Mn2+-doped CsPbX3 (X=Cl, Br and I) perovskitenanocrystals and their applications
LIU Hui-wen, YAO Dong, LIU Yi, ZHANG Hao
(State Key Laboratory of Supramolecular Structure and Materials,College of Chemistry,Jilin University,Changchun 130012,China)
*Corresponding author, E-mail:hao_zhang@jlu.edu.cn
Abstract: Colloidal Mn2+ doped CsPbX3(X=Cl, Br, I) nanocrystals(NCs) are being explored extensively as alternative emitting materials, wherein highly efficient optical and optoelectronic processes can be achieved. Mn2+ doping in perovskite NCs also reveals several new fundamental aspects of doping and new dopant-induced optical properties through different methods of synthesis. Mn2+ doping exists in wide-band-gap perovskite hosts where the excitation energy is transferred to an Mn d-state, resulting in short-range tunable yellow-orange d-d emissions. Enormous efforts have been expended on understanding the doping process and designing highly efficient doped NCs. The unique electronic and fluorescent properties endow these Mn2+ doped perovskite NCs with various optoelectronic applications in light-emitting diodes(LEDs) and solar cells. Combining all these facts, this review focuses on the recent progress in synthesis methods, emission mechanism, and potential applications of Mn2+ doped CsPbX3 perovskite NCs and provides an outline for plausible future studies.
Key words: perovskite;fluorescence;Mn2+ doped;CsPbX3 perovskite nanocrystals
1 Introduction
Colloidal all-inorganic cesium lead halide(CsPbX3) perovskite nanocrystals(NCs) have become a subject of intense research in recent years due to their superb luminescence properties and facile chemical tunability of the bandgap, which have shown great potential for outperforming commonly used II-VI and III-V NCs in their ability to harvest photons, create charge carriers, and efficiently generate photons from the recombination of charge carriers[1-13]. As an already-demonstrated strategy for controlling over the electronic and optical properties of semiconducting NCs, doping impurity ions into NC hosts has been applied in traditional II-VI and III-V NCs[14-19]. Specifically, doping with transition metal ions has been extensively explored as a way to introduce the possibility of creating a charge and size imbalance center in the host lattice with new optical, electronic, and magnetic properties, making them much more functional than their host NCs[20-22]. A variety of impurity dopants, including Mn2+, Cu2+, Ag+, Co2+, and Eu2+, have been incorporated to improve their original properties[23-25]. For instance, Mn2+can generate intense sensitized dopant luminescence and create a magnetically coupled excitonsstate[16-19]. These new properties of Mn2+-doped NCs result from the exchange coupling between the charge carriers of the host semiconductor and d electrons of the dopant, which opens new pathways of energy exchange or forms new coupled electronic states between the exciton and dopant[14-15]. The above properties make Mn2+doped NCs attractive for light-emitting diodes(LEDs), luminescent solar concentrators, and related photonic technologies[26-28].
Based on the increased understanding of the doping mechanism, Mn2+doping is now performed in CsPbX3(X=Cl, Br and I) perovskite NCs[29-31]. Mn2+ions, occupying the substitution position, can also be doped in CsPbX3NCs stably with sufficiently strong exchange coupling between the charge carriers. This would preferably be incorporated during perovskite NCs formation, which introduces new optical, electronic and magnetic properties[32]. Importantly, the intrinsic ionic characteristics and flexibility of the perovskite crystal structure of CsPbX3NCs allow for the strong possibility of Mn2+ions being doped in hosts to tune their properties, which has already attracted extensive interest[33-34]. The first successful doping of Mn2+ions in CsPbX3NC host was demonstrated in CsPbCl3, which was achieved by a simple modification of the usual hot-injection synthesis method,i.e., adding MnCl2as an additional reactant[29]. Subsequently, various other methods were developed to synthesize Mn2+-doped CsPbX3NCs in a wider range of doping levels[35-38]. Similarly, the high-energy host emission is switched to Mn yellow-orange emission; however, they reveal different doping paths and several new findings[39-45]. Until now, synthesis of Mn2+-doping in CsPbX3NCs remains one of the key issues for developing new optical and electronic properties, which attracts increasing attention[45-48].
In this review, we mainly focus on the progress and challenges of Mn2+doped CsPbX3perovskite NCs. Recent developments, applications, and setbacks of this new class of materials are summarized. Firstly, we give a brief description of the selection of Mn sources for dopant and preparation methods. Secondly, we emphasize their optoelectronic properties, doping and emission mechanisms and stability. Thirdly, the various applications of this new class of materials are reviewed. Finally, we summarize the existing challenges facing this research and give an outlook on probable ways to mitigate such challenges with our vision for the future of the Mn2+-doped perovskite NCs.
2 Synthesis Methods of Mn2+-doped CsPbX3 NCs
The most widely reported and most extendly used doping strategy in traditional semiconductor NCs is growth doping in which dopants are allowed to be absorbed onto host NCs during NCs growth[19,49-50]. Another widely accepted doping protocol is diffusion doping performed mostly via thermal annealing, in which added dopant ions substitute host ions by ion exchange and reside in the crystal lattice[16,51-52]. However, recently developed strategies for doping in perovskites suggest that a dopant precursor is required at the beginning of the reaction, which does not follow conventional nucleation doping[29-31]. In the case of doped perovskite NCs, the most widely used strategy is reportedly simultaneous formation[29-35]. Hence, Mn precursors are introduced along with Pb precursors at the beginning. Mn2+-doped CsPbX3NCs can be achieved using various methods similar to the traditional semiconductor NCs, which are summarized as follows.
2.1 Hot-injection Synthesis
Colloidal synthesis of halide perovskite NCs at relatively high temperatures of 140-200 ℃ was firstly reported by Protesescuetal.[53]. This synthesis process is illustrated in Fig.1(a). This method takes advantage of the ionic nature of chemical bonding in CsPbX3compounds and the majority of crystal growth occurs within the first 3 seconds after the injection of Cs-oleate into the mixture of PbX2and octadecene(ODE) owing to the fast nucleation and growth kinetics[53]. This can be completed by many groups, including our group. The synthesis of Mn2+-doped CsPbX3NCs where MnX2, PbX2and oleic acid(OA), oleylamine(OLA), and ODE were completely dissolved in crude solution with the subsequent injection of Cs-oleate solution[29-31]. Our group also achieved different Mn substitution ratios by manipulating the molar feed ratios and reaction temperatures, such as a high Mn substitution ratio of 46% with high photoluminescence(PL) efficiencies of 54%[31].
2.2 Room-Temperature Synthesis
2.2.1 The Ligand-Assisted Reprecipitation Method(LARP)
The LARP at room temperature for the fabrication of halide perovskite NCs was developed by Zhang and co-workers(Fig.1(b))[10]. Specifically, halide perovskite NCs were simply obtained by vigorously stirring a halide perovskite precursor solution including PbX2(X=Cl, Br, and I), CsX, DMF, and long-chain organic ligands such as n-octylamine and oleic acid in a poor solvent such as toluene or hexane[10]. In this way, an Mn-precursor will be added together into the DMF solution. In detail, CsX, MnX2and PbX2, OA, and OAm are dissolved in DMF or DMSO, and toluene is dropped into the resulting mixture. The lower solubility of ions compared to DMF in the toluene induces rapid recrystallization and simultaneous Mn2+doping into the NCs, where surface ligands control the size and morphology of NCs. Li and coworkers developed this room temperature synthesis method to achieve Mn2+-doped cesium lead halide quantum dots(QDs) with a high Mn substitution ratio. The low-temperature reaction prefers to occur in the metastable phase[54]. Furthermore, the as-prepared perovskite QDs exhibits bright orange emission owing to an ultrahigh level of Mn2+doping[54].
2.2.2 Post-Synthesis Strategy
Nagetal. developed a post-synthesis strategy for Mn2+doping in colloidal CsPbX3NCs(Fig.1)[55]. Firstly, CsPbX3NCs with the desired composition, size, and shape were prepared following reported protocols[53]. Then, a post-synthesis doping methodology using the precursor with MnBr2dissolved in a mixture of acetone and toluene was used, employing a 1 minute reaction at room temperature[55]. This post-synthesis doping protocol provides a unique opportunity to achieve different dopant concentrations, which eliminates most of the synthesis-related inhomogeneity to make the study of the effect of dopant concentration more reliable. Besides, this 1 min post-synthesis doping procedure can probably be extended to other sets of dopants and perovskite NCs.
2.3 Microwave-Assisted Synthesis
The microwave-assisted method plays an important part in the synthesis of various traditional semiconductor NCs, which is usually highly efficient but time- and energy-consuming(Fig.1(d))[56-60]. Our group proposed this microwave-assisted method for the synthesis of Mn-doped CsPbCl3[61]. Cesium acetate (CsOAc), bis(2,4,4-trimethylpentyl) phosphinic acid(TMPPA), PbCl2, MnCl2, and ODE can be put in one beaker with certain microwave power to initiate the reaction. It should be known that the higher reaction activity of CsOAc plays an important role in the fast reaction rate of the microwave-assisted method[61]. The Mn substitution ratio can reach 27% with the microwave-assisted power of 100 W. Other groups also developed the microwave-assisted methods for achieving high PL efficiency CsPbX3NCs covering the fully visible spectrum[62-64].
2.4 Solvothermal Synthesis
The solvothermal method has been considered as the most promising route for the preparation of various NCs due to its simple procedure, precise control over morphology, high crystallinity and easy reproduction[65-68]. Li′s group developed a facile solvothermal strategy to synthesize Mn2+-doped CsPbCl3NCs, which shows better stability than those fabricated by hot injection(Fig.1(e))[69].
2.5 Photo-Induced Synthesis
Inspired by the developed CH2X2(X=Cl, Br) photo-induced anion exchange, Qiaoetal. demonstrated that photo excitation results in the cation exchange and the formation of Mn2+-doped CsPbX3NCs in the presence of a small amount of dissolved Mn acetate in CH2X2[70].
Overall, the two-step standard hot-injection synthesis method has reached its maturity and can now be used to produce various monodisperse Mn-doped perovskite NCs with excellent control over the shape of the NCs. While the aforementioned hot-injection methods are particularly appropriate for producing Mn-doped perovskite NCs samples with a high degree of control, they have two main draw backs:the synthesis needs to be performed in air-free conditions and it is hard to employ them for large-scale production. These problems can be avoided by employing alternative synthesis routes, such as the LARP and the heat-up “related” approaches(microwave and solvothermal synthesis methods), which can yield NCs ingram scale even under air atmosphere. More in details, the heat-up, solvothermal, photo-induced and microwave techniques can easily be used to produce mainly Mn-doped CsPbX3NC systems in large quantities and with high PLQYs.
3 Selection of Mn Precursor Sources for Doping
In the synthesis of Mn2+-doped perovskite NCs, Mn precursor powders are usually introduced along with Pb precursor at the beginning of the procedure[29-31]. The proper Mn precursor seems to be essential for the doping process. Firstly,among various manganese(II) salts, MnX2(X=Cl, Br, and I) is given priority in doping perovskite NCs due to its ability to easily break the Mn-X bond for further doping[29-35]. The MnX2, MnCl2was proved to be the superior precursor for doping in CsPbCl3(Fig.2(a)). Our group used MnCl2to achieve the Mn2+doped CsPbCl3NCs with cube-shaped tetragonal CsPbCl3nanostructures(Fig.2(b))[31,61]. We also noticed that with higher doping efficiency, the crystalline shape of the host diminishes[31,61]. Nevertheless, we also achieved a very high Mn∶Pb precursor ratio(10∶1) for 46% Mn2+doping at 210 ℃[31]. Interestingly, Liu et al. reported that this can also be directly synthesized using MnBr2and PbCl2to achieve Mn∶CsPbCl3-xBrxNCs because the weaker Mn-Br bond would more easily be broken compared to MnI2and PbCl2[29]. On the other hand, direct doping was observed to be difficult for CsPbBr3and CsPbI3irrespective of using MnBr2and MnI2as a dopant precursor. At this point, Parobeketal. have achieved MnBr2-doped CsPbBr3NCs, but did so with an excess of HBr to supply the amount of Br(Fig.2(c))[71]. However, methods of directly doping the CsPbI3NCs with MnI2as dopant precursor have not yet been published. Rather, Br and I were incorporated via anion exchange on Mn:CsPbCl3or the synthesis was carried out in mixed halides(discussed later). Secondly, Xuetal. synthesized the Mn2+-doped CsPbCl3perovskite NCs utilizing MnAc2as an Mn precursor. HCl was an important and necessary raw material to be added in the room-temperature reactions to effectively motivate the formation of Mn-Cl bond(Fig.2(d))[72]. Thirdly, Linetal. used Mn-stearate as the Mn-precursor for the doping of Mn2+into perovskite CsPbCl3QDs via a facile colloidal hot-injection approach(Fig.2(e))[39]. Recently, high reaction activity halide precursors like benzoyl halide is used for the synthesis of high PL efficiency CsPbX3NCs(Fig.2(f))[73], and we found that high reaction activity halide sources make it possible for various Mn precursors(manganese acetate, manganese acetylacetonate, and manganese halidesetc.) to participate in the doping process. These can also achieve high PL efficiencies and high Mn substitution ratios. The selection of the Mn precursor seems to play an important part in the synthesis of Mn2+-doped CsPbX3NCs. Based on the above Mn2+-doped examples, as Mn substituted the Pb in the crystal lattice, it was evident that Mn-X bond strength in the Mn-precursor should be comparable to the Pb-X bond strength in CsPbX3for successful doping. To this point, the Mn-Cl bond is most benefit to the bond broken and further diffused into perovskite NCs lattice. Based on the above discussion, excess chloride ions mostly facilitated Mn2+doping in CsPbCl3NCs and chloride ions were the key for promoting the insertion of Mn2+in perovskite nanocrystals for room-temperature reactions. Therefore, if the Mn sources are chosen without MnX2, the extra halide ions should be added as the supplement to guarantee the Mn-doped process.
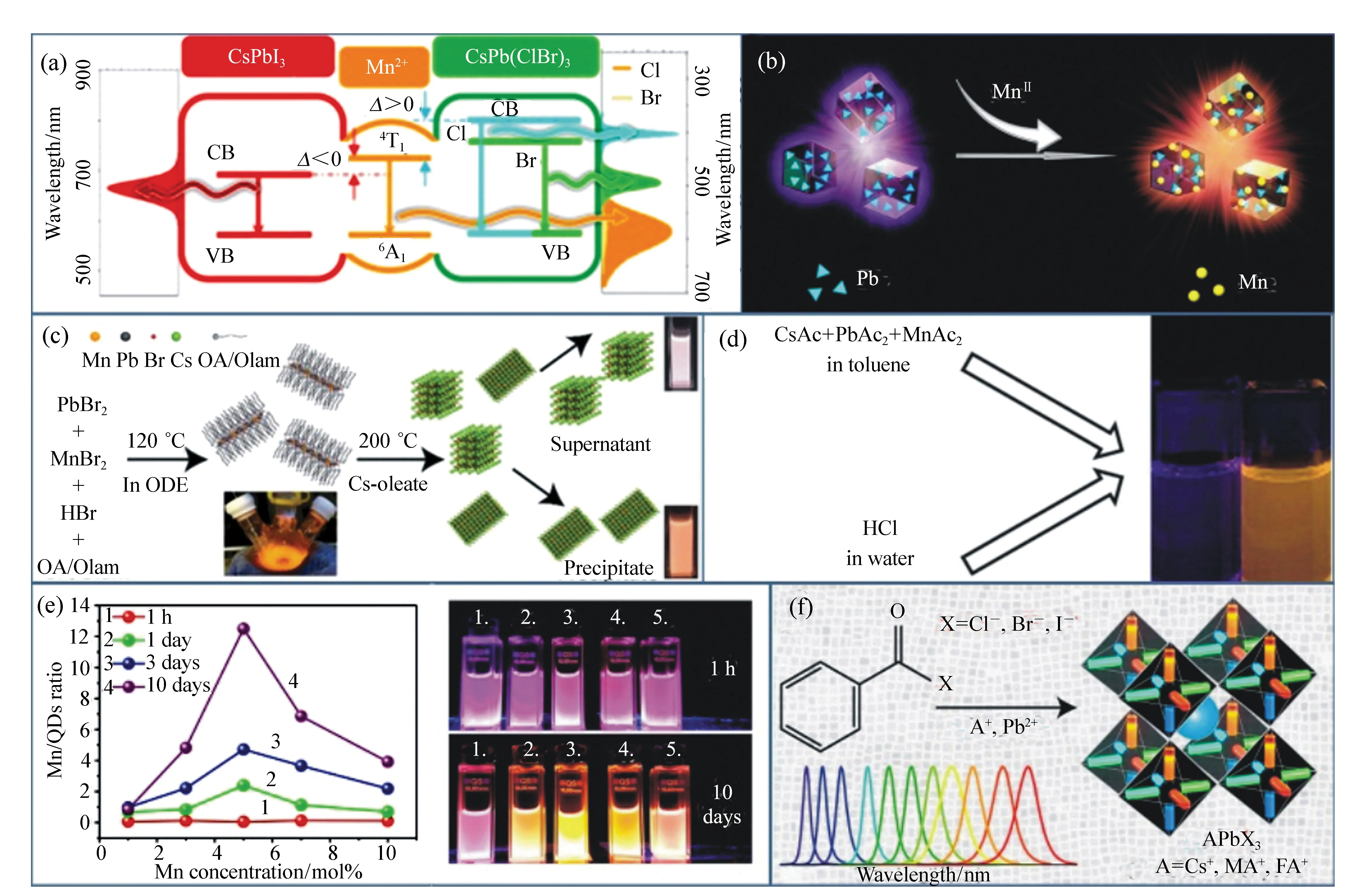
Fig.2 Summary of the selection of Mn sources for various synthesis methods of the Mn2+-doped CsPbX3 NCs. The most used MnCl2(a) and (b)MnBr2 with the aid of HBr(c) MnAc2 with the aid of HCl(d) Mn-stearate(e) manganese acetate, manganese acetylacetonate, and manganese halides with the aid of benzoyl halide(f) as the Mn sources participated in the reaction
4 Mn2+-Doped into Various Low-Dimensional Morphologies
Tremendous progress has been made in this method over the past two years. Various low-dimensional morphologies, including zero-dimensional(0D) morphologies, such as QDs and nanoparticles; one-dimensional(1D) morphologies such as nanowires and nanorods; and two-dimensional(2D) morphologies perovskite, such as nanoplatelets(NPLs) and nanosheets(NSs)(Fig.3(a) and 3(d)) are developed by more and more researchers[74-75]. In this section, we discuss the Mn2+-doped perovskite NCs that have been reported recently with controlled morphologies, such as QDs, NPLs, NSs,etc.
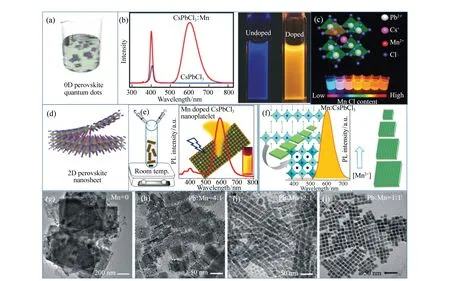
Fig.3 (a)Sketch of 0D CsPbX3 QDs, (b) and (c)Mn2+-doped 0D CsPbX3 QDs, (d)sketch of 2D CsPbX3NSs, (e) and (f)Mn2+-doped 2D CsPbX3NSs or NPLs, (g-j) TEM images of NCs with different Mn2+ substitution ratios(color version please see in the journal website)
4.1 0D Morphologies Perovskite Doping:QDs
The first synthesis of cesium lead halide(CsPbX3, X=Cl, Br, I) perovskite QDs was reported by Kovalenkoetal. in 2015 based on the traditional hot-injection methods in the presence of OLA and OA as ligands[53]. Inspired by this, Mn2+ions doping NCs are mainly focused on the 0D perovskite QDs, usually obtained by the solution phase synthesis methods. Parobek′s group, Liu′s group, and our group almost simultaneously reported colloidal Mn2+-doped CsPbCl3nanocubes, and the morphologies of as-prepared NCs showed no difference after Mn doping (Fig.3(b) and 3(c))[29-31]. Totally, Mn2+-doping into CsPbX3QDs exhibited strong dopant luminescence characteristic in the d-d transition of Mn2+ions resulting from the exciton-to-Mn energy transfer, which is similar to the case of Mn2+-doped II-VI QDs[32-33].
4.2 2D Morphologies Perovskite Doping:Nanoplatelets and Nanosheets
Perovskite materials have been included in the class of 2D semiconductor materials, mainly in the form of NPLs. However, unlike other materials of this type, which are covalent semiconductors, these 2D morphologies perovskites are also ionic materials, endowing them with special properties. Because of this, developing the Mn2+-doped 2D morphologies perovskites has the potential for unexpected energy or electron transfer owing to 2D morphologies perovskites, which can enhance fluorescence emission decay rates and higher exciton binding energies. The fabrication of perovskite NPLs through both solution-phase synthesis and vapor phase deposition techniques have been reported. For Mn2+-doped NCs, the methods mostly used are solution-phase synthesis. Nagetal. reported the colloidal Mn2+-doped cesium lead halide perovskite NPLs, which was the first report of Mn2+doping in a CsPbX3host exhibiting strong quantum confinement of charge carriers[76]. The fluorescent properties are similar to the Mn2+-doped perovskite nanocubes(Fig.3(e))[76]. These Mn2+-doped CsPbX3NPLs are suitable candidates for exploring the effects of quantum confinement on dopant-carrier exchange interaction and exhibiting interesting magneto-optic properties[76]. Kundu et al. reported Mn2+-doped 2D morphologies perovskites could be a suitable material to tune dopant-exciton exchange interactions and further explore their magneto-optoelectronic properties[77]. The successful production of these perovskite NPLs has introduced a new family of 2D semiconductors for nanoscale and printable optoelectronic devices[77]. Also, Pradhan et al. reported the dimension tunability governed by the amount of Mn composition retained in the layered structure(Fig.3(f))[78]. Totally, as Mn2+doping in perovskites have opened up a new window of tuning the optical properties for perovskites, the discussed new physical process of doping and the evolution of the dopant emission will certainly help better understand doping and its mechanism.
5 PL Emission Properties and Mechanism of the Mn2+-Doped CsPbX3NCs
5.1 Doping Emission Mechanism and Energy Transfer Process
In all the above-discussed reports of Mn2+-doped CsPbX3nanocubes, the samples all show a broad PL with a peak at around 580 nm because of the Mn2+d-d transition, along with the excitonic PL of CsPbCl3with a peak at about 405 nm(Fig.3(b)). Subsequently, we are most concerned about the doping and emission mechanism of Mn2+-doped CsPbCl3NCs. This Mn2+d-d emission is a spin-forbidden4T1to6A1transition with microsecond to millisecond lifetimes[32-33]. The host NC absorbs UV excitation light to form photo-generated excitons, which then transfer their energy to the dopant ions (Mn2+ions) forming the excited4T1state of Mn2+d-electrons, followed by4T1to6A1de-excitation process, emitting orange-red emission light[32-33]. Lietal. further illustrates the respective partial density of state(PDOS) of CsPbCl3, CsPb0.875Mn0.125Cl3and CsPb0.75Mn0.25Cl3, which shows that the conduction band and the upper valence band are mainly dominated by the electrons of the Pb(4p) and Cl(3p) orbits, while Cs seems to have no apparent contribution. This may explain the band-edge emission of CsPbCl3QDs(Fig.4(b))[54]. It also shows that the contribution of Mn(d) orbits is obvious to both the conduction band and the upper valence band. Additionally, the energy of the d-d transition in Mn2+ions is lower than the energy gap of CsPbCl3, causing energy transfer between excitons and Mn2+ions[54]. The calculation results imply that the dual-color emission is most likely caused by the band-edge emission of CsPbCl3QDs combined with the d-d transition in Mn2+ions, which is consistent with the results of the PL spectra.
Energy transfer(ET) from the exciton to Mn2+is orders of magnitude slower than that in Mn2+-doped traditional semiconductor NCs(CdS/ZnS:Mn2+). Even for high Mn2+doping concentrations, exciton emission from the perovskite host is still present. The inefficient ET has been attributed to the more ionic character of the perovskite NCs and the weaker confinement which reduces ET. To further understand the exciton-to-Mn2+ET process, Xuetal. investigate the evolution of the exciton-to-Mn2+ET efficiency as a function of composition and temperature in CsPbCl3-xBrx∶Mn2+NCs, which shows a strong dependence of the transfer efficiency on Br-content[41]. An initially fast increase is followed by a decrease for higher Br- concentrations, which are explained by a reduced exciton decay rate and faster exciton-to-Mn2+ET upon Br-substitution. Further addition of Br-makes back-transfer from Mn2+to the CsPbCl3-xBrxhost possible and lead to a decrease in Mn2+emission[41]. The full understanding of the ET transfer dynamics of Mn2+-doped perovskite NCs will aid to optimize the ET and Mn2+luminescence efficiency.
5.2 Mn2+-Doping Sites Distribution
Samanta′s group concluded that, as indicated by the electron paramagnetic resonance(EPR) spectra, the distribution of Mn2+in doped CsPbCl3NCs is uniform up to a Mn-concentration of 2% and for the higher Mn-content(>15.5%), doped Mn2+remains inside the NCs rather than on the surface[79]. In this regard, our group also gets the same conclusion in the characterization of XRD analysis[31]. When the Mn2+doping concentration is higher, Mn2+ions may also diffuse into the CsPbCl3lattice and further occupy the Pb sites. Besides, by combining the EPR pattern and Mn2+PL decay behavior, it can be concluded that in addition to the crystal field effect, the Mn-Mn exchange interaction also contributes to the red-shift of the Mn PL band at higher Mn2+dopant concentrations[79].
5.3 PL Dynamic Analysis and Photoluminescence Quantum Yields(PLQYs)
The PLQY is an important factor in determining the performance of NC-based devices. Defects and traps in NCs can act as nonradiative recombination centers to reduce the PLQYs. Luminescence decay curves can provide insights into the PLQYs, because nonradiative recombination from the excited state shortens the luminescence lifetime, and inhomogeneities or other features may result in multiexponential decay. Meijerink et al. found that elongated Mn2+PL decay and a change from multiexponential decay to single exponential decay have been observed upon growth of CsPbCl3shells around the Mn2+:CsPbCl3NCs, which separates the Mn2+ions from the NC surfaces[41].
As to the influence of PLQYs of Mn2+-doped perovskite NCs, both the Mn2+concentration and temperature have an impact on the emission of Mn2+:CsPbCl3NCs. The PLQYs of Mn2+emission in Mn2+doped CsPbX3NCs has been described in several studies and all the studies found that Mn2+concentration will influence the PLQYs. Klimov′s group shows the PLQYs were increased to a maximum value of 27% by increasing doping levels to a B-site Mn2+concentration of 9.6%[29]. Our group also reported that Mn2+∶CsPbCl3NCs showed a peak Mn2+PLQYs of 54% at 27% for Mn2+concentration, but NC crystallinity was found to deteriorate at higher Mn2+loading while the PLQY decreased[31]. So, proper Mn2+concentration plays an important part in the contribution of the doped perovskite NCs to the PLQYs. Secondly, temperature is an important variable of PL. Variable-temperature experiments can elucidate fundamental features of an NCs excited-state dynamics. Meijerinketal. reported that upon raising the temperature, the peak intensity of the Mn2+doped CsPbCl3NCs shows a continuous decrease, losing nearly 50% of peak intensity at 383 K[41]. Cui's group also shows that the increased temperature from 278 to 323 K leads to gradual PL quenching(as a result of the thermally activated trapping of charge carrier) and emission red-shift due to the thermal expansion of the crystalline lattice[44].
5.4 Anion Exchange and the Impact on Dopant Emission
Though Mn2+doping is successful in nanocubes and NPLs of CsPbCl3, it was found to be difficult in other halides counterparts like CsPbBr3and CsPbI3. Consequently, the anion exchange process is employed for converting Mn2+-doped CsPbCl3NCs to other halide systems with partial success. The doping mechanism of Mn2+in different halide compositions of CsPbX3NCs is also a key issue to be solved. The exciton energy transfer to Mn2+d-states typically depends on the host band gap and relative positions of Mn2+4T1and6A1states[32-33]. As shown in Fig.4(a), CsPbCl3is ideal for accomplishing Mn2+emission. Br incorporation, to some extent, red-shifts the absorption, but it would still be possible for Mn2+ion energy transfer[33].
Band gap and optical properties of CsPbX3perovskites are typically tuned with halide ion exchange. Hence, doping Mn2+is also extended to CsPbBr3and CsPbI3NCs, and the possible exciton energy transfer inducing Mn2+emission in these nanostructures is investigated. For Br systems, Parobeketal. achieved directly MnBr2-doped CsPbBr3NCs through an excess of HBr to supply an efficient amount of Br[71]. It can also be established by the anion exchange reaction from CsPbCl3NCs, whose ions exchange from Cl to Br with retaining Mn2+in host NCs and also reflects the change in PL spectra showing dual emission during this process. Importantly, as to the MnBr2-doped CsPbBr3or CsPbClxBr1-xNCs, the QY of dopant emission was also observed to be significantly lower than in the Cl system[29-31,71]. In comparison to CsPbCl3, the energy differences remain at a minimum between the excited stated of CsPbBr3and Mn2+. This might be one of the reasons the exciton energy is not efficiently transferred to Mn2+states, leading to poor dopant emission. On the other hand, the exciton emission intensity is significantly enhanced in Mn2+doped CsPbBr3. Klimovetal. analyzed this energy gap and correlated the possibility of both forward and backward transitions between the host and dopant states. Similar observations were also recorded for I-exchange where dopant emissions were finally quenched. These results suggest that the confined lead chloride is the most appropriate perovskite host for transferring the exciton energy to the dopant state which results in intense dopant emission.
5.5 Other Doping Phenomenon and Emission Properties
5.5.1 Phase-Transition Process
Zhao′s group developed a novel high concentration doping method based on the transformation from Cs4PbBr6NCs, when reacted with divalent metal bromide MBr2(M=Mn, Zn, and Eu), to CsPbxM1-xBr3NCs(Fig.4 )[80]. This work achieves a high M doping concentration and endows perovskite NCs with new magnetic and electron features by inserting various divalent and even trivalent metal ions.
5.5.2 Metal Ions Co-Doped Perovskite NCs
Songetal. were first to report dual ion Bi3+/Mn2+co-doped CsPbCl3perovskite NCs through the hot injection method, in which the doping concentrations for the Bi3+and Mn2+ions were carefully controlled and measured[81]. When the Bi3+and Mn2+ion doping concentrations were set at 8.7% and 2.5%, respectively, white light emission was achieved[81]. It shows great potential of single-component perovskite NCs in lighting and displays, especially for white light emission. Furthermore, co-doped CsPbX3NCs can also endow new optical-electronic properties, which paves the way for further study of metal-doped CsPbX3NCs.
5.5.3 Enhanced Stability
Mannaetal. reported fluorescent alloy CsPbxMn1-xI3perovskite NCs with high structural and optical stability. We all know the fact that CsPbI3NCs are still limited in their further application because of their phase instability as they can easily degrade into the yellow non-emitting δ-CsPbI3phase within a few days(Fig.4(e))[82]. Methods with Mn2+-doped into the CsPbI3have essentially the same optical features and crystal structure as the parent α-CsPbI3system but they are stable in films and in solutions for periods of over a month. Mannaetal. noticed that the stabilization stemming from a small decrease in the lattice parameters slightly increases the tolerance factor combined with an increase in the cohesive energy[82]. The improvement of stability is undoubtedly a major break through in the development of CsPbX3NCs, which shows the considerable advantages of Mn sources as the dopants in the perovskite NCs.
5.5.4 Oxygen sensing capability
Chenetal., first investigated the effect of O2on the luminescence of Mn∶CsPbCl3NCs and the degree of the host-dopant energy transfer process in NCs(Fig.4(d))[83]. Importantly, the near-surface Mn2+dopants are the sensitive sites whose ligand field can be temporarily disturbed by O2and thereby influences the Mn2+emission(4T1→6A1). This interesting sensing phenomenon can be seen to stimulate many new properties for Mn-doping perovskite NCs.
6 Electronic, Optical and Optoelectronic Applications
In this section, we will summarize different applications using colloidal Mn2+doped perovskite NCs. Interestingly, compared with the PLQYs of undoped CsPbCl3NCs, Mn2+-doped CsPbCl3NCs shows higher PLQYs. Besides, exciton confinement and exciton energy transfer to the Mn2+ion state endows NCs with extraordinary electronic and optical properties.
6.1 Mn2+-Doped CsPbX3 NCs as Color-Converting Materials
Our group prepared LEDs based on hot-injection-processed colloidal Mn2+doped CsPbCl3NCs, which were used as the color-converting material(Fig.5(a) and 5(b))[31,61]. We made a down-conversion LED by coating a mixture of curable resin and Mn2+-doped CsPbCl3NCs on top of the UV(365 nm GaN-based) LEDs[31,61]. Furthermore, various perovskite composites were produced to improve the stability and extra photoelectric property of doped-perovskite NCs. Zhang′s group synthesized Mn2+-doped CsPbCl3embedded in a cage of Zeolite-Y as a new composite phosphor for the white light-emitting diode(WLED), which significantly improved the resistance to both elevated temperature and water(Fig.5(c))[84]. The device possesses a CIE coordinate of (0.34, 0.36), a correlated color temperature of 5 336 K and a color rendering index of 81. Furthermore, other coated materials, such as SiO2, polydimethylsiloxane(PDMS), SiO2/Al2O3monolith(SAM),etc, show higher stability both in the solution and as an LED color-conversion materials. The PL emission spectra show nearly no variation after 24 hours of operation[35,37]. Interestingly, Zhangetal. proposed that strong Mn2+emission using high Br-concentrations. Cs(Pb1-x-zZnz)(ClyBr1-y)3∶xMn2+perovskite NCs were first realized through ion exchange reaction with the aid of ZnBr2[84]. As a result, white light-emitting perovskite NCs could be obtained using ion exchange engineering and be used as new color conversion materials in the LED prototypes.
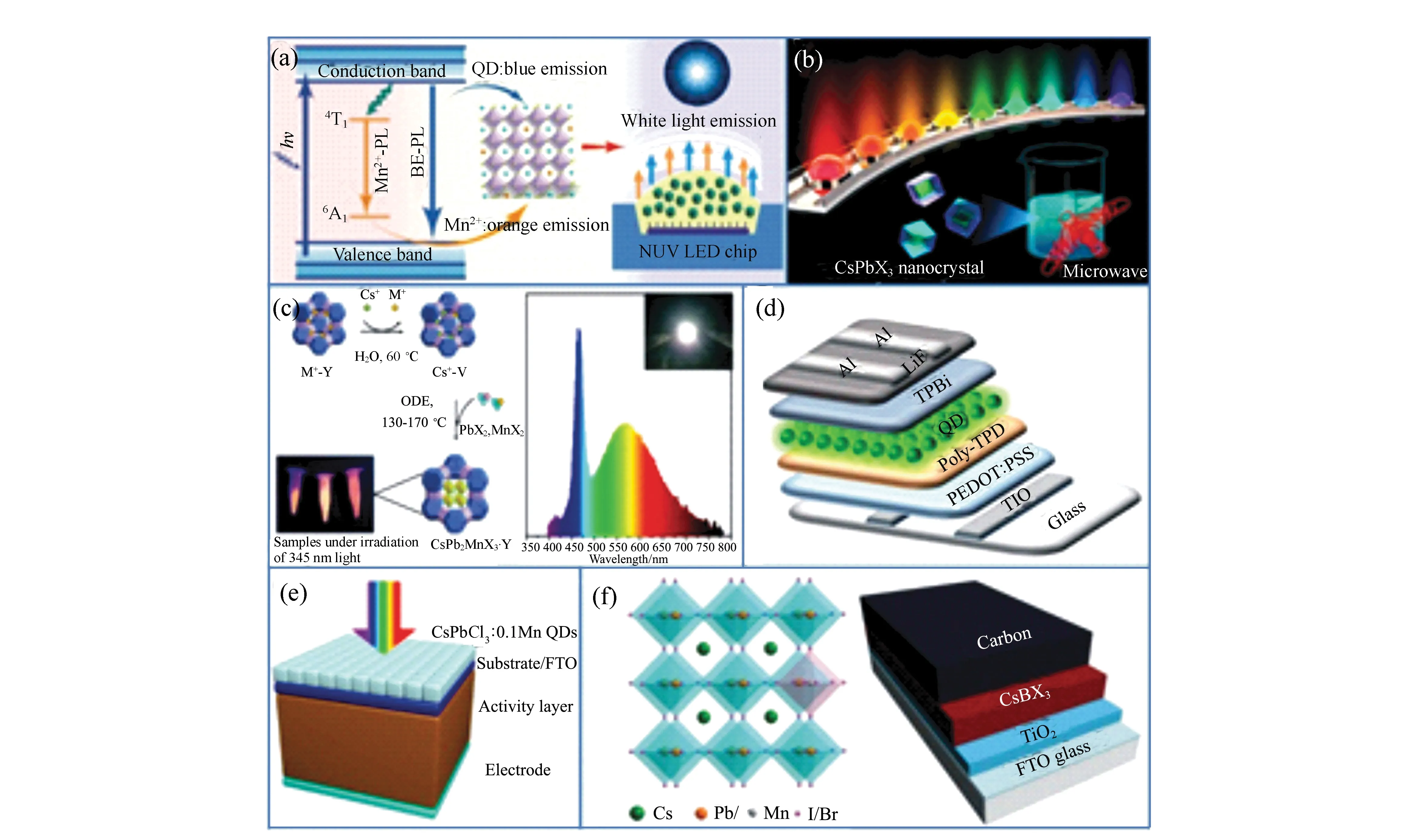
Fig.5 (a) and (b) Mn2+-doped CsPbX3 NCs as color-converting materials for LEDs. (c)LEDs with white color emission. (d)Mn2+-doped CsPbBr3 NCs for electroluminescent LED devices. (e) and (f) Mn2+-doped CsPbX3 NCs for solar cells
6.2 Electroluminescent(EL) LEDs
Benefitting from greatly improved thermal stability and optical performance through effective Mn2+substitution strategies, Zeng′s group fully utilized Mn2+-doped CsPbX3QDs as efficient light emitters toward the fabrication of high-performance perovskite LEDs(PLEDs), which show high EQE of 1.49% and CE of 6.40 cd/A, in comparison to devices using pure CsPbX3QDs as light emitters(Fig.5(d))[85]. The improved stability along with higher device performance reveals that such a Mn2+-substitution strategy might eventually open up a new avenue for the fabrication of efficient optoelectronic devices with excellent long-term stability. Summary of the performance of perovskite-based LEDs with different Mn-substitution ratio is shown in Tab.1.
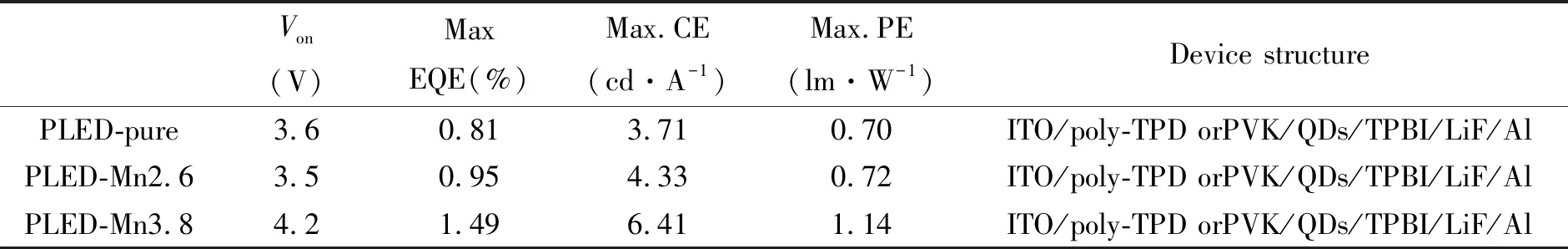
Tab.1 Comparison of the performance parameters of PLEDs based on different Mn-substitution ratio
6.3 Perovskite Solar Cells(PSCs)
Liuetal. focused more on applications in perovskite solar cells utilizing Mn2+-doped CsPbX3NCs. The group firstly developed interstitial Mn2+ions doped in a CsPbI2Br film providing a passivating effect. The champion device shows an increased open circuit voltage of 1.172 V with an overall power conversion efficiency(PCE) of 13.47%[86]. Detailed PSCs parameters are shown in Tab.2. Subsequently, Liu′s group further applied the as-prepared Mn2+doped CsPbCl3NCs as the light conversion materials onto the front side of the perovskite solar cells by converting UV light to visible light(Fig.5(e))[87]. The results show that Mn2+-doped CsPbCl3NCs would respectively increase the PCE to 3.34% of the device, which is the best recorded enhancement. Meanwhile, the stability of perovskite solar cells under UV irradiation has also been improved[87]. The above work shows Mn2+-doped CsPbX3NCs have significant potential to applications in photovoltaics. The key parameters of the perovskite solar cells coated with different concentrations of CsPbCl3:0.1Mn QD layers are summarized in Tab.3. Liang and coworkers also developed all-inorganic perovskite solar cells(PSCs) based on CsPb1-xMnxI1+2xBr2-2xfilms, and when the doping concentrationxwas 0.005, PSC based on CsPb0.995Mn0.005I1.01Br1.99film displayed the highest PCE of 7.36%(Fig.5(f))[88]. Based on the above discussion about Mn2+-doped perovskite NCs applied in the solar cells, it can be seen that there is enhanced PCE and stability in the PSC devices, which is mainly ascribed to small bandgap and ideal band structure of Mn2+-doped NCs. After effective doping, the NCs firstly exhibited more uniform morphology and better crystallinity providing a smooth path for charge-transfer in PSCs, improved light harvesting ability and reduced energy loss in hole transfer[88]. It not only reveals the potential for achieving high photovoltaic properties in the Mn2+-doped perovskite NCs, but also opens the door for further studies on other dopants in perovskite materials.
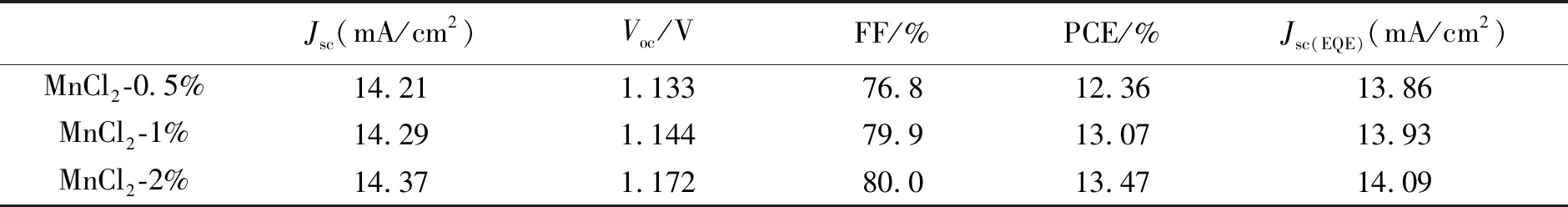
Tab.2 Comparison of the performance parameters of PSCs based on different CsPbBrI2 films
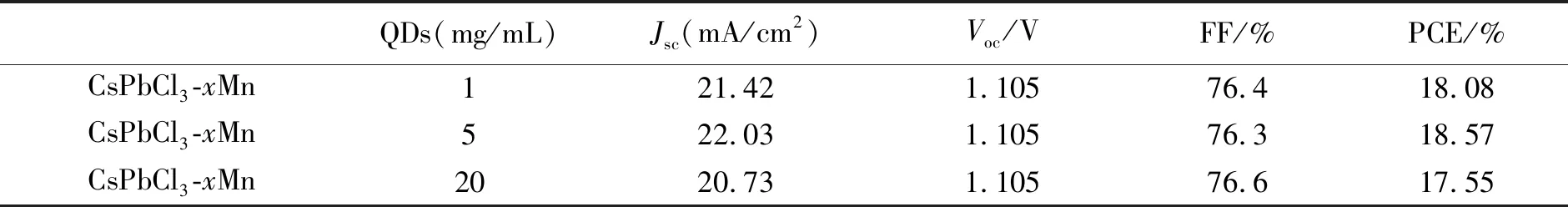
Tab.3 Key J-V parameters of PSCs with different coated layer thicknesses of CsPbCl3∶0.1Mn QDs
7 Conclusions and Future Challenges
Here we discussed the progress made in synthesis, optical properties, fluorescence mechanisms and applications(electronic, optical, and optoelectronic) of colloidal Mn2+-doped CsPbX3NCs. The emerging new findings on Mn2+ions doping in perovskite nanostructures provide several new fundamental insights for understanding the doping process, exciton confinement and exciton energy transfer to a dopant state. Furthermore, Mn2+-doped CsPbX3NCs endows higher stability and more excellent fluorescent properties, which shows outstanding properties in LED and solar cell applications. However, further investigation of Mn2+doping chemistry and physics is still required.
Firstly, new doping methods for efficient Mn2+doping are required, which would be derived from more ideas on the synthesis methods of various traditional semiconductor NCs. More investigation of the various doping processes is also required to understand more about doping mechanisms. Secondly, the magneto-optic properties of Mn2+-doped CsPbX3NCs is an interesting point to be studied. It will endow the doped perovskite NCs with more excellent properties, which tailors the magnetic properties in perovskite systems. Thirdly, highly toxic lead component is a key issue restricting its applicability, so many efforts have been devoted to the lead-free perovskite NCs. Similarly, Mn2+-doped non-lead perovskite NCs have also made some progress. Nagetal. and Mannaetal. both reported the Mn2+-doped Cs2AgInCl6double perovskite[89-90], but the Mn2+doping ratio and PLQYs are relatively low at only 16%, which urges us to pay more attention to designing better methods of synthesis to achieve excellent fluorescent properties. Other lead-free perovskite NCs, such as Cs2NaBiCl6and Cs2NaBi1-xInxCl6, can also be suitable doping host. Fourthly, while Mn2+-doping into the 0-dimension, 2-dimension and 3-dimension perovskite NCs have been intensively explored, extending the doping into 1-dimension perovskite NCs is also a key breakthrough. Fifthly, the development of the synthesis of doped perovskite NCs with pure dopant emission without any excitonic emission is also required, which is possible for more confined NCs in which the entire energy can be transferred to the dopant state and would result in highly intense dopant emission. Sixthly, Xuetal. have predicted Co-doped and Fe-doped CsPbBr3perovskites could be a promising candidate for CO2reduction from DFT calculation, which paves the way for designing a new doped CsPbBr3system[91]. This simulation opens new ideas for further doped perovskite NCs with more catalytic applications. Seventhly, further improving the EQE of the electroluminescent LEDs is also a key issue needed to be paid attention. Accordingly, we first notice that postligand engineering is used to exchange or remove the ligand of the NCs after the synthesis process but this can also affect the stability of NCs, which seems to be an excellent way to further improve the EQE of LEDs. In this regard, replacing long ligands(here, oleylamineand oleic acid) with shorter ligands and reducing the concentration of surface ligands using ligand postengineering are worthwhile ventures. Furthermore, the fabrication of uniform NCs polycrystalline layers, in situ preparation of Mn2+-doped NCs thin films, suppression of luminescence quenching inside NCs layers and defect passivation also require further attention in research, which can hopefully improve EQE of LEDs greatly.

