Modified Adsorption Capacity to Space Molecular Pollutant of Zeolite via Interface Engineering with Atomic Layer Deposition
Xianghua Gong, Yang Li, Zhen Zhang and Xiaohong Wu
(School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150000, China)
Abstract: When spacecraft operate in space, most organic materials will release small molecular hydrocarbons and large molecular organic gases due to gas evolution effect under high vacuum conditions. These gases can deposit on the surface of spacecraft, adversely affecting its performance. Adsorption becomes the first choice for removing such organic pollutants in the space environment. Zeolite material has a stable and dense porous structure, and has been widely used in the field of pollution adsorption. In this work, Al2O3 was deposited on the surfaces of 5A zeolite for the first time by atomic layer deposition (ALD) technology. As a result, the adsorption performance of Al2O3 coated 5A zeolite (zeolite@ Al2O3) was significantly modified. The corresponding adsorption process was clarified via adsorption kinetics study.
Keywords: zeolite; Al2O3 coating; space contamination control; ALD; kinetics
1 Introduction
Space science and technology have been developed for decades[1-3]. With the development of aerospace industry, the higher performance of spacecraft is required. However, contaminants in the space environment such as space molecular pollution can attach to the surface of spacecraft, undermining their performance. For instance, contamination on thermal control surfaces will change absorptance/emittance ratios, contamination deposited on solar arrays will decrease power output, and contamination in optical instruments will reduce signal strength. In the 1970s and 1980s, twenty American satellites were scrapped due to such pollution problems. Therefore, effective contamination control is important for the successful operation of spacecraft. Studies on the space environmental pollution are currently focused on the effective prevention and control of pollutants.
One of the main problems encountered in the prevention and control of the molecular contamination in space is the molecular contamination which is difficult to capture due to spacecraft’s high speed. In order to eliminate the space molecular pollution, surface scrubbing and high-pressure solvent spraying methods are adopted in Europe and the United States, but they have certain defects[4-7].
Adsorption method can effectively remove the gasous pollution, and thus has been used in the field of space environmental pollution prevention[8]. Because the space environment is very complex, the adsorbent materials used in space should have strong adsorption capacity, high mechanical strength, good stability under extreme temperatures, and good reusability. Zeolite adsorption materials become a potent candidate for their large specific surface area, high stability, and adaptability to the extreme space environment[9]. The United States has fabricated molecular adsorption coating (MAC) composed of the highly porous zeolite materials to capture degassed organic molecular contaminants such as hydrocarbons and silicone resins[10-12]. Wang et al.[13]developed a molecular pollution monitor for space applications based on a ground molecular pollution monitoring equipment. Li et al.[14]proposed a molecular adsorption on-orbit removal technique that uses zeolite adsorber thin film to adsorb organic molecular contaminants. The commonly used zeolites are A-type, X-type, and Y-type. The average pore size of 5A zeolite is similar to the aerodynamic diameter of organic molecules, which is suitable for adsorbing space molecular pollution. However, zeolite has certain defects, such as poor mechanical property, poor adsorption capacity for small molecules, and weak specific adsorption capacity. Therefore, surface modification of the zeolite is necessary to increase its surface active sites, enhance the adsorption capacity, and achieve selective adsorption.
Some materials have been used for modification of zeolite. Al2O3is non-toxic and biocompatible, and has good stability. In addition, metallic oxide thin films show unique surface properties such as special wettability[15-17]. Catana et al.[18]obtained Al2O3/zeolite material showing a preferential adsorption of V4+onto the Al2O3film, suggesting that this method could be useful for vanadium passivation of catalysts. Kovalevskiy et al.[19]studied the photocatalytic oxidation reactions of ethanol and diethylsulfide by using TiO2/zeolite composite photocatalyst. For the metallic oxide/zeolite composite, zeolite not only carries metallic oxide but also provides the adsorption capacity. As a result, the composite effectively removes the contaminants. The commonly used preparation methods for the metallic oxide modified zeolite composites include sol-gel method, sintering method, liquid phase deposition method, and coupling method[20]. These methods usually have complex and variable preparation process.
The advantages of coatings on the materials obtained by atomic layer deposition (ALD) technology consist of enhanced selectivity, homogeneous dispersion, greater resistance to contamination, and longer lifetime. Due to the sequential vapor phase nature of ALD, the coatings with tailored thickness (i.e. <0.1 nm) can conformally cover porous materials, which have high surface areas and high aspect ratio[21-22]. Moreover, ALD process is simple and easy, and has no destruction on the porous structures of the materials[23-24].
In this work, the Al2O3coated 5A zeolite material is prepared by using ALD, which has strong mechanical binding force and presents effective adsorption for the organic pollutants. Kinetic study was also performed to clarify the underlying adsorption process[25].
2 Materials and Methods
2.1 Materials
5A zeolites was purchased from Aladdin. Nanda 703 silicone glue as the source of molecular pollutant, was purchased from Liyang Kangda New Material Co., Ltd. Trimethylaluminum (TMA) as precursor of Al was purchased from Kemicro Jiaxing. All other chemicals were purchased from Sigma-Aldrich. All chemicals were used without further purification.
2.2 Synthesis
An ALD system (TALD-150 Kemicro Jiaxing) was employed to deposit the Al2O3coatings on the 5A zeolite. Trimethylaluminum (TMA) and distilled-water (H2O) were used as precursors of Al and O. High purity N2(99.99%) gas was used as the carrier and purging gas. Before Al2O3deposition, the 5A zeolite was vacuum annealed at 200 ℃ for 2 h in tube furnace. When the 5A zeolite was exposed to trimethylaluminum and H2O at 0.03 s and 0.02 s, respectively, at a working pressure of 0.15 torr, then it was purged with N2for 40 s. The growth of Al2O3in ALD was self-limiting because only the precursor molecules chemisorbed on the surface can participate in the growth of the membrane. During the Al2O3deposition process, the sample temperature was kept at 150 ℃ and the water was at room temperature.
2.3 Characterization
Infrared spectrum of organic pollutants was carried out by Fourier transform infrared spectrometer (FTIR) (Thermo Fisher Scientific, IS 50) with wavelength of 500- 4 000 cm-1. X-ray photoelectron spectroscopy (XPS, TSC K-Alpha, AlKa) was adopted to investigate the elemental states of Al and O. The crystalline structure of the 5A zeolite and Al2O3coated 5A were confirmed by X-ray diffraction (XRD, Rigaku Ultima IV, Cu-Kα radiation). The material morphology was observed by Transmission Electron Microscopy (TEM) test before and after 5A zeolite coating.
2.4 Adsorptive Property
Nanda 703 silicone glue was used as the source of molecular pollutant and heated in a sealed tank in the laboratory. Weighing difference of method was used to study the effect of experimental conditions on adsorption performance. A blank test was set up to eliminate the influence of air. A group of heating tanks with the same amount of zeolite@Al2O3was prepared. The heating tanks without 703 glue were set as the blank experiments. The weight of molecular pollutant adsorbed on modified Al2O3coated 5A molecular sieve with temperature and time was measured by ex-situ weighing with precision balance. (Since molecular sieves tend to adsorb air components, especially water vapor in the air, the weighing process should be operated as quickly as possible to minimize the chance of molecular sieves coming into contact with air to ensure the accuracy of the experimental data.)
2.5 Kinetic Method
Adsorption kinetic study can reveal adsorption rates and the factors affecting the rates. In addition, the adsorption kinetics can predict the adsorption rates and speculate adsorption principles, so that the adsorption scheme can be elaborately designed. In this work, the quasi-first-order kinetic model, quasi-second-order kinetic model, and Weber-Morris intragranular diffusion model were used to explore the adsorption of organic gas by zeolite@Al2O3over time.
(1) Quasi-first-order kinetic model.
The quasi-first order kinetic model is based on the theory of membrane diffusion, where the driving force of the adsorption process is the difference between the equilibrium adsorption amount and the instantaneous adsorption amount, and the adsorption rate is determined by the concentration of the adsorbate in the adsorbent.
In(qe-qt)=Inqe-k1t
whereqe(mg/g) is the theoretical adsorption capacity,qt(mg/g) is the adsorption capacity oft(min),k1(min-1) is the first-order adsorption rate constant, andt(min) is the adsorption time.
(2) Quasi-second-order kinetic model.
The quasi-second-order kinetic modal assumes the driving force of the adsorption process is active sites on the surface of the adsorbent, and the adsorption rate is determined by the concentration of the adsorbate and the number of active sites of the adsorbent. It is usually used to describe the adsorption process involving the chemical adsorption. The expression is as follows:
wherek2(g/mg·min) is the second adsorption rate constant.
(3) Weber-Morris intragranular diffusion model.
The intragranular diffusion model is
qt=kpit0.5+C
whereqtrefers to the amount of organic gas adsorbed at timet, mg/g;kpirefers to the intragranular diffusion rate constant, mg/(g·min0.5); andCis a constant.
3 Results and Discussion
The ALD method for preparing Al2O3coated 5A zeolite material includes four steps. In the first step, N2carries water vapor to form hydroxyl groups on the surface of the 5A zeolite. In the second step, excess water vapor and by-products are blown away. In the third step, N2carries trimethylaluminum into the cavity and reacts with hydroxyl groups on the surface of the zeolite to generate Al2O3and by-product methane. In the fourth step, N2blows away excess gas and by-products. The above four steps are repeated many cycles, and Al2O3film layer is deposited on the surface of the 5A zeolite.
3.1 Material Characterization
The XRD patterns of the 5A zeolite and zeolite@Al2O3samples are shown in Fig.1. It can be seen from the XRD patterns that the peak positions of the two samples did not shift, but the peak strength of the zeolite@Al2O3became weaker. This is because the Al2O3deposited by ALD are amorphous and the Al2O3was coated on the surface of the zeolite, which affected the peak strength.
The adsorption capacity of the porous material is affected by many factors such as specific surface area, pore size distribution, and surface properties. Surface characteristic is one of the key factors affecting the adsorption capacity, which is associated with the surface chemical composition.

Fig.1 XRD patterns of 5A zeolite and zeolite@Al2O3
XPS was used to determine the surface chemical composition (Fig.2). According to Fig.2(a), the full spectrum of the Al2O3shows Al, O, C elements. The C element probably originates from the contamination of some organic compounds like unreacted TMA and/or carbon-based compounds formed during ALD. From the Al 2p spectrum, the peaks at 74.2 eV are attributed to Al-O (Fig.2(b)). As for the O 1s spectrum, the peak at 530.7 eV is related to typical Al-O bond (Fig.2(c)). The above XPS analysis indicates the deposition of the Al2O3coating contains a certain amount of active surface hydroxyl groups.
The TEM observation was performed to explore the morphology of the 5A zeolite and zeolite@Al2O3(Fig.3). It can be seen that the size of the 5A zeolite particles is about 3 μm (Fig.3(a)). After Al2O3coating, a very thin layer appeared on the surfaces of the 5A zeolite (Fig.3(c)). At higher resolutions, the lattice fringes of 5A zeolite can be seen at the edges (Fig.3(b)), while the lattices fringes vanished in zeolite@Al2O3(Fig.3(d)). This is because the Al2O3deposited by ALD was amorphous and the Al2O3was coated on the surface of the zeolite, which affected the surface topography as shown in Figs.3(e)-(f).
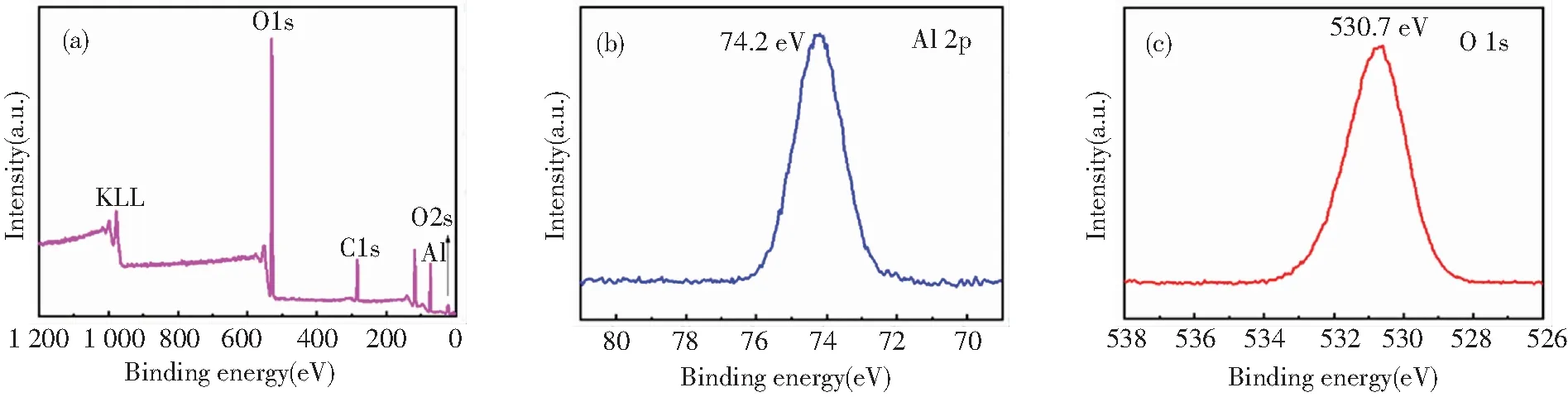
Fig.2 XPS spectra of the zeolite@Al2O3 (a) full spectrum (b) Al 2p (c) O 1s
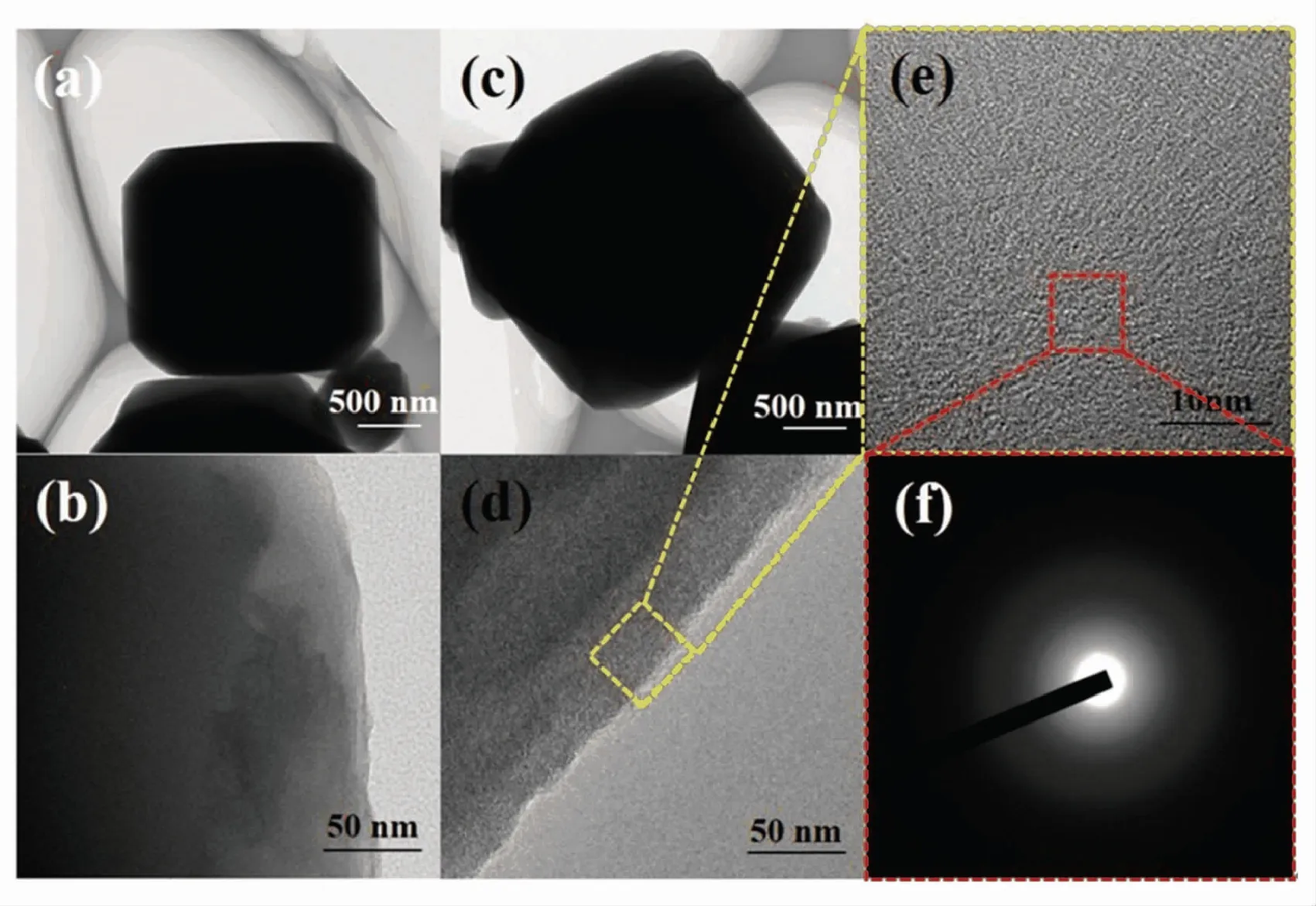
Fig.3 TEM images of (a,b) 5A zeolite and (c,d,e,f) zeolite@Al2O3
3.2 Adsorption Performance
Under the process pressure of 0.15 torr, the zeolite@Al2O3was prepared at the process temperatures of 100 ℃, 150 ℃, and 200 ℃, respectively. The adsorption capacity of the zeolite@Al2O3was evaluated as shown in Fig.4. When spacecraft is in orbit, organic nonmetallic material such as adhesive, electric wire, and insulating material will release small molecular and affect the performance of spacecraft. Nanda 703 silicone glue was widely used in spacecraft as adhesive, so it was selected as the typical target material of molecular pollutant. These zeolite@Al2O3presents different adsorption capacity, which is related to the process temperature. For example, the lowest and highest adsorption capacity (12.9 and 17.7 mg/g) correspond to the process temperatures of 200 ℃ and 150 ℃, respectively. In this work, the optimal process temperature was 150 ℃.
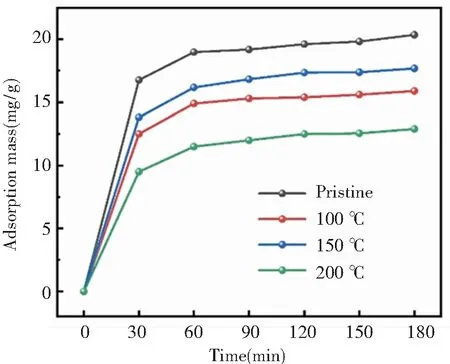
Fig.4 The adsorption of 5A zeolite and zeolite@Al2O3at different temperature
When the process temperature was at 100 ℃, the reaction of metal organic precursor was incomplete, and residual precursor reduced adsorption. When the process turns to high temperature, the porous structures of the zeolite may be destroyed, leading to decreased adsorption ability. In addition, at the high process temperature, the gases in the chamber become more active, so as to adhere to the surfaces of the zeolite, adversely affecting the subsequent formation of Al2O3coating.
Under the process pressure of 0.15 torr and at the deposition temperature of 150 ℃, zeolite@Al2O3was prepared for the cycle periods of 150 cycles and 350 cycles, respectively. The corresponding adsorption capacity was shown in Fig.5. Al2O3surface modification can improve the mechanical property of zeolite, but sacrifice certain adsorption performance at the same time.
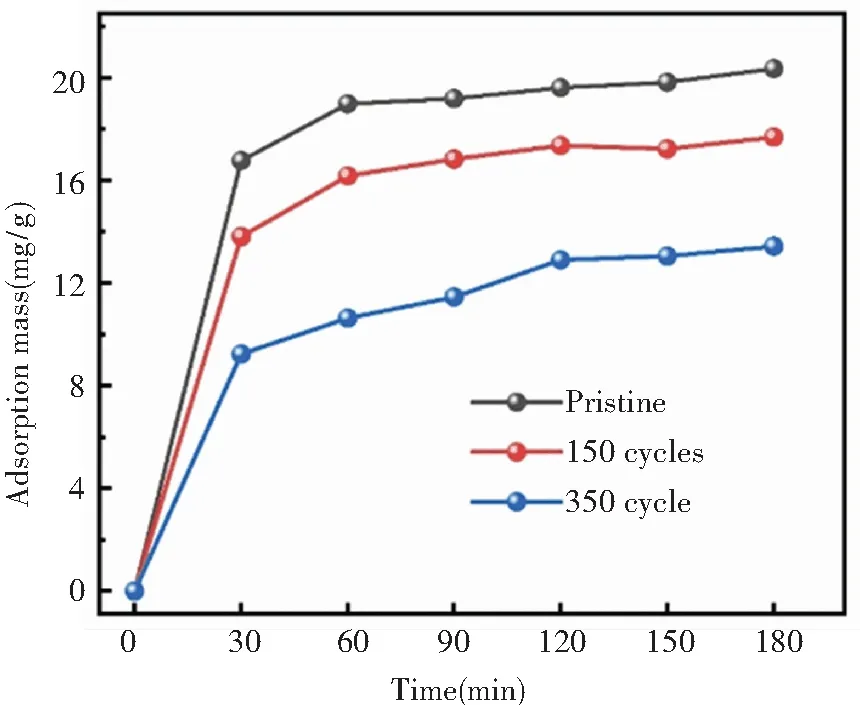
Fig.5 The adsorption of 5A zeolite and zeolite@Al2O3at different cycle
As can be seen, the adsorption capacity of the zeolite@Al2O3slightly decreases with the increasing of deposition cycle. The optimal number of cycles is 150 cycles for the balance of the mechanical property and adsorption capacity.
In order to identify the chemical composition of the heated gases released from Nanda 703 glue, and the organic gases which were adsorbed by zeolite, infrared spectrometer was used. Fig.6 shows the corresponding infrared spectrum. As can be seen, there are many same peaks between the two curves, for instance, the stretching vibration peaks of CH3and CH2group appear at 2 960 cm-1, 2 925 cm-1, and 2 852 cm-1, the stretching vibration of C=C group corresponds to the peak at 1 644 cm-1and 1 602 cm-1, the in-plane bending vibration peak of CH group locates at 1 450 cm-1, the stretching vibration peaks of ether C-O group are at 1 263 cm-1, the stretching vibration of Si-O-Si group corresponds to the peak at 1 038 cm-1, the stretching vibration peaks of ether Si(CH3)2group and SiCH2are at 801 cm-1and 702 cm-1, respectively[26]. From the infrared spectroscopic analysis, the major components of the released organic gases from Nanda 703 glue under heating include siloxane compounds.
In addition,the strong peaks at 672 cm-1possibly correspond to the characteristic chemical bonding between siloxane compounds and Al2O3.
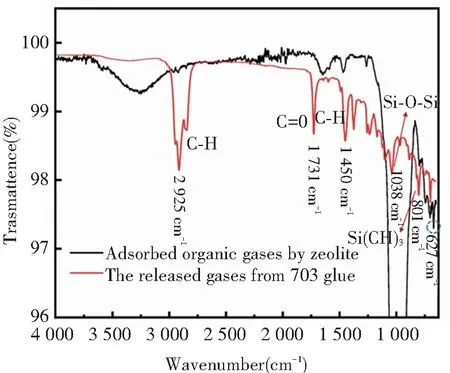
Fig.6 The infrared spectrum of adsorbed organic gas and released gas from 703 glue
For space organic gas molecular pollutant control technology, hydrophobic surface characteristic of Al2O3via ALD was beneficial to the modified adsorbent material design. Adsorption property of zeolite@Al2O3to organic gas molecular and water molecular are shown in Fig.7. After Al2O3modification, the decrement of adsorption of zeolite@Al2O3to organic gas was 14.08%, while the value to water molecular was markedly turned to 57.03%. Significantly reduced water absorption indicates zeolite@Al2O3has great application potential for space organic molecular pollutant control.
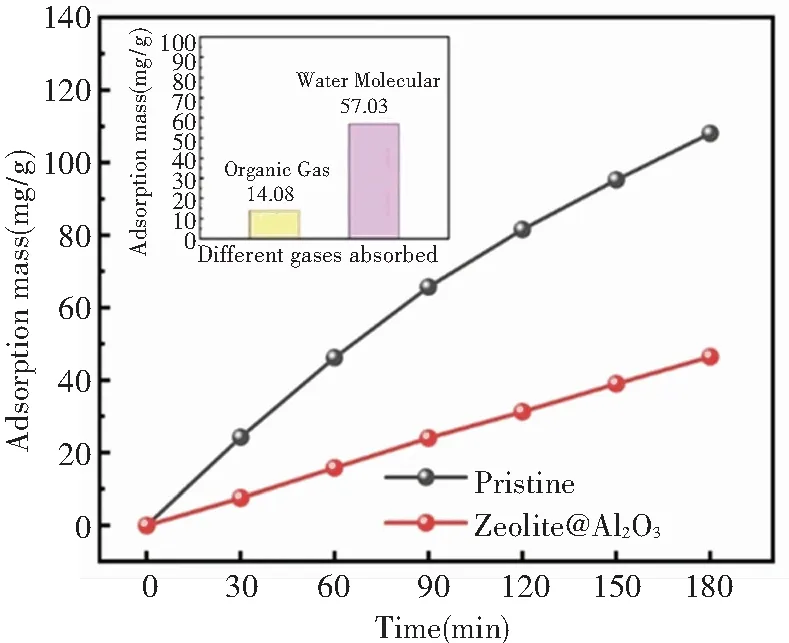
Fig.7 The adsorption of pristine 5A zeolite and prepared zeolite@Al2O3(the insert: the absorption decrement of zeolite@Al2O3)
3.3 Adsorption Kinetics
Kinetics studies the relationship between the adsorption rate and the factors affecting the adsorption rate. The adsorption kinetics of zeolite@Al2O3can be described by quasi-first-order kinetic model, quasi-second-order kinetic model, and intragranular diffusion model. Studying the adsorption kinetics is very important for the application of materials.
In this work,the zeolite@Al2O3adsorption process was fitted through the model and the values of R2simulated by quasi-first stage and quasi-second stage were compared. The results are shown in Fig.8 and Fig.9.
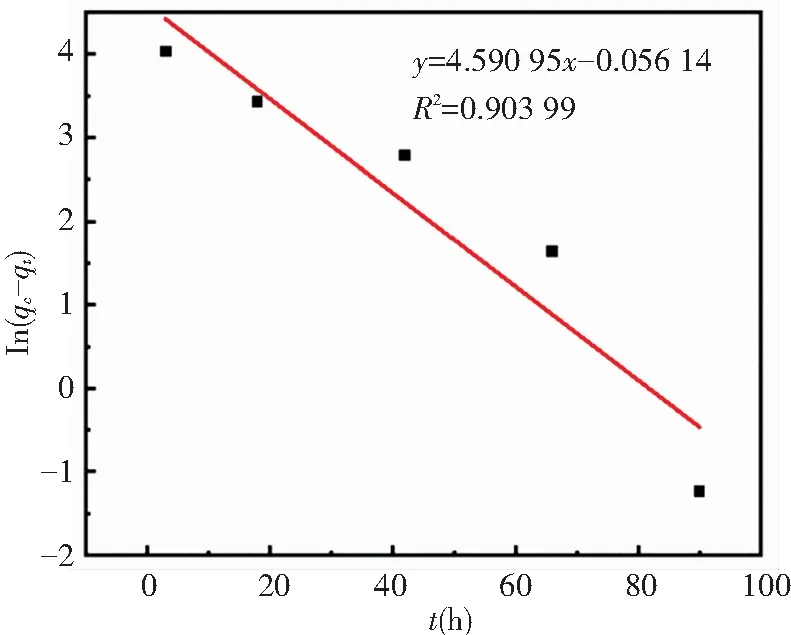
Fig.8 Quasi-first-order kinetic fitted straight line of zeolite@Al2O3adsorbing organic gas
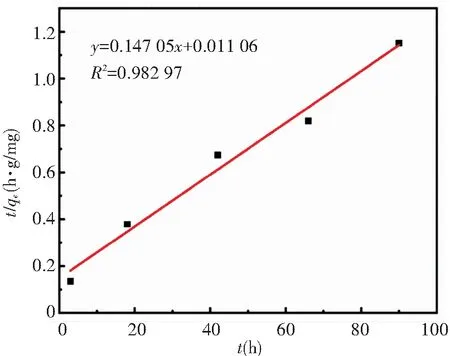
Fig.9 Quasi-second-order kinetic fitted straight line of zeolite@Al2O3adsorbing organic gas
For the curves fitted by the two models, largerR2indicates better fitting effect. TheR2fitted by the quasi-first-order kinetic and the quasi-second-order kinetic fitting is 0.903 99 and 0.982 97, respectively. Thus the fitting effect of the quasi-second-order kinetic model is better. This means that the zeolite@Al2O3absorbs organic gases through not only physical adsorption but also chemical adsorption.
In the intragranular diffusion model, as shown in Fig.10,theqtandt0.5present good linear relationship during the entire adsorption process. The straight line does not pass through the origin, indicating that intra-particle diffusion is not the rate-limiting step to control the adsorption process. In other words, the adsorption process is determined by other adsorption stages.

Fig.10 Fitted straight line of heating time to intragranular diffusion of zeolite@Al2O3adsorbing organic gas
4 Conclusions
In summary,5A zeolite was successfully modified by Al2O3via ALD. The improved adsorption performance of 5A zeolite can be attributed to the hydrophobic surface characteristic of Al2O3. The zeolite@Al2O3adsorption process follows the quasi-second-order kinetic model, suggesting that the adsorption process has both physical and chemical adsorption. Moreover, the adsorption is controlled by multiple factors instead of only intra-particle diffusion.
 Journal of Harbin Institute of Technology(New Series)2020年3期
Journal of Harbin Institute of Technology(New Series)2020年3期
- Journal of Harbin Institute of Technology(New Series)的其它文章
- Evolution Toward Artificial Intelligence of Things Under 6G Ubiquitous-X
- Evaluation Method of Output Waveform Quality for Neutral-Point- Clamped Three-Level Converter
- Review: Scalable Fabrication of Polymeric Nanofibers from Nano- Spinning Techniques to Emerging Applications
- Controlled Movements in Superlubric MEMS
- Review: Recent Progress on Poly(ether ether ketone) and Its Composites for Biomedical, Machinery, Energy and Aerospace Applications
- Random Low Patch-rank Method for Interpolation of Regularly Missing Traces
