Plasma-assisted abatement of SF6 in a packed bed plasma reactor:understanding the effect of gas composition
Xiaoxing ZHANG (張曉星),Yuan TIAN (田遠(yuǎn)),Zhaolun CUI (崔兆侖),3 and Ju TANG (唐炬)
1 School of Electrical Engineering and Automation,Wuhan University,Wuhan 430072,People’s Republic of China
2 School of Electrical and Electronic Engineering,Hubei University of Technology,Wuhan 430068,People’s Republic of China
3 Authors to whom any correspondence should be addressed.
Abstract
Keywords:SF6,PBR,plasma,gas composition
1.Introduction
Sulfur hexafluoride (SF6) is a potent greenhouse gas with extremely stable chemical properties.Research has showed that its global warming potential (GWP) is 23 000 times that of carbon dioxide (CO2),which could exist for more than 3200 years in the atmosphere[1–3].Meanwhile,SF6has been widely applied to the power industry,semiconductor and metallurgy sectors due to its excellent dielectric strength,arcextinguishing properties and chemical inertness.Recently,the potential impact on the global climate has attracted tremendous attention.Nowadays,more than 10 000 tons of SF6are produced every year,over 80% of which are used in gasinsulated switchgears [4].However,green insulating gas still should be further studied,which means that humans will face the problem of SF6treatment for a long time [5,6].For instance,the SF6issues would appear when the equipment was broken or repaired.In addition,the concentration of SF6applied to semiconductor sectors and the metallurgy industry is usually extremely low,which is difficult for recycling.Those problems pose serious hazards to the ecological system.Therefore,how to abate SF6waste gas has become a hot issue in the industrial environmental protection field.

Figure 1.Schematic of the experimental setup.
Compared with traditional methods,such as pyrolysis and photolysis,no-thermal plasma (NTP) technology has exhibited high potential in addressing environmental pollutants due to the presence of abundant energetic electrons and chemical reactive species [7–15].Dielectric barrier discharge(DBD),as one of the methods,has the advantage of generating NTP compared with other types of discharge approaches due to the simplicity and scalability of its experimental setup [16].In DBD plasma,free electrons are highly energetic with a typical electron energy of 1–10 eV,which is sufficient to activate reactant molecules (SF6) to produce chemically reactive species for the initiation and propagation of chemical reactions arising from the radical chain mechanism[17].Furthermore,it is worth noting that the overall gas temperature of the DBD can be as low as room temperature and less heat loss can be achieved.Therefore,it has been widely applied to the treatment of industrial waste gas including volatile organic compounds (VOCs) and greenhouse gases [18–23].
However,the plasma behavior is still poorly understood owing to the influence of so many processing parameters on its performance.Much research on this issue has been studied and many enlightening conclusions are obtained.For example,research showed that some packing materials in the reactor could intensify the micro-discharge and surface discharge in the system,and finally the removal efficiency was improved [24–29].A packed-bed plasma reactor was applied(PBR,DBD with packing materials) to abate SF6and CF4[29].The results showed that PBRs exhibited a higher energy efficiency than traditional DBD reactors,which was easier to remove waste gas molecules.Furthermore,studies showed that gas composition also has a huge impact on the SF6treatment.Zhang et al found that water vapor,oxygen and ammonia (NH3) could promote the degradation of SF6in DBD reactors[30–32].The above studies are very crucial and the results are very instructive.However,until now,few studies were focused on the combination of packing materials and gas composition.
Water,as a common substance,can be obtained easily.Research demonstrated that lots of H and OH radicals could be generated by water vapor in the plasma area,which can react with those dissociative fragments resulting from the decomposition of SF6and help to remove SF6[31].However,the influence of water vapor concentration on the degradation process has not been investigated comprehensively,as well as its effect on the by-products.Many researchers have studied the impact of different applied gases on products selectivity,but the influence of the concentration of the applied gases on products selectivity was ignored.Investigation into the products concentration and selectivity under different conditions provides a better point of view to understand the effect of those parameters on SF6abatement.Furthermore,this work also could provide ideas of the treatment of other exhaust gases.
In this study,the removal of SF6was conducted in a PBR using argon (Ar) as a carrier gas.The influence of different plasma processing parameters (water vapor concentration,SF6inlet concentration and pre-heating temperature) on removal efficiency and products selectivity of SF6was investigated comprehensively.
2.Experiment
2.1.Experimental setup
The experiments were performed in a glass beads packed bed,non-thermal atmospheric pressure plasma reactor,which was shown schematically in figure 1.The experimental setup consisted of a gas source,a plasma generator,detecting equipment and exhaust gas treatment modules.The gas source was applied to supply particular gas composition of the system including water vapor generator (FD-HG,Furande,Suzhou,China),Gas sample compounder (GC500,Tunkon Electrical Technology Co.,Ltd),SF6and Ar(above 99.999%,Newradargas Co.,Ltd,Wuhan).The H2O concentration was measured by a mirror dewpoint meter (GE600,Henan Relations Co.,Ltd).The water vapor generator contained a heating module inside,which could preheat the gas input from the gas distributor.Ar was the carrier gas,which brought SF6andwater vapor into the reactor and then went through detecting apparatus.
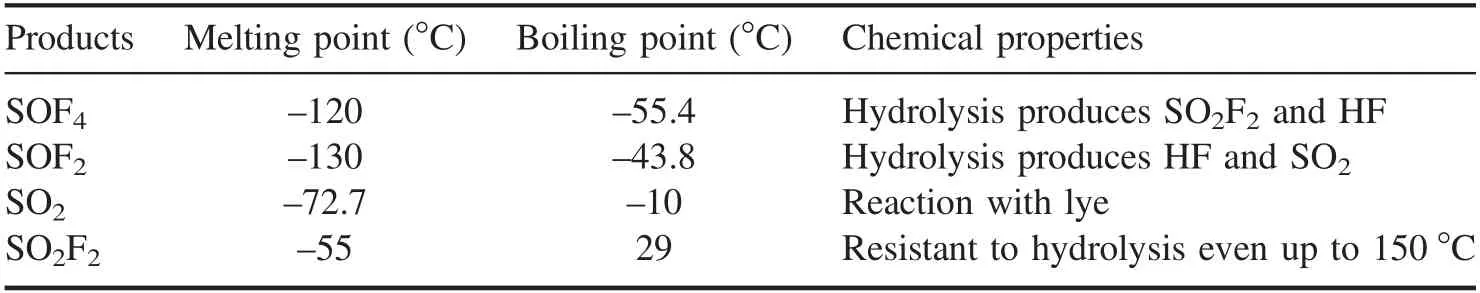
Table 1.The physical and chemical properties of the four products [30].
The power supply (CTP-2000K,Nanjing Suman Electronics) was applied to change the single-phase electric supply into high frequency and high voltage alternative currency and applied it on the reactor to generate plasma.The discharge reactor employed in the present work was a coaxial cylindrical reactor.A stainless-steel mesh (high-voltage electrode) was wrapped tightly around a quartz tube with an external diameter of 24 mm and an inner diameter of 20 mm.The inner ground electrode was a copper rod with an external diameter of 4 mm,which was installed along the axis of the quartz tube.The discharge length was about 200 mm with a discharge gap of 6 mm,the thickness of the quartz barrier layers inside and outside the reactor was 2 mm.Glass beads with a diameter of 2.5 mm were filled in the reactor as the packing material.
Detecting equipment included an oscillograph (Tektronix TDS 320),emission spectrometer (MX2500+,Ocean Optics Co.,Ltd,USA),gas chromatograph (GC,GC-450,Shanghai Huishi Instrument) and gas chromatography-mass spectrometry (GCMS,Shimadzu Ultra 2010 plus with CP-Sil 5 CB column,SHIMADZU Co.,Ltd).The oscillograph was applied to record the applied voltage and discharge currency of the PBR.The SF6concentration before and after treatment was detected by GC.The emission spectrometer was a threechannel one in which a grating was used as a beam splitting element and a CCD array as a beam splitting element detector.It could measure the wavelength from 300 nm to 800 nm with 0.1 nm optical resolution.Four kinds of sulfur-containing byproducts were quantitatively detected by GCMS.
The exhaust gas processed module included lye and a molecular sieve.The water-soluble products (SOF4,SOF2,SO2) were absorbed by the lye,while the remaining waterinsoluble product (SO2F2) was adsorbed by the molecular sieve [33–35].The properties of the four products are shown in table 1.
The experiment conditions were summarized as follows:the input power was fixed at 100 W,the electric source frequency was fixed at 8.7 kHz,the mixed gas flow rate was determined to be 150 ml min-1,all tests were conducted at room temperature (298 K) under atmosphere pressure (101.3 kPa).Additive water vapor flow rate was 0.15–3 ml min-1(the water vapor concentration was corresponding 0.1%–2%),the concentration of SF6ranged from 1% to 8%.Each set of experiments was performed three times.
2.2.Measurement
The destruction and removal efficiency(DRE)was calculated as follows:

wherecinandcout(ppm,parts per million) refers to the SF6concentration before and after treatment respectively.
Energy yield (EY) was defined as the mass (g) of SF6degradation per unit of energy (kWh).It was calculated as follows:
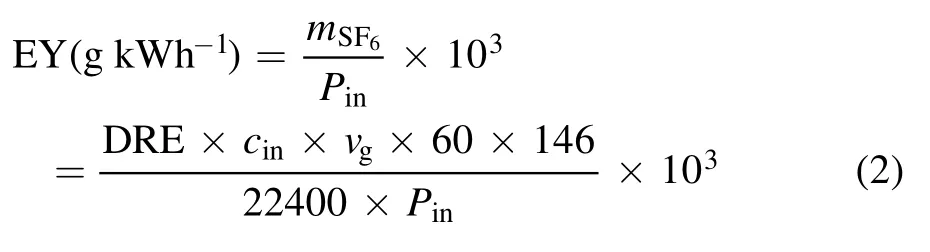
wheremSF6denotes the mass of SF6abated in unit time (h).Pinrefers to the input power(W)of the power supply.vgrefers to the velocity (ml min-1) of the mixed gas.
SK(selectivity)is the selectivity of a particular byproduct(K),wherecKrefers to the byproduct concentration.
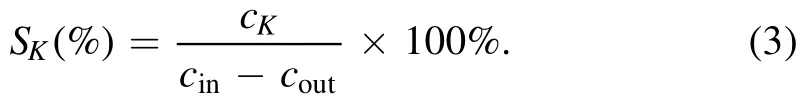
3.Results and discussion
3.1.Removal efficiency
Figure 2 presents the DREs and EY as a function of water vapor concentration,SF6inlet concentration and pre-heating temperature.In figure 2(a),the DRE of 2%SF6increased first and then dropped slightly.This tendency indicated that there was an optimal water vapor concentration range contributing to the promoted effect on DRE at 2% SF6in the system.Moreover,figure 2(b) shows that the DREs decreased as the SF6concentration decreased.But the DRE in the 1% H2O group was always higher than that of the 2% H2O group.It suggested that higher SF6concentration had an adverse effect on DRE.Furthermore,a better promoting effect on DRE was achieved in 1% group compared with the 2% H2O group under the same conditions.Figure 2(c)demonstrates the DRE as a function of pre-heating temperature.Compared with figure 2(a),warming up the mixed gas before discharge was beneficial to the abatement of SF6obviously.The 3% difference of the highest DRE was obtained before and after preheating.However,a negligible tendency began to be observed as the pre-heating temperature raised up to 120°C.Figure 2(d) shows that the EY increased from 5.87 g kW-1h-1to 24.01 g kW-1h-1as the concentration of SF6increased.It implies that the application of higher SF6concentration made better use of energy.
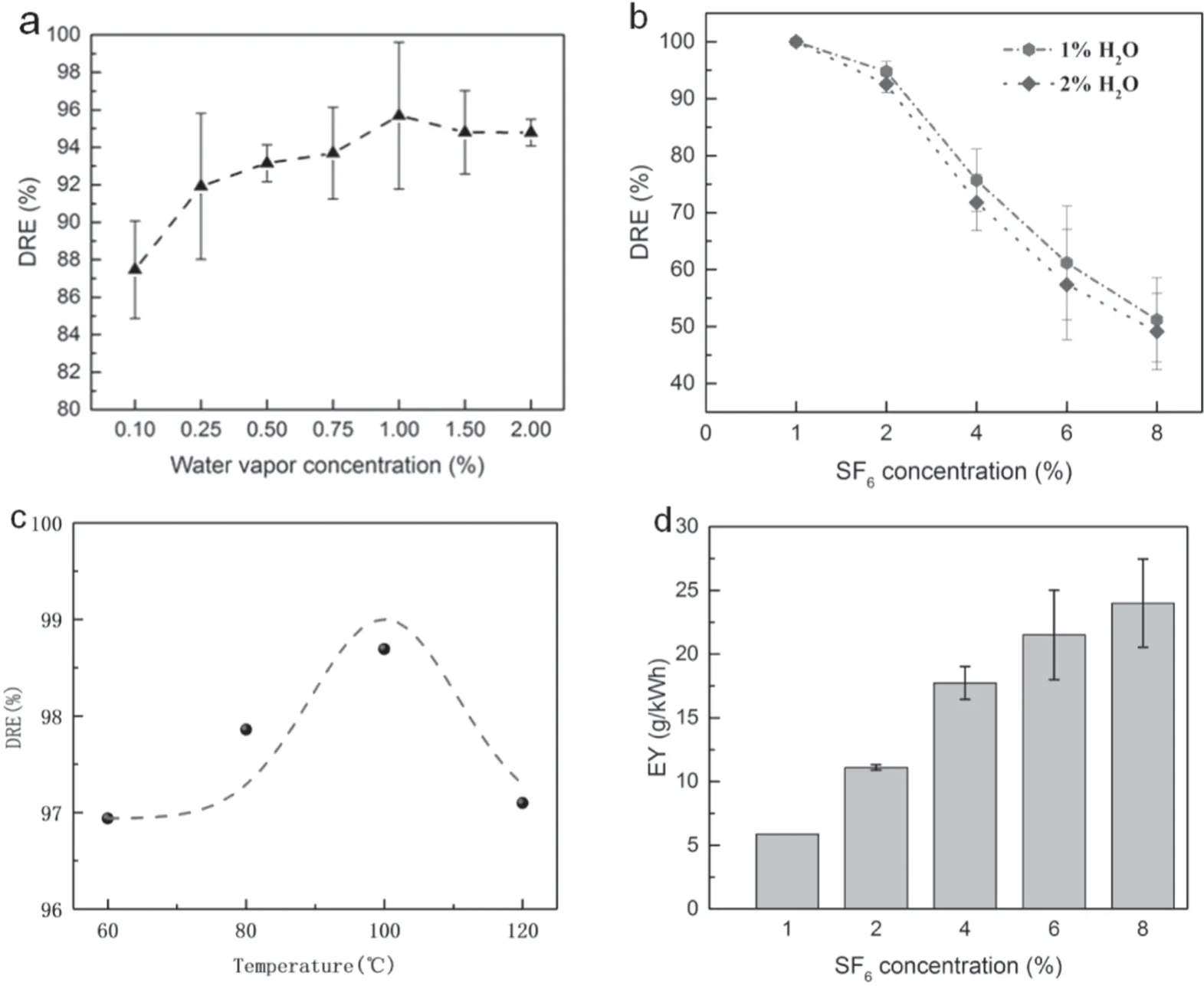
Figure 2.(a) DRE under different water vapor concentrations (2% SF6,100 W,150 ml min-1),(b) DREs under different SF6 inlet concentrations (100 W,150 ml min-1),(c) DRE at different pre-heating temperatures (2% SF6 + 1% H2O,100 W,150 ml min-1),(d) EY under different SF6 inlet concentrations (1% H2O,100 W,150 ml min-1).
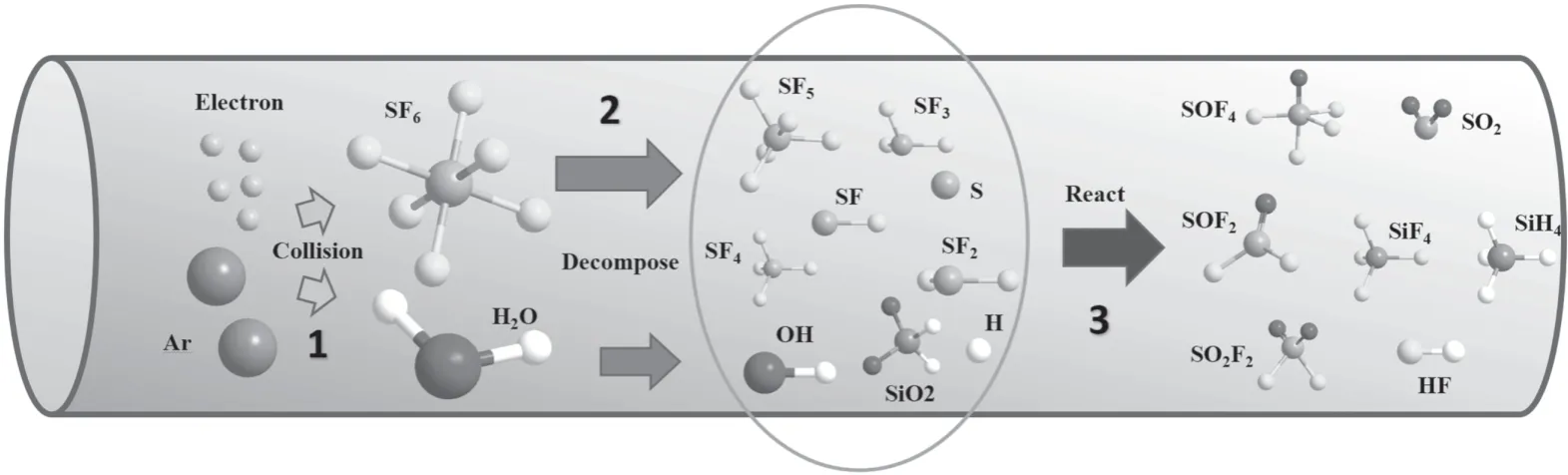
Figure 3.SF6 degradation process.
Similar conclusions have been obtained in previous reports.For example,in[30],Zhang et al suggested the DRE could increase first and then decrease with the increment of NH3concentration.Chen et al demonstrated that DRE could decrease with the increment of SF6inlet concentration [10].Based on our previous work,the SF6removal process was divided into three parts as shown in figure 3 [32].
Three parts occurred during the treating process including collision,decomposition and reaction [32].The first step happens first because it provides raw materials for the latter reactions.Actually,collision consists of the preparation of excited atoms (equation (4)) and free electrons.The reaction threshold will be lower because of those reactive species.Then,with the increment of the electric field,those particles will speed up to obtain high energy.When they collided with SF6or H2O molecules,those molecules will be decomposed into SFx,OH and H,as the equations (5)–(7) show.In this paper,Ar was used as carrier gas because it can generate lots of free electrons by the Penning ionization(references[1]and[36]reported this effect).After this stage,those products will recombine to generate SF6again if there is no intervention(equation (8)).However,those radicals will react with SFxbecause they are very active in the plasma region.Therefore,the existence of H,OH can inhibit this reverse reaction.In this way SF6can be abated completely.
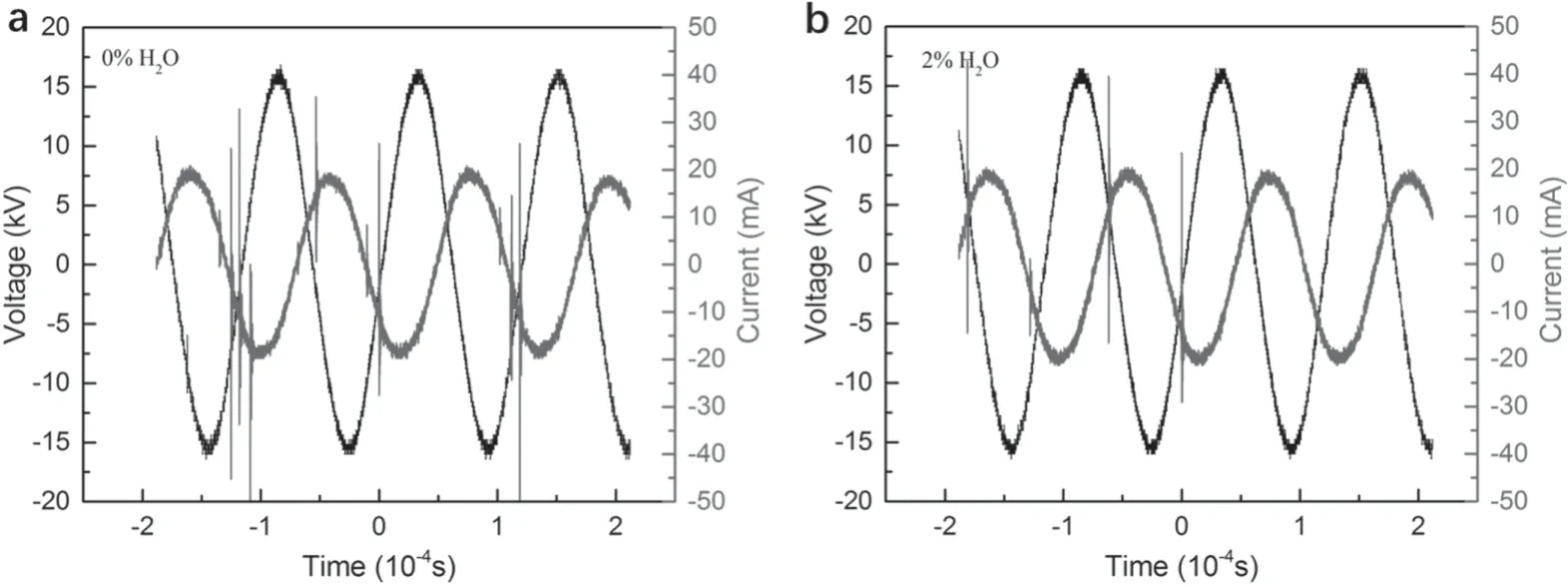
Figure 4.(a) The applied voltage and current waveform of the PBR under no H2O (2% SF6/Ar,100 W,150 ml min-1),(b) the applied voltage and current waveform of the PBR under 2% H2O concentration (2% H2O + 2% SF6,100 W,150 ml min-1).
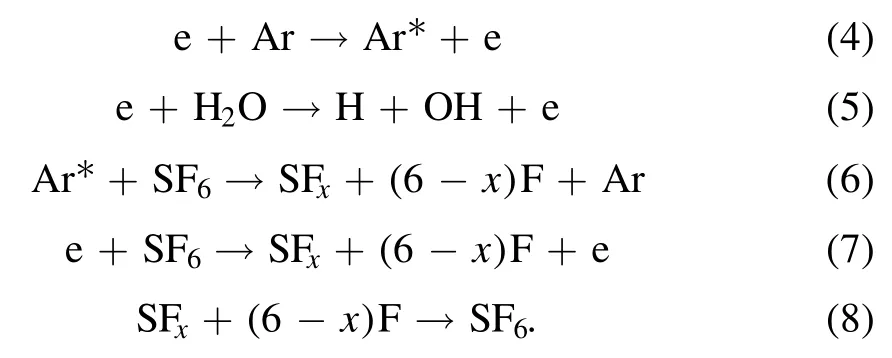
When the water vapor was lower than 0.25%,DRE climbed quickly with the increment of water vapor concentration.However,when the water vapor concentration continued to increase,this promoted effect slowed down and even disappeared.When the water vapor concentration was 2%,the DRE was a litter lower than that of 1% H2O.However,a higher DRE was still obtained compared with the situation of no H2O.This result is in accordance with that by Lee et al [13].It clearly indicated that there was a tradeoff between the positive and adverse influence of H2O.Firstly,water vapor can prevent sub-fluoride recombination,which was helpful to improve the DREs and EY.However,water vapor was also an electronegativity gas.It can absorb free electrons in the ambient,which will weaken collision and decomposition.References [13]and [37]also reported the similar conclusion that the highest removal efficiency has been obtained under an optimized amount of oxygen condition.However,the oxygen concentrations were not same in the two pieces of research,which may be due to the difference between their working conditions.Therefore,the optimized H2O concentration of plasma application is determined by the specific work conditions rather than a fixed value.
Figure 2(b) shows DREs as a function of SF6concentration.Similar to water vapor,SF6is also an electronegativity gas,high concentration of SF6could suppress the development of micro-discharge and lower the DRE.Therefore,its DRE dropped from 100%at 1%SF6to 51.2%at 8%SF6.Moreover,1% of the H2O group had a better abatement efficiency,which indicated that it has a better tradeoff in this system.The degradation efficiency of SF6was improved when H2O concentration between 0.10% and 0.25% was added.In addition,it is easy to understand that the DRE increased under higher temperatures as higher temperatures led to extensive molecules movement and intensified collisions between reactive species.However,if the temperature continued to increase,opposite results would occur because the higher temperatures will enlarge the volume of mixed gas,leading to the increased flow rate and the decreased average residence time,and this effect decreased the DRE[17].As shown in figure 2(d),the total mass of SF6treated will increase with the SF6concentration.In [30],Zhang et al pointed out that the mass of degraded SF6increasing with flow rate,because the increased flow rate and concentration would lead to the increment of the number of SF6molecules.This effect would also appear when the SF6inlet concentration increased because both bring more SF6molecules into the reactor.When the concentration of SF6was low,the averaged number of collisions of the SF6molecule was higher,thus leading to more sufficient degradation of SF6molecules(higher DRE),but the total amount of degradation was less (lower EY).In contrast,the averaged number of collisions of the SF6molecule decreased,although the degradation was not sufficient (lower DRE),more molecules participated in the reaction (higher EY).Therefore,the increment of SF6concentration would also increase the SF6degradation.The above investigation revealed that increasing SF6concentration was a feasible method to improve EY.
Similar voltage and current amplitude are shown in figure 4,which implies that the addition of water vapor will not change the electric parameter of the system.However,it can be found that the number of micro-discharge channels is quite different.The intensity of the micro-discharge can reflect the movement of charged particles in the air gap [36].Under a higher humidity environment,the micro-discharge in the tube was much less than the group without water.Because water molecules can adsorb free electrons during the process of ionization.This process will result in lower energy applied to the SF6decomposition due to the finite total energy.Consequently,the micro-discharge was difficult to happen,thus decreasing the number of discharge pulse.
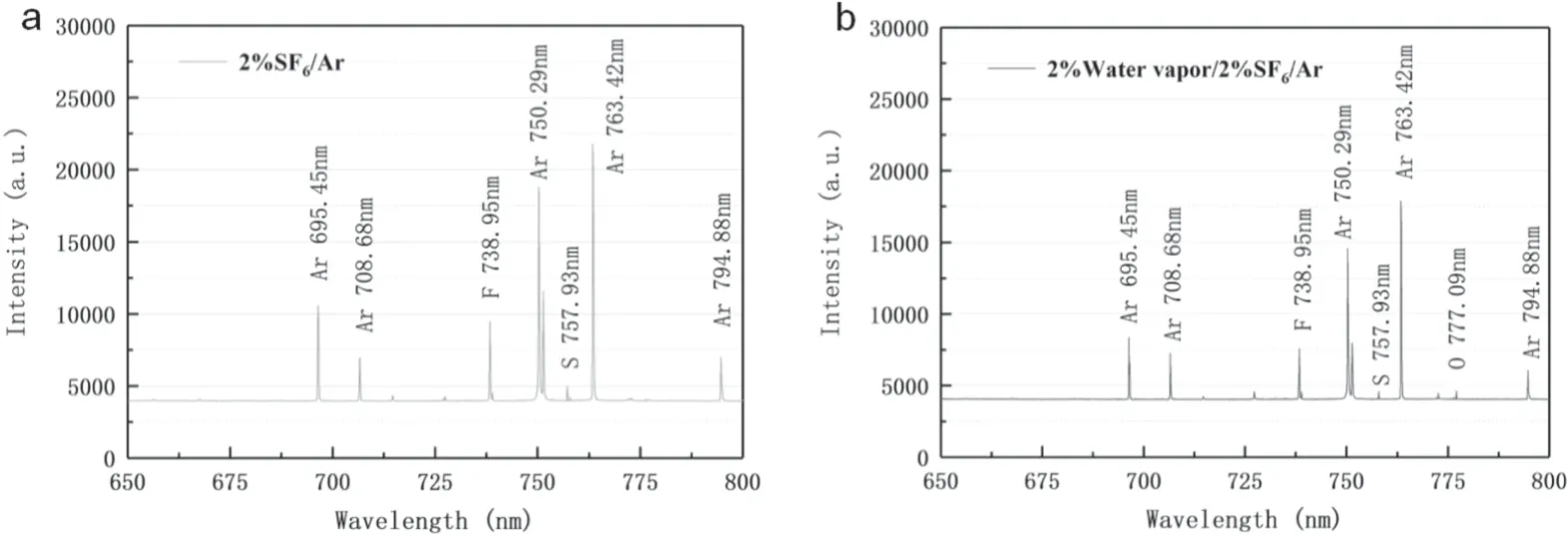
Figure 5.(a) Emission spectrum (2% SF6,100 W,150 ml min-1),(b) emission spectrum.(2% SF6 + 2% H2O,100 W,150 ml min-1).
When the kinetic energy of a free electron colliding with an atom or molecule is greater than their excitation energy,they will be excited and corresponding characteristic spectra were also emitted.The active substances involved in the reaction can be studied by these lines,as shown in figure 5.In this paper,quartz glass was applied as a barrier medium to collect the emission spectrum.By comparing with the NIST database[38],several main atomic lines of the Ar/SF6as well as Ar/SF6/H2O plasma emission spectra were initially diagnosed (Ar 695.45 nm,Ar 708.68 nm,Ar 750.29 nm,Ar 794.88 nm,F 738.95 nm,S 757.93 nm,O 777.09 nm).Among them,the intensity of Ar lines was much greater than those of other elements,because argon was the main component of the mixed gas.A previous study demonstrated that the kinetic energy of free electrons was as high as 11.5 eV in the DBD system,which could excite argon atoms in the mixed gases[23].Moreover,both F and S atoms appeared in the emission spectrum due to the break and decomposition of many SF6molecules under plasma conditions,and the emission intensity of F was much larger than S.Firstly,the number of F atoms was more than S atoms;Secondly,a single S atom can appear only when the SF6molecule was completely decomposed.Furthermore,O was not detected under anhydrous conditions,but a weak line was observed under 2% H2O,indicating that water molecules were also involved in the decomposition of SF6.H2O and SiO2were two sources of O in the 2% H2O system.However,in the anhydrous system,the O atoms could only be obtained from the SiO2(etching reaction) [31].The emission line of the O atoms did not appear in the system,which may be due to the slow progress of the etching reaction.In addition,the intensity of the emission spectrum decreased significantly after the addition of water vapor,evidenced by the intensity of the overall emission spectrum.A similar phenomenon was also found from previous research [39],in which the+CO2emission spectrum intensity in pure CO2was much smaller than that of the diluted gas.Therefore,this phenomenon may be due to the absorption of more electrons and energy by the H2O as mentioned above(equations(9)–(12)).Its adsorption capacity for free electrons was strong although the moisture was relatively low,and this adsorption may induce decreased energy for exciting Ar atoms and made the intensity of Ar lines descend.
3.2.Products analysis
Figure 6 presents the concentration and selectivity of four products as a function of water vapor concentration.As shown in figure 6(a),the concentration of SOF2decreased dramatically while SO2content went up significantly as the water vapor concentration increased from 0.1% to 2%.In addition,the other two products were relatively stable.Clearly,among the four products,SO2and SOF4were sensitive to water vapor concentration,the concentration of SO2increased from 2613.6 ppm to 8543.7 ppm and the concentration of SOF4dropped from 790.9 ppm to only 8.6 ppm,respectively.In addition,[31]showed that 0.5% H2O could significantly enhance the selectivity of SO2,which is consistent with the results from this paper.However,there is a big difference in the selectivity of SOF4.This may be due to the higher energy in the PBR system,which led to the inability of the primary product SOF4to exist in large quantities.Furthermore,[37]showed that SO2F2kept stable when the O2concentration changed,but the SOF2decreased significantly.This may be due to the different sensitivity of SOF2to H2O and O2.
Equations(13)–(18)indicate that the concentration of SFxshould decrease with the increment of x.This will result in a higher concentration of SOF4compared with the SOF2because the raw materials of the former are much abundant than that of SOF2(equations (19)–(22)) [1].By contrast,figure 6(a) shows the opposite trend.It implied that most of the SOF4would react further to be transformed into other substances,as equations (23) and (24) display.When the water vapor concentration was 0.1%,the concentration of SOF4was 790.9 ppm;but the concentration of SOF4dropped to only 8.6 ppm as the concentration of water vapor increased to 2%.This fact demonstrated that water vapor exhibited a strong suppression effect on SOF4.That may be due to the fact that H2O could react with SOF4to form SOF2and SO2F2.
The concentration of SOF2cannot fluctuate largely compared with SOF4.When the water vapor concentration was 0.1%,the concentration of SOF2was 3451.8 ppm with the lowest value.Furthermore,when H2O concentration increased to 0.75%,the highest concentration of 4136.0 ppm for SOF2was achieved.Equations (21)–(26) reveal that H2O has a double effect on SOF2concentration because it is not only a reactant to form SOF2,but a reactant to consume its raw materials.Therefore,when the water vapor concentration was higher than 0.75%,SOF2started to decrease.
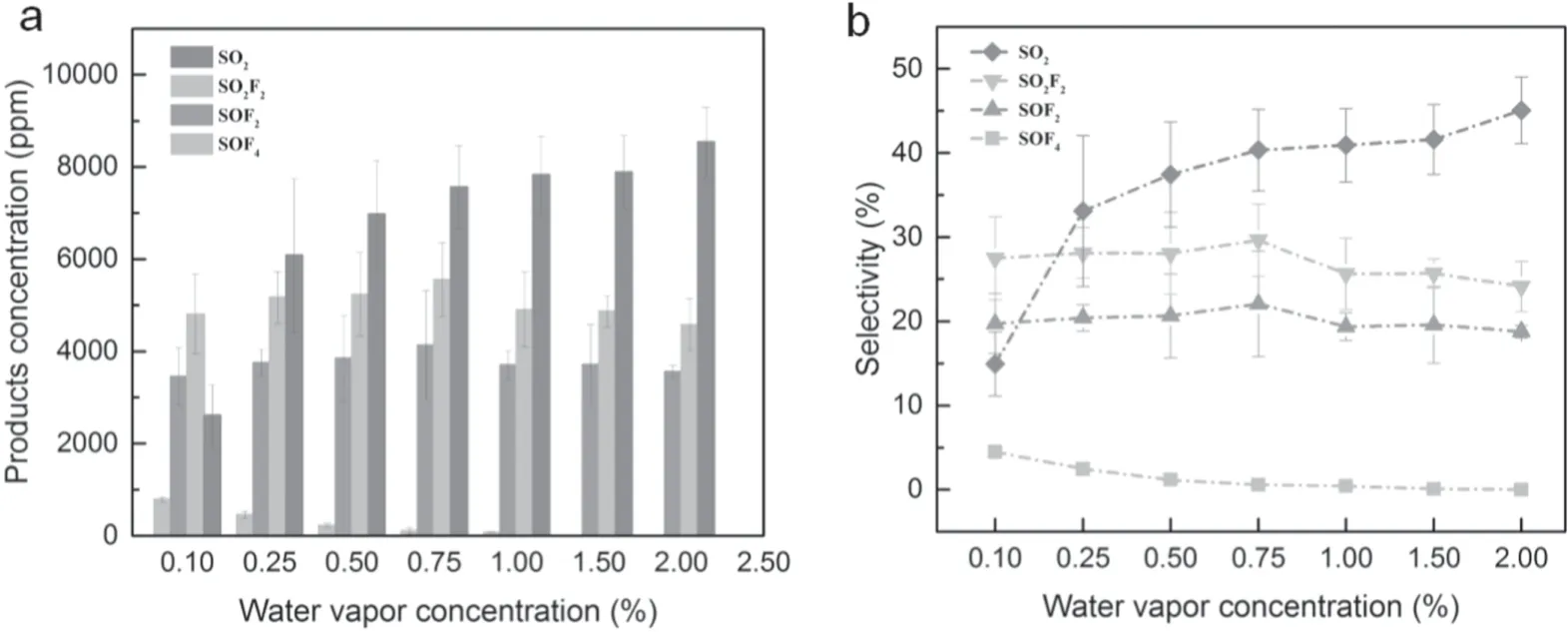
Figure 6.(a)Product concentration under different water vapor concentrations(2%SF6,100 W,150 ml min-1),(b)product selectivity under different water vapor concentrations (2% SF6,100 W,150 ml min-1).

Table 2.Formulas in the degradation process [1,30].
Similar to SOF2,the SO2F2increased first and then decreased with the H2O concentration.The lowest SO2F2concentration value of 4811.9 ppm was achieved when 0.1%concentration of the water vapor concentration was applied.Furthermore,when H2O concentration reached 0.75%,the highest concentration of 5554.3 ppm was obtained.When the H2O concentration was lower,most of the H2O molecules collide with free electrons to generate OH and H radicals(shown in (5)).Those active radicals generated SO2F2easily,so the SO2F2concentration climbed up with the H2O concentration.However,most H2O existed in the form of molecules as the increment of water vapor concentration,which has an adverse effect on SO2F2.Finally,SO2F2decreased.
There is a great correlation between SO2concentration and H2O concentration.As the water vapor concentration increased from 0.1% to 2%,SO2concentration increased beyond threefold and became the highest product among those four products.This was consistent with previous work.In [31],Zhang et al compared the SO2production of SF6/Ar/O2and SF6/Ar/H2O systems.The results showed that the selectivity of SO2in the latter system was much higher than that of the former.Furthermore,in [40],Zeng et al also reported that the addition of water vapor was beneficial to the formation of SO2.As H2O concentration increased from 0 to 3000 ppm,the production of SO2increased by more than 10 times.Actually,this effect is helpful for the treatment of the exhaust gas because SO2is soluble in lye.Plenty of H radicals were consumed by the packing material and glass barriers (equations (27)and(28)).Equation(29)shows that more O radicals were produced with the increment of water vapor concentration,which contributed to the formation of SO2.At the same time,the concentration of SOF2and SO2F2gradually decreased,indicating that SO2may be a secondary product of SOF2and SO2F2(equations (25) and (26)).
Figure 6(b) displays the distribution of four products’selectivity as a function of water vapor concentration.Similar to figure 5(a) the selectivity of SOF4ranged from 0.05% to 4.51%,which was always the lowest.This distribution means that SOF4was the most unstable product of this environment,which could not exist in a large amount.Furthermore,SOF2and SO2F2were relatively stable,since the selectivity of the two products did not change much under different H2O concentration conditions.Selectivity of SOF2and SO2F2was similar.SO2F2exhibited a higher selectivity due to its higher stability compared with SOF2.In addition,SO2was extremely sensitive to H2O concentration.When the water vapor concentration exceeded 0.25%,it has the highest selectivity among those four products.There are two reasons accounting for this result.Firstly,SO2has a superior stability as a final product.Besides,H2O concentrations exhibited a very positive influence on the generation of SO2.All the potential SF6degradation paths were summarized in figure 7 and the reaction equations were summarized in table 2.
Figure 8 presents the product concentration and selectivity as a function of SF6concentration at 1% H2O.Higher SF6concentration was accompanied by higher products concentration since more SF6molecules were degraded under higher SF6inlet concentrations.SOF4concentration was extremely low (<100 ppm) when the concentration of SF6was not more than 2%,but its concentration raised to 13 218.6 ppm (the highest value) as the concentration of SF6further increased.Meanwhile,SO2F2increased as SF6concentration increased,while SOF2concentration remained relatively stable when SF6concentration was higher than 1%.
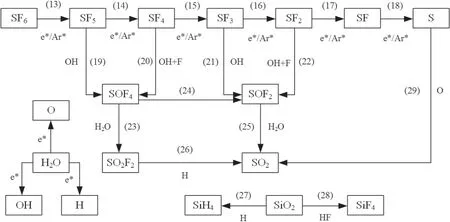
Figure 7.SF6 degradation paths.
However,the selectivity of these products changed distinctly compared with figure 6(b).The selectivity of SOF4was the lowest when SF6concentration was lower than 2%.In contrast,as the SF6inlet concentration increased to 8%,SOF4became the most abundant product.SO2also dramatically changed as its selectivity was significantly influenced by SF6inlet concentration.Although SOF2and SO2F2were still keeping stable during this process,it should be noted that the selectivity of SO2F2was much higher than that of SOF2.
According to the previous analysis,SOF4can be generated in large quantities.However,SOF4would continue to react accompanying with a series of other compounds due to the further addition of H2O molecules,thereby reducing its concentration.However,when the concentration of SF6reached 4% or higher,the H2O content was relatively lower,which caused most SOF4to lack the raw materials for further reactions,resulting in an increment of SOF4concentration.
When the concentration of SF6was 1%,the concentration of SOF2was only 154.2 ppm,which was consistent with the above inference,because SOF2has a similar reaction path with SOF4,except that SOF4can react with H radical to form SOF2.Thus,SO2with relatively surplus H2O can be generated easily,resulting in a lower SOF2concentration.Therefore,the concentration change of SOF2is ahead of the concentration change of SOF4,where a higher concentration has already achieved when the concentration of SF6is 2%.Therefore,the concentration range of the SF6is not as large as further increased SF6concentration,which indicated that the SOF2concentration was not influenced by SF6concentration variation,when SF6was excessive (>2%).It can be noted from equations (21),(22),(24),and (25) that the number of decomposed SF6molecules increased as the increased SF6concentration,but the number of SF2and SF3did not definitely increase,because when the concentration of SF6is low,the same SF6molecule can be subjected to multiple collisions and multiple decompositions,thereby generating more SF2and SF3,but the averaged number of collisions per SF6molecule will decrease upon the increased number of SF6molecules in the system.It will increase the number of SF4and SF5,and reduce SF2and SF3.Eventually,the produced SOF2does not increase with the increment of SF6.
The concentration of SO2F2increased with the increased SF6concentration.Firstly,a rapid increase in the concentration of SOF4will provide sufficient feedstock to form it.Secondly,the relative content of H2O will be insufficient for the increased concentration of SF6,which will limit the consumption path of SO2F2.Finally,SO2F2concentration increased due to its stable chemical property.Therefore,as the SF6concentration increased from 1% to 8%,the concentration of SO2F2ranged from 385.6 ppm to 11 014.8 ppm.
When the SF6concentration increased from 1% to 2%,the SO2concentration increased from 1686.7 ppm to 7832.6 ppm.SO2is a relatively stable product combined with the previous analysis.When the concentration of SF6was doubled,four times of concentration was achieved.However,with the augment of SF6concentration,the growth rate of SO2production gradually decreased.Finally,when the concentration of SF6was 8%,the concentration decreased slightly.This may be due to the fact that the increment of SF6led to the decreased SOF2concentration and increased SO2F2concentration,while SOF2is the raw material for SO2generation.In addition,the SO2F2augment will increase the concentration of HF in the system,which also has an inhibitory effect on SO2formation.
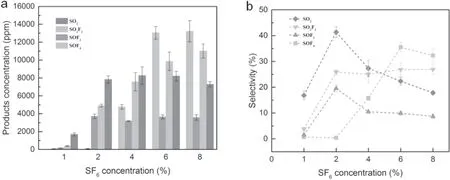
Figure 8.(a) Product concentration under different SF6 inlet concentrations (1% H2O,100 W,150 ml min-1),(b) product selectivity under different SF6 inlet concentrations (1% H2O,100 W,150 ml min-1).
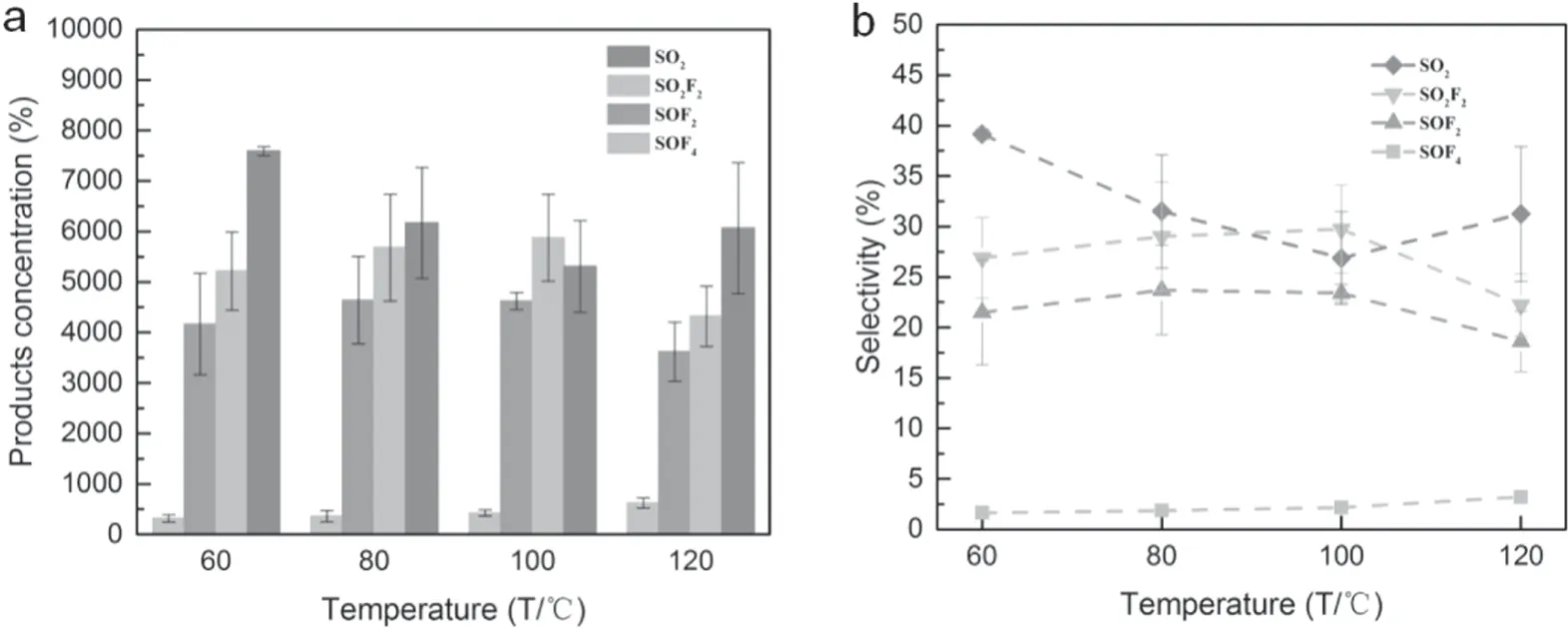
Figure 9.(a)Product concentrations at different pre-heating temperatures(2%SF6 + 1%H2O,100 W,150 ml min-1),(b)product selectivity at different pre-heating temperatures (2% SF6 + 1% H2O,100 W,150 ml min-1).
The performance of different products under different pre-heating temperatures was studied,as shown in figure 9.It can be clearly seen that SOF4was positively correlated with temperature.Its concentration increased from 318.5 ppm at 60°C to 624.2 ppm at 120°C.However,pre-heating temperature on the product resulted in much lower selectivity of SO2compared with the un-preheated condition.
Only SOF4and SO2showed different trends from the previous one,according to the influence of temperature on the products.Among them,SOF4increased with the temperature.Referring to its generation and consumption paths,it can be found that the increment of temperature may have a certain inhibitory effect on its further transformation.SO2decreased first and then raised with the temperature,which may be associated with the stability of SO2and its raw materials at different temperatures.Reference [17]studied the methanol conversion product distribution characteristics at different pre-heating temperatures.As pre-heating temperature increases,some products changed significantly.However,[41]pointed out that the temperature changes below 400°C would not influence SF6degradation.Therefore,the mechanism for the temperature effect on the products is still unknown.Finally,it can be noted that the increment of temperature causes a slight decrease in the total amount of these four products,the reduced portion may be converted into other compounds.
In our previous work,we have investigated the effects of three parameters on SF6degradation.Water molecules could participate in the physical and chemical reactions of the plasma region,which deeply influences the SF6removal efficiency and product distribution.Primarily,the addition of water vapor significantly improved the processing efficiency of SF6,the degree of improved efficiency varied as the water vapor concentration changed.The analysis demonstrated that the effect of water vapor concentration was subject to specific conditions,so the position of the optimal equilibrium point is not fixed.In addition,electrical signals and emission spectra have shown that the entire process was mainly influenced by the participation of water molecules in chemical reactions during the SF6process,and the changes in electrical parameters were negligible.The water vapor concentration mainly exhibited an influence on the selectivity of SOF4and SO2,which indicated that adjusting its concentration should be considered preferentially where the product treatment is mainly concerned.The main change induced by the increment of SF6concentration was the increased stability and primary products such as SO2F2and SOF4.This may be due to the increased energy utilization.The pre-heating temperature further increased the efficiency of DBD treatment of SF6and only affected the production of SOF4.Therefore,pre-heating the mixed gas is a feasible method under the high DRE demand condition.In summary,the proper water vapor concentration and pre-heating temperature play a great crucialrole in the improvement of the DRE;but for EY,the inlet concentration of SF6is the most important.

Table 3.Products and parameters relationship.
Finally,the relationship between the products and the parameters were defined in table 3.√indicates that a particular product is susceptible to a particular parameter.
4.Conclusions
In this study,the degradation of SF6in a packed bed plasma reactor was investigated comprehensively.Removal efficiency and products selectivity have been discussed under different parameter conditions,including the water vapor concentration,SF6inlet concentration and pre-heating temperature.The best promoted effect was achieved by 1% H2O when the water concentration was in the range of 0.1%–2%,and its DRE reached 95.7% at 2% SF6.Under 100°C preheating,this value further reached 98.7%.Therefore,an appropriate amount of moisture addition and pre-heating temperature could increase the removal efficiency of SF6.Moreover,the EY at different initial concentrations demonstrated that higher SF6 concentration had a favorable effect on EY.The current waveform of the PBR tube revealed that the presence of moisture influenced the filament discharge process within the reactor.The emission spectrum data suggested that water molecules participated in reactions,which confirmed that the dissociation of H2O molecules consumed a certain amount of discharge energy.Furthermore,the variation of the four products was also presented by adjusting the above three parameters.Among the four products,the negligible influence of water vapor concentration,initial concentration of SF6and pre-heating temperature on most stable SO2F2was achieved;although SO2is also a relatively stable product,its concentration is greatly affected by water vapor concentration and pre-heating temperature;SOF2is greatly affected by the initial concentration of SF6;SOF4as the most unstable product was affected by all the three parameters.
The inlet concentration of SF6has the greatest influence on EY,which should be considered preferential when higher EY was needed.Pre-heating and water vapor can result in complete degradation of SF6,and it is also an effective way to promote SF6abatement.The distributions of the products indicated that certain products can be obtained by manipulating experimental conditions for subsequent further processing.In addition,DBD is only suitable for processing SF6gas under low concentrations and flow rates due to the limitation of existing technologies.Subsequent studies can be carried out from these two aspects.This article provides a deeper understanding of the performance of PBRs in SF6abatement and makes a preparation for its largescale application.
Acknowledgments
This study is funded by National Natural Science Foundation of China (No.51777144) and State Grid Science and Technology Project (SGHB0000KXJS1800554).
 Plasma Science and Technology2020年5期
Plasma Science and Technology2020年5期
- Plasma Science and Technology的其它文章
- Vickers hardness change of the Chinese low-activation ferritic/martensitic steel CLF-1 irradiated with high-energy heavy ions
- Comparison of discharge characteristics and methylene blue degradation through a direct-current excited plasma jet with air and oxygen used as working gases
- Evaluation of influence of cold atmospheric pressure argon plasma operating parameters on degradation of aqueous solution of Reactive Blue 198 (RB-198)
- Plasma-induced graft polymerization on the surface of aramid fabrics with improved omniphobicity and washing durability
- The far-field plasma characterization in a 600W Hall thruster plume by laser-induced fluorescence
- Experimental study of a low-pressure hybrid RF discharge
