Plant distribution and pharmacological activity of flavonoids
Shao-Hui Wang, Yan-Lan Hu, Tong-Xiang Liu*
Plant distribution and pharmacological activity of flavonoids
Shao-Hui Wang1, 2, 3, 4, Yan-Lan Hu3, 4, Tong-Xiang Liu3, 4*
1Medical College, Qingdao Binhai University, Qingdao 266555, China.2Affiliated Hospital of Qingdao Binhai University, Qingdao 266555, China.3Key Laboratory of Ethnomedicine (Minzu University of China), Ministry of Education, Beijing 100081, China.4School of Pharmacy, Minzu University of China, Beijing 100081, China.
Flavonoids are natural organic compounds that are widely found in nature, their structural types are complex, and they mainly include flavonoids, flavonols, dihydroflavonols, isoflavones, dihydroisoflavones, chalcones, orange ketones, flavanoids, anthocyanidins, and biflavonoids. This review covers the plant distribution and pharmacological activities of flavonoids. Flavonoids are mainly distributed inand, and they are abundant in plants such as,,,, and. Because of their wide distribution and variety, researchers have found that flavonoids have diverse biological activities, mainly focusing on anti-inflammatory, antibacterial and antitumor activities. Mechanistically, the anti-inflammatory effects are mainly related to the NF-κB and MAPK (mitogen-activated protein kinase) signaling pathway and then the inhibition of the production of inflammatory cytokines and mediators. The antibacterial activity is mainly manifested as inhibitory effects on many strains, including,, and,via destroying the stability of the microbial membrane, inhibiting the invasion of virulent bacteria into host cells, promoting the apoptosis of bacteria, inhibiting bacterial fatty acid synthesis, etc. The antitumor activity of flavonoids is related to their inhibition of cell proliferation and induction of apoptosis via the mitochondria-mediated, endoplasmic reticulum-mediated, and death factor and its receptor-mediated signal transduction pathways. Understanding the plant distribution and pharmacological activity of flavonoids not only reveals the importance of identifying such valuable flavonoids in another genus or family but also provides a basis for fully exploiting the therapeutic potential of flavonoids.
Flavonoids, Plant distribution, Pharmacological activity, Antitumor, Anti-inflammatory, Antibacterial.
This review covers the plant distribution and pharmacological activities of flavonoids, stressing the importance of identifying such valuable flavonoids in another genus or family while providing a basis for fully exploiting the therapeutic potential of flavonoids.
Flavonoids are found in some traditional Chinese medicines that function to clear heat and dampness, some pathological products resulted from diseases. The most representative drugs among them are Huangqin (), Chuanhuangbai (), and Kushen (). As early as the Donghan dynasty of China, these three herbs were recorded in an ancient book of Chinese medicine called.
Background
Flavonoids are an important class of natural organic compounds that includes more than 4,000 polyphenolic compounds that are widely found in various natural plants [1]. Flavonoids are also present in some traditional Chinese medicines. The most representative drugs among them are Huangqin (), Chuanhuangbai (), and Kushen (). As early as the Donghan dynasty of China, these three herbs were recorded in an ancient book of Chinese medicine called.
The C6-C3-C6skeleton is formed in plantsby the condensation of three malonyl residues with hydroxy cinnamic acid [2], labeled as rings A, B, and C. Currently, flavonoids are roughly classified into 10 categories based on their structure, including flavonoids, flavonols, dihydroflavonols, isoflavones, dihydroisoflavones, chalcones, orange ketones, flavanoids, anthocyanidins, and biflavonoids. In addition, the 5 and 7 positions of ring A; 3', 4', and 5' positions of ring B; 3 and 2 positions of ring C of flavonoids are often replaced by hydroxyl groups, these readily form glycosides with a variety of five- or six-carbon sugars through a β glycoside bond, most of which are oxyglycosides, such as 7-o-glycosides in flavones, flavanones, and isoflavones. Compared with oxygen glycosides, which have been widely studied, there are relatively few studies on carbon glycosides in the literature [3]. Carbon glycosides can be directly connected to the skeleton through acid-resistant C-C bonds, and they mainly occur at the C6and C8positions.The basic structures and glycosylation sites of common flavonoids are summarized in Figure 1.
Because of their wide distribution and variety, flavonoids have been found to possess diverse biological activities, such as antioxidation, antibacterial, antihypertensive, liver protection, antitumor, and neuroprotection effects. In this review, the plant distribution and pharmacological activities of flavonoids are systematically summarized, and the results not only reveal the importance of identifying such valuable flavonoids in another genus or family but also provide a basis for fully exploiting the therapeutic potential of flavonoids.
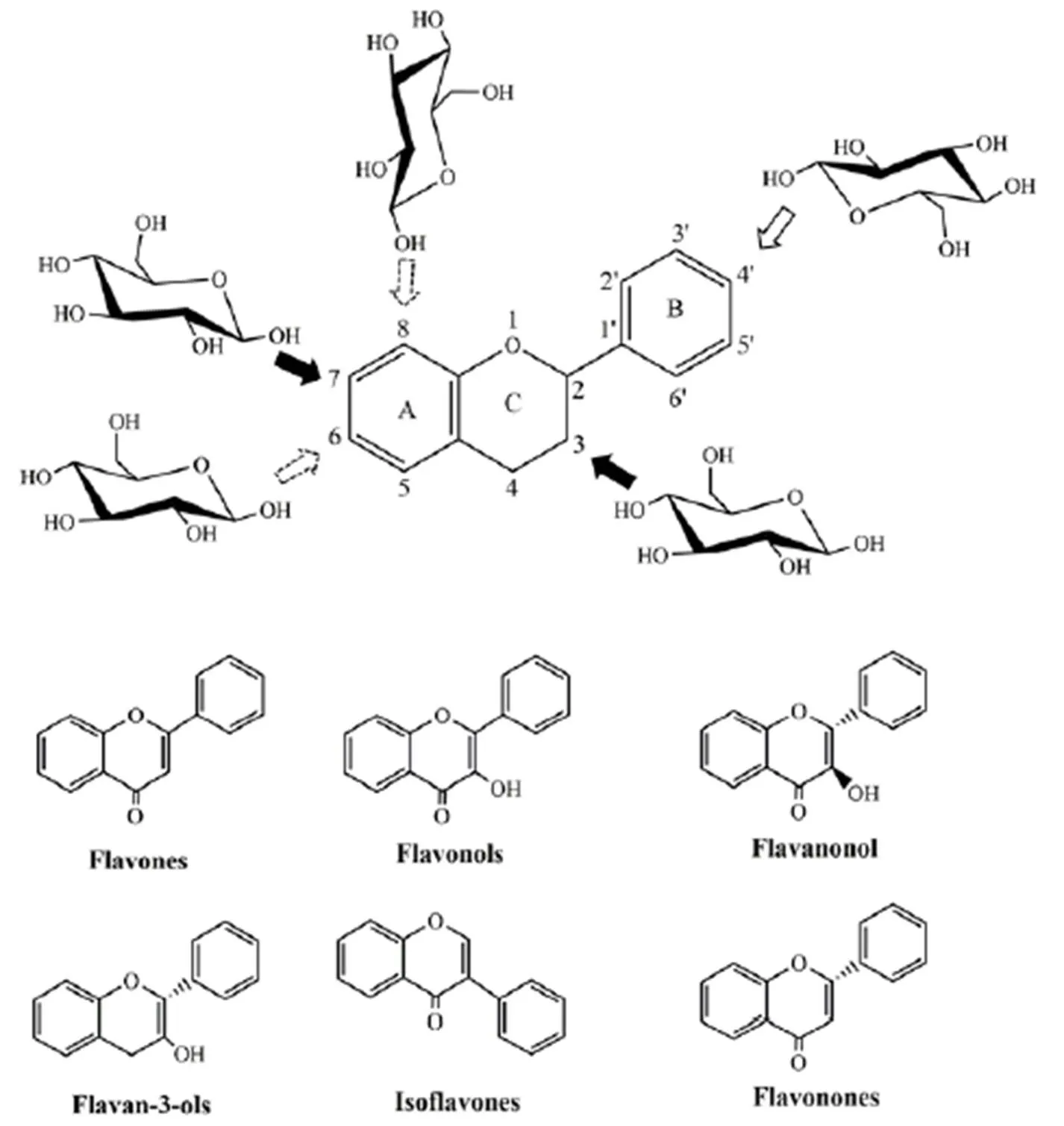
Figure 1 Basic structure of the common classes of flavonoids and the common points of glycosylation
Common glycosylation points are C3and C7(black arrows); B ring glycosylation is also observed in some plants (hollow arrow); C-glycosides are the rarest in plants (arrow with dotted lines);C-glycosylation mostly occurs at the C6and C8positions.
Plant distribution of flavonoids
A search in(http://phenol-explorer.eu/) produced 492 different flavonoids and isoflavone components in 400 types of food; thus, there are even more flavonoids distributed in plants. By sorting through the plant sources of flavonoids, we found that the distribution of flavonoids was the highest in the families of,,,,,, and(Table 1). Kaempferol, quercetin, apigenin, luteolin, isorhamnetin, and quercetin are the most common chemical constituents. In addition, many new compounds that have been isolated, purified, and identified were all modified from the existing flavonoids such as kaempferol and luteolin.

Table 1 Plant sources of flavonoids
Pharmacological Activity of Flavonoids
Anti-inflammatory activity
Inflammation is not only a defensive protective response but also a stimulus to various injury factors. It is dedicated to the defense and elimination of pathogenic factorsand. However, persistent and intense inflammation can cause damage to human tissues and cells. Macrophages are important immunoregulatory cells that secrete different inflammatory factors, stimulate inflammatory responses, clear pathogens, and maintain the balance of the internal environment. LPS stimulates the RAW 264.7 monocyte/macrophage-like cell line, a commonly used inflammatory model, and many flavonoids provide good resistance to this stimulation. It was reported that luteolin blocked the activation of NF-κB and inhibited the expression of NO, PGE2 (prostaglandin E2), inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)2 , TNF-α, and interleukin (IL)-6 in a dose-dependent manner under LPS stimulation in a RAW264.7 cell inflammation model[87]. Alpinetin can block the phosphorylation of IκBα protein, p65, p38, and ERK (extracellular signal-regulated kinase) and significantly inhibit the production of TNF-α, IL-6, and IL-1β [96]. Baicalein was shown to upregulate the expression of estrogen receptor ERα/ERβ in an LPS-induced RAW264.7 cell inflammation model, downregulate,, andmRNA, inhibit NO and cytokine production in cells, and ultimately regulate the NF-κB pathway and estrogen-like activity to inhibit LPS-induced inflammatory cytokine production, thereby preventing inflammation-related diseases [110]. It can also play an anticomplement role through classical and alternative approaches[64]. Total flavonoids of Sangshen () can inhibit the expression of IL-6, induce iNOS, phosphorylate p65 and IκB, increase the expression of IL-10, and exert anti-inflammatory and analgesic effects by inhibiting the pro-inflammatory cytokines iNOS and NF-κB [18]. Also acting on the classical NF-κB inflammatory signaling pathway, the total flavonoids of Qingma () can reduce the content of COX2, NO, IL-1β, IL-6, and TNF-α, increase the concentration of IL-10, inhibit the mRNA expression and phosphorylation of p65, and regulate the expression of inflammation-related factors through the NF-κB and MAPK (mitogen-activated protein kinase) signaling pathways, in turn affecting the process of inflammation [107].
Acacetin inhibits 5-lipoxygenase activity in a concentration-dependent manner, which exerts an anti-inflammatory effect through inhibiting the activity of 5-lipoxygenase and preventing the production of leukotrienes, and it also inhibits the biosynthesis of TNF-α and NO [6]. In addition, some scholars have foundanti-inflammatory experiments that luteolinand the ethyl acetate fraction of Suanjiang () can significantly alleviate edema in a carrageenan-induced acute rat inflammation model [87].In the chronic rat inflammatory model, luteolin was also found to inhibit cotton ball-induced granuloma formation at 110 mg/kg and 50 mg/kg, and its anti-inflammatory effect was comparable to that of 50 mg/kg indomethacin [87].Compared with 2 mg/kg aspirin, 5 mg/kg and 10 mg/kg of the ethanol extract of Youbingshiwei () were shown to exert significant anti-inflammatory activity against xylene-induced ear swelling in mice, and the maximum inhibition rate was as high as 67%[10].Apigenin, quercetin, kaempferol, and their analogues have similar anti-inflammatory activities [87].
Antibacterial activity
Since Fleming discovered penicillin, antibiotics have been used extensively in the clinic to reduce the prevalence and spread of a large number of diseases, but the struggle between humans and infectious diseases is endless. Despite the tremendous progress we have made, the emergence of various drug-resistant germs due to the abuse of antibiotics has made it difficult to treat certain infectious diseases. Faced with such a dilemma, researchers have turned their attention to the natural chemical ingredient flavonoids. Studies have found that the flavonoids fromcan bind to soluble proteins outside the bacteria, destroying the stability of the microbial membrane [85]. Baicalin increases alkaline phosphatase activity and attaches to and penetrates the cell membrane of bacteria, causing the surface of the membrane to sag, changing the permeability, and increasing the susceptibility to various antibiotics [111].Another study reported that baicalein could penetrate bacterial biofilm, promote the hydrolysis of the quorum sensing regulatory protein TraR protein, interfere with cell signaling, and inhibit bacterial quorum sensing[112]. In addition, baicalein can inhibit bacterial virulence by inhibitinginvasion into host cells by covalently binding to the protein substrates SipA/B/C/D and SopB ofSPI-1 T3SS [60]. It has been shown that the flavonoids from Paotongguo () can activate the apoptotic pathway of Leishmania and methicillin-resistantand have synergistic effects with oxacillin and tetracycline [66]. Some scholars have reported that luteolin can inhibit the activity of enoyl-ACP reductase and inhibit bacterial fatty acid synthesis in a non-competitive manner and then exert its antibacterial effect [108, 113]. Interestingly, 1 mg/mL quercetin reduced the F-ATPase activity ofby 47.37% and 0.5 mg/mL kaempferol reduced it by 49.66%; they significantly inhibited the production of acid by, suggesting that quercetin and kaempferol may have potential for the prevention and treatment of dental caries [114].Apigenin alone or in combination with LysGH15, a lysin from phage GH15, which exhibits a highly efficient and extensive cleavage profile for MRSA (methicillin-resistant Staphylococcus aureus), was found to reduce rabbit erythrocyte lysis, protect lung tissue from mice withpneumonia, and reduce the number of the bacteria in the lungs and blood, and TNF-α, IL-1β, and IL-6 levels were very similar to those in healthy mice [115].Other studies have found that flavonoids have antimicrobial activity, but the mechanisms of their antimicrobial activity have not been thoroughly studied. For example, the total flavonoids of Sangshen ()[18], the total flavonoids of Qingma () [107], catechin[108], quercetin [108, 114], L-epicatechin [108], the ethyl acetate fraction of the ethanol extract of Leigongteng () [109], and kaempferol [114] were all found to have inhibitory effects on many strains of,,,,,,,and(Table 2).
Antiviral effects
In one study, 120-360 mg/kg of the total flavonoids from Shiqizhu () was used to treat influenza A virus-infected mice for 5 days, and the results indicated that the total flavonoids from Shiqizhu () upregulatedthe expression of(),,(),,,),),and()mRNAs and downregulated the expression of Caspase-3, NF-κB, and p65 proteins. In addition, the total flavonoids from Shiqizhu () reduced the levels of IL-6, TNF-α, and IL-1β in serum and increased the expression of IFN-α. These results clearly demonstrate that the total flavonoids from Dendrobium can significantly alleviate lung inflammation, apoptosis, and water transport abnormalities induced by influenza A virus, which may be achieved by regulating the TLR7, RIG-1, and AQP5 signaling pathways[116].
Immunomodulatory effects
The total flavonoids extracted from Yinyanghuo (Maxim) were found to downregulate the expression ofandmRNAs, upregulate the level of BCL-2 mRNA, and decrease the apoptosis rate of T cells; moreover, the activities of caspase-8 and caspase-3 in T cells of corticosterone rats were significantly inhibited, and the number of T cells was maintained [117]. Another study found that the total flavonoids extracted from Yinyanghuo (Maxim) significantly enhanced phagocytosis by the monocyte-macrophage system in normal mice, increased the level of serum hemolysin antibody formation, and antagonized the inhibition of monocyte-macrophage phagocytosis by cyclophosphamide, reducing serum hemolysin antibody levels and delayed-type hypersensitivity[30].
Antioxidant activity
There is a complete oxidation system in cells, including ROS/RNS (reactive nitrogen species), O2, HO, HO2, H2, NO, NO+, and N2O3. Endogenous products mainly originate from mitochondria, peroxisomes, NADPH oxidase, cytochrome P450, and the xanthine redox enzyme system, and exogenous products are mainly related to ultraviolet and ionizing radiation. Low levels of ROS provide a stable microenvironment for maintaining the normal function of various intracellular biological macromolecules. On the contrary, high levels of free radicals can destroy the redox environment in cells and cause cell damage and death, and a persistent imbalance will eventually lead to aging and related diseases. Many flavonoids are excellent free radical scavengers because they can be used as hydrogen or electron donors. HORAC (Hydroxyl radical antioxidant capacity), TEAC [6-hydroxy-2, 5,7,8-tetramethylchroman-2-carboxylic acid (Trolox)-equivalent antioxidant capacity], ABTS [2, 2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt], FCR (Folin-Ciocalteu), ORAC (oxygen radical absorbance capacity), 1,1-diphenyl-2-picrylhydrazyl (DPPH), FRAP (ferric reducing antioxidant power), POV (peroxide value), and others are commonly used indicators to detect antioxidant activity, especially DPPH (Table 3). Compared with the commonly used antioxidants such as vitamin C and ascorbic acid, quercetin [85] inand flavonoids[21] in the methanol extract of peony have the same antioxidant activity as vitamin C. At the same concentration, the total flavonoids of Taojinniang () [76] and Maobaiyang ()stamen [15] were shown to be better than ascorbic acid at scavenging DPPH. The levels of SOD and GSH-Px (glutathione peroxidase) increased and that of MDA (malonic dialdehyde) were found to decrease in the plasma of Kunming mice after administration, which indicated that flavonoids could promote the expression of antioxidant enzymes and protect the body from peroxidation [76].
Antitumor effect
Inhibit the proliferation of tumor cells. The first step in cancer research is to study antiproliferation, which forms the basis for subsequent research. Both plant total flavonoid extracts, such as total flavonoids from Shuli (), total flavonoids extracted from Shiye (), and the N-butanol extract of Shiwei (), and monomeric compounds, such as 6,7,3'-trimethoxy-3,5,4'- trihydroxyflavone, genistein, and rutin, all have good inhibitory effects on the growth of various tumor cell lines. Their sources and corresponding IC50values are shown in Table 4.
Minimum inhibitory concentration (MICs);amixture;bpositive control.
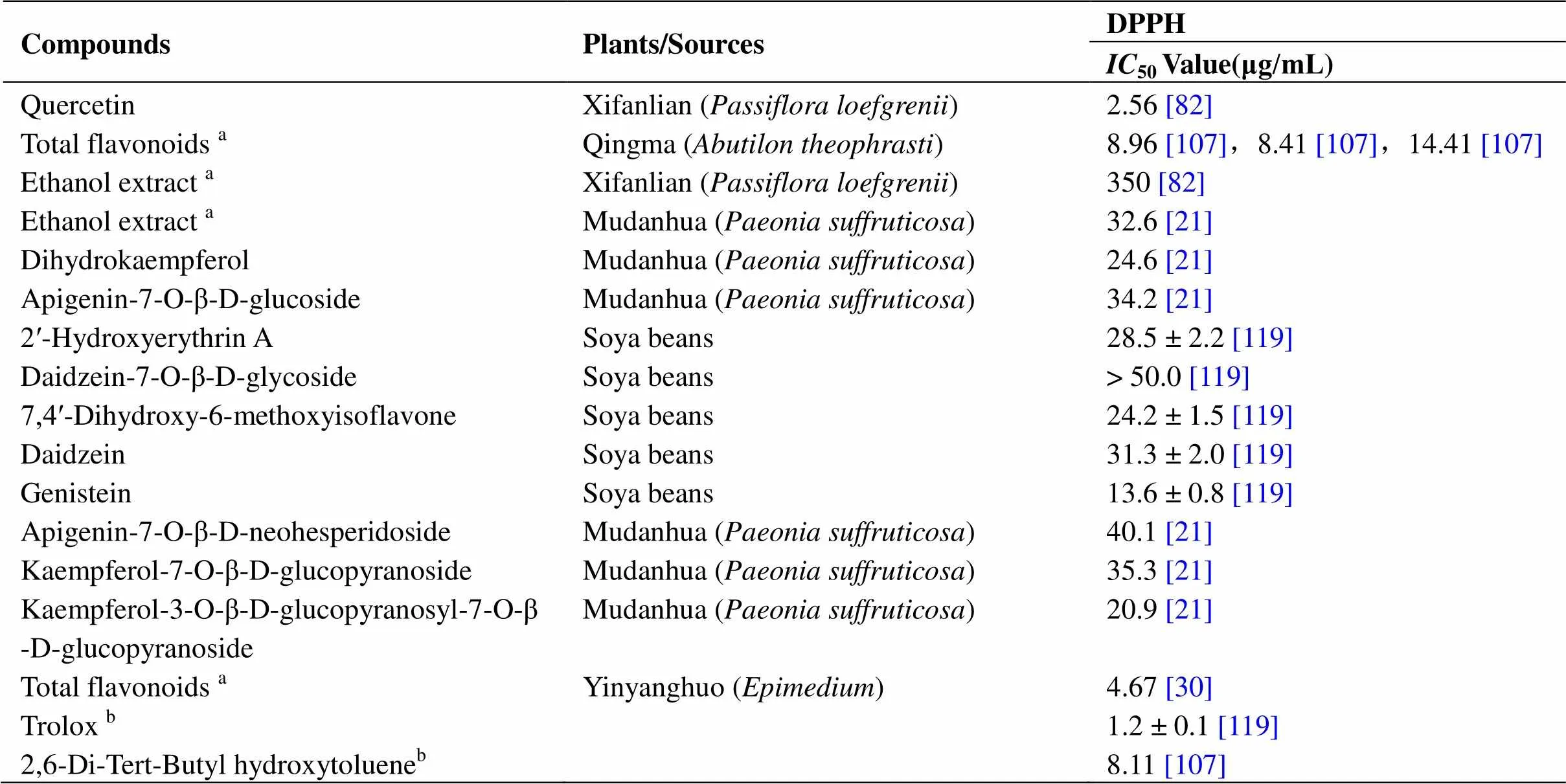
Table 3 Determination of the antioxidant capacity of flavonoids
DPPH: 1,1-Diphenyl-2-picrylhydrazyl.amixture;bpositive control.
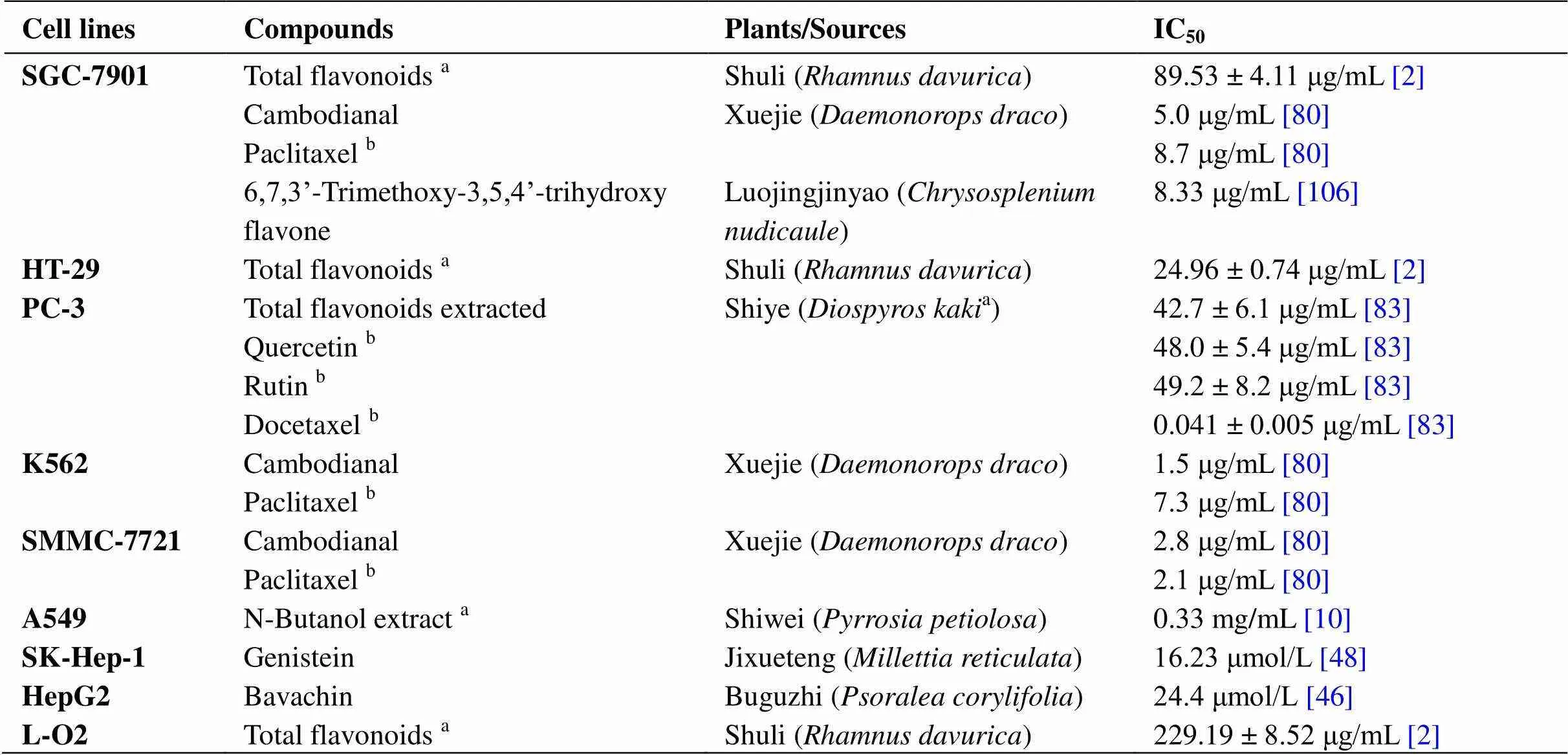
Table 4 IC50 values of flavonoids that inhibit the proliferation of tumor cell lines
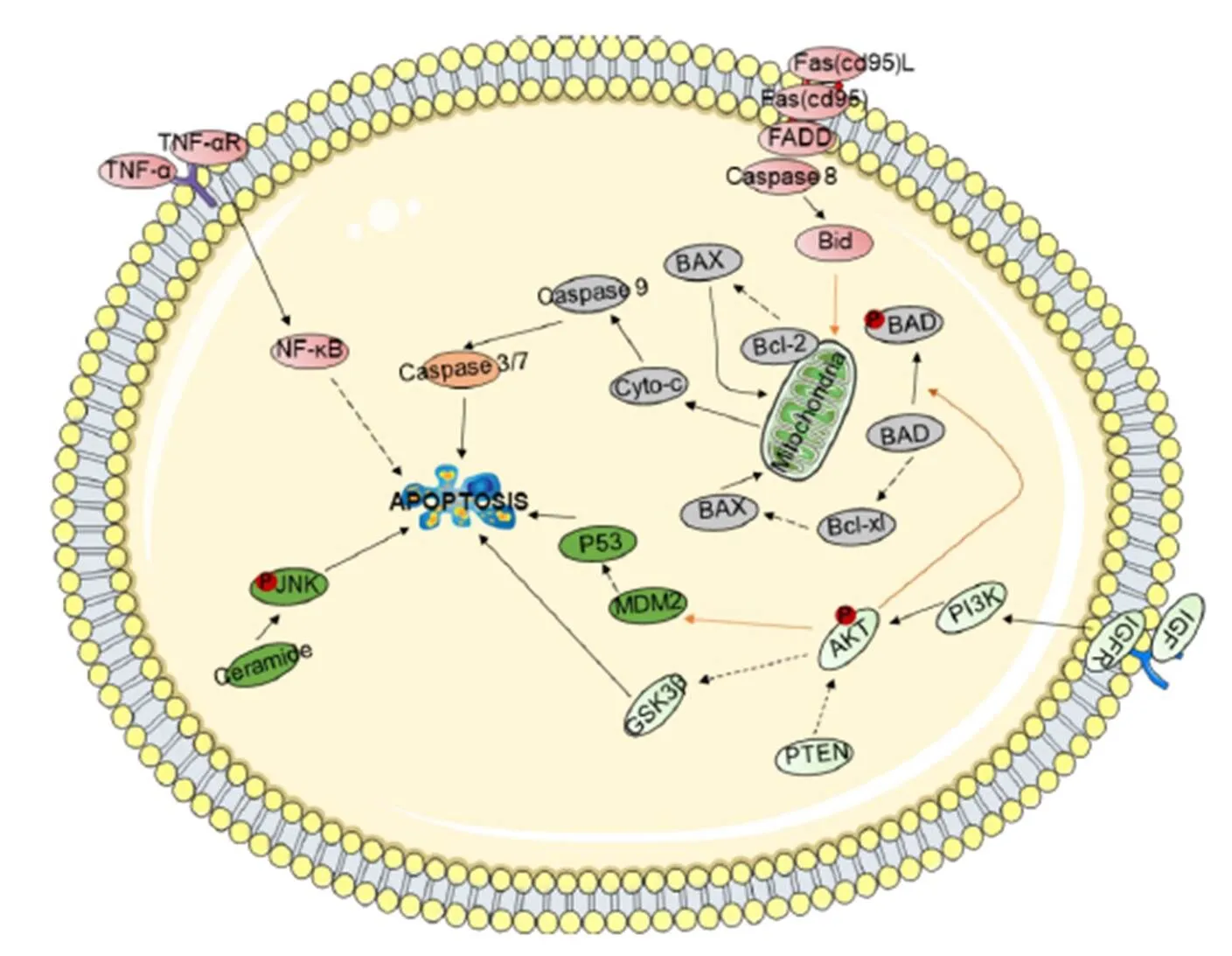
Promote apoptosis of tumor cells. Apoptosis, known as programmed cell death, is an autonomous and orderly procedure for death controlled by genes. Apoptosis not only participates in the regulation of the number of cells in the body but also can remove cells in the body that are not functional, cells that are harmful to the body, mutated cells, and cells that cannot survive after being damaged. Moreover, apoptosis plays an important role in the development and homeostasis of the body. Therefore, promoting the apoptosis of tumor cells can effectively inhibit the occurrence and development of tumors. There are three main apoptosis-related signal transduction pathways (Figure 2).
.Some scholars have found that the total flavonoids extracted from Shiye ()[83], 7-hydroxy-2',2'-dimethyl-2'H,4H-3,6'-bichromen-4-one [46], Baicalein [123], quercetin, and resveratrol [124] can reduce mitochondrial membrane potential, change the mitochondrial morphology, induce the production of large amounts of reactive oxygen species (ROS), reduce the ratio of B-cell lymphoma-2 (Bcl-2)/ Bcl-2 associated X protein (BAX), promote the release of cytochrome C from mitochondria, and then induce the activationofcaspase-9andcaspase-3,followedby cleavage of poly (ADP-ribose) polymerase (PARP), leads to DNA fragmentation and ultimately to tumor cell apoptosis.In addition, studies have reported that the combination of quercetin and resveratrol can affect the expression of mammalian target of rapamycin (mTOR), p-protein kinase B (Akt), p-mTOR, NF-κB, matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-2 (MMP-9), and TIMP-2 (tissue inhibitor of metalloproteinases-2) proteins, which may further enhance the antitumor activity of the drug by targeting mitochondria, Akt/mTOR, and NF-κB signaling pathways[124].
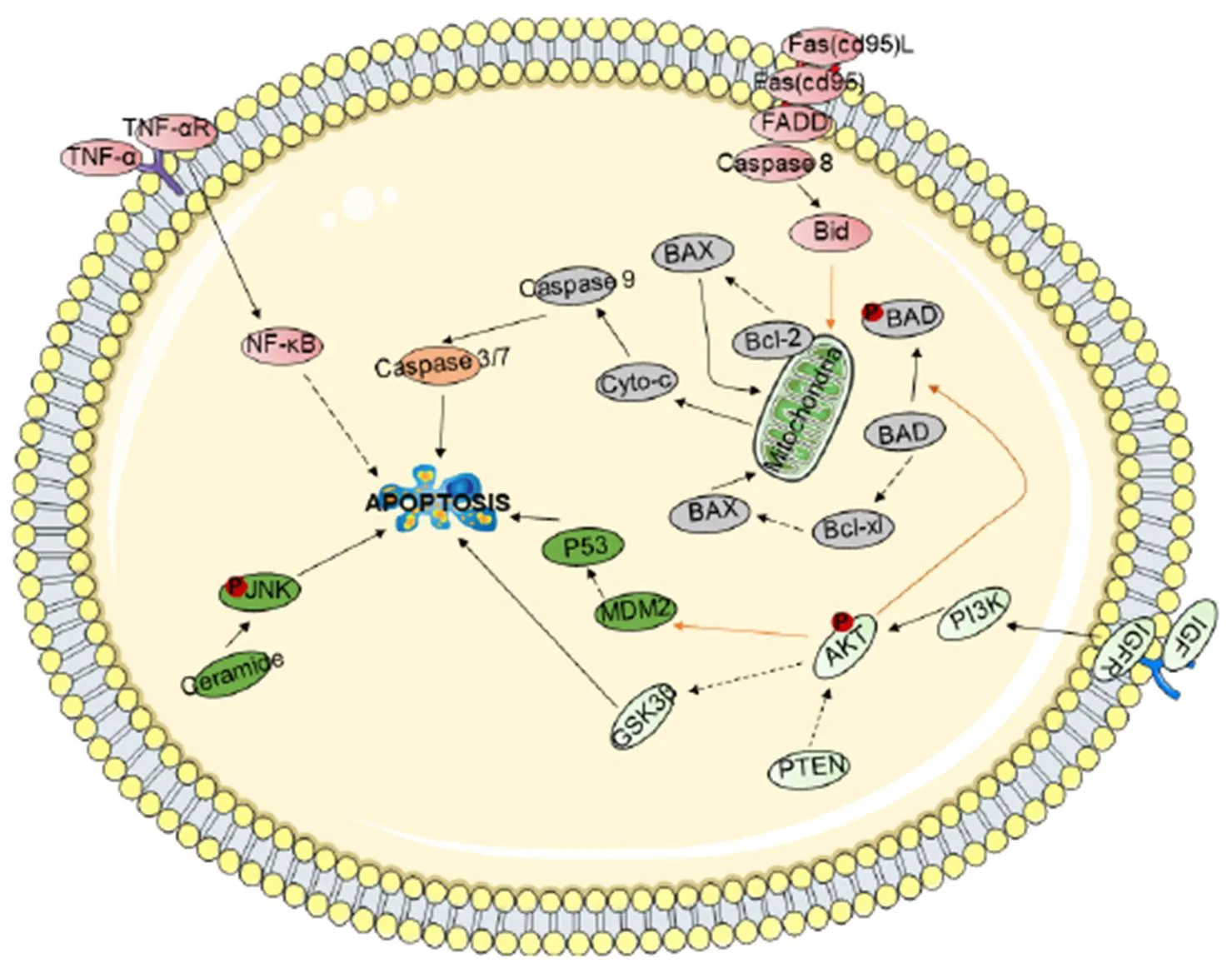
Figure 2 Schematic representation of the cell apoptosis signaling pathway
Shown are the extrinsic (receptor-mediated, red) and intrinsic (mitochondria-driven, gray) apoptosis pathways, which act in opposition to the survival proteins such as the PI3K/Akt signaling circuitry (light green). Other signaling loops (dark green) and executioner caspases (orange) are activated in both the extrinsic and intrinsic pathways; inter-talk between pathways (arrows) and the activation and suppression effects are indicated by solid arrows and dashed arrows, respectively.
It has been shown in the literature that the treatment of HepG2 cells with 20 μmol/L Bavachin can activate activating transcription factor (ATF)4, the transcription factors CHOP(C/EBP-homologous protein) and X-box binding protein1 (XBP1), and protein kinase R-like ER kinase (PERK)-ATF4, inositol-requiring enzyme1(IRE1)-XBP1s, and ATF6. Three pathways induce endoplasmic reticulum stress. Bavachin induces endoplasmic reticulum stress by inducing PERK-ATF4, IRE1-XBP1s, and ATF6; moreover, the depletion of Mfn2 (mitochondrial fusion 2) aggravates ER stress by phosphorylating Akt, activating apoptosis-responsive proteins such as death receptors, and inducing tumor cell apoptosis [46].
Studies in the literature have shown that genistein can upregulate the expression of Fas, FasL, and p53 proteins in SK-Hep-1 cells, subsequently induced the activation of caspase-9 and caspase-3,which was followed by cleavage of PARP, leading to DNA fragmentation and ultimately to apoptosis [48]. Baicalin can block cells in G0/G1 phase; decrease mitochondrial membrane potential; activate caspase-3, caspase-8, and caspase-9; initiate FasL/Fas expression; and induce apoptosis significantly through internal and external sources. Moreover, the proto-oncogeneand its target gene,(human telomerase reverse transcriptase) are downregulated to inhibit telomerase activity, ultimately inhibiting the proliferation of tumor HL-60 cells[125]. Some scholars have found through miRNA microarray analysis that baicalin downregulates many oncomiRs such as miR-10a, miR-23a, miR-30c, miR-31, miR-151a, and miR-205and, inhibitingexpression. It effectively promotes apoptosis in the HT-29 colon cancer cell line in dose- and time-dependent manners and inhibits tumor growth in corresponding transplanted nude mice[126].
Antihypertensive effect
In one study, 26 mg/kg Epimedium flavonoids was injected into the lateral ventricle of mice, and epimedium flavonoids were found to increase the secretion of the amino acid neurotransmitter r-aminobutyric acid in the periventricular system and increase its affinity for receptors; strengthen the inhibition of the central sympathetic nervous system; expand coronary blood vessels, the femoral artery, and the cerebrovascular system; and reduce peripheral resistance, thus lowering blood pressure [30].
Reduce obesity
The occurrence and development of various diseases are related to obesity, and in recent years, the incidence of diabetes, hyperlipidemia, hypertension, and endocrine disorders in obese patients has increased significantly. Obesity has become an important cause of cardiovascular and cerebrovascular diseases, hypertension, and diabetes. Some scholars found that the total flavonoids extracted from Yinyanghuo (Maxim) increased the appetite and exercise volume of rats, but the weight of the rats was lower than that of untreated rats after 24 months. Moreover, the concentration of age-related metabolites returned to a level seen in younger animals; the amount of unsaturated fatty acids decreased; saturated fatty acids, deoxycholic acid, triglycerides, and total cholesterol increased; and their fur was smoother and more lustrous. These results suggest that the total flavonoids extracted from Yinyanghuo (Maxim) may have potential body weight controlling and anti-aging effects [117].
Role in liver protection
Dihydromyricetin can significantly improve the abnormal expression of cytochrome P450 2E1, Kelch-like ECH-binding protein 1, and heme oxygenase-1 in the liver of mice with alcoholic liver disease, improve the disordered nuclear localization of NF-κB and nuclear factor erythroid-2-related factor 2 (Nrf2), and play a role in liver protection. Further exploration of the mechanism revealed that dihydromyricetin may mediate the activation of Nrf2 via the autophagy pathway, upregulate p62 positive feedback Nrf2 activation, reduce inflammation in liver steatosis and the pathological progression of alcoholic liver disease, and partially restore liver pathological changes [127].
Neuroprotection
Some researchers have found that in mice, isobavachalcone can inhibit the excessive activation of microglia, reduce the expression of IL-6 and IL-1β in the brain of Parkinson’s disease mice, prolong the residence time of mice on a Rota-rod, reduce neuronal necrosis, and effectively alleviate Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine.experiments showed that isobavachalcone inhibited NF-κB by blocking LPS-induced NF-κB subunit transfer from mouse microglia BV-2 cytoplasm to the nucleus, reduced the LPS-induced oxidative stress response to inflammatory cytokine expression, and protected nerves through antagonism[44].
Other effects
In one study, the total flavonoids extracted from Yinyanghuo (Maxim) (150 mg/kg) were administered to young rats by intraperitoneal injection for 7 days. It was found that the total flavonoids extracted from Yinyanghuo (Maxim) significantly increased the weight of the anterior pituitary, epididymis, and seminal vesicles in young rats; increased testosterone, estradiol, and luteinizing hormone levels; and promoted the secretion of testosterone by rat stromal cells[30]. In addition to promoting the male reproductive system and reproductive endocrine activity, the total flavonoids extracted from Yinyanghuo (Maxim) were shown to promote the proliferation of primary osteoblasts and osteoblast-like MAGR106 cells, enhance the extent of mineralized tuberculosis in vitro, and enhance the mRNA expression of(human Bone morphogenetic protein 2),(human Bone morphogenetic protein 4),(runt-related transcription factor 2),, and, as well as promote the osteogenic differentiation of human bone marrow mesenchymal stem cells[30]. Icariin can significantly increase the expression of eNOS (endothelial nitric oxide synthase) and cGMP in corpus cavernosum smooth muscle, inducing the relaxation of corpus cavernosum smooth muscle and penile erection[28]. Another study reported that baicalin increased the concentration of cAMP and AFC (alveolar fluid clearance) in a dose-dependent manner in rats with acute lung injury, and baicalin prevented the reduction of AFC by upregulating α-ENaC (α-epithelial Na+ channel) protein expression, which was activated by stimulating the cAMP/PKA (cyclic adenosine monophosphate/protein kinase A) signaling pathway [128].
Prospective
The structure of flavonoids in plants is diverse, and many plant families contain them. The research topics mentioned in this review include flavonoid monomer components, different polar extraction sites, and total flavonoids. Flavonoids have a variety of pharmacological effects, including anti-oxidation, antitumor, weight loss, antibacterial, antihypertensive, liver protective, anti-inflammatory, and neuroprotective activities. In short, plant-derived flavonoids are widely used as a bulk component, and whether the monomer is administered alone, monomers are administered in combination, or the total extract is administered directly, good effects have been obtained. However, the research on the mechanisms of the various activities of flavonoids is still in its infancy. Therefore, further studies are needed on the mechanisms by which flavonoids affect diseases and their long-term effects. Such studies will provide a sound scientific basis for the development and application of natural medicines, bringing relief to patients with various diseases.
1. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016, 5: e47.
2. Chen G, Li X, Saleri F,. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21: 1275.
3. Gonzales GB. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: questions, considerations and future perspectives. Proc Nutr Soc 2017, 76: 175-181.
4. Ma YF, Shang FD. Distribution of flavonoid in medicinal plants. J Biol 2003, 20: 35-39.
5. Zou H, Xu KP, Li FS,Unciflavones A-F, six novel flavonoids from Selaginella uncinata (Desv.) Spring.2014, 99: 328-333.
6. Fan SY, Zeng HW, Pei YH,The anti-inflammatory activities of an extract and compounds isolated from Platycladus orientalis (Linnaeus) Franco in vitro and ex vivo. J Ethnopharmacol 2012, 141: 647-652.
7. Ogo Y, Mori T, Nakabayashi R,. Transgenic rice seed expressing flavonoid biosynthetic genes accumulate glycosylated and/or acylated flavonoids in protein bodies. J Exp Bot. 2016, 67: 95-106.
8. Uddin SJ, Grice D, Tiralongo E. Evaluation of cytotoxic activity of patriscabratine, tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum. Pharm Biol 2012, 50: 1276-1280.
9. Wang N, Wang JH, Li X,. Flavonoids from Pyrrosia petiolosa (Christ) Ching. J Asian Nat Prod Res 2006, 8: 753-756.
10. Cheng D, Zhang Y, Gao D,. Antibacterial and anti-inflammatory activities of extract and fractions from Pyrrosia petiolosa (Christ et Bar.) Ching. J Ethnopharmacol 2014, 155: 1300-1305.
11. Wang T, Xiao J, Hou H,Development of an ultra-fast liquid chromatography-tandem mass spectrometry method for simultaneous determination of seven flavonoids in rat plasma: Application to a comparative pharmacokinetic investigation of Ginkgo biloba extract and single pure ginkgo flavonoids after oral administration. J Chromatogr B Analyt Technol Biomed Life Sci 2017, 1060: 173-181.
12. Chen S, Xing XH, Huang JJ,. Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzyme Microb Technol 2011, 48: 100-105.
13. Yu XA, Azietaku JT, Li J,Simultaneous determination of eight flavonoids in plasma using LC-MS/MS and application to a pharmacokinetic study after oral administration of Pollen Typhae extract to rats. J Chromatogr B Analyt Technol Biomed Life Sci 2017, 1044: 158-165.
14. Al-Rimawi F, Abu-Lafi S, Abbadi J,. Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by LC/PDA and LC/MS and their antioxidant activity. Afr J Tradit Complement Altern Med 2017, 14: 130-141.
15. Wan P, Sheng Z, Han Q,. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J Chromatogr B Analyt Technol Biomed Life Sci 2014, 945: 68-74.
16. Tao XY, Zhang DW, Chen RD,Chemical constituents from cell cultures of Morus alba. Chin J Chin Mater Med 2012, 37: 3738-3742.
17. Ti H, Wu P, Lin L,. Stilbenes and flavonoids from Artocarpus nitidus subsp. lingnanensis. Fitoterapia 2011, 82: 662-665.
18. Chen H, Yu W, Chen G,. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23: 4.
19. Zhang CF, Chen J, Zhao LQ,. Three new flavonoids from the active extract of Fallopia convolvulus. J Asian Nat Prod Res 2011, 13: 136-142.
20. Sait S, Hamri-Zeghichi S, Boulekbache-Makhlouf L,HPLC-UV/DAD and ESI-MS(n) analysis of flavonoids and antioxidant activity of an Algerian medicinal plant: Paronychia argentea Lam. J Pharm Biomed Anal 2015, 111: 231-240.
21. Zhang H, Li X, Wu K,Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules 2017, 22: 5.
22. He C, Peng B, Dan Y,. Chemical taxonomy of tree peony species from China based on root cortex metabolic fingerprinting. Phytochemistry 2014, 107: 69-79.
23. Xu L, Zhang X, Lin LM,. Two new flavonol glycosides from the Tibetan medicinal plant Aconitum tanguticum. J Asian Nat Prod Res 2013, 15: 737-742.
24. Yu Y, Yi ZB, Liang YZ. Validate antibacterial mode and find main bioactive components of traditional Chinese medicine Aquilegia oxysepala. Bioorg Med Chem Lett 2007, 17: 1855-1859.
25. Wang R, Li YX, Quan QM. The contents changes of polysaccharides and total flavonoids during flower bud differentiation of Epimedium sagittatum. J Chin Med Master 2009, 32: 1511-1514.
26. Chen J, Xu Y, Wei G,Chemotypic and genetic diversity in Epimedium sagittatum from different geographical regions of China. Phytochemistry 2015, 116: 180-187.
27. Li WK, Zhang RY, Xiao PG. Flavonoids from Epimedium wanshanense. Phytochemistry 1996, 43: 527-530.
28. Li C, Li Q, Mei Q,. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci 2015, 126: 57-68.
29. Peng YD, Huang WH, Guo BL. Research on quality of Epimedium extract in market. Chin J Chin Mater Med 2007, 32: 1858-1861.
30. Ma H, He X, Yang Y,. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol 2011, 134: 519-541.
31. Vidovic M, Morina F, Milic S,Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Physiol Biochem 2015, 93: 44-55.
32. Wang L, Mei Q, Wan D. Simultaneous determination by HPLC of quercetin and kaempferol in three Sedum medicinal plants harvested in different seasons. J Chromatogr Sci 2014, 52: 334-338.
33. Zhang S, Liu C, Bi H,. Extraction of flavonoids from Rhodiola sachlinesis A. Bor by UPE and the antioxidant activity of its extract. Natur Prod Res 2008, 22: 178-187.
34. Chou TH, Chen JJ, Lee SJ,. Cytotoxic flavonoids from the leaves of Cryptocarya chinensis. J Natur Prod 2010, 73: 1470-1475.
35. Olennikov DN, Kashchenko NI, Chirikova NK,. Iridoids and Flavonoids of Four Siberian Gentians: Chemical Profile and Gastric Stimulatory Effect. Molecules 2015, 20: 19172-19188.
36. Yin C, Xie L, Guo Y. Phytochem Anal and antibacterial activity of Gentiana macrophylla extract against bacteria isolated from burn wound infections. Microb Pathog 2018, 114: 25-28.
37. Wu SK, Zhang N, Shen XR,. Preparation of total flavonoids from loquat flower and its protective effect on acute alcohol-induced liver injury in mice. J Food Drug Anal 2015, 23: 136-143.
38. Thongnest S, Lhinhatrakool T, Wetprasit N,. Eriosema chinense: a rich source of antimicrobial and antioxidant flavonoids. Phytochemistry 2013, 96: 353-359.
39. Huang M, Wang W, Wei S. Investigation on medicinal plant resources of Glycyrrhiza uralensis in China and chemical assessment of its underground part. Chin J Chin Mater Med 2010, 35: 947-952.
40. Lou H, Yuan H, Yamazaki Y,. Alkaloids and flavonoids from peanut skins. Planta medica 2001, 67: 345-349.
41. Song S, Zheng X, Liu W,. 3-Hydroxymethylglutaryl flavonol glycosides from a Mongolian and Tibetan medicine, Oxytropis racemosa. Chem Pharm Bull 2010, 58: 1587-1590.
42. Woo ER, Kwak JH, Kim HJ,. A new prenylated flavonol from the roots of Sophora flavescens. J Natur Prod 1998, 61: 1552-1554.
43. Tan Q, Zhang S, Shen Z. Flavonoids from the roots of Campylotropis hirtella. Planta medica 2011, 77: 1811-1817.
44. Jing H, Wang S, Wang M,. Isobavachalcone Attenuates MPTP-Induced Parkinson's Disease in Mice by Inhibition of Microglial Activation through NF-kappaB Pathway. PLoS One 2017, 12: e0169560.
45. Jiang H, Hu JR, Zhan WQ,. Screening for fractions of Oxytropis falcata Bunge with antibacterial activity. Natur Prod Res 2009, 23: 953-959.
46. Yang Y, Tang X, Hao F,Bavachin Induces Apoptosis through Mitochondrial Regulated ER Stress Pathway in HepG2 Cells. Biol Pharm Bull 2018, 41: 198-207.
47. Zhang L, Qiang P, Yu J,Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer. Autophagy 2018: 1-16.
48. Fang SC, Hsu CL, Lin HT,. Anticancer effects of flavonoid derivatives isolated from Millettia reticulata Benth in SK-Hep-1 human hepatocellular carcinoma cells. J Agric Food Chem 2010, 58: 814-820.
49. Ma L, Wu F, Chen RY. Advance of chemical constituents and bioactivity of Saururuaceae plants. Chin J Chin Mater Med 2003, 28: 196-198.
50. Chen SD, Li T, Gao H,Anti HSV-1 flavonoid derivatives tethered with houttuynin from Houttuynia cordata. Planta medica 2013, 79: 1742-1748.
51. Zheng CJ, Li HQ, Ren SC,Phytochemical and Pharmacological Profile of Vitex negundo. Phytother Res 2015, 29: 633-647.
52. Liu X, Luo Y, Wu H,Systematic analysis of O-methyltransferase gene family and identification of potential members involved in the formation of O-methylated flavonoids in Citrus. Gene 2016, 575: 458-472.
53. Adaszynska-Skwirzynska M, Dzieciol M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Natur Prod Res 2017, 31: 2575-2580.
54. Yu C, Qu F, Mao Y,Different extraction pretreatments significantly change the flavonoid contents of Scutellaria baicalensis. Pharm Biol 2013, 51: 1228-1235.
55. Huang ZH, Jiang DX, Lai XP. Study on the enrichment and purification of total flavonoids in Schizonepeta tenuifolia by macroporous adsorption resin. J Chin Med Master 2010, 33: 1476-1480.
56. Gao Z, Huang K, Yang X,. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochimica et biophysica acta 1999, 1472: 643-650.
57. Hu HW, Xie XM, Zhang PZ,. Study on the flavonoids from Mosla chinensis “jiangxiangru”. J Chin Med Master 2010, 33: 218-219.
58. Broncel M. Antiatherosclerotic properties of flavones from the roots of Scutellaria baicalensis Georgi. Wiad Lek 2007, 60: 294-297.
59. Guan Z, Li S, Lin Z,Identification and quantitation of phenolic compounds from the seed and pomace of Perilla frutescens using HPLC/PDA and HPLC-ESI/QTOF/MS/MS. Phytochem Anal 2014, 25: 508-513.
60. Tsou LK, Lara-Tejero M, RoseFigura J,Antibacterial Flavonoids from Medicinal Plants Covalently Inactivate Type III Protein Secretion Substrates. J Am Chem Soc 2016, 138: 2209-2218.
61. Leung KC, Seneviratne CJ, Li X,Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials 2016, 138: 2209-2218.
62. Zhu L, Zhao L, Wang H,Oroxylin A reverses P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by G2/M arrest. Toxicol Lett 2013, 219: 107-115.
63. Bai Y, Xia B, Xie W,Phytochemistry and pharmacological activities of the genus Prunella. Food Chem 2016, 204: 483-496.
64. Xing S, Wang M, Peng Y,. Simulated gastrointestinal tract metabolism and pharmacological activities of water extract of Scutellaria baicalensis roots. J Ethnopharmacol 2014, 152: 183-189.
65. Shi QQ, Dang J, Wen HX,. Anti-hepatitis, antioxidant activities and bioactive compounds of Dracocephalum heterophyllum extracts. Bot Stud 2016, 57: 16.
66. Navratilova A, Nesuta O, Vancatova I,C-Geranylated flavonoids from Paulownia tomentosa fruits with antimicrobial potential and synergistic activity with antibiotics. Pharm Biol 2016, 54: 1398-1407.
67. Li MX, He XR, Tao R,. Phytochemistry and pharmacology of the genus pedicularis used in traditional Chinese medicine. Am J Chin Med 2014, 42: 1071-1098.
68. Borgognone D, Cardarelli M, Rea E,. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J Sci Food Agric 2014, 94: 1231-1237.
69. Pereira C, Barros L, Carvalho AM,. Infusions of artichoke and milk thistle represent a good source of phenolic acids and flavonoids. Food Funct 2015, 6: 56-62.
70. Qiu J, Gao F, Shen G,Metabolic engineering of the phenylpropanoid pathway enhances the antioxidant capacity of Saussurea involucrata. PLoS One 2013, 8: e70665.
71. Liu QM, Zhao HY, Zhong XK,. Eclipta prostrata L. phytochemicals: isolation, structure elucidation, and their antitumor activity. Food Chem Toxicol 2012, 50: 4016-4022.
72. Yang GE, Bao L, Zhang XQ,Studies on flavonoids and their antioxidant activities of Artemisia annua. J Chin Med Master 2009, 32: 1683-1686.
73. Zhou CX, Wu DY, Li XP,Research progress in Laggera medicinal plants. Chin J Chin Mater Med 2006, 31: 1133-1140.
74. Xing XD, Zhang Q, Feng F,. Chemical constituents from stems of Ilex pubescens. J Chin Med Master 2012, 35: 1429-1431.
75. Goodger JQ, Seneratne SL, Nicolle D,. Foliar Essential Oil Glands of Eucalyptus Subgenus Eucalyptus (Myrtaceae) Are a Rich Source of Flavonoids and Related Non-Volatile Constituents. PLoS One 2016, 11: e0151432.
76. Wu P, Ma G, Li N,. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa(Ait.) Hassk. Food Chem 2015, 173: 194-202.
77. Perez-Gregorio MR, Regueiro J, Simal-Gandara J,. Increasing the added-value of onions as a source of antioxidant flavonoids: a critical review. Crit Rev Food Sci Nutr 2014, 54: 1050-1062.
78. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol 2014, 4: 64.
79. Hua S, Zhang Y, Liu J,Ethnomedicine, Phytochemistry and Pharmacology of Smilax glabra: An Important Traditional Chinese Medicine. Am J Chin Med 2018, 46: 261-297.
80. Wang H, Jiang HM, Li FX,Flavonoids from artificially induced dragon's blood of Dracaena cambodiana. Fitoterapia 2017, 121: 1-5.
81. Gao SM, Liu JS, Wang M,Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J Ethnopharmacol 2018, 219: 50-70.
82. Argentieri MP, Levi M, Guzzo F,. Phytochem Anal of Passiflora loefgrenii Vitta, a rich source of luteolin-derived flavonoids with antioxidant properties. J Pharm Pharmacol 2015, 67: 1603-1612.
83. Ding Y, Ren K, Dong H,Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem Biol Interact 2017, 275: 210-217.
84. Bei WJ, Xu AL, Li CY,. Flavonoids from Diospyros kaki inhibit the adhesion between lymphocyte and dorsal root ganglion.J Chin Med Master 2009, 32: 740-744.
85. Djouossi MG, Tamokou JD, Ngnokam D,Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa Forssk. (Salicaceae). BMC Complement Altern Med 2015, 15: 134.
86. Zhou XL, Shi T, Yang L,Research on the effect and mechanism of total ginkgo flavones-glycoides on hepatocyte apoptosis in rats with nonalcoholic fatty liver disease. Chin J Integ Trad West Med Dig 2017.
87. Li AL, Chen BJ, Li GH,Physalis alkekengi L. var. franchetii (Mast.) Makino: An ethnomedical, phytochemical and pharmacological review. J Ethnopharmacol 2018, 210: 260-274.
88. Wang Y, Wang SL, Zhang JY,Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J Ethnopharmacol 2018, 211: 197-206.
89. Kurepa J, Nakabayashi R, Paunesku T,Direct isolation of flavonoids from plants using ultra-small anatase TiO(2) nanoparticles. The Plant Journal 2014, 77: 443-453.
90. Luan N, Li D. Study on supercritical CO2extraction of flavonoids from Cynomorium songaricum. J Chin Med Master 2010, 33: 1167-1171.
91. Cui Z, Guo Z, Miao J,The genus Cynomorium in China: an ethnopharmacological and phytochemical review. J Ethnopharmacol 2013, 147: 1-15.
92. Li JS, Zhao YY, Wang B,. Separation and identification of the flavonoids from Buddleia officinalis Maxim. Acta Pharmaceutica Sinica 1996, 31: 849-854.
93. Peng XJ, Li C. Study on the flavanone constitutes of Buddleja davidii. J Chin Med Master 2011, 34: 1534-1537.
94. Xu XH, Yang NY, Qian SH,. Stduy on flavonoids in Ligustrum lucidum.J Chin Med Master 2007, 30: 538-540.
95. Jia J, Zhang F, Li Z,. Comparison of Fruits of Forsythia suspensa at Two Different Maturation Stages by NMR-Based Metabolomics. Molecules2015, 20: 10065-10081.
96. Qu J, Yan X, Li C,Comparative Evaluation of Raw and Ripe Fruits of Forsythia suspensa by HPLC-ESI-MS/MS Analysis and Anti-Microbial Assay. J Chromatogr Sci 2017, 55: 451-458.
97. Ye M, Li Y, Yan Y,. Determination of flavonoids in Semen Cuscutae by RP-HPLC. J Pharm Biomed Anal 2002, 28: 621-628.
98. Sun Q, Wang D, Li FF,Cytotoxic prenylated flavones from the stem and root bark of Daphne giraldii. Bioorg Med Chem Lett 2016, 26: 3968-3972.
99. Lu CL, Zhu L, Piao JH,. Chemical compositions extracted from Wikstroemia indica and their multiple activities. Pharm Biol 2012, 50: 225-231.
100. Pan L, Hu H, Wang XInhibitory effects of neochamaejasmin B on P-glycoprotein in MDCK-hMDR1 cells and molecular docking of NCB binding in P-glycoprotein. Molecules 2015, 20: 2931-2948.
101. Jaiswal R, Muller H, Muller A,. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry 2014, 108: 252-263.
102. Yuan Y, Song L, Li M,Genetic variation and metabolic pathway intricacy govern the active compound content and quality of the Chinese medicinal plant Lonicera japonica thunb. BMC genomics 2012, 13: 195.
103. Zhang FS, Lv YL, Zhao Y,. Promoting role of an endophyte on the growth and contents of kinsenosides and flavonoids of Anoectochilus formosanus Hayata, a rare and threatened medicinal Orchidaceae plant. J Zhejiang Univ Sci B 2013, 14: 785-792.
104. Zhang J, Wang LS, Gao JM,. Rapid separation and identification of anthocyanins from flowers of Viola yedoensis and V. prionantha by high-performance liquid chromatography- photodiode array detection-electrospray ionisation mass spectrometry. Phytochem Anal2012, 23: 16-22.
105. Wang A, Lin L, Wang Y. Traditional Chinese Herbal Medicine Penthorum chinense Pursh: A Phytochemical and Pharmacological Review. Am J Chin Med 2015, 43: 601-620.
106. Luo Y, Yu H, Yang Y,A flavonoid compound from Chrysosplenium nudicaule inhibits growth and induces apoptosis of the human stomach cancer cell line SGC-7901. Pharm Biol 2016, 54: 1133-1139.
107. Tian C, Zhang P, Yang C,Extraction Process, Component Analysis, and In Vitro Antioxidant, Antibacterial, and Anti-Inflammatory Activities of Total Flavonoid Extracts from Abutilon theophrasti Medic. Leaves. Mediators Inflamm 2018.
108. Li Y, Xia H, Wu M,Evaluation of the Antibacterial Effects of Flavonoid Combination from the Leaves of Dracontomelon dao by Microcalorimetry and the Quadratic Rotary Combination Design. Front Pharmacol 2017, 8: 70.
109. Chen Y, Zhao J, Qiu Y,Prenylated flavonoids from the stems and roots of Tripterygium wilfordii. Fitoterapia 2017, 119: 64-68.
110. Fan GW, Zhang Y, Jiang X,Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-kappaB-dependent pathways. Inflammation 2013, 36: 1584-1591.
111. Zhao QY, Yuan FW, Liang T,Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. J Dairy Sci 2018, 101: 2415-2422.
112. Zeng Z, Qian L, Cao L,Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl Microbiol Biotechnol 2008, 79: 119-126.
113. Yao J, Zhang Q, Min J,. Novel enoyl-ACP reductase (FabI) potential inhibitors of Escherichia coli from Chinese medicine monomers. Bioorg Med Chem Lett 2010, 20: 56-59.
114. Guan X, Zhou Y, Liang X,. Effects of compounds found in Nidus Vespae on the growth and cariogenic virulence factors of Streptococcus mutans. Microbiol Res 2012, 167: 61-68.
115. Xia F, Li X, Wang B,Combination Therapy of LysGH15 and Apigenin as a New Strategy for Treating Pneumonia Caused by Staphylococcus aureus. Appl Environ Microbiol 2016, 82: 87-94.
116. Yu CH, Yu WY, Fang J,Mosla scabra flavonoids ameliorate the influenza A virus-induced lung injury and water transport abnormality via the inhibition of PRR and AQP signaling pathways in mice. J Ethnopharmacol 2016, 179: 146-155.
117. Yan S, Wu B, Lin Z,Metabonomic characterization of aging and investigation on the anti-aging effects of total flavones of Epimedium. Mol Biosyst 2009, 5: 1204-1213.
118. Zhang HX, Lunga PK, Li ZJ,. Flavonoids and stilbenoids from Derris eriocarpa. Fitoterapia 2014, 95: 147-153.
119. Wang T, Liu Y, Li X,. Isoflavones from green vegetable soya beans and their antimicrobial and antioxidant activities. J Sci Food Agric 2018, 98: 2043-2047.
120. Jiang J, Wang RP, Hou MH,. Hydromethanolic extract of Rehum emodi exhibits significant antimicrobial activity against acute gastroenteriti bacterial strains. Microb Pathog 2018, 115: 179-182.
121. Zhou Z, Han N, Liu Z,The antibacterial activity of phytochemically characterised fractions from Folium Syringae. Natur Prod Res 2014, 28: 1495-1498.
122. Njume C, Gqaza BM, Rozani C,. Studies on bioactivity and secondary metabolites of crude extracts of Bidens pilosa L. (Asteraceae): A medicinal plant used in the Transkei region of South Africa. Pak J Pharm Sci 2016, 29: 877-885.
123. Mu J, Liu T, Jiang L,The Traditional Chinese Medicine Baicalein Potently Inhibits Gastric Cancer Cells. J Cancer 2016, 7: 453-461.
124. Xia J, Rong L, Sawakami T,Shufeng Jiedu Capsule and its active ingredients induce apoptosis, inhibit migration and invasion, and enhances doxorubicin therapeutic efficacy in hepatocellular carcinoma. Biomed Pharmacother 2018, 99: 921-930.
125. Ren X, Zhang Z, Tian J,The downregulation of c-Myc and its target gene hTERT is associated with the antiproliferative effects of baicalin on HL-60 cells. Oncol Lett 2017, 14: 6833-6840.
126. Tao Y, Zhan S, Wang Y,Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci Rep 2018, 8: 14477.
127. Qiu P, Dong Y, Li BDihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett 2017, 274: 31-41.
128. Deng J, Wang DX, Liang AL,. Effects of baicalin on alveolar fluid clearance and alpha-ENaC expression in rats with LPS-induced acute lung injury. Can J Physiol Pharmacol 2017, 95: 122-128.
:
This study was financially supported by the Autonomous Foundation of Key Laboratory of Ethnomedicine (Minzu University of China), Ministry of Education (No. KLEM-ZZ201902), the National Natural Science Foundation of China Grants (No. 81973977), and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (No. GCYS2018110001).
:
ROS, Reactive oxygen species; Bcl-2, B-cell lymphoma 2; BAX, Bcl-2 associated X protein; PERK, Protein kinase R-like ER kinase; ATF, Activated transcription factor; IRE1, Inositol-requiring enzyme-1; XBP1, X-box binding protein 1; MMP-2: Matrix metalloproteinase-2, MMP-9: Matrix metalloproteinase-2; PARP: Poly (ADP-ribose) polymerase; Akt: Protein kinase B; DPPH, 1,1-Diphenyl-2-picrylhydrazyl; TLR: Toll-like receptor;RIG, Retinoic acid-inducible gene; AQP, Aquaporin; iNOS, Inducible nitric oxide synthase; COX, Cyclooxygenase; IL, Interleukin; AFC, Alveolar fluid clearance; Nrf2: Nuclear factor erythroid-2-related factor 2.
:
The authors declare that they have no conflict of interest.
:
Shao-Hui Wang, Yan-Lan Hu, Tong-Xiang Liu. Plant distribution and pharmacological activity of flavonoids. Traditional Medicine Research 2019, 4 (5): 269-287.
:Nuo-Xi Pi.
:2 August 2019,
26 August 2019,
:5 September 2019.
10.12032/TMR20190824131
Tong-Xiang Liu, Key Laboratory of Ethnomedicine (Minzu University of China), Ministry of Education, No. 27 Zhongguancun South Street, Haidian District, Beijing 100081, China. E-mail: tongxliu123@hotmail.com.
 Traditional Medicine Research2019年5期
Traditional Medicine Research2019年5期
- Traditional Medicine Research的其它文章
- Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity
- Quantitation of phytochemical constituents of Fumaria vaillantii L. with different extract methods
- Prescribing Chinese patent medicines without traditional Chinese medicine training is now banned in China
- Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A
- Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements
- Antitumor applications of nano-traditional Chinese medicine
