Organic anion transporters also mediate the drug–drug interaction between imipenem and cilastatin
?
a Department of Clinical Pharmacology, College of Pharmacy, Dalian Medical University, Dalian 116044, China
b College (Institute) of Integrative Medicine, Dalian Medical University, Dalian 116044, China
c Provincial Key Laboratory for Pharmacokinetics and Transport, Dalian Medical University, Dalian 116044, China
d Department of Pharmacy, First Affiliated Hospital of Dalian Medical University, Dalian 116011, China
Keywords: Imipenem/cilastatin Renal dipeptidase Organic anion transporters Drug–drug interaction
ABSTRACT This study aimed to clarify that organic anion transporters (OATs) mediate the drug–drug interaction (DDI) between imipenem and cilastatin.After co-administration with imipenem,the plasma concentrations and the plasma concentration-time curve (AUC) of cilastatin were significantly increased,while renal clearance and cumulative urinary excretion of cilastatin were decreased.At the same time,imipenem significantly inhibited the uptake of cilastatin in rat kidney slices and in human OAT1 (hOAT1)-HEK293 and human OAT3(hOAT3)-HEK293 cells.Probenecid,p-aminohippurate,and benzylpenicillin inhibited the uptake of imipenem and cilastatin in rat kidney slices and in hOAT1-and hOAT3-HEK 293 cells,respectively.The uptakes of imipenem and cilastatin in hOAT1-and hOAT3-HEK 293 cells were significantly higher than that in mock-HEK-293 cells.Moreover,the K m values of cilastatin were increased in the presence of imipenem with unchanged V max,indicating that imipenem inhibited the uptake of cilastatin in a competitive manner.When imipenem and cilastatin were co-administered,the level of imipenem was higher compared with imipenem alone both in vivo and in vitro .But,cilastatin significantly inhibited the uptake of imipenem when dehydropeptidase-1 (DPEP1) was silenced by RNAi technology in hOAT1-and hOAT3-HEK 293 cells.In conclusion,imipenem and cilastatin are the substrates of OAT1 and OAT3.OAT1 and OAT3 mediate the DDI between imipenem and cilastatin.Meanwhile,cilastatin also reduces the hydrolysis of imipenem by inhibiting the uptake of imipenem mediated by OAT1 and OAT3 in the kidney as a complement.
1.Introduction
Currently,antibiotics seem to be the most commonly used drugs in the treatment of severe systemic infections,and their use is on the rise.Imipenem/cilastatin is an intravenousβlactam antibiotic that has a vital role in the treatment of infections not easily treated with other antibiotics.Imipenem exhibits a broad spectrum of antibacterial activity against aerobic and anaerobic gram-positive and gram-negative microorganisms [1].When imipenem is administered alone,it is quickly degraded by dehydropeptidase-1 or renal dipeptidase (also termed DPEP1),which is a kidney membrane enzyme that hydrolyses a variety of dipeptides.DPEP1 is responsible for the hydrolysis ofβ-lactam antibiotics,such as penem and carbapenem [2].Cilastatin is not an antibiotic by itself,but it inhibits DPEP1,which is responsible for degradation of the antibiotic imipenem.Thus,cilastatin is intravenously combined with imipenem to protect it from DPEP1 and prolong its antibacterial potency as a traditional theory [3].
Organic anion transporters (OATs) are expressed on the basolateral membrane of proximal tubules and have been shown to take part in the renal secretion of a wide range of anionic xenobiotics,such asp-aminohippurate (PAH),benzylpenicillin (PCG),probenecid,antiviral drugs,and ?lactam antibiotics [4].However,it has been reported that probenecid,a potent inhibitor of OATs,could increase the plasma concentration-time curve (AUC) of imipenem and also significantly increase T 1/2? [5].Piperacillin is capable of inhibiting renal transport of several ?-lactam antibiotics possibly mediated by OATs.A likely benefit of combination therapy of imipenem and piperacillin over imipenem or piperacillin monotherapy is that piperacillin interferes with the renal transport of imipenemviaan OAT,retards the renal clearance of imipenem,and maintains the high blood concentration of imipenem.It is also likely that the combination therapy of imipenem and piperacillin is beneficial in reducing this nephrotoxicity via OATs [6].Therefore,it has been implied that imipenem might be a substrate of a renal OAT.At the same time,cilastatin is an inhibitor OAT1 and OAT3 [7].However,whether cilastatin is a substrate or just an inhibitor of OATs has not been clarified.In addition,OAT-mediated drug–drug interaction (DDI) between imipenem and cilastatin has not been reported.
The purpose of the present study is to elucidate the involvement of OATs in the interaction between imipenem and cilastatin in the kidney.The DDI,which is mediated by OATs or DPEP1,contributes to the efficacy of imipenem and reduces the nephrotoxicity of imipenem.Therefore,this study may be a supplement to the pharmacological mechanism of imipenem/cilastatin.To our knowledge,this study demonstrated for the first time that OATs also mediated the DDI between imipenem and cilastatin.
2.Material and methods
2.1.Chemicals
Imipenem was supplied from Dalian Meilun Biology Technology Co.,Ltd.(Dalian,China).Cilastatin was extracted from imipenem and cilastatin sodium for injection.Probenecid,PAH,PCG,estrone-3-sulfate (ES),and tetraethyl ammonium (TEA) were purchased from Sigma (St.Louis,MO,USA).All other reagents and chemicals in this study were of analytical purity grade and were commercially available.
2.2.Animals
Male Wistar rats weighing 200–220 g were purchased from the Experimental Animal Centre of Dalian Medical University(Dalian,China) for pharmacokinetic studies (permit number SCXK 2013-0003).The rats were fed in a temperatureand humidity-controlled room with free access to water and standard rat chow.Before each experiment,rats were fasted overnight with water available before surgery and anaesthetised by pentobarbital (60 mg/kg,intraperitoneal injection) at the beginning of each experiment.All animal experiments were performed according to local institutional guidelines.
2.3.Pharmacokinetic interaction in vivo in rats
In the pharmacokinetic interaction studies,rats were randomly divided into five groups (n=4):(1) cilastatin alone(45 mg/kg);(2) cilastatin+probenecid (45 mg/kg for cilastatin and 100 mg/kg for probenecid);(3) cilastatin+imipenem(45 mg/kg for both);(4) imipenem alone (45 mg/kg);and(5) imipenem+probenecid (45 mg/kg for imipenem and 100 mg/kg for probenecid).Blood samples were collected from the jugular vein at 1,5,10,30,60,120,240,360,and 480 min after administration.The bladder was cannulated with polyethylene tubing,the distal end of which flowed into an Eppendorf tube resting on a small pad of ice.Urine was collected directly from the bladder at 2,4,6,and 8 h after administration.The concentration of imipenem and cilastatin was measured by the liquid chromatography-tandem mass spectrometry (LC–MS/MS).The cumulative urinary excretion and renal clearance was calculated.
2.4.In vitro uptake in rat kidney slices
A ZQP-86 tissue slicer (Zhixin Co.Ltd.,China) was used to cut kidney cortical tissues into slices as previously described [8].After pre-incubation for 3 min under a carbogen atmosphere at 37 °C in 6-well culture plates with gentle shaking,kidney slices were transferred to 24-well culture plates containing 1 ml fresh oxygenated buffer with imipenem (100 μM) and/or cilastatin (100 μM) for further incubation at 37 °C or 4 °C and gently shaken.In the inhibition assay,probenecid (200 μM),PAH (200 μM),PCG (200 μM),and TEA (200 μM) were added to buffer with imipenem (100 μM) or cilastatin (100 μM).The uptake of imipenem and cilastatin was measured at 0,1,5,10,15,and 20 min.At the end of the incubation period,kidney slices were washed with ice-cold Hanks’ balanced salt solution (HBSS;pH 7.5),then dried with filter papers.After homogenisation,the accumulated concentrations of these drugs in kidney slices were determined as described following.The Krebs-bicarbonate slicing buffer consisted of 120 mM NaCl,16.2 mM KCl,1 mM CaCl2,1.2 mM MgSO4,and 10 mM NaH2PO4/Na2HPO4,adjusted to pH 7.4.
2.5.In vitro transporter uptake assays
Human OAT1 (hOAT1)-HEK293 and human OAT3 (hOAT3)-HEK293 transfected cells and mock cells were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) with antibiotics in an atmosphere of 5% CO2/95% air at 37 °C.Cultured cells were washed three times and pre-incubated in the transport buffer (containing 118 mM NaCl,23.8 mM NaHCO3,4.8 mM KCl,1.0 mM KH2PO4,1.2 mM MgSO4,12.5 mM HEPES,5.0 mM glucose,and 1.5 mM CaCl2,pH 7.4) for 15 min at 37 °C.The uptake assay was initiated by removal of the medium and the addition of 1 ml transport buffer containing imipenem (100 μM) and/or cilastatin (100 μM) in or not in the presence of PAH (200 μM)and PCG (200 μM),then gently shaken at 37 °C.At 1,3,5,10,15,and 30 min,the medium was removed,and the cells were washed three times with 1 ml of ice-cold Hank’s balanced salt solution (HBSS) to terminate the uptake assay.Subsequently,the cells were lysed and collected.According to the uptake of imipenem and cilastatin in the indicated time in hOAT1-and hOAT3-HEK293 cells,1 min was selected as the linear uptake time.The time of 1 min was used to examine the concentration-dependence uptake of imipenem and cilastatin and the effects of imipenem on the uptake of cilastatin.Samples were then analysed by LC–MS/MS.The uptake of PAH (10 μM) and ES (10 μM) in hOAT1-and hOAT3-HEK293 transfected cells and mock cells at 1,3,5,10,and 15 min was detected to investigate the cellular functions.
2.6.Small interfering RNAs (siRNA) assay
siRNA oligos for DPEP1 were designed and synthesised by General Biosystems,Inc.(Anhui,China).For knockdown experiments,siRNA oligos were diluted to a concentration of 50 nM and transfected by lipofectamine 2000 (Invitrogen)according to the manufacturer’s protocol.The sequence of DPEP1 siRNA was as follows:
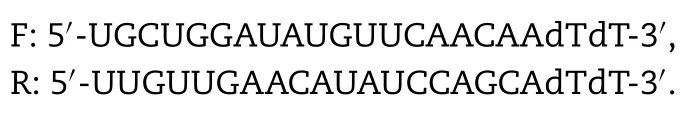
After 48 h,the expression of DPEP1 mRNA and protein in hOAT1-and hOAT3-HEK293 transfected cells and mock cells was measured.Transporter uptake assays in hOAT1-and hOAT3-HEK293 transfected cells and mock cells were conducted using the aforementioned methods to detect cellular functions and the changes of uptake of imipenem when imipenem and cilastatin were co-administered.
2.7.Quantitative real-time PCR (qRT-PCR)
Gene expression was examined by qRT-PCR as described previously [9].5 ×105hOAT1-and hOAT3-HEK293 transfected cells and mock cells were seeded in each well of 6-well plates,and then the indicated drugs were added for 48 h.Total RNA was extracted by the RNAReagent Kit(Takara Biotechnology,Japan),and 1 μg total RNA was applied to reverse transcription for cDNA.cDNA was amplified and detected using thePremix Ex TaqTMKit (Takara Biotechnology,Japan) by ABI7500 Real-Time PCR System (Applied Biosystems,USA).Relative quantification of gene expression of DPEP1 was calculated by the 2?△△ Ctmethod.The primers used for qRT-PCR were as follows:
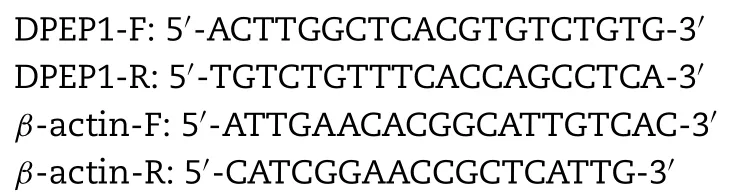
2.8.Western blotting analysis
Protein expression was detected by western blot analysis following a previous description [7].5 ×105/well hOAT1-and hOAT3-HEK293 transfected cells and mock cells were seeded in 6-well plates and treated with indicated drugs for 48 h.Cell lysates were prepared,separated by sodium dodecylsulfate-polyacrylamide gel electrophoreseis (SDSPAGE),transferred onto nitrocellulose membranes,and immunoblotted with anti-β-actin,anti-DPEP1.The protein bands were detected by the ChemiDocTM XRS+Imaging system (Bio-Rad).Quantification of protein expression was analysed with Image LabTMSoftware (Bio-Rad).
2.9.Biological sample preparation and data analysis
Various biological samples were prepared as previously described [10].A 50-μl aliquot sample (plasma,urine,kidney homogenated samples,or cell lysates) was added to 200 μl of acetonitrile and was mixed and vortexed for 1 min and centrifuged at 15 000 rpm for 15 min to remove the protein precipitate.The upper layer was transferred into a new polythene tube and evaporated to dryness under a gentle stream of nitrogen at 37 °C.Then,the dried residue was redissolved with 200 μl of the mobile phase solution.Urine samples were diluted 20 times with the same mobile phase.Kidney slices were mixed with 300 ml of normal saline after weighing,and were homogenised (IKA-T10 homogeniser) on ice.Finally,a 10-μl aliquot was used for LC–MS/MS analysis.
The main pharmacokinetic parameters of imipenem and cilastatin were calculated automatically using the Practical Pharmacokinetic Program (3P97) edited by the Chinese Mathematical Pharmacological Society.
2.10.LC–MS/MS analysis
The Agilent HP1200 liquid chromatography system (Agilent Technology Inc.,CA,USA) and API 3200 triple-quadrupole mass spectrometer (Applied Biosystems,CA,USA) operated with a TurboIon spray interface in positive ion mode were used for LC-MS/MS analysis.Chromatographic separation was performed on an Eclipse XDB-C8column(150 mm ×4.6 mm,5 μm;Agilent Technology Inc.,CA,USA)at ambient temperature.The mobile phase consisted of acetonitrile and water with 0.1% (v/v) formic acid (40:60,v/v)at a flow rate of 0.5 ml/min.The parameters of the ESI source were optimised under the actual chromatographic conditions.Multiple reactions monitoring (MRM) mode was used to detect the compound of interest.The selected transitions ofm/zwerem/z300.10 → 126.10 for imipenem,m/z359.20 → 97.40 for cilastatin,m/z193.00 → 149.00 for PAH,andm/z348.90 → 268.90 for ES.Data acquisition and analysis were performed with Analyst software (version 1.4.1).
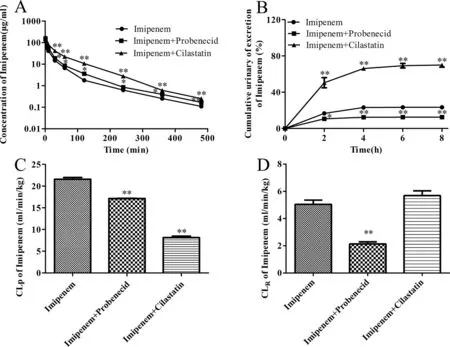
Fig.1–Mean plasma concentration-time curves (A),cumulative urine excretion curves (B),plasma clearances (CL P) (C) and renal clearances (CL R) (D) of imipenem after intravenous administration of imipenem and probenecid or cilastatin in rats.Data are expressed as mean ±SD (?P < 0.05;??P < 0.01 compared with control;n=4).
2.11.Statistical analysis
Statistical analysis was performed using the SPSS 13.0 software.All test results were mean ±standard deviation (SD)One-way analysis of variance (ANOVA) was used to test for statistically significant differences among various groups.In all statistical analyses,P<0.05 orP<0.01 was considered to be statistically significant.
3.Results and discussion
3.1.In vivo pharmacokinetic DDI between imipenem and cilastatin in rats
Antibiotics are used to prevent,treat,and control,bacterial infection.They are often combined with other drugs to treat disease more effectively [11].Thus,they are often prepared as compound preparations based on beneficial DDI.For instance,piperacillin/tazobactam,an intravenousβ-lactam/βlactamase inhibitor combination,is widely used to treat various infections [12].Tazobactam inhibitedβ-lactamase is used to reduce the hydrolysis of piperacillin,resulting in the increased plasma concentrations of piperacillin [13].In addition to the DDI mediated byβ-lactamases,the DDI that is mediated by OAT1 and OAT3 contributes to the efficacy of piperacillin/tazobactam [14].This indicated that the transporters had a significant effect in compound preparations,which was often ignored.
Most antibiotics are eliminated by kidney and biliary excretions,and this process mainly depends on renal or biliary tubular secretion aided by transporters [15].OATs mediate drug and toxicant disposition and affect pharmacokinetics and pharmacodynamics [16].The OATs are considered to be one major group of transporters central to these renal DDIs[17].For instance,probenecid is used to decrease the OAT1-and OAT3-mediated renal elimination of penicillin and otherβ-lactam antibiotics [18].
A combination of the carbapenem antibiotic imipenem and DPEP1 inhibitor cilastatin has been used for many years as a potent antibacterial combination for the treatment of serious infections [19].Probenecid could increase the AUC of imipenem and reduce the renal excretion of imipenem.Whereas,cilastatin is an inhibitor of OAT1 and OAT3.However,whether there is a DDI between imipenem and cilastatin mediated by OAT1 and OAT3 remains unclear.This study,which focused on transporters,was conducted to find a supplement to the traditional mechanism of inhibiting DPEP1.
To determine whether probenecid changed the plasma concentrations and the cumulative urinary excretions of imipenem and cilastatin,imipenem and probenecid,and cilastatin and probenecid,were co-administered intravenously.The plasma concentrations of imipenem and cilastatin were increased markedly compared with that in the imipenem or cilastatin alone group (Fig.1 A,and Fig.2 A).Furthermore,the area under theAUCand T 1/2βof imipenem or cilastatin in the co-administration groups increased (Table 1),and the plasma clearance rate (CLp) of imipenem or cilastatin in the co-administration groups decreased (Table 1;Figs.1 C,and 2 C).Cumulative urinary excretions over 8 h and renal clearance rate (CLR) of imipenem or cilastatin with probenecid were significantly decreased compared with the imipenem or cilastatin alone group (Figs.1 B,2 B,1D and 2D;Table 1).These findings indicated that probenecid inhibited the eliminations of imipenem and cilastatin.Some of the results were the same as the findings of Norrby SR [5].These findings indicated that the eliminations of imipenem and cilastatininvivocould be related to OATs.To examine the interaction between imipenem and cilastatin,the drugs were co-administered intravenously.When imipenem and cilastatin were coadministered,the plasma concentrations and theAUCof cilastatin were significantly increased (Fig.2 A;Table 1);renal clearance and cumulative urinary excretion of cilastatin were decreased significantly compared with the cilastatin alone group (Fig.2 B and 2D;Table 1).These results suggested that imipenem inhibited the renal excretion of cilastatin.When imipenem and cilastatin were co-administered intravenously,the plasma concentrations and the AUC of imipenem were also significantly increased (Fig.1 A;Table 1);however,the cumulative urinary excretion of imipenem was increased significantly compared with the imipenem alone group(Fig.1 B).This is possibly because cilastatin inhibited DPEP1 and avoided the hydrolysis of imipenem.The content of imipenem in plasma and urine was increased.However,theCLRof imipenem was not influenced by the co-administration(Fig.1 B and 1D).This was because its AUC was also increased.
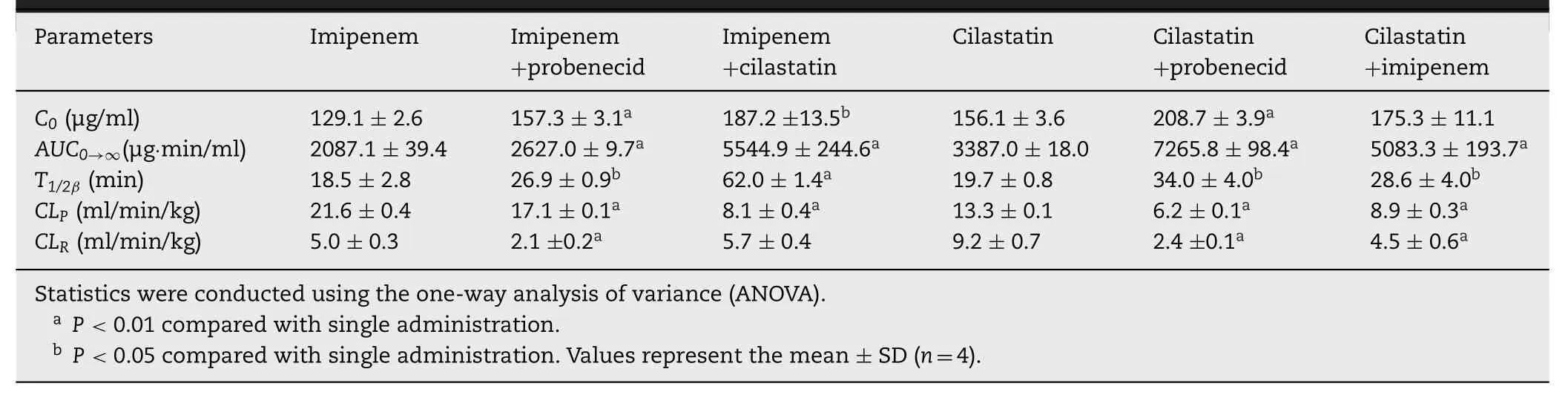
Table 1–Pharmacokinetic parameters of imipenem and cilastatin following i.v.administration.
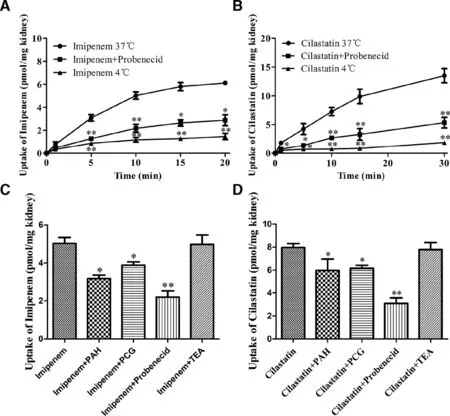
Fig.3–The uptake of imipenem and cilastatin in rat kidney slices.The inhibition effect of probenecid (200 μM) on the uptake of imipenem (100 μM) (A) and cilastatin (100 μM) (B) in kidney slices in time-dependent manner.Inhibition effects of PAH (200 μM),PCG (200 μM),probenecid (200 μM) and TEA (200 μM) on the uptake of imipenem (100 μM) (C) and cilastatin(100 μM) (D) in kidney slices.Data are expressed as mean ±SD (?P < 0.05;??P < 0.01 compared with control;n=3).
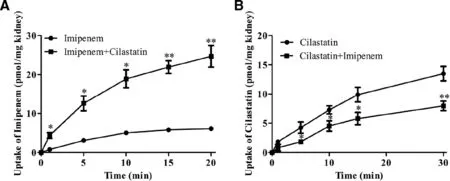
Fig.4–The DDI between imipenem (100 μM) and cilastatin (100 μM) in rat kidney slices.Data are expressed as mean ±SD(?P < 0.05;??P < 0.01 compared with control;n=3).
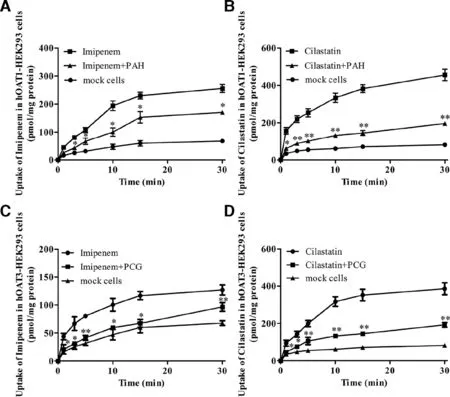
Fig.5–The time-dependent inhibitory effects of PAH (200 μM) and PCG (200 μM) on imipenem (100 μM) and cilastatin(100 μM) uptake in mock,hOAT1-HEK293 cells (A and B) and hOAT3-HEK293 cells (C and D).Data are expressed as mean ±SD (?P < 0.05;??P < 0.01 compared with control;n=3).
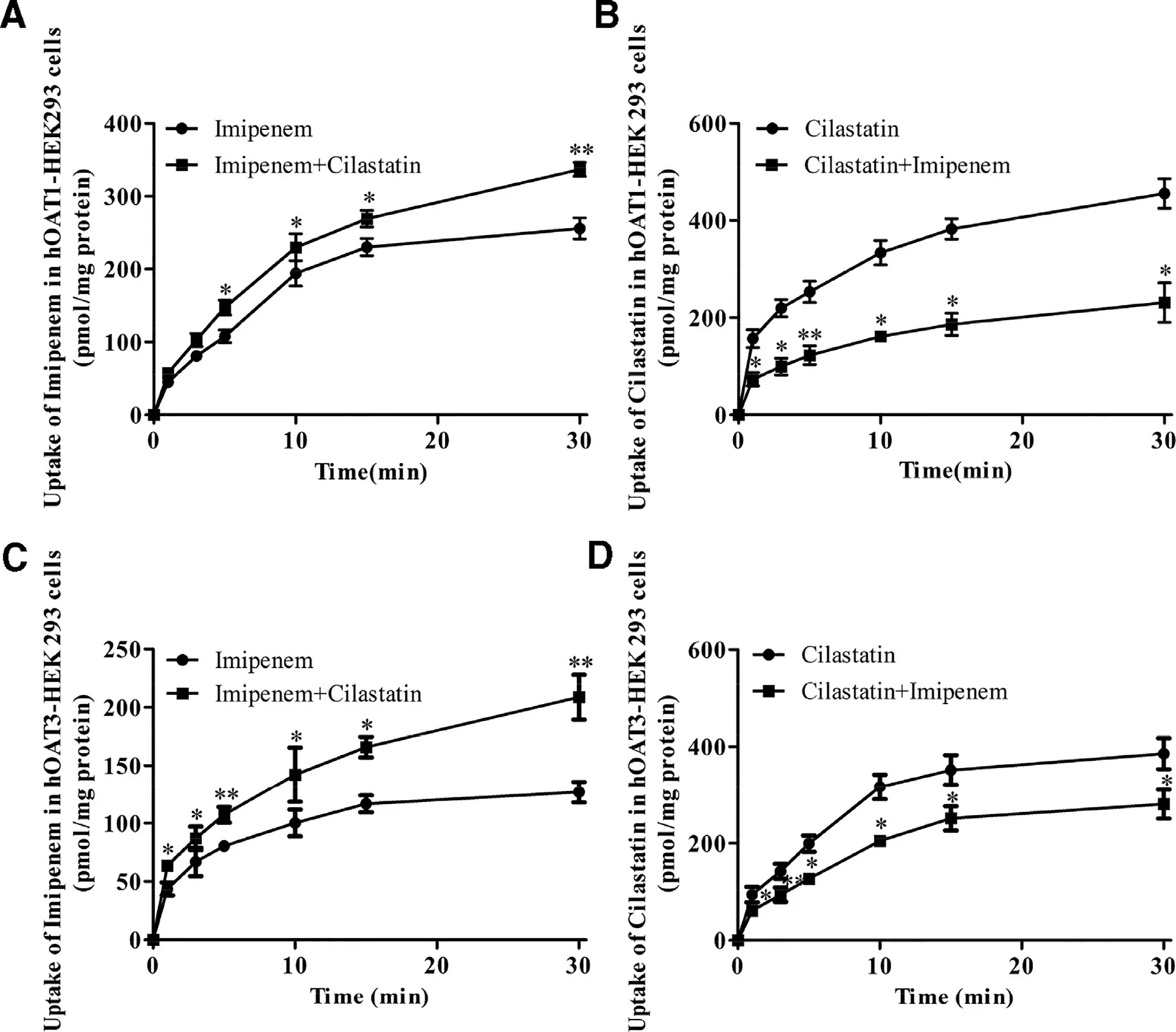
Fig.6–The DDI between imipenem (100 μM) and cilastatin (100 μM) in hOAT1-HEK293 cells (A and B) and hOAT3-HEK293 cells (C and D).Data are expressed as mean ±SD (?P < 0.05;??P < 0.01 compared with control;n=3).
3.2.In vitro uptake DDI between imipenem and cilastatin in rat kidney slices
To further investigate whether the target of the DDI is located in the kidney,fresh rat kidney slices were used to investigate the uptake of imipenem and cilastatin.The uptakes of imipenem and cilastatin were significantly higher at 37 °C compared with 4 °C and inhibited by probenecid (Fig.3 A and B).This indicated that the uptake processes of imipenem and cilastatin in rat kidney slices were temperature-related,and OATs-mediated transportation might be involved in the processes.PAH (a substrate of OAT1),PCG (a substrate of OAT3),and TEA (a substrate of OCTs) [20]were employed to clearly find out which transporters mediated the transport of imipenem and cilastatin.The results indicated that PAH,PCG,and probenecid but not TEA inhibited the uptake of imipenem (Fig.3C) and cilastatin (Fig.3 D).Therefore,the uptake of imipenem and cilastatin was mediated by OATs rather than OCTs in rat kidneys,at least in part.
When imipenem and cilastatin were co-administered compared with the imipenem or cilastatin alone groups,the uptake of cilastatin was decreased (Fig.4 B).But,the uptake of imipenem was increased (Fig.4 A).The results were consistent with the experimentinvivo.DPEP1,known as membrane dipeptidase,microsomal dipeptidase,or renal dipeptidase,is a zinc-dependent metallopeptidase that hydrolyses a variety of dipeptides and is present in a number of tissues including the kidney,lungs,and intestine [21,22].We indicated that cilastatin inhibited DPEP1 to increase the stability of imipenem;thus,the uptake of imipenem in kidney was increased (Fig.4 A).
3.3.DDI between imipenem and cilastatin in hOAT1 and hOAT3-HEK293 cells
First,to examine whether imipenem and cilastatin were transported by hOAT1 and hOAT3,we used mock hOAT1-and hOAT3-HEK293 cells to investigate the uptakeof imipenem and cilastatin.The uptake of imipenem(Fig.5 A and 5C) and cilastatin (Fig.5B and 5D) in hOAT1-and hOAT3-HEK293 cells was significantly higher than that in mock cells and was significantly inhibited following the addition of PAH and PCG.The results both in kidney slices and in hOAT1-and hOAT3-HEK293 cell experiments confirmed that imipenem and cilastatin were substrates of hOAT1/3.
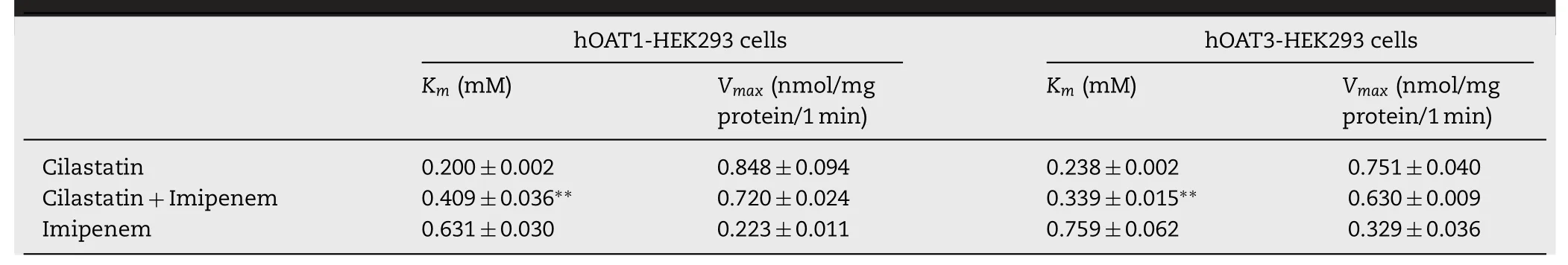
Table 2–The K m and V max values of imipenem and cilastatin in hOAT1/hOAT3-HEK293 cells.Values are mean ±SD(??P < 0.01 vs control,n=3).
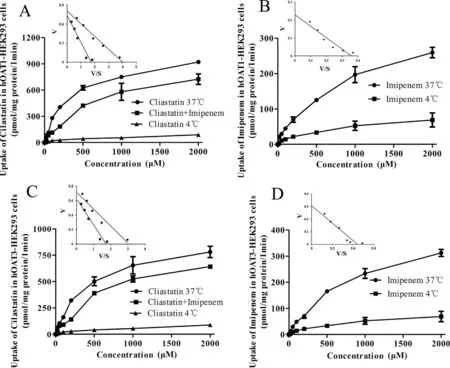
Fig.7–The inhibitory effect of imipenem (100 μM) on the concentration-dependent profile of uptake of cilastatin(0–2000 μM) (A and C) and the concentration-dependent profile of the uptake of imipenem (0–2000 μM) (B and D) by hOAT1/3-HEK293 cells.Insets:Eadie-Hofstee plots of imipenem and cilastatin uptake,V,uptake rate (nmol/mg protein/min);S,concentration of imipenem and cilastatin (mM).Data are expressed as mean ±SD (n=3).
The DDI between imipenem and cilastatin was also reviewed in hOAT1-and hOAT3-HEK293 cells.When imipenem and cilastatin were co-administered compared with the imipenem or cilastatin alone group,the uptake of cilastatin in hOAT1-and hOAT3-HEK293 cells was decreased by imipenem (Fig.6 B and 6D).But,the uptake of imipenem was increased by cilastatin in hOAT1-and hOAT3-HEK293 cells (Fig.6 A and 6C).As we know,hOAT1-and hOAT3-HEK293 cells were the human embryonic kidney 293 (HEK293) cells stably transfected with hOAT1 or hOAT3 [14],and DPEP1,a kidney membrane enzyme,was highly expressed in the kidney.Therefore,the increase of imipenem may be due to inhibiting DPEP1 by cilastatin.The results were consistent with that in kidney slices (Fig.4 A).
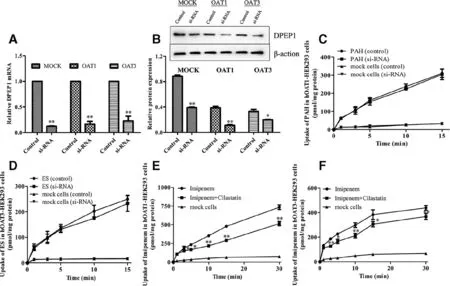
Fig.8–DDI mediated by OAT1/3 between imipenem and cilastatin in mock/hOAT1-/ hOAT3-HEK 293 cells with DPEP1 siRNA.mRNA (A) and protein (B) expression of DPEP1 in mock/ hOAT1-/hOAT3-HEK293 cells.Cells were treated with si-RNA of DPEP1 for 48 h.The uptake of PAH (10 μM) and ES (10 μM) in mock/hOAT1 (C) and hOAT3-HEK293 cells (D).The uptake of imipenem (100 μM) in the presence and absence of cilastatin (100 μM) in mock/hOAT1 (E) and hOAT3-HEK293 cells (F) when DPEP1 was silenced.Values are expressed as mean ±SD (?P < 0.05;??P < 0.01 compared with control;n=3).
Subsequently,to clarify the affinity of imipenem and cilastatin on hOAT1 and hOAT3 and the inhibitory type of imipenem on cilastatin,the concentration-dependent uptake of imipenem in hOAT1-and hOAT3-HEK293 cells and the effect of imipenem on the concentration-dependent uptake of cilastatin in hOAT1-and hOAT3-HEK293 cells were examined and Eadie–Hofstee plot analysis (Fig.7).The K m and Vmax values of imipenem for hOAT1 were 0.631 ±0.030 mM and 0.223 ±0.011 nmol/mg protein/min,and for hOAT3 were 0.759 ±0.062 mM and 0.329 ±0.036 nmol/mg protein/min,respectively (Fig.7 B and 7D;Table 2).The K m values of cilastatin uptake by hOAT1-and hOAT3-HEK293 cells were significantly increased with unchanged Vmax values in the presence of imipenem (Fig.7 A and 7C;Table 2).This indicated that imipenem inhibited the uptake of cilastatin in hOAT1-and hOAT3-HEK293 cells in a competitive way as a substrate of hOAT1 and hOAT3.Of course,when we calculated the K m of imipenem for hOAT1 and hOAT3,we ignored the metabolic effects.In fact,DPEP1 is also expressed on the cell membrane.If enzyme metabolism is excluded,imipenem’s K m may be smaller.Overall,hOAT1 and hOAT3 were the target transporters involved in DDIs between imipenem and cilastatin in the kidney.
3.4.DDI mediated by hOAT1 and hOAT3 between imipenem and cilastatin in mock/hOAT1-/hOAT3-HEK293 cells with DPEP1 siRNA
To understand the effect of cilastatin on the uptake of imipenem in mock/hOAT1-/hOAT3-HEK293 cells by removing the impact of DPEP1,we used RNAi technology to silence DEPE1.The expressions of DPEP1 mRNA (Fig.8 A) and protein(Fig.8 B) were significantly decreased when DPEP1 was silenced by siRNA oligos for 48 h in mock/hOAT1-/hOAT3-HEK293 cells.
To examine whether DPEP1 siRNA affected cellular functions,the uptake of PAH and ES (a typical substrate of hOAT3) [23]in mock/hOAT1-/hOAT3-HEK293 cells with or without DPEP1 siRNA for 48 h was detected.The results showed that there is no difference in the uptake in cells(Fig.8C and 8D).Then,imipenem and cilastatin were coadministered in mock/hOAT1-/hOAT3-HEK293 cells when DPEP1 was silenced.Compared with the imipenem alone group,the uptake of imipenem in the co-administrated group was decreased by cilastatin in hOAT1-and hOAT3-HEK293 cells when DPEP1 was silenced (Fig.8E and 8F).The results were not the same as before (Fig.6 A and 6C),indicating that cilastatin inhibited the uptake of imipenem by hOAT1 and hOAT3.
Above all,these results indicated that besides the traditional pharmacological mechanism of inhibiting DPEP1 in imipenem/cilastatin compound preparation,imipenem could increase theAUCof cilastatin by inhibiting the renal elimination of cilastatin mediated by OATs.Then,the increase of cilastatin further enhanced the stability of imipenem by inhibiting the uptake of imipenem in kidney in a competitive manner and avoiding the hydrolysis of DPEP1.
Our findings show the DDI mediated by OATs between imipenem and cilastatin,which is a useful supplement to the traditional theory and can affect the clinical safety and rational application of imipenem in at least the following two aspects.First,be alert to the DDI mediated by OATs.The instructions indicate that the dose of imipenem/cilastatin needs to be adjusted for renal function.This can be explained by our findings.Factors such as disease and age can reduce the expression and function of OATs [24–26],thereby reducing the intake of imipenem/cilastatin and leading to the accumulation of drugs in the circulation and the generation of clinical adverse events.In addition,the drugs such asβ-lactam antibiotics,angiotensin-converting enzyme inhibitors (ACEIs),and sartan can inhibit the renal excretion of imipenem/cilastatin mediated by OATs [6].Therefore,our findings clarify the critical role of OATs in renal disposal of imipenem/cilastatin and avoid the potential adverse reactions mediated by OATs.Second,OATs may be involved in imipenem’s neurologic adverse reactions.The study found that OAT3 was involved in the brain efflux process of the substrates [27].However,cilastatin may inhibit OATs and lead to imipenem’s retention in the brain.This also explains the high incidence of adverse reactions of imipenem in the nervous system,especially when combined with renal dysfunction.Therefore,we should be fully aware of the advantages and disadvantages of the DDI mediated by OATs between imipenem and cilastatin.In clinical application,factors such as renal function and combination of drugs were considered to achieve individualised administration.
Under physiological conditions,due to the full metabolism of imipenem,the role of OAT is easy to overlook.In fact,ingestion is often a rate-limiting process for kidney and liver disposal.The DDI mediated by OATs between imipenem and cilastatin slows down imipenem’s kidney clearance process.The retention time of imipeneminvivois prolonged and the antibacterial effect of imipenem is improved.On the other hand,the DDI based on DPEP1 increases the recovery rates of imipenem,making it possible for imipenem to be applied to urinary system infections.Therefore,we think both mechanisms are important to the efficacy of imipenem.Based on the current results,it is difficult to account for the respective contribution rates of OATs and DPEP1 in DDI.We will design animal models of normal and kidney infection and clarify the respective contribution rates of OATs and DPEP1 in DDI throughinvitroandinvivoexperiments.This is the topic that we will continue to study in the future.
4.Conclusions
In conclusion,this research demonstrated that imipenem and cilastatin are substrates of OAT1 and OAT3.We also found that OAT1 and OAT3 mediate the DDI between imipenem and cilastatin.It may be a supplement to the traditional pharmacological mechanism of imipenem/cilastatin compound preparation.Our findings provide to the possibility of making a compound preparation through the DDI mediated by transporters to increase efficacy and reduce side effects.
Conflict of interest
The authors declare that we have no conflicts of interest.
Acknowledgments
The work was supported by a grant from the National Natural Science Foundation of China (No.81874324,81473280,U1608283),the Natural Science Foundation of Liaoning (No.20170540293) and Dalian Science and technology innovation fund (No.2018J12SN065).The authors thank Prof.Yuichi Sugiyama (Sugiyama Laboratory,RIKEN,Japan) and Dr.Likun Gong (Shanghai Institute of Materia Medica,Chinese Academy of Science) for kindly providing mock/hOAT1/3-HEK293 cells.
 Asian Journal of Pharmacentical Sciences2020年2期
Asian Journal of Pharmacentical Sciences2020年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- Special topic:Emerging role of transporters in drug interaction and delivery
- The influence of genetic polymorphisms in drug metabolism enzymes and transporters on the pharmacokinetics of different fluvastatin formulations
- Chronic exposure to excess iron promotes EMT and cancer via p53 loss in pancreatic cancer
- Research and development of drug delivery systems based on drug transporter and nano-formulation
- Glutamine transporters as pharmacological targets:From function to drug design
- Amino acid transporters:Emerging roles in drug delivery for tumor-targeting therapy
