoptosis, and/or survival through ROS.
ROS are also related to immunogens and tolerogenic processes[24].The increased systemic immunity or enhanced tumor immunity induced by PDT may also be mediated by ROS[25].Currently, although little is known about whether or how ROS are involved in stem cell immunity, it has been determined that identifying the mechanism by which ROS metabolism affects the fate of stem cells will promote the necessary understanding to apply PDT to inhibit the spread of distant cancer stem cells.Although PDT is used to treat superficial malignancies, its immunogenicity has the potential to eliminate systemic CSCs.But in a counterintuitive outcome, some research found that low-dose PDT promotes tumor cells metastasis[25,26].The epithelialmesenchymal transition (EMT) has been shown to be one of the causes of cancer cell migration and invasion[27].PDT can induce EMTin vitro[28].EMT may be closely related to the metabolic reprogramming of CSCs and cancer cells[28].ROS can induce stemness and metabolic changes in cancer cells.Therefore, PDT appears to induce the EMT and promote CSC phenotype acquisition by regulating cellular metabolism.The EMT, stemness, and oncogenic metabolism are known to be associated with resistance to PDT.Therefore, understanding PDT-induced metabolism and the molecular mechanism of the EMT is also conducive to accurately generating the appropriate level of ROS and enhance the efficacy of PDT.Therefore, in this review, we present the differences in ROS produced by the two types of photochemical reactions induced by PDT, the metabolic processes of endogenous ROS, and the similarities and differences in the biological effects of different ROS.We analyze the effects of ROS on cells at different sites and explain how they might affect the fate of stem cells.Finally, in view of some controversial characteristics of CSCs, we propose how to leverage the advantages of PDT to manipulate the fate of CSCs.
PRODUCTION AND METABOLISM OF ROS DURING PDT
Generation of exogenous ROS
PDT is a chemical reaction between a PS and oxygen under laser energy; when the three are combined, ROS are produced.Under normal circumstances, the PS is in the ground singlet state, where all the electrons rotate in pairs in low-energy orbits.When irradiated with the wavelength of the PS absorption peak, one of the electrons in the highest occupied molecular orbitals of the PS moves to the lowest unoccupied molecular orbital, which places PS into a transient and unstable activated singlet state, leading to a series of events[29,30].Due to the aromatic nature of many PSs, the energy difference between highest occupied molecular orbitals and lowest unoccupied molecular orbital is quite small.The light emitted at the excitation wavelength is usually in the visible or near-infrared parts of the spectrum.The activated singlet of the PS reverses the rotation of the activated electrons to generate a triplet, which is a process that enables triplets to cross between PS systems.The PS triplet has a lower energy and longer life than the singlet because the activated electrons spin parallel to the previous paired electrons and are not easily separated to create a singlet.To enter a more stable state, the triplet electron excited by the PS either enters the correct rotating orbit, is released to fall to the ground singlet state, and emits fluorescence (slow process) or directly interacts with molecules in the environment to return to the singlet state.The interaction between triplet states is reversible, but the interaction between the triplet state and the singlet state is irreversible.Therefore, the activated PS triplet can only interact with the molecules in the triplet rotation.In the cell, the triplet state of O2has obvious double radical properties in the ground state; therefore, energy is easily transferred from the triplet PS to oxygen molecules.This process is the basis of the chemical reaction of PDT.
The effectiveness of PDT is due to free radicals and electronically activated oxygen species, and their production depends on whether a PDT type I or II reaction occurs[31](Figure 1).As mentioned earlier, the outermost layer of the ground state triplet has two unpaired orbits that rotate in parallel; therefore, when the triplet state is excited by PS, the reactions of different molecules are quite different[29,32].In a type I reaction, PS directly removes an electron to generate a superoxide anion (O2?).O2?can quickly form other substances, including hydroxyl radicals and hydrogen peroxide.In the type II reaction, the energy that excites the PS triplet state is transferred to O2.The spin of the outermost electron of O2thus flips and moves to an orbit that previously contained unpaired (natural) electrons with opposite spins; this describes a singlet oxygen (1O2) in the active state.1O2is not a free radical because all the electrons spin in pairs, but they are very active and short lived[33,34].
Generation of endogenous ROS
Under normal circumstances, approximately 90% of the ROS in the body are produced by the mitochondrial electron transfer chain[35].The yield of ROS produced by the ETC increase during hypoxia, light stimulation, ischemia-reperfusion, aging, and mitochondrial respiratory depression.Over 90% of the oxygen in mitochondria is reduced by cytochrome oxidase to water molecules, while only 0.1%-0.2% of O2forms ROS through electron flow, mainly through electron transport chain complexes I and III[15,36].The rate of the ROS produced by mitochondria is mainly affected by mitochondrial membrane potential (MMP) regulation.
In theory, PDT also has the ability to increase endogenous ROS by changing the MMP[37].Generally, it is difficult for exogenous ROS to directly induce MMP changes to produce more endogenous ROS, but it can be accomplished through endoplasmic reticulum-mitochondria crosstalk.Briefly, PDT induces endoplasmic reticulum stress (ER stress, ERS), which mainly transmits death signals from the endoplasmic reticulum to the mitochondria by the proteinkinase R-like ER kinase (PERK) pathway.In this process, Ca2+influx from the endoplasmic reticulum to mitochondria induces a decrease in the MMP and promotes ROS production[38,39].In more detail, the mitochondria-associated ER membrane (MAM) may explain this phenomenon.MAMs whose contacting is increased in CSCs are connected by protein-binding complexes[40].These two membranes are 10-25 nm apart and communicate through the calcium ionrelated pathway[40].Mitofusion2 (Mfn2) maintains MAM distance and prevents ER and mitochondria from being too close and thus prevent Ca2+-mediated MMP changes and apoptosis.When 5-aminolevulinic acid mediated PDT is performed, Mfn2 expression was reduced, suggesting an production of endogenous ROS[41].In addition, phosphofurin acidic cluster sorting protein 2 (PACS2) is an essential protein that mediates MAM-Ca2+overload[42].PACS2 promotes the cleavage of BCR-associated protein(BAP)-31, which is an important factor in the caspase-8 apoptosis pathway activated by Ca2+.During PDT, BAP31 is cleaved before the mitochondria changes and mediates the release of ERS and Ca2+[43].From the point of view of enzyme catalysis, PDT regulates flavin adenine dinucleotide, ubiquinones, and cytochrome in mitochondrial respiratory chain complexes I and III to produce ROS[44-46]; mitochondrial triphosphopyridine nucleotide(NADPH) oxidase and xanthine oxidase catalyze the production of O2?[47]; mitochondrial myeloperoxidase myeloperoxidase (MPO) catalyzes the production of OH; and protein kinase C catalyzes the production of H2O2[48,49].Because the dynamic changes in ROS are rapid, in trace amounts, and complex, it is difficult to accurately detect the process by which ROS are produced, from exogenous to endogenous ROS, by PDT within a short period.Therefore, although we believe that PDT may induce endogenous ROS, very few studies have addressed or clarified this process.
Metabolism of ROS during PDT
According to the type of PDT photochemical reaction, ROS produced by subsequent metabolism can also be generally divided into two parts.The most active free radical molecule is ?OH, which is converted into stable hydroxide ions by receiving electrons and generating water and protons.O2-receives electrons to form peroxide ions (O22-) and then is rapidly protonated to form H2O2.O2-is inert in biological systems because the antioxidant action of superoxide dismutase converts O2-to H2O2and O2.H2O2is converted into water and oxygen molecules[50,51].Nonetheless, H2O2may react with very low concentrations of electron-deficient substances[52], such as ferrous ions (Fe2+), which cause the oxygen and oxygen bonds of H2O2to break, producing ferric iron (Fe3+), hydroxide, and ?OH (Fenton reaction)[53].?OH cannot be catabolized by enzymes but can be broken down by antioxidant peptides (such as glutathione) or antioxidant vitamins (such as vitamin C)[54].PDT type I reaction products can also indirectly cause the formation of reactive nitrogen because O2?reacts with nitric oxide (NO) to generate peroxynitrite anion (ONOO?)[17,55,56].ONOO-is very active and has a short life span.Rapid homogeneous fission forms ?OH and nitrogen dioxide (NO2).ONOO-also reacts with carbon dioxide to form carbonate anion radicals (CO3-) and N O2.All the resulting free radicals are destructive and they continue to move in the cell until they are paired (free-radical pairing).Although not a free radical,1O2reacts with macromolecules in several different ways.1O2can act as a dienophile in the Diels–Alder cycloaddition reaction, and it can react with the aromatics and the diene of the conjugated system, leading to the degradation of many lipids and proteins.Disulfide bonds and other electron-rich substances may also attack1O2[57](Figure 1).In contrast to free radicals,1O2cannot be destroyed by enzymes but can be inactivated by antioxidants (such as carotenoids).
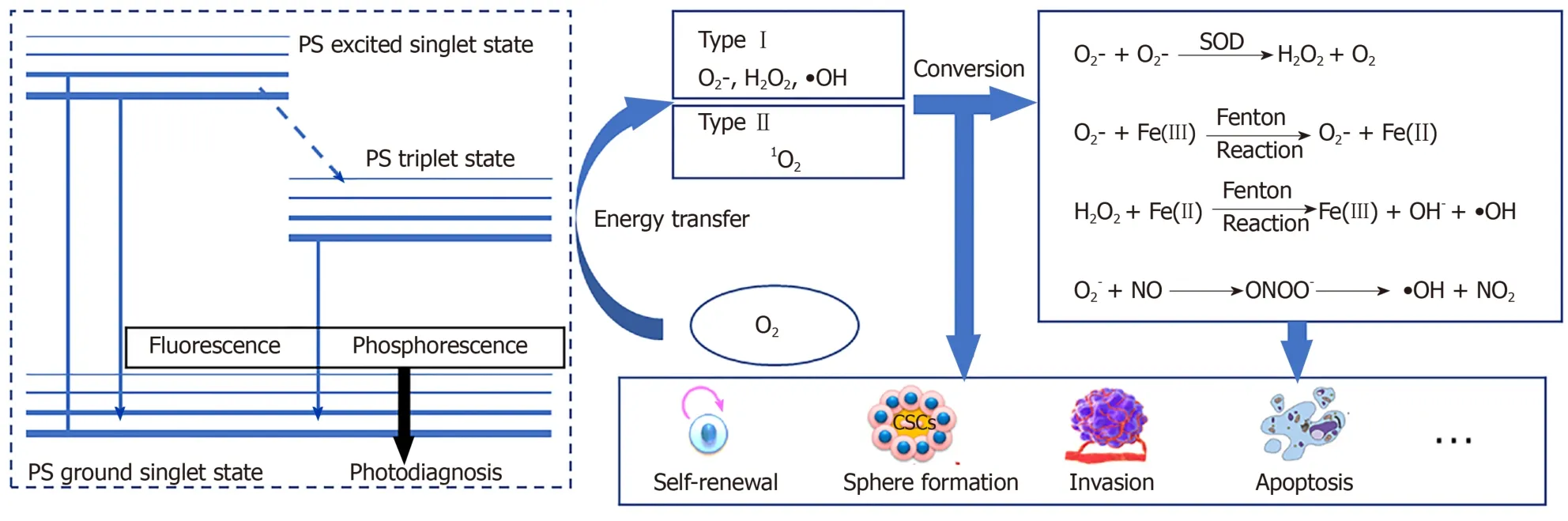
Figure 1 The process of reactive oxygen species production and transformation in cancer stem cells.
IMPACT OF ROS FROM PDT ON CSCS
Location of photosensitizers in CSCs
The body has a complex antioxidant system.Normally, the production and removal of ROS are maintained in a dynamic balance so that they do not cause damage to the body[58].Disruption of stem cell metabolism can directly determine whether stem cells are at rest, self-renewing, or differentiating[59-61], and controlling ROS levels is one of the feasible ways in which metabolism is disrupted.After PDT treatment, intracellular ROS accumulation increases, causing excessive oxidation of proteins, DNA, and lipids, which may be a direct means of controlling the fate of stem cells.ROS bind to proteins to generate carbonyl derivatives[62], alter the tertiary structure of the proteins, and promote protein/DNA[63]-protein cross-linking, leading to changes in the protein activity of CSC marker proteins such as octamer binding transcription factor 4 and sex determining region Y box 2 (Sox2)[64].ROS directly attack DNA bases and easily cause deoxyguanosine modifications to one carbon atom (that is, 8-OHdG)[62], which may be one of the causes of point mutations in proto-oncogenes or tumor suppressor genes, such as Ras and p53[65,66].Free radicals generate lipid peroxides through oxidation, which can damage cell membranes and promote ferroptosis.
However, whether ROS oxidize lipids, inactivate proteins, or damage DNA in CSCs largely depends on where the ROS are generated (because the half-life of the ROS produced by PDT ranges from 3.5 μs to 5 s, and the1O2diffusion distance is approximately 40 nm)[14].Some people think that the killing effect of a PS in mitochondria is significantly higher than that of the same PS is in other organelles, and the importance of PS positioning is even higher than the ROS yield[67].Therefore, before describing the effects of PDT and ROS on CSCs in detail, it is necessary to summarize the subcellular localization of commonly, recently used PSs.Of course, in special cases, PDT undergoes different localization and interacts with unique effector sites before activation.For example, in our previous studies, we observed that a mitochondrial PS induced ERS-mediated apoptosis, which suggested that intercellular organelle crosstalk was involved in the ROS-regulated CSCs[68].In this review, PS mapping for tumor PDT can be roughly divided into three areas: Mitochondria[69-78], endoplasmic reticulum[12,68,79-82], and lysosomes[83-88].These PSs can be roughly summarized into porphyrin-based photosensitizers, such as porphyrins[89-92], chlorins[80,93,94], phthalocyanines, and naphthalocyanines[95-99], and non-porphyrin-based photosensitizers, such as cyanine, methylene blue, Nile blue, rhodamine, triarylmethane, and acridine[68,100-105].These PSs and their derivatives are summarized by category in Table 1.Therefore, to more accurately control and eliminate CSCs, the effects of ROS on PDT in different cell structures should be classified and explained.
By the way, it is worth mentioning that photochemical internalization is a special CSC targeting strategy though PDT does not play a major role in this approach.In photochemical internalization, PS are modified by CSC biomarkers (such as CD133, CD44, CSPG4, and EpCAM)[106-109]that are first anchored to the cell membrane and then are endocytosed into intracellular vesicles.Finally, the drugs carried into the cell are released through PDT.For more details, please refer to a previous review[110].
ROS and mitochondria in CSCs
The effect of ROS on the function of mitochondria has always been one of the research focuses of CSCs.ROS produced through PDT can induce apoptosis through increased mitochondrial membrane potential[111].Studies have shown that mitochondrial photooxidative stress can cause a large number of lipid peroxidation reactions in the mitochondrial membrane, leading to rapid changes in the MMP, which stimulates pressure-dependent anion channels [voltage-dependent anion channels (VDACs)][112]and promotes the opening of the permeability transition pore complex[113,114], thereby releasing cytochrome C (Cyt C)[115,116]into the cytoplasm.Cyt C combines with caspase-1 to form a multimer, which initiates apoptosis in CSCs in a caspase-dependent manner; in the non-caspase-dependent apoptosis pathway, other proteins are activated and released, such as apoptosis inducing factor, Omi/HtrA2, and endonuclease G during PDT[117-119].The release of these enzymes depends on the cleavage of calpain[120].These factors all directly lead to the apoptosis of CSCs.
B-cell leukemia 2 (BCL-2) family protein interactions are important in the induction of apoptosis induced by changes to the MMP in CSCs.And they are also important mitoROS modulators.In the canonical pathway, BCL-2 associated X and K proteins are located on the cytoplasmic side and the mitochondrial side of the outer mitochondrial membrane under normal conditions, respectively.When the MMP is increased, BCL-2 associated X protein is transported and inserted into the outer mitochondrial membrane, and the local conformation of BCL-2 associated K protein forms homooligomers or hetero-oligomers, which release Cyt C in the membrane space and initiate CSC apoptosis[121].In the non-canonical pathway, BCL-2 may be involved in the regulation of the cell redox state without antioxidant characteristics.First, there is a physical interaction between BCL-2 and CcOVα (cytochrome c oxidase subunit Vα).Overexpression of BCL-2 causes an increase in mitochondrial localized CcOVα, which is conducive to CcO total enzyme assembly and the ETC process[122,123].Second, the BCL-2 BH3 domain interacts with glutathione (GSH)in vitro, suggesting that BCL-2 functions in regulating mitochondrial GSH content[124].Finally, the mitochondrial localization of GTPase-Rac1, which is associated with stem cell deletion, and its interaction with BCL-2 suggest that Rac1 plays an important antioxidant role[125].These results suggest that BCL-2 may be a bridge connecting mitochondrial apoptosis and ROS in CSCs.
Energy metabolism is one of the main functions of mitochondria, and this process is closely related to the stemness of tumor cells.Research on energy metabolism in CSCs at various stages is rife with controversy.Early studies found that CSCs have more obvious anaerobic glycolytic characteristics than are expressed in differentiated cancer cells; that is, CSCs have increased expression of glycolytic enzymes, increased production of lactic acid, and decreased or resting mitochondrial function[126,127], and their ROS levels are usually lower than those of cancer cells.Recent studies have suggested that mitochondria in CSCs have increased mass and membrane potential, and their mitochondrial function reflects higher mitochondrial ROS levels and enhanced oxygen consumption rates[128].In any case, mitochondrial function and oxygen concentration are essential for maintaining CSC function[129,130].It has been inferred that under hypoxic conditions, some CSCs preferentially undertake oxidative phosphorylation for survival and maintenance of stemness and convert to glycolytic metabolism during differentiation[131].The production of ROS by PDT consumes a large amount of oxygen, which forces CSCs to change from oxidative phosphorylation in the “stem cell” state to anaerobic glycolysis during differentiation, suggesting that PDT can effectively control the differentiation of CSCs.In addition, ROS can reduce the expression of caveolin-1 in cancer-associated fibroblasts, the major component of tumor stroma.The reduction of caveolin-1 stabilizes HIF-1α (which forms a heterodimer), enhances glycolysis to adapt to hypoxic conditions, and leads to a further increase in ROS production[132-134].These studies indicate that oxygen depletion in mitochondria caused by PDT mediated ROS production inhibits stemness of cancer cells.
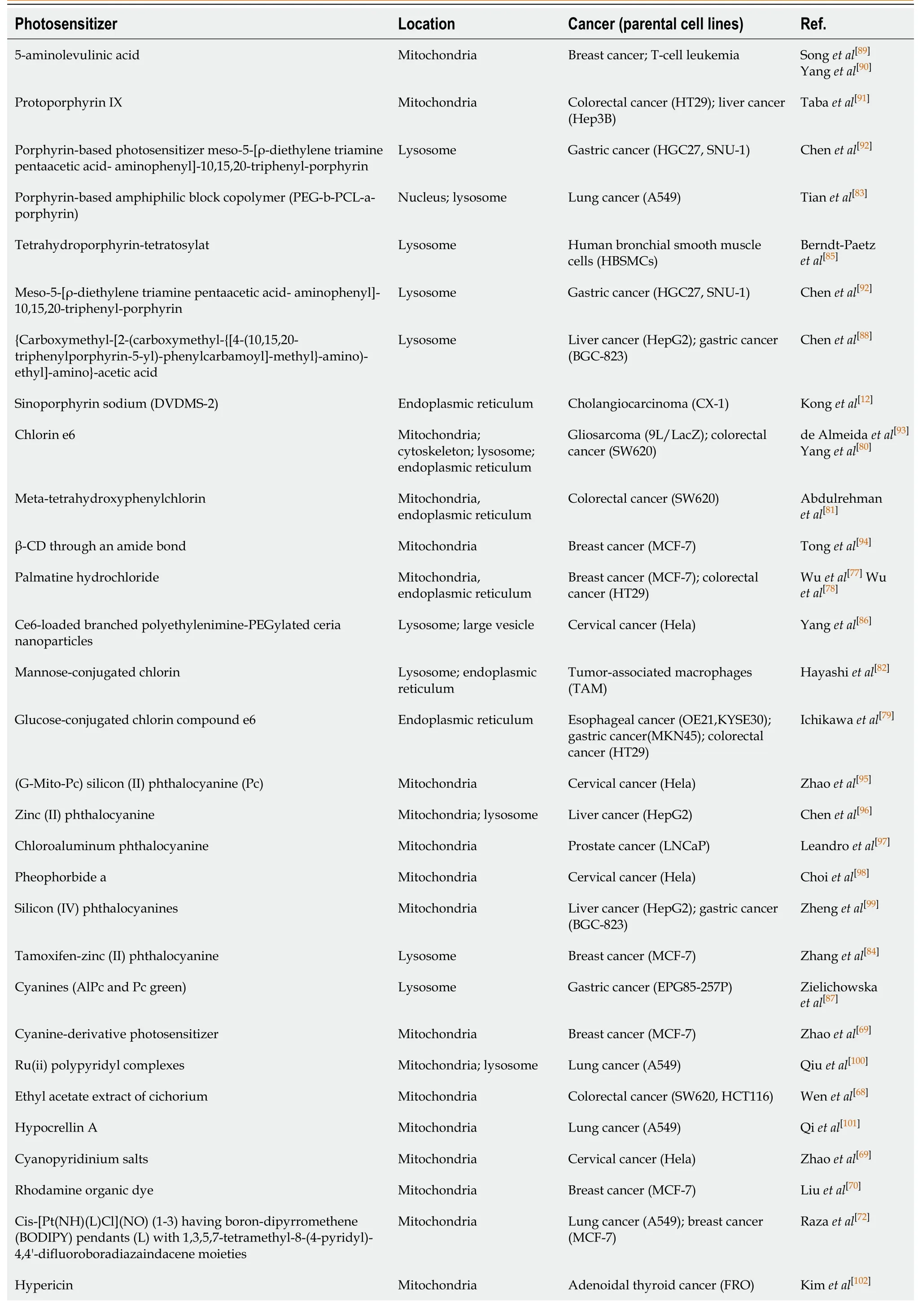
Table 1 Subcellular localization of photosensitizers in different cancer stem cells
Platinum(II) complexes [Pt(L)(R-BODIPY)]Cl Mitochondria Lung cancer (A549); breast cancer (MCF-7); cervical cancer (Hela)Ramu et al[103]Cis-[Pt(NH)(L)Cl](NO), where L is an imidazole base conjugated to 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)Mitochondria Breast cancer (MCF-7)Raza et al[72]Triphenylphosphonium Mitochondria Lung cancer (A549)Rui et al[74]Triphenylphosphonium Mitochondria Cervical cancer (Hela)Choi et al[98]Methyl-functionalized derivatives of the drug carrier triphenylphosphonium Mitochondria Cervical cancer (Hela); gastric cancer (FU97)Hu et al[75]Hypocrellin B Mitochondria Breast cancer (MDA-MB-231)Jia et al[76]Aloe-emodin Mitochondria; endoplasmic reticulum Osteosarcoma (MG63)Li et al[104]BODIPY-Appended 2-(2-Pyridyl) benzimidazole Platinum (II) Catecholates Mitochondria Keratinocytes (Hacat)Mitra et al[105]Acetylatedglucose-conjugated chlorin Endoplasmic ruticulum Esophageal cancer (OE21, KYSE30); gastric cancer (MKN45);Ichikawa et al[79]
Some key genes may mediate both of these effects.PDT inhibits the Wnt pathway[135]which plays an important role in CSCs[136,137], inducing mitochondrial repression and glycolytic conversion by activating Dlx-2 and Snail[138-140].This mitochondrial suppression is mediated by the inhibition of mitochondrial complex IV[140].Wnt also directly targets pyruvate dehydrogenase kinase, thereby inhibiting mitochondrial respiration and promoting glycolytic conversion[141].Therefore, the Wnt pathway may regulate CSCs through the above two functions at the same time.Currently, little is known about the relationship between typical stemness markers and the regulation of CSC metabolism, but researchers have shown that the stem cell marker CD44 may be crucial in the regulation of glycolytic metabolism[142,143].The direct interaction between CD44 and the GSH transporter--solute carrier family 7 member 11 has been reported in multiple PDT articles[144,145], also suggesting the ability of PDT to manipulate CSCs through redox and energy metabolism.
Mitochondrial autophagy also plays a protective role against ROS in CSCs.Currently, it is widely recognized that mitochondrial autophagy counteracts PDT.For details, please refer to our previous review[146].
ROS and the endoplasmic reticulum in CSCs
The most widespread and common effect of PDT-me diated photooxidative stress is the unfolded protein response, secondary to endoplasmic reticulum stress (UPRER, UPR).Increased endoplasmic reticulum-related ROS in PDT have been shown to cause upregulation of various ER molecular chaperones, such as calcium-binding proteins (GRP78/Bip and GRP94) and protein disulfide isomerase.These key proteins lead to the accumulation of unfolded proteins in the ER cavity, leading to PERK-, IRE1-, and ATF6-mediated UPR[147-149].In the intestine, activation of UPR by PERK kinase leads to differentiation of intestinal epithelial stem cells and colon CSCs, and the absence of X box binding protein 1 results in increased stemness and adenoma formation.X box binding protein 1 activation results in reduced cell proliferation and intestinal epithelial cell stemness due to cross-activation of the PERK-eIF2α signaling[150].In pancreatic cancer, GRP78 downregulates stem cell clone formation and self-renewal characteristics, suggesting that the UPR plays a role in inhibiting stemness in pancreatic cancer[151].The contradictory results mentioned above can be explained by the dual nature of UPRERin both survival and apoptosis signaling.The fate of cells with respect to these two signal cascades depends on the intensity of the photooxidative stress.Severe ER photooxidative stress can stimulate more cascades that are transducing death-promoting signals (such as apoptosis), such as that stimulated by CCAAT/enhancer binding protein homologous protein, which is a key pro-apoptotic transcription factor in ERS[152,153].A small or low level of ER photooxidative stress can stimulate more promoting survival signaling cascades (such as autophagy, p38 mitogen-activated protein kinase(MAPK) signaling, antioxidant signaling, and amino-terminal kinase JNK signaling)[154,155].
In addition to the UPR mechanism described above, ROS can affect the fate of CSCs through endoplasmic reticulum-mitochondrial crosstalk.PDT treatment causes the release of internal Ca2+of the endoplasmic reticulum into the cytoplasm and mitochondria, inducing MMP-mediated apoptosis.In the above process, MAM (mitochondria-associated membrane), which is a solid-state connection between mitochondria and the endoplasmic reticulum, overexpress in CSCs.Its state and efficiency of the coupling are among the primary regulatory characteristics by which factors influence Ca2+concentration in mitochondria[38-40].Therefore, ROS generated by PDT may affect MAMs by various genes, such as P53, PML, ERO1, and p66Shc.P53 proved to be differentially expressed in PDT is involved in the regulation of Ca2+-mediated apoptosis in a transcription-independent manner.Among the proteins that accumulate in the MAMs, p53 activates the pathway to cell death, while p53 deletion leads to a Ca2+decrease in the endoplasmic reticulum[156].PML (promyelocytic leukemia) proved to be another tumor suppressor involved in regulating the endoplasmic reticulum-mitochondrial Ca2+dialog[157].The endoplasmic reticulum oxide protein endoplasmic oxidoreductin 1(ERO1)-Lα regulates the release of Ca2+, and the generation of ROS, from the endoplasmic reticulum through inositol 1,4,5-triphosphate receptor type 1 and thioredoxin domain containing 4[158,159].It is involved in the formation of disulfide bonds with protein disulfide isomerase and therefore plays an important role in protein folding.p66Shc is an ROS-generating protein located in MAMs.When it undergoes oxidative stress, p66Shc is phosphorylated at Ser36 and then is translocated to mitochondria and/or MAMs.It is involved in ROS generation and apoptosis-related signaling pathways[160,161].
By integrating some studies, we also speculate that some factors are involved in the regulation of ERS and MAM at the same time after PDT treatment.First, PERK is highly expressed after PDT and can activate UPR.PERK is found to be localized to MAMs and promotes endoplasmic reticulum-mitochondria coupling[162].Second, Mfn2, a kind of skeleton in MAMs, can regulate the endoplasmic reticulum associated autophagy and apoptosis by downregulating the activity of PERK[163,164].PACS2, another important component of MAM, also participates in the autophagy process.In PACS2-knockout and Mfn2-knockout cells, the accumulation of autophagic markers and the translocation of endoplasmic reticulum-related proteins were significantly reduced, indicating that MAMs play specific roles in the formation of autophagosomes and ERS[165].This shows that there is mutual regulation between ERS and MAM.Based on the research of ER-mitochondria crosstalk, the efficacy of autophagy and ERS inhibitors/activators combined with PDT in the treatment of CSCs has been verified in multiple studies, regardless of whether the photosensitizer is localized to the endoplasmic reticulum or mitochondria.
ROS and lysosomes in CSCs
Lysosome status also directly or indirectly affects CSCs, such as through apoptosis initiation or autophagy flux.However, in addition to apoptosis and autophagy, lysosomes have recently been found to an play important role in the switch of eukaryotic cells to deep quiescence[166].The dormant state that can be reversed by the stimulation of growth signals is called cell resting.In contrast, it is irreversible in state of senescence.The depth of the resting state of the cells is directly related to the difficulty of maintaining the stemness of CSCs and re-entering the proliferative state.Fujimakiet al[166]found that resting depth-related genes were significantly enriched in the lysosome pathway.By measuring the autophagy flux that characterizes lysosomal function, it was found that, as the resting state of the cells deepened, lysosomal function gradually decreased.The exogenously expressed transcription factor microphthalmia associated transcription factor enhanced lysosomal function in cells, and an increase in lysosomal function reduced the concentration of the ROS in the cell to prevent deepening of the resting state.The “switch” works by regulating the concentration of ROS in cells.The ROS produced by PDT may also regulate the resting state of cells through lysosomes, which may interfere with the stemness of CSCs and play a therapeutic role.
PDT mediates the activation of lysosomal-related PS, which can significantly induce the production of autophagy and mediate the release of cathepsin and lysosomalmitochondrial crosstalk.The proliferation of colonic CSC spheres depends on the key autophagy related mTORC kinase, which is activated by the ROS produced by the NADPH oxidase (NOX)[167].NOX1 is colocalized with mTORC1 in vacuolar assembly protein (VPS)41-/VPS39- lysosomes, where mTORC1 binds S100A9 (a member of the S100 calcium-binding protein) in an ROS-dependent manner, and S100A9 can thus be oxidized by ROS.This finding indicates that ROS in VPS41-/VPS39- lysosomes mediate S100A9 oxidation and mTORC1 activation, which are essential processes for colonic CSC proliferation and colon cancer progression[168].Moreover, NOX1/2 and ROS-containing endosomal compartment co-localize with RAS and associated protein (RAB) 5/7[169,170], which is involved in the lysosomal-mitochondrial crosstalk pathway and plays a key role in maintaining CSC survival[171].The investigators determined that RAB5/7 overexpression can effectively inhibit CSCs.Further, they found that mefloquine hydrochloride (an autophagy inhibitor) is involved in the endolysosomal pathway by targeting RAB5/7, and this effect is dependent on the mitochondrial autophagy key protein PTEN induced putative kinase 1/parkinson disease protein 2.Although there is no direct evidence, it can be speculated that ROS produced by NOX may mediate lysosomal-mitochondrial crosstalk.
Fe2+plays an important role in lysosomal-mitochondrial crosstalk.The substance to be degraded in the cytoplasm is entrapped in autophagic vesicles, and then, the contents of autophagic vesicles and lysosomes are degraded under the action of lysosomal enzymes.Autophagy leads to a large amount of redox-active iron (Fe2+) accumulating in the lysosome cavity.These high concentrations of active iron can cause membrane instability when the lysosome is undergoing slight oxidative stress.PDT that targets lysosomes can directly result in the release of a large number of proteases upon photooxidative stress and thus promote activation of endogenous apoptosis-related protein[172,173].In addition, in the study of the antitumor effect of bafilomycin (an autophagy inhibitor) combined with phthalocyanine 4 (Pc4, photosensitizer), the lysosome was found to be alkalized by bafilomycin, causing the collapse of the pH gradient and the release of Fe2+.Subsequently, mitochondria accumulate a large amount of active iron (Fe2+) through the one-way Ca2+transport channel, thus generating OH through the Fenton reaction with H2O2.Thus, the apoptosis pathway is initiated.This study shows that autophagy inhibitors combined with Fe2+can further increase the effect of PDT on CSCs.In fact, the Fenton reaction is very commonly used in the design and synthesis of nano-photosensitizers.Currently, some studies have used the Fenton reaction to increase the concentration of O2[174]or hydroxyl radicals[175]in the tumor microenvironment, and others used it to directly kill CSCs.PDT combined with the Fenton reaction[176]is a feasible treatment method for CSCs showing strong drug resistance.In addition, a new type of programmed cell death, ferroptosis, is also closely related to Fe2+and the Fenton reaction[177].Some researchers have found that ferricin chelates lysosomal iron[178].These compounds induce iron depletion by preventing iron transport, leading to the dissolution of ferritin.Enzymatic degradation, followed by ROS formation and iron release, mediates the lysosomal ferroptosis pathway.In the development of PS, some scientists have induced ferroptosis by artificially reducing Fe2+or releasing Fe2+in the lysosome, creating a potential therapeutic mechanism for killing apoptosis-resistant CSCs (Figure 2).
ROS and cell membranes in CSCs
Excessive lipophilic dyes, anionic dyes, and photosensitizers (especially localized on biological membranes) can affect different unsaturated phospholipids and membrane cholesterol, leading to excessive lipid peroxidation[179], which directly leads to a wide range of membrane changes, loss of membrane integrity and fluidity, and inactivation of the membrane protein system, causing accidental cell necrosis.This effect can be induced with all types of PDT under sufficient ROS.The exogenous pathway of apoptosis is mediated by death receptors on the cell membrane.Tumor necrosis factor type-I receptor (TNFR1), Fas/CD95, DR3, and TNF-related apoptosis-inducing ligands R1 and R2 (TRAIL-R1 and TRAIL-R2) were found to be differentially expressed upon PDT, and their death domains are necessary for exogenous apoptosis.Exogenous apoptosis induced by PDT is usually activated by cytokines released by photooxidative stress or dead cells[180,181].A very low dose of sulfathiophene (2.0 μg/mL) combined with radachlorin-PDT (0.5 μg/mL) showed a synergistic effect of exogenous and endogenous apoptosis[182].The CSCs in the CD44 (+)/CD24 (-/low) subgroup were more sensitive to Fas- and TRAIL-mediated exogenous apoptotic pathways; therefore, the PDT-induced exogenous receptor apoptosis pathway may be very sensitive in this subgroup[183].Through methotrexate-mediated epigenetic enhancement of ALA-PDT, Salva and colleagues restored sensitivity to death receptorrelated pathways and increased the effect in subgroups of cells with low expression of Fas[184].Exogenous and endogenous apoptosis induced by PDT generally does not happen separately.In many studies, PDT mediated by phenalenone[181], coralyne[185], ALA[186], hypericin[187], dipyrromethene boron difluoride(BODIPY)[188], and zinc phthalocyanine[189]can cause both endogenous and exogenous apoptosis.This dual apoptosis phenomenon is generated primarily by signaling molecules, including p38 MAPK, Janus kinase (JAK)-2, and signal transducer and activator of transcription(STAT)-1.
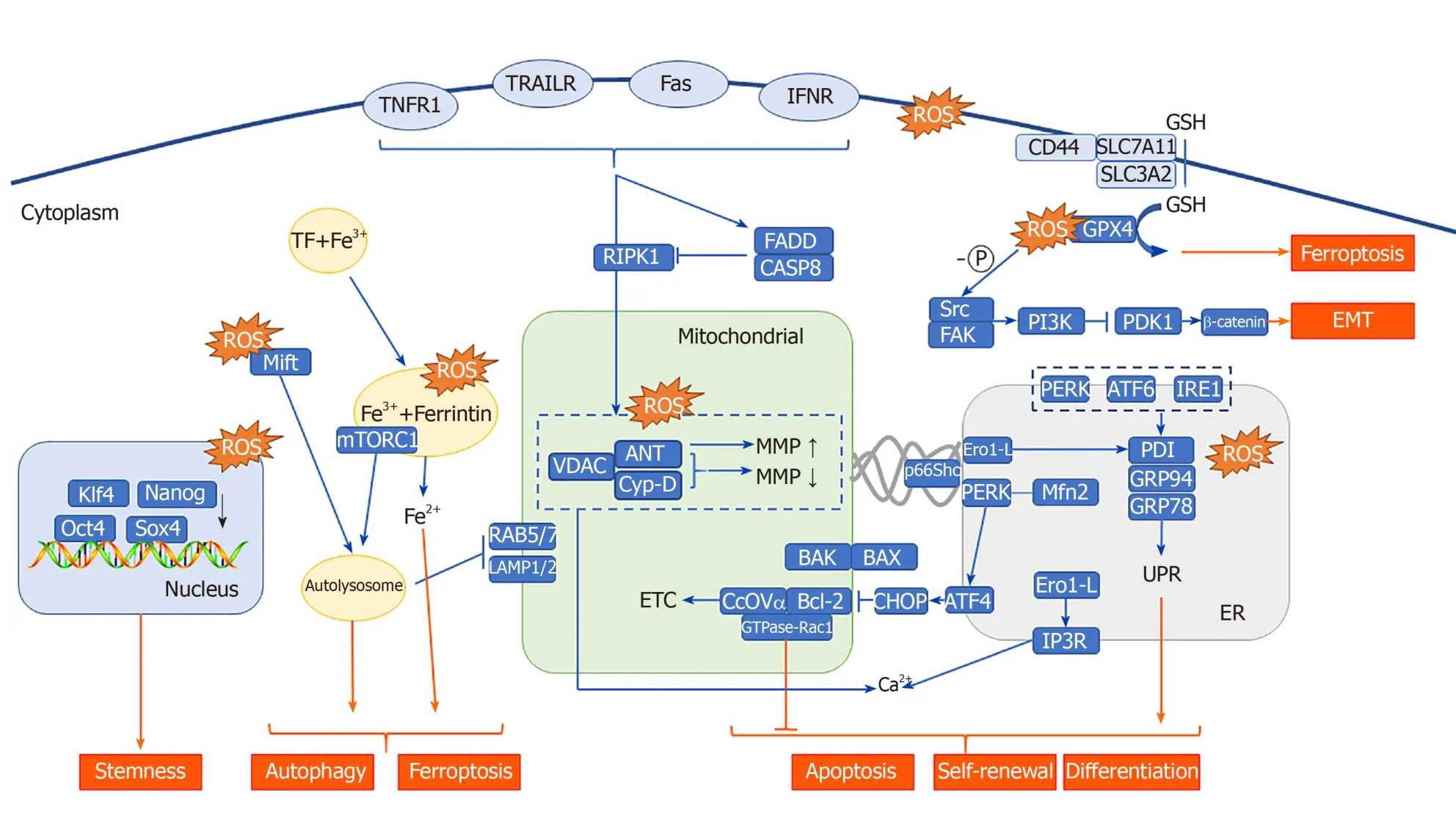
Figure 2 Reactive oxygen species located in different subcellular structures affect the stemness, self-renewal, differentiation, apoptosis, autophagy, ferroptosis, and epithelial-mesenchymal transition of cancer stem cells through a variety of molecules.
ROS-induced immunogenicity and CSCs
PDT is a treatment method that mainly induces apoptosis, and most forms of apoptosis are based on immune silencing or immune tolerance, that is, tolerogenic apoptosis[190].This immune tolerance to apoptosis is an important host protection mechanism.After photooxidative stress, most apoptotic cells express chemotaxis signals, such as intercellular cell adhesion molecule, phosphatidylethanolamine, phosphatidylinositol, and low-density lipoprotein, and signals that inhibit antiphagocytosis proteins, such as CD31 and CD47, to promote the phagocytosis of tolerogenic cells by macrophages, prevent immune responses, and exhibit tolerant immunobiological characteristics[191-194].
However, in a significant portion of PDT samples, lysates of necrotic or apoptotic cells enhance immunogenicity[173].Through this PDT-induced enhanced immunogenicity, CSCs may be cleared by immunocytes.Immunogenic apoptosis has all the biochemical markers of tolerogenic apoptosis, but tolerogenic apoptosis does not have the following two main characteristics: (1) Exposure to or secretion of important immunogenic signaling proteins or damage-associated molecular patterns (DAMPs); and (2) The ability to activate the host's immune system.DAMPs can be secreted or expressed on the cell surface after cell injury or death to promote inflammation[195,196].It has been found that photooxidative stress, especially mitochondrial photooxidative stress, can cause DAMPs to be expressed on the cell surface (ecto-) or released outside the cell (exo-), such as calreticulin (CRT) and GRP78[197,198].IFN-1 has recently been recognized as a new type of DAMP that links innate and adaptive immunity, and it has been hypothesized to be the basic requirement for inducing immunogenic cell death, especially through the activation of dendritic cells.Me-ALA-induced PpIX (endogenous PS) was found to upregulate IFN-1 expression in B16-OVA melanoma cells[199].This upregulation of α/β transcripts coincided with the interferon regulatory factor-3 phosphorylation that activated STAT1 and increased the expression of ligand receptors and interferon- stimulated genes (ISGs, like chemokine (C-X-C motif) ligand 10, interferon-induced GTP-binding protein Mx1, and ubiquitin-like protein ISG15).In this sense, PDT-treated melanoma cells induce IFN-1-dependent phenotype maturation of dendritic cells by enhancing costimulatory signals (CD80 and MHC-II molecules) and tumor-directed chemotaxis.Based on the discovery of enhanced immunogenicity, there have been studies combining PDT and immunotherapy, loading si-PD-L1[200], docetaxel (DTX)[201], PD-L1 monoclonal antibody[202], and anti-PD-L1 peptide[203], among others, to enhance the antitumor effect.
Compared with those of specific drug-induced immunogenicity, PDT-mediated immunogenic activation also has certain targeting advantages.Although mitoxantrone, mitomycin C, 5-fluorouracil, camptothecin, cisplatin, oxaliplatin, ultraviolet radiation, and γ-radiation can induce ROS-mediated ERS[204-207], in most cases, ERS induction is nontargeted and not completely efficient.In the pre-apoptotic stage, ERS-mediated immunogenic apoptosis is accompanied by ecto-CRT and exo-ATP production[197,201].Another study found that Photofrin-PDT mainly produces mitochondrial photooxidative stress and a small amount of ER photooxidative stress.ER photooxidative stress mediated by hypericin-PDT can induce immunogenic apoptosis through targeted oxidative stress[208].The expression of pre-apoptotic ecto-CRT in the mitochondria of Hyp-PDT-treated cells is not as high as it is under ERS targeting[197].This finding suggests that photosensitizers targeting the ER may have a better effect in inducing immunogenicity.
ROS-mediated EMT in CSCs
The activation of cancer cell invasion and metastasis is one of the characteristics that changes the normal function of cells to acquire enhanced malignant growth, and it is also the main obstacle for humans to overcome cancer[209].The EMT refers to the biological process in which epithelial cells are transformed into cells with interstitial phenotypes under special physiological or pathological conditions; that is, the epithelial cells lose their original phenotype connected with the basement membrane and acquire resistance to the phenotype of interstitial cells, such as the ability to undergo apoptosis or degrade the extracellular matrix, and higher migration and invasion[210].The weakening of tumor cell adhesion and the enhancement of tumor cell movement are the basis of invasion and metastasis.The EMT provides the conditions for invasion and metastasis of tumor cells of epithelial origin.Therefore, the EMT is closely related to tumor invasion and metastasis.The relevant molecular mechanism of the EMT in tumor cells is the loss of cell epithelial morphology and related markers (including E-cadherin, desmosome smoothelin, Muc-1, cytokeratin 18, occludins, claudins, and ZO-1) and the acquisition of mesenchymal markers (including Ncadherin, vimentin, fibronectin, vitronectin, alpha smooth muscle actin [α-SMA], and FSP1)[27].This process is affected by the regulatory effects of Snail, Slug, ZEB1, and Twist1 in the tumor microenvironment.Among all the factors that promote tumor cell migration, ROS play key roles by activating signals that cause cell migration, such as activating the proto-oncogene tyrosine protein kinase (Src) and focal adhesion kinase (FAK)[211].The EMT is the initial step of ROS-activated tumor cell migration.During the ROS generation process, the EMT is also affected by the regulation of Src and FAK, resulting in tumor cell migration.Studies have found that para-cresyl sulfate can promote tumor cell migration by activating ROS/Src/FAK signaling in bladder cancer tumor cells[212].Gouletet al[213,214]found that IL6 can regulate cancer-associated fibroblast-induced EMT through the STAT3/AKT signaling pathway in bladder cancer, induce myeloma cell proliferation through Src, and regulate the Src/STAT3 signaling pathway in lymphatic endothelial cells.Therefore, IL6 may be a regulator of ROS-activated Src/FAK signaling.
The EMT plays an important role in the process of acquiring stemness by tumor stem cells.Transcription factors that regulate EMT, such as Snail, ZEB1, and Twist1, are involved in the process of conferring stemness to CSCs.For example, Snail can induce a CSC phenotype in colorectal cancer cells, in which it enhances stemness properties and resistance to radiotherapy[215].ZEB1 inhibits the expression of miRNAs, including miR-183, miR-200c, and miR-203, and thus upregulates the stem cell-related factors Sox2 and Klf4.Knocking out ZEB1 prevents not only the EMT and cell invasion and metastasis but also the emergence of stem cell phenotypes[216].Twist1 can induce the EMT and stem cell properties by increasing Bmi-1 expression and acting synergistically with it.EMT induced by TGF-β1 plays a key role in the generation of CSCs and is involved in maintaining the characteristics of CSCs, such as self-renewal and differentiation[217].Most cholangiocarcinoma CSCs have epithelial and mesenchymal characteristics and express EMT markers.IL6, which may be involved in ROS-activated Src/FAK signaling, can regulate stem cell self-renewal.In breast cancer, head and neck squamous cell carcinoma, gastric cancer, and glioma, IL-6 promotes stem cell self-renewal through the classic IL-6R/gp130/STAT3 signaling pathway, while IL-6 also increases N-cadherin, E-cadherin, Twist, Snail, and Vimentin expression to accelerate the tumor cell EMT, which leads to cancer metastasis.Studies have shown that ROS may activate the NF-κB pathway and cause gastric cancer cells and cancer-associated fibroblast cells to release IL-6, thereby mediating tumor metastasis and CSC self-renewal and maintenance[218,219].It can be seen from the above literature that PDT mainly inhibits CSCs through IL-6/SRC/FAK mediated EMT mechanism.
CONCLUSION
Although PDT is promising in the treatment of CSCs, several pitfalls will have to be overcome in order to take a step forward in clinical application.First, despite the obvious tumor targeting of PS, the heterogeneity of CSCs determines that it is difficult for a single targeted PS to eliminate all CSCs.The single-cell sequencing will be very useful for exploring molecular subtypes common to different CSCs.Second, CSCs account for a small proportion of tumors and are widely distributed.In order to enhance the killing effect against deep CSCs by PDT, it is necessary to design the PS that can absorb longer wavelengths.The dosimetry of PDT is also one of the existing challenges.The ROS yield of PS determines whether the CSCs will metastasize and recur.Developing different PS-PDT treatment criteria from different subcellular localizations can help reduce adverse outcomes.Finally, in view of the immune activating effect of PDT, PDT combined with immunotherapy has shown good experimental results.However, how to safely and effectively use PDT to activate immunity is still a difficult problem in clinical treatment design.Based on these considerations, although PDT exhibits anti-tumor (including CSCs) effects, further research is needed to expand clinical application.
ACKNOWLEDGEMENTS
We thank Dr.Xiao-Xue Li for her comments and suggestions.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases
- Application and prospect of adipose stem cell transplantation in treating lymphedema
- Role of stem cell therapies in treating chronic wounds: A systematic review
- Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
