Application and prospect of adipose stem cell transplantation in treating lymphedema
Zhu-Jun Li, Elan Yang, Yun-Zhu Li, Zheng-Yun Liang, Jiu-Zuo Huang, Nan-Ze Yu, Xiao Long
Zhu-Jun Li, Elan Yang, Yun-Zhu Li, Zheng-Yun Liang, Jiu-Zuo Huang, Nan-Ze Yu, Xiao Long, Department of Plastic and Reconstructive Surgery, Peking Union Medical College Hospital of Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China
Abstract
Key words: Lymphedema; Adipose-derived stem cells; Animal model; Clinical trial; Vascular endothelial growth factor-C; Lymphangiogenesis
INTRODUCTION
As the second circulatory system of the body, the lymphatic system functions to transport tissue fluid in the interstitial space back to the venous circulation system and maintain fluid homeostasis.Hypoplasia or dysfunction of lymphatic vasculature may result in “l(fā)ymphedema”[1], which is characterized by retention of lymphatic fluid in the interstitial space, leading to a series of pathological changes, including tissue swelling, chronic inflammation, lipid deposition, and tissue fibrosis[2].
There are two types of lymphedema according to etiology[3].Primary lymphedema comes from developmental or congenital abnormalities of the lymphatic system resulting in dysfunctional lymphatics, which could be symptomatic at birth or more commonly in adolescence.Secondary lymphedema is more common and results from trauma, obstruction, surgery, or infection involving the lymphatic system.Up to 250 million people in developing countries suffer from lymphedema, with the parasitic disease filariasis as the most prevalent cause[4]; lymphadenectomy and radiation therapy for cancer treatment are usually the major causes of lymphedema in developed countries.
Chronic lymphedema affects 0.13%-2% of the global population[5].It is estimated that one out of six patients undergoing treatment for a solid tumor will eventually develop lymphedema[6].For patients with breast cancer, 24%-49% of those receiving mastectomy will develop upper extremity lymphedema[7].The persistence of the disease, burden of treatment, and likelihood of progression press heavy medical and socioeconomic burdens onto patients.Therefore, development of effective therapies for lymphedema is of vital importance.
Lymphedema can be treated conservatively, surgically, or by a combination of them[2].Conservative therapies consist of complex decongestive therapy, manual lymphatic drainage, and exercise[8].Surgical therapies include liposuction, wedge resection, Charles procedure (radical excision for limb lymphedema), and lymphatic reconstruction or bypass techniques[9].However, there is a lack of effective and feasible therapies which could radically cure lymphedema[10].Therefore, elucidating the underlying pathophysiological mechanisms of lymphedema holds promise for the treatment of lymphedema.
Progenitor or stem cell-based therapies, which treat diseases through regeneration, have represented an alternative treatment method not only for lymphedema but for a wide spectrum of other diseases as well.Due to their abundant resources, easy access, pluripotent capacity, and harboring of few ethical and immunological issues, the adipose-derived stem cells (ADSCs) are considered as one of the most promising seed cell types for regenerative medicine[11-15].In addition, ADSCs exhibit paracrine immunomodulatory and trophic effects in their local microenvironment.Emergingin vitrostudies have investigated the possible mechanisms and benefit of ADSCs in the treatment of lymphedema, such as their capacity for differentiation into lymphatic endothelial cells (LECs)[16,17]and paracrine secretion of cytokines[18], chemokines[19]and exosomes[20], thereby promoting angiogenesis and modulating the immune response.Takedaet al[21]reported that, by secreting lymphangiogenic factors, ADSCs promote proliferation, migration, and tube formation of LECsin vitro.Denget al[22]demonstrated that overexpression of Prox1 in human ADSCs (vialentiviral vectors) induces the differentiation of human ADSCs into stable lymphatic endothelial-like cellsin vitroand that the differentiated cells form tube-like structures (as shown in tube formation assay).Yenet al[23]showed that ADSCs promote lymphangiogenesis under stimulation of vascular endothelial growth factor-C (VEGF-C), a key lymphangiogenic factor, or in response to inhibition of TGF-β1; moreover, stimulation of ADSCs with VEGF-C was found to markedly increase cellular proliferation and cellular survival afterin vivoimplantation and to induce the expression of podoplanin, a lymphangiogenic cell marker.Sunet al[19]reported that interleukin-7 enhanced the differentiation of ADSCs into LECsviaAKT signaling pathways.Most recently, Saijoet al[24]revealed that paracrine effects of ADSCs promoted lymphangiogenesis in irradiated LECs.They reported that coculture with ADSCs and the use of ADSCconditioned medium improved proliferation, migration, and tube formation of nonirradiated LECs.Furthermore, they demonstrated that irradiated ADSCs can exert similar alleviative effects to irradiated human dermal LECs[24].
Collectively, these research findings have suggested that ADSCs might serve as suitable seed cells for lymphatic tissue engineering and secondary lymphedema therapy.The aim of this review is to systematically summarize the application of ADSCs for lymphedema treatment in animal studies and in clinical trials.In addition, the future perspectives of ADSCs in lymphedema therapy are discussed.
MATERIALS AND METHODS
A systematic search was performed on four databases (PubMed, Clinicaltrials.gov, the evidence-based Cochrane library, and OVID) using the following search string: (“l(fā)ymphedema” or “l(fā)ymphoedema” or “l(fā)ymphangiogenesis”) and (“adipose-derived stem cells” or “adipose-derived stromal cells” or “adipose-derived regenerative cells”).After duplicate removal, all studies were screened based on title and abstract.Furthermore, full-text versions of included studies were read for further evaluation.A manual search was also performed by skimming the references of included studies.The search process is presented in Figure 1.
The inclusion criteria were animal studies and clinical trials using adipose-derived cells for treatment of any kind of lymphedema, which had been published no later than November 2019.The exclusion criteria were non-English language, reviews, orin vitrostudies.For animal studies, data retrieved were year of publication, first author, type of animal models, cell type used (freshly isolated or culture-expanded as well as autologous or allogeneic), cell dosage, cell characterization (cell count/viability, surface marker analysis,etc.), means and routes of transplantation (alone or in combination with growth factors, scaffolds,etc.), assessment types, and results.For clinical trials, data retrieved were year of publication, country of origin, disease treated, study design (randomized controlled trial, nonrandomized study, or case series/pilot study), number of participants, cell type used (freshly isolated or cultureexpanded as well as autologous or allogeneic), cell dosage, cell characterization (cell count/viability, surface marker analysis,etc.), means and routes of transplantation (alone or in combination with growth factors, scaffolds,etc.), assessment types, and outcomes.
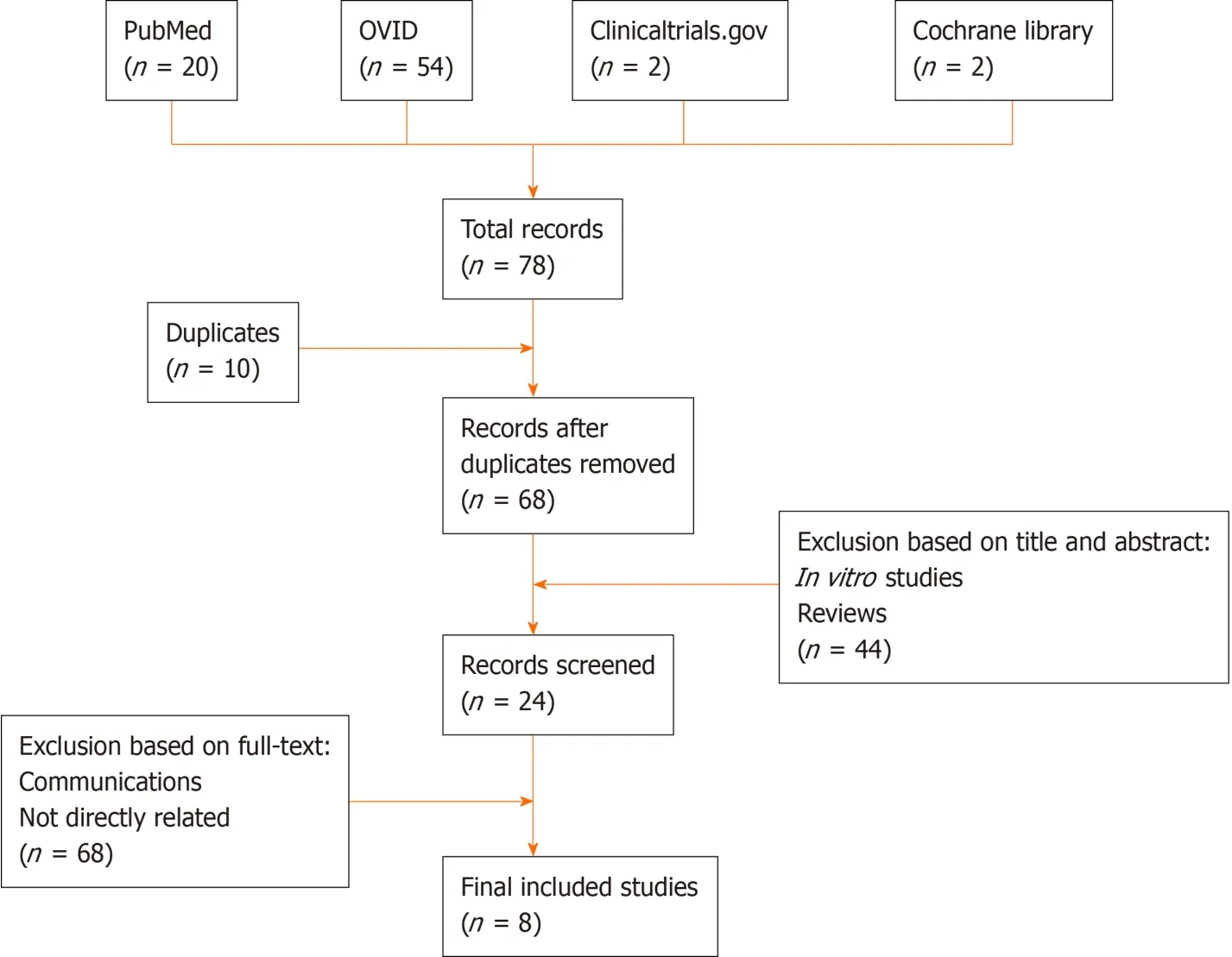
Figure 1 Search process of articles regarding adipose-derived stem cell-based treatment of lymphedema studied in animal models and clinical trials.
RESULTS
A total of eight research articles published before November 2019 met the inclusion criteria for this analysis.These included five articles focused on animal studies (Table 1) and three focused on clinical trials (Table 2).
Animal studies
A total of five animal studies were included in the analysis.Commonly-used animal models were mouse hindlimb or tail model of lymphedema.The surgical procedures were circumferential incision with or without radiation.The number of cells used for injection varied from 104to 1010each time.Various treatments were used in combination with cell injection, including controlled-release VEGF-C, platelet-rich plasma (PRP), or vascularized lymph node transfer.Treatment outcomes were evaluated by circumference, dermal edema depth, imaging (lymphangiography and photodynamic dye), and histochemical and immunohistochemical staining (for CD31, LYVE1, and VEGF receptor).
Hwanget al[25]established a mouse hindlimb model of lymphedema by electrocauterizing the lymph vessels in the thigh following a circumferential incision.In vivostudy demonstrated that combination of human (h) ADSCs and VEGF-C hydrogel markedly alleviated dermal edema and increased lymphatic vessel density when compared with results achieved with hADSCs or VEGF-C hydrogel alone at various post-treatment time points (from 3-4 d to 4 wk post-treatment).In addition, the authors demonstrated the existence of hADSCs in all of the implantation sites in the hADSC/VEGF-C group with LECs phenotype.Their results also suggested that, in conjunction with hADSCs, VEGF-C-containing hydrogels could serve as suitable delivery vectors to improve lymphangiogenesis.
Shimizuet al[26]reported on the establishment of a mouse tail model of lymphedema.They made a 2 mm-wide circumferential excision on the skin 10 mm distal to the tail base and excluded a 4 mm2dermal flap at the ventral side.They indicated that local injection of 2 × 106freshly isolated autologous ADSCs could reduce lymphedema and accelerate lymphangiogenesis at the congestive lymphedema region.The authors also revealed that ADSCs could release VEGF-C to stimulate lymphangiogenesis and recruit bone marrow-derived M2 macrophages to serve as lymphatic endothelial progenitor cells.
Ackermannet al[27]assessed the effects of PRP and adipose stem cells on angiogenesis, microcirculation, lymphangiogenesis, microvascular architecture, and wound healing in a mouse tail lymphedema model.They found that treatment with PRP and adipose stem cells could accelerate wound healing and increase epithelialization.PRP application induced a remarkably improved lymphangiogenesis.Ultimately, the authors drew the conclusion that PRP and adipose stem cells can affect lymphangiogenesis and lymphedema development.
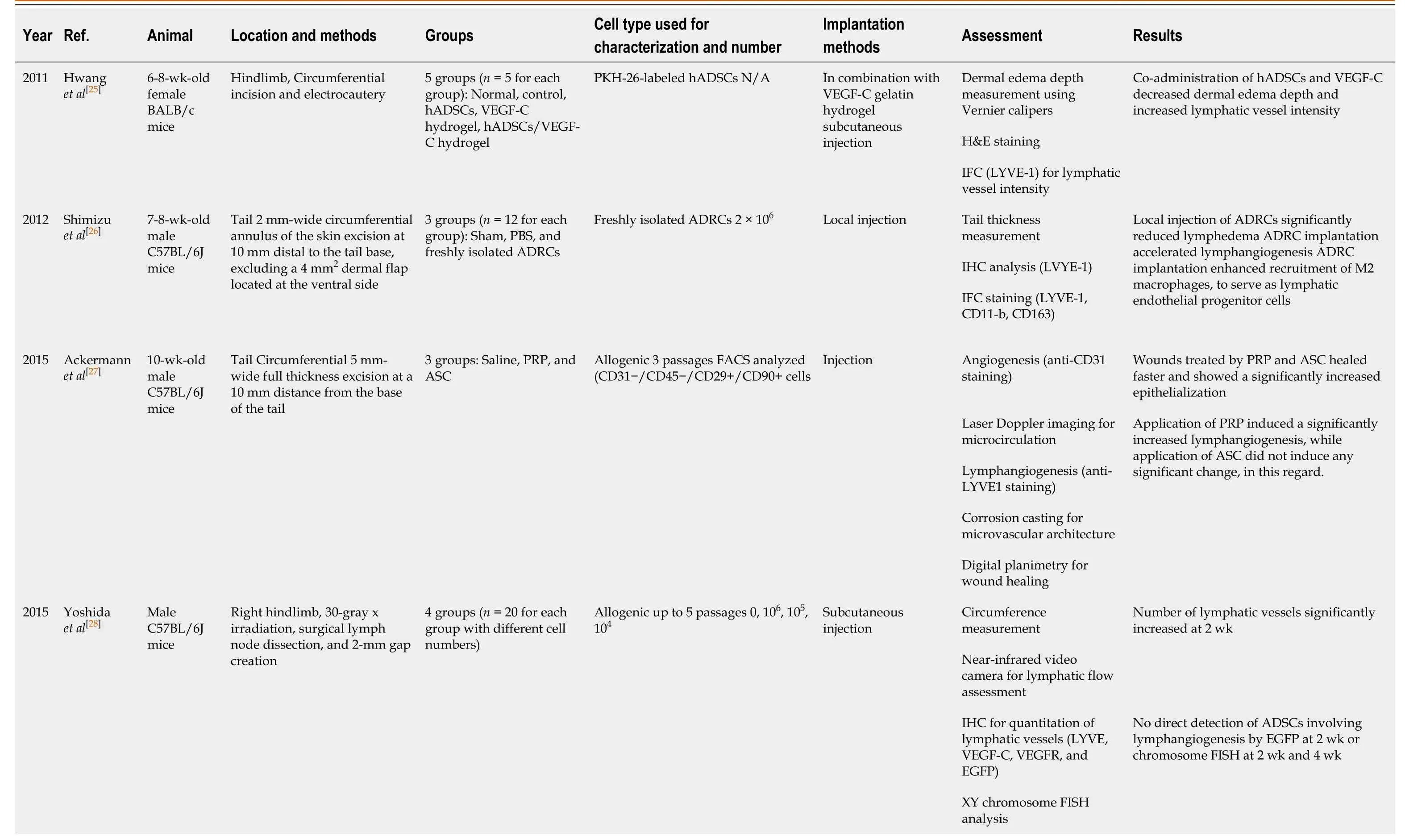
Table 1 Adipose-derived stem cell-based treatment of lymphedema in animal studies

ADRCs: Adipose-derived regenerative cells; ADSCs: Adipose-derived stem cells; ASC: Adipose stem cells; EGFP: Enhanced green fluorescent protein; FISH: Fluorescent in situ hybridization; H&E: Hematoxylin and eosin; hADSCs: Human adipose-derived stem cells; IFC: Immunofluorescence; IHC: Immunohistochemistry; N/A: Not applicable; PBS: Phosphate-buffered saline; VEGF-C: Vascular endothelial growth factor-C; VEGF-R3: Vascular endothelial growth factorreceptor 3; VLNT: Vascularized lymph node transfer.
Yoshida et al[28]reported on the establishment of a mouse right hindlimb secondary lymphedema model, using 30 Gy X-ray irradiation 7 d before surgery to create a circumferential incision and a gap of approximately 2 mm, left open.Circumferential measurement, lymphatic flow assessment, and quantification of lymphatic vessels demonstrated that at 14 d post-treatment of local injection with 106ADSCs, 105ADSCs, or 104ADSCs, the numbers of lymphatic vessels were significantly increased in the transplanted groups; the authors concluded that in secondary lymphedema, ADSCs could increase collecting vessels and reconstruct the lymphatic vascular network.A more recent study from the same research group[29]demonstrated that local implantation of 104ADSCs in combination with vascularized lymph node transfer decreased tissue volume, increased lymphatic vessels density, and improved the lymphatic function in a slightly different mouse hindlimb model (left vs right hindlimb, 5 mm- vs 2 mm-wide gap, left open).
Clinical trials
A total of three clinical trials were included in the analysis.All these studies were conducted by the same research group in Denmark[25-27], focusing on upper limb and breast cancer-related lymphedema (BCRL), using freshly isolated autologous ADSCs with fat grafting.The follow-up period ranged from 4 mo to 1 year.
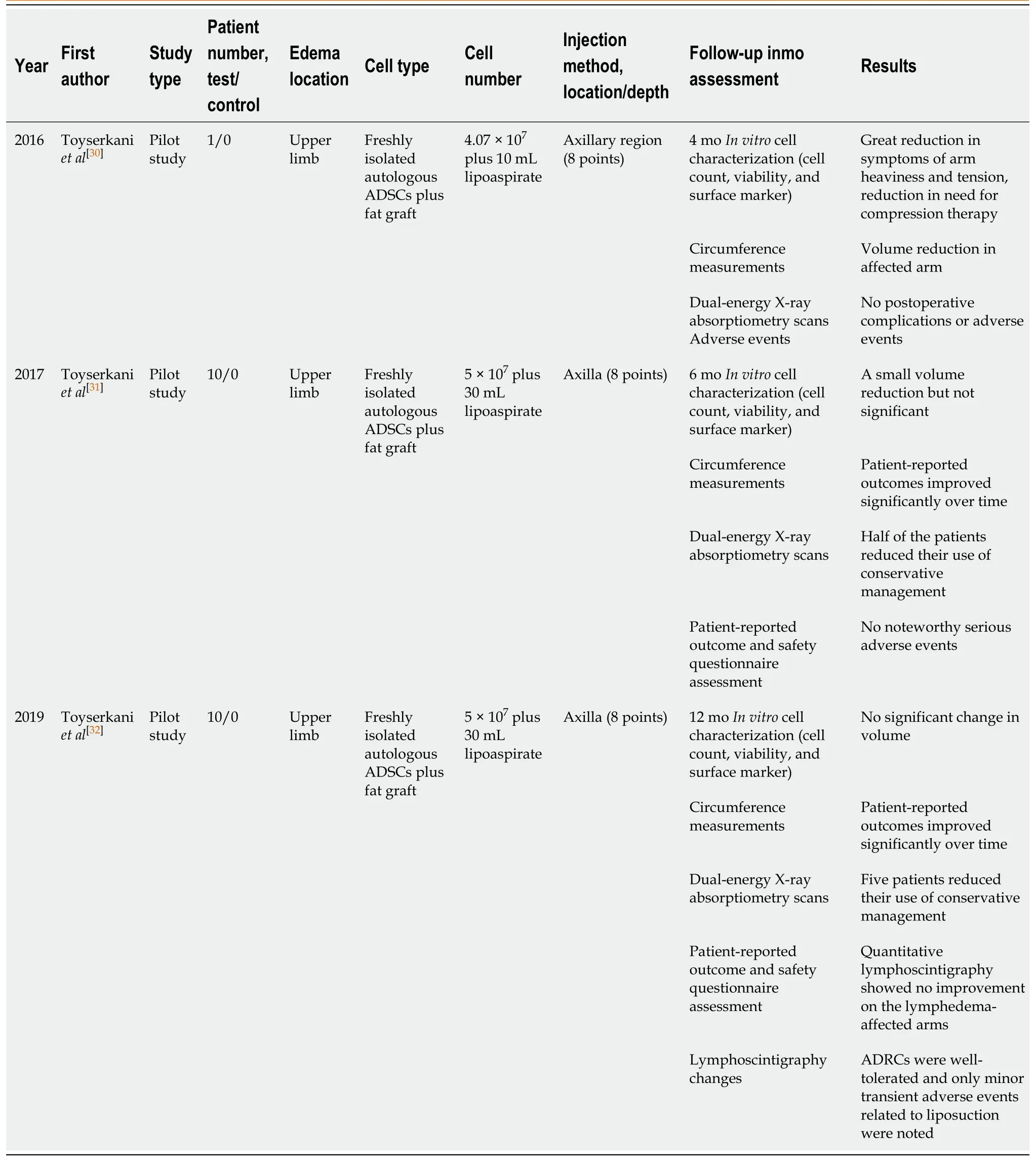
Table 2 Adipose-derived stem cell treatment of lymphedema in clinical trials
In 2016, Toyserkaniet al[30]reported a pilot study using ADSC-assisted lipotransfer to treat lymphedema.A 48-year-old female patient developed BCRL after lymphadenectomy and radiation therapy.A total of 4.07 × 107freshly isolated autologous ADSCs were administered, along with 10 mL of lipoaspirate (for fatgrafting), to the axillary region.At 4 mo post-treatment, the authors noted that the daily symptoms of arm heaviness and tension were greatly relieved, the needs for compression therapy were reduced, and the volume of the affected arm was decreased without postoperative complications or adverse events.
Furthermore, the authors had also performed a larger study to validate the feasibility and safety of this procedure in 2007[31], which was registered at Clinicaltrials.gov under the identifier NCT02592213 at phase 2 stage.In this pilot study, ten BCRL patients were included.Combined with a scar-releasing fat graft (about 30 mL) procedure, approximately 5 × 107freshly isolated adipose-derived regenerative cells (ADRCs) were injected into the axillary region.During a 6-mo follow-up period, there was a small but not significant volume reduction.Five of the patients showed a reduced need for conservative treatment, and patient-reported follows-up improved significantly over time.Slight, temporary adverse events were observed, but were more likely caused by liposuction procedures rather than ADRC injection.Ultimately, the ADRCs were deemed as well-tolerated.
In 2019, the results of lymphoscintigraphic evaluation with 1-year follow-up were reported[32].Consistent with the results of the 6-mo follow-up, ADRC injection improved lymphedema, as revealed by patient-reported outcomes without serious adverse events.However, there was no improvement in lymphoscintigraphic evaluation and no change in arm volume after the ADRC treatment.Now, this research group is recruiting patients for a randomized phase 3 trial, which is registered at Clinicaltrials.gov under the identifier NCT03776721.This study started December 17, 2018 and the estimated study completion date is September 1, 2021, designed to recruit 80 participants with a parallel assignment to evaluate the efficacy and safety of implantation of freshly isolated adipose-derived stromal cells in combination with fat grafting at the affected axillary region.
DISCUSSION
Lymphedema, characterized by tissue swelling, lipid deposition, and fibrosis due to excess accumulation of interstitial fluid and inadequate lymphatic drainage, affects 0.13%-2% of the global population[5]and remains a chronic, debilitating and incurable disease.Stem cell-based regenerative medicine has shown great promise for refractory diseases, such as inflammatory bowel diseases[33], heart failure[34], osteoarthritis[35], rheumatoid arthritis[36], and graft-versus-host disease[37].With the properties of selfrenewal, multipotential differentiation, paracrine, immunomodulatory, and trophic effects, and low immunogenicity alongside their practical advantages, ADSCs have become one of the most promising candidates for regenerative medicine.
ADSCs are isolated from the aqueous fraction – known as the stromal vascular fraction (SVF) – by means of enzymatic digestion of lipoaspirate (liposuction product)[38,39].As the major source of ADSCs, SVF is a heterogeneous cell group composed of ADSCs, endothelial precursor cells, endothelial cells, macrophages, smooth muscle cells, lymphocytes, pericytes, and pre-adipocytes derived from fat tissue.Recent advances have shown the role and efficacy of SVF and ADSCs in improvement of tissue regeneration, especially in plastic reconstruction[40], such as breast reconstruction[41,42], wound healing[43,44], scars[45,46], and soft tissue defects[47].The SVF is more easily obtained, regardless of cell separation and culture conditions.Therefore, therapeutic cellular products are obtained immediately with minimal contact with reagents, making it not only technically easier but also relatively safer.At the same time, the unique heterogeneous cell components of SVF may achieve better treatment results in comparative animal studies.It is worth noting that SVF might be suitable for autologous therapy only, due to the existence of various cell types that might cause immunological rejection[48], whereas ADSCs are useful in both allogenic therapy and autologous therapy.
As mentioned in the first part of this review, emergingin vitrostudies have already definitively demonstrated the advantage of ADSCs in lymphangiogenesis and treatment of lymphedema.All five animal studies included in this systematic review were performed on mouse models and the ADSCs were injected immediately or shortly after lymphedema induction.These studies examined the effect of ADSCs on acute lymphedema and exhibited improvement at 4-6 wk.However, the anatomy, physiology, and healing capacity are quite different between mice and human beings, bringing up the need for larger animal models in order to simulate chronic lymphedema in human beings more accurately.Further preclinical studies with better animal models are needed to determine whether ADSC-based therapies could fulfil expectations and be extrapolated to clinical use in patients.As is commonly known, to observe lymphatics in mice, it is usually necessary to visualize the blue dye-stained lymphatic vessels in dissected tissues or tissue sectioning for immunohistological staining for the lymphatic markers, such as VEGFR-3, PROX1, and LYVE1, to quantitatively identify lymphatic vessel intensity and lymphangiogenesis.Advances in near-infrared fluoroscopy lymphatic imaging[49], magnetic resonance imaging agents based on nanotechnology, and gene reporter technologies have paved roads for depicting functions of lymphatic vasculature.Though not all lymphatic imaging methods are suitable for both clinical and preclinical trials, their effective combination will provide new tools for lymphedema translational medicine[50].
Therapy of lymphedema has benefitted substantially from the most recent advances in lymphatic vessel engineering and regenerative medicine, including cell-seeded scaffolds for vessel reconstruction, implantation of stem cells, prolymphangiogenic growth factors, or a combination of these technologies.As mentioned above, when implanted into the injury site in a lymphedema mouse model, the gelatin hydrogel containing controlled-release VEGF-C could encapsulate hADSCs, increase vessel density, and improve dermal edema, leading to efficient application in lymphatic vessel regeneration[25].It has been reported that SVFs and ADSCs could improve wound healing when used alone or in combination with PRP and hyaluronic acid, specifically in healing of lower-extremity soft- and hard-tissue wounds[43]and in severe hidradenitis suppurativa wounds[47].Many other studies confirmed that SVFs and ADSCs can improve wound healing when used alone or in combination with PRP and fat graft[41,45,51,52].Considering the similar biological processes and biomolecular pathways shared between wound healing and lymphedema, such as inflammation and angiogenesis, the efforts towards establishing ADSCs-based therapy for lymphedema will benefit from these studies.Certainly, the research efforts towards development of cell isolation and expansion methods and of three-dimensional scaffolds[53], nanoparticles, and targeted delivery will help to optimize and maximize the efficacy of ADSC-assisted therapy.Standardized platforms with minimized manipulations and maximized efficacy will facilitate both up-scaling and the ease of clinical translation.
Over the past few years, concerns regarding the safety of stem cell application have also been raised.Indeed, Toyserkaniet al[54]performed a systematic review, including over 1400 patients who received ADSC treatment and were followed for 4 wk to 3 years, in order to assess the safety of ADSC therapy, concentrating on the risks of thromboembolical, immunological, and oncological safety concerns.They reported that the observed adverse outcomes were relevant to liposuction, injuries from implantation, or the underlying condition, rather than the ADSC therapy itself.Although the ADSC therapy has shown a favorable safety performance, more reliable and rigorous safety assessment approaches are still encouraged for further study and clinical practice.
In conclusion, the results ofin vitrostudies, animal models, and clinical trials characterize ADSC-based treatment as a promising option and one that can be used within biologically rational and controllable environments for the treatment of lymphedema.However, further investigations in larger animal models and largerscale, multicenter randomized clinical trials with more reliable and rigorous safety assessments are needed to develop more effective and durable therapeutic strategies.
ARTICLE HIGHLIGHTS
Research background
Lymphedema is a chronic, debilitating and incurable disease that affects 0.13%-2% of the global population.Emerging evidence indicates that adipose-derived stem cells(ADSCs) might serve as suitable seed cells for lymphatic tissue engineering and lymphedema therapy.Here, we appraise the in vivo evidence for the application of ADSCs for lymphedema treatments.
Research motivation
Emerging research findings have suggested that ADSCs might serve as suitable seed cells for tissue engineering of lymphatic vessels in vitro and potentially in the treatment of secondary lymphedema in vivo.It is critical that we systematically summarize the application of ADSCs for lymphedema treatments in animal studies and in clinical trials and discuss the future perspectives.
Research objectives
The main objectives of this review are to systematically summarize the application of ADSCs for lymphedema treatments as shown in animal studies and clinical trials.In addition, the future perspectives of ADSCs in lymphedema therapy are discussed.
Research methods
A systematic search was performed on four databases – PubMed, Clinicaltrials.gov, the evidence-based Cochrane library, and OVID – using the following search string: (“l(fā)ymphedema” or “l(fā)ymphoedema” or “l(fā)ymphangiogenesis”) and (“adipose-derived stem cells” or “adipose-derived stromal cells” or “adipose-derived regenerative cells”).A manual search was performed by skimming the references of relevant studies.Animal studies and clinical trials using adipose-derived cells for the treatment of any kind of lymphedema were included.
Research results
A total of eight research articles published before November 2019 were included for this analysis.Five articles focused on animal studies and another three focused on clinical trials.ADSC transplantation therapy was demonstrated to be effective against lymphedema in all studies.The animal studies found that coadministration of ADSCs and controlled-release vascular endothelial growth factor-C or platelet-rich plasma could improve the effectiveness of ADSC therapy.Three sequential clinical trials were conducted on breast cancer-related lymphedema patients, and all showed favorable results.
Research conclusions
The results ofin vitrostudies, animal models, and clinical trials characterize ADSCbased treatment as a promising option and one that can be used within biologically rational and controllable environments for the treatment of lymphedema.
Research perspectives
Further preclinical studies in larger animal models and large-scale, multicenter randomized controlled clinical trials with more reliable and rigorous safety assessments are needed to develop more effective and durable therapeutic strategies.Advances in lymphatic imaging methods will provide opportunities for lymphedema translational medicine as well.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases
- Role of stem cell therapies in treating chronic wounds: A systematic review
- Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
