Quantitation of phytochemical constituents of Fumaria vaillantii L. with different extract methods
Fahimeh Mohajerani, Zeinab Pourjabbar, Fatemeh Zamani Mazdeh, Roja Rahimi, Gholam-Reza Amin, Tayebeh Toliyat, Sareh Kargar, Mannan Hajimahmoodi, *
Quantitation of phytochemical constituents ofL. with different extract methods
Fahimeh Mohajerani1, 2, Zeinab Pourjabbar2, Fatemeh Zamani Mazdeh3, Roja Rahimi1, Gholam-Reza Amin2, Tayebeh Toliyat4, Sareh Kargar2, Mannan Hajimahmoodi1, 2, 3*
1Persian Medicine and Pharmacy Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1416753955, Iran.2Drug and Food Control Department,Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1416753955, Iran.3Food and Drug Administration, Tehran University of Medical Sciences, Tehran 1416753955, Iran.4Pharmaceutical Department, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1416753955, Iran.
: The genus fumaria includes more than 40 species in the world. The aim of this study was to quantify the phytochemical constituents ofL. aerial parts and compare the different methods of extraction. Total phenol, total flavonoid, total alkaloid, ascorbic and organic acids (oxalic, maleic, citric, succinic and fumaric acids) yields were evaluated in terms of the temperature effect, type of solvent and maceration time.Dried plant samples were extracted by different procedures. Total phenolic, total flavonoid, total alkaloid and ascorbic acid yields were determined by spectrophotometric methods. Also, the organic acid yields were analyzed using high performance liquid chromatography method.: With the same extraction method, the natural flora extract was showed more yields of oxalic, maleic and citric acids than the commercial one, while the commercial extract was showed more yields oftotal phenol,ascorbic, succinic and fumaric acids than the natural flora one. The water-boiled extract was showed more yields of total phenol and total flavonoid. The macerated in ethanol 80% extract was also demonstrated more amounts of total alkaloid and ascorbic acid. Among different aqueous macerated extracts of the commercial sample, as the maceration time increased, total phenol, total flavonoid, oxalic, maleic, succinic, fumaric and ascorbic acids yields decreased. Macerated commercialdried fumitory in double-distilled water for 24 hrs resulted in an extract with the highest possible fumaric acid yield.:It can be concluded that both water-boiled and macerated in ethanol 80% extracts can be used as rich sources of total phenolic and total flavonoid, which are considered as the important antioxidants.
Total phenolic, Total flavonoid, Total alkaloid, Organic acid,L
Total phenolic, total flavonoid, total alkaloid, ascorbic and organic acids yields inL. aerial parts with different extract methods are evaluated and compared by spectrophotometric and HPLC methods.
The genus fumaria (Fumariaceae or Papaveraceae) includes more than 40 species in the world. Seven species are found in Iran.L. is one of the species which grow in a wide variety of areas of Iran with the common name of fumitory or earth smoke. The aerial parts of the plant which harvested during flowering time are used for medicinal purposes.

Background
The genus fumaria (Fumariaceae or Papaveraceae) includes more than 40 species in the world [1]. Seven species are found in Iran [2].L. is one of the species which grow in a wide variety of areas of Iran with the common name of fumitory or earth smoke [3]. The aerial parts of the plant which harvested during flowering time are used for medicinal purposes. The whole plant are claimed to be anti-allergic drug, antipyretics, choleretic, diuretic, laxative and blood-puri?er in traditional and folkloric medicine and used for controlling of hepatobiliary, dermatological, and gastrointestinal disorders [4, 5]. Today, fumaria extracts are components of several phytotherapeutic preparations, which are used mostly in cases of minor hepatobiliary dysfunction, gastrointestinal diseases and skin disorders [6].
Typically, reactive oxygen species (ROS) are often by-products of biological and metabolic reactions in body cell or due to external factors [7]. ROS can cause tissue damage which results in inflammatory, cardiovascular, neurodegenerative diseases and even cancers. Antioxidants are capable of inhibiting or delaying oxidative activity of free radicals [8]. In recent decades, there has been an increasing interest for natural antioxidants especially herbal ones due to carcinogenic potential of synthetic antioxidants [9]. The protective action of medicinal plants against oxidative stress is due to the presence of antioxidants, especially phenolic, flavonoids compounds and antioxidant vitamins [10]. Different antioxidant compounds have been reported from fumaria species [11]. These compounds include alkaloids (often referred to as fumitory alkaloids or protopine-like alkaloids), flavonoids, glycosides, tannins, saponins, anthraquinones, steroids and triterpenoids [12]. Moreover, the presences of organic acids in different parts of the fumitory plant have been established by several recent studies [13-15]. Organic acids have basic functions in plant organisms such as respiration, reproduction, storage, cell division and growth [16]. Likewise, organic acids have been identified as the metabolites of Krebs cyclein the plants [17]. They are naturally found in vegetables and fruits [18]. The profile and concentration level of organic acids depend on plant variety, region, extracting techniques and aging process [19, 20].
In the present study, total phenolic, total flavonoid, total alkaloid and ascorbic acid yields in different extracts ofL.aerial parts were determined by spectrophotometric methods. Furthermore, simultaneous determination of organic acids including oxalic, maleic, citric, succinic and fumaric acids in these extracts was developed using a cation-exchange column high performance liquid chromatography (HPLC) method.
Methods
Chemicals and reagents
All solvents, reagents and standards were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA) and Merck (Darmstadt, Germany).
Plant material
Two types ofL. were used in this study. The herbal market sample was purchased from the herbal drug market of Tehran, Iran (originated from Shiraz city, Fars province, Iran),which is commonly used in traditional medicine of Iran. The natural flora sample was collected from the natural flora of Alamut Mountain, Qazvin Province, in the north of Iran in April 2015 when the aerial parts of the plant were at the flowering and fruit setting stage. The natural flora sample was compared with the herbal market one extracted by same method in terms of targeted phytochemical properties. They were authenticated at the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran and were the same species of fumaria. Their voucher specimens were deposited at the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran (Reference number: PMP-335 for the commercial sample and 6530-THE for the natural flora one). The plant was dried in shade at room temperature and ground to get a coarse powder.
Sample preparation
All the samples were dried and ground. The commercial sample (100 g) was extracted through different methods including boiling in double distilled water for 15 mins (extract no.1), maceration in ethanol 80% for 24 hrs (extract no.2) and maceration in double distilled water (600 mL) for 72, 48 and 24 hrs (extracts no.3, no. 4 and no.5). The natural flora sample was only extracted by maceration in double distilled water for 24 hrs (extract no.6) in order to be compared with the commercial one with the same extraction method. In all the extraction methods, the solvent to material ratio was 6 : 1. Aqueous extraction was investigated in 24, 48 and 72 hrs in order to determine the effect of maceration time on the yields of targeted phytochemical components. The ethanolic extracts were prepared in order to be compared with the distilled water extracts in the same extraction condition (maceration for 24 hrs). One method of extraction was only used for the fresh sample in order to be compared with the herbal market extract with the same extraction condition (maceration for 24 hrs in aqueous solvent). All the extracts were filtered through sieve no.20 (20 mesh size) and concentrated in a rotary evaporator under reduced pressure and low temperature (45°C) conditions. Drying aqueous extracts was done by using freeze dryer. Then, the yield value of each extract was calculated.
Spectrophotometric analysis
Determination of total phenolic yield. Total phenolic yield was determined according to the Folin-Ciocalteau method with slight modification [21]. 0.1 g of dried extract was transferred to a 10 mL volumetric flask and diluted with 50% methanol solution (dilution factor = 100). After being sonicated for 15 mins, the solution was filtered through Whatman paper. A set of gallic acid standard solutions covering the concentration range between 25 and 125 μg mL-1were prepared (diluted with 50% methanol solution). 200 μL of the dilute solution (50% methanol solution) and each of the standard solutions were separately transferred into test tubes. After adding 1.5 mL of Folin-Ciocalteu reagent (previously diluted 1 : 10 with double distilled water) into all the tubes, they were incubated at room temperature for 5 mins. Then, 1.5 mL of sodium bicarbonate solution (60 g L-1) was added to all the tubes and incubated again in darkness at room temperature for 90 mins. All the samples were sonicated for 15 mins to evacuate the gases. To prepare the blank, 400 μL of double distilled water was transferred to a test tube. Next that, 3 mL of Folin-Ciocalteu reagent were added to the tube, and were incubated at room temperature for 5 mins. Then, 3 mL of sodium bicarbonate solution (60 g L-1) was added to the tube and incubated again in darkness at room temperature for 90 mins. The absorbance was measured against the blank at 725 nm with an UV/VIS spectrophotometer. All determinations were performed in triplicate. Total phenol yield was expressed as mg of gallic acid equivalent (GaE)/g of dried plant.
Determination of total flavonoid yield. Total flavonoid yield was measured by using aluminum chloride colorimetric method with some modification. This method is based on the formation of flavonoid-aluminum complex [22]. 0.05 g of dried extract was transferred to a 10 mL volumetric flask and diluted with 50% methanol solution (dilution factor = 200). The solution was sonicated for 15 mins and then was filtered through Whatman papers. A set of quercetin standard solutions covering the concentration range between 5 and 100 μg mL-1were prepared (diluted with 50% methanol solution). 10 mL of 50% methanol solution and each of the standard solutions were separately transferred to 25 mL volumetric flasks. To prepare the blank, 10 mL of 50% methanol solution was transferred to a 25 mL volumetric flask. After adding 1 mL of aluminum chloride reagent 2% (w/v) to all volumetric flasks, they were diluted with methanolic acetic acid 5% (v/v). All samples were incubated at room temperature for 30 mins. Next they were sonicated for 15 mins to evacuate the gases. The absorbance was measured against the blank at 415 nm with an UV/VIS spectrophotometer. All determinations were performed in triplicate. Total flavonoid yield was expressed as mg of quercetinequivalent (QE)/g of dried plant.
Determination of total alkaloid yield. Total alkaloid yield was determined by using a UV spectrophotometer method with some modification. This method is based on the reaction between alkaloid and bromocresol green [23, 24]. The concentration range of standard atropine solutions was between 10 and 50 μg mL-1. 0.1 g of dried extract was transferred to a 10 mL volumetric flask and diluted with chloroform (dilution factor = 100). All experiments were performed in triplicate. Total alkaloid yield was expressed as mg of atropine equivalent (AtE)/g of dried plant.
Determination of ascorbic acid yield. Ascorbic acid yield was measured by using the 2,4-dinitrophenyl hydrazine method [25]. For preparing 2,4-dinitrophenyl hydrazine, thiourea, copper sulphate (DTC solution), 3 g of powdered 2,4-dinitro-phenyl hydrazine, 0.4 g of tiourea and 0.05 g of copper sulfate were transferred to a 100 mL volumetric flask and then diluted with sulfuric acid 9 N solution. This solution was stable for at least one week.
0.1 g of ascorbic acid was transferred to a 100 mL volumetric flask and diluted with trichloroacetic acid 5% solution. A set of reference standard solutions of ascorbic acid covering the concentration range between 5 and 50 μg mL-1were prepared (diluted with methanol). 0.1 g of dried extract was transferred to a 10 mL volumetric flask and diluted with double distilled water (dilution factor = 100). After being sonicated for 15 mins, the solution was filtered through Whatman paper. 400 μL of the extract solution and each concentration of standard solutions were separately transferred into the test tubes. To prepare the blank, 200 μL of double distilled water was transferred to a test tube. Next, 80 μL of DTC solution (2, 4-dinitrophenyl hydrazine, thiourea, copper sulphate) added into all the tubes and they were incubated in water bath (37°C) for 3 hrs. The samples were then placed in ice for 10 mins and then 600 μL of sulfuric acid 65% solution was added to all the tubes and were shaken vigorously. All the tubes were incubated at room temperature for 30 mins. The absorbance was measured against the blank at 520 nm with an UV/VIS spectrophotometer. All determinations were performed in triplicate. Ascorbic acid yield was expressed as mg of ascorbic acid/g of dried plant.
HPLC analysis
Apparatus and chromatographic conditions. Organic acids including oxalic, maleic, citric, succinic and fumaric acids were analyzed by using a Knauer HPLC system (Germany) coupled to a UV Detector K-2500 monitored at the 210 nm, equipped with a degassor, an injection valve (10-μL sample loop), a quatry pump, an oven Knauer, a cation-exchange Eurokat H, vertex plus column (300 × 8 mm, 10 μm) as the stationary phase and the Chem32 software. 10 μL aliquots of the diluted samples were injected into the system. Separation was performed at the pressure of 3 MPa with an isocratic flow rate of 0.7 mL min-1of 0.003 N sulfuric acid in deionized water (pH 3.2)as the mobile phase. In order to achieve complete separation of peaks in the chromatogram, this analysis would need to be run at 60°C.
Standard preparation.The standard organic acids were precisely weighed (5 mg) and dissolved in 5 mL of deionized water. A set of serial dilutions of each organic acid was prepared. The concentration ranges of standard solutions were 1-30 μg mL-1for fumaric acid and oxalic acid, 5-60 μg mL-1for citric acid and succinic acid and 0.5-20 μg mL-1for maleic acid. Then, a 10-μL aliquot of each serial dilution was injected into the HPLC system.
Determination of yield of organic acid. The organic acids were analyzed in different dried extracts of, 10 mg of each dried extract was precisely weighed and dissolved in 5 mL of deionized water in a 5 mL volumetric flask by using a sonicator for 2 mins (dilution factor = 500). The solution was filtered through a 0.45 μm PVDF membrane. Then, it was injected into the HPLC system.
Statistical analysis
The obtained data were considered with the SPSS statistical package, version 21 (SPSS Inc. Chicago, IL, USA). Analysis of variance (ANOVA) was applied for evaluation the differences of distribution between different methods of extraction.
Results
Determination of yield for different methods of extraction
According to the results (Table 1), the highest extractive value (43.9%) was obtained in the extraction method of boiling at 100°C for 15 mins. Among the different maceration methods, the ethanolic (80%) extracthad slightly higher extractive value (20.3%) in comparison with the aqueous macerated extracts. Furthermore, in the method of maceration with double distilled water, as the maceration time increased, the amount of extractive value decreased from 24 to 48 hrs, and then remained approximately consistent from 48 to 72 hrs.
Yields of total phenolic, total flavonoid, total alkaloid, ascorbic acid and organic acids
In the present study, we investigated the yields of total phenolic, total flavonoid, total alkaloid, ascorbic acid and organic acidsin different extracts ofL. growing in Iran. Calibration equations, correlation coefficients and validation parameters for different analysis methods were shown in Table 2 for the yields of total phenolic, total flavonoid, total alkaloid and ascorbic acidand in Table 3 for the organic acids.
The results for yields of total phenolic, total flavonoid, total alkaloid and ascorbic acid extracted with different methods were presented in Table 4. These results show mg of the active ingredient per 1 g of dried plant [mg/g dried weight (DW)]. According to One way ANOVA analysis, there are significant differences between the yields of total phenol, flavonoid, alkaloid and ascorbic acidin various methods of extraction (all< 0.001).The water-boiled extract is showed more yields of total phenol and total flavonoid (< 0.001).The macerated in ethanol 80% extract is also demonstrated more amounts of total alkaloid and ascorbic acid (< 0.001). Flavonoid has not significant difference in commercial samples macerated in double distilled water for 72 hrs and 48 hrs.
The results for organic acids were demonstrated in Tables 5. These results show mg of the active ingredients per 1 g of dried plant (mg/g DW). One way ANOVA analysis approves that the yields of fumaric, citric, succinic, oxalic and maleic acids had significant differences in various methods of extraction (all< 0.001). Macerated commercialdried fumitory in double-distilled water for 24 hrs results in an extract with the highest possible yield of fumaric acid (< 0.001).
Discussion
Regarding different methods of extraction, the yields of total phenolic, total flavonoid, total alkaloid and ascorbic acid in dried commercial sample ofL. ranged in the limits of 0.999-5.481 mg GaE/g DW, 0.558-5.585 mg QE/g DW, 0.266-1.009 mg AtE/g DW and 0.106-0.737 mg ascorbic acid/g DW, respectively. Water-boiled extract had the highest yields of total phenol and total flavonoid among all the investigated extracts when the extractive values were considered in different extraction methods. Furthermore, the highest ascorbic acid and alkaloid yield was found in ethanolic extract. With the same extraction method, the commercial extract showed higher yields of total phenol andascorbic acid than natural flora one.
According to Sou?ek, methanolic extract ofL showed a high yield of total phenol as 20.41 mg GaE/g DW [6]. Orhan. found that total phenolic and flavonoid yields in the ethanolic extracts of four fumaria species includingHausskn.,DC.,Jordan andLam., growing in Turkey, were in the range of 0.015-0.030 mg GaE/g DW and 0.006-0.017 mg RutinE/g DW, respectively [26]. Abbasi. was carried out a research on total phenolic yield in the aqueous extract ofand was reported 0.047 mg GaE/g DW for total phenolic yield [9]. In a recent study, Jaberian. indicated that the methanolic extract ofhamore total phenol in comparison to methanol-water extract (10.5 compared to 4.27 mg GaE/g DW), whereas total flavonoid yield of the methanol-water extract was higher than the methanolic extract (3.11 in comparison with 2.07 mg QE/g DW) [1]. Ivanov. reported high total phenolic and flavonoid yields for ethanolic extracts ofand, growing in Bulgaria, which ranged in the limits of 20.20-30.30 mg GAE/g DW and 8.70-16.62 mg QE/g DW, respectively [21].

Table 1Yields in the extraction of 100 g of dried aerial parts
LOD: Limit of detection; LOQ: Limit of quantitation.
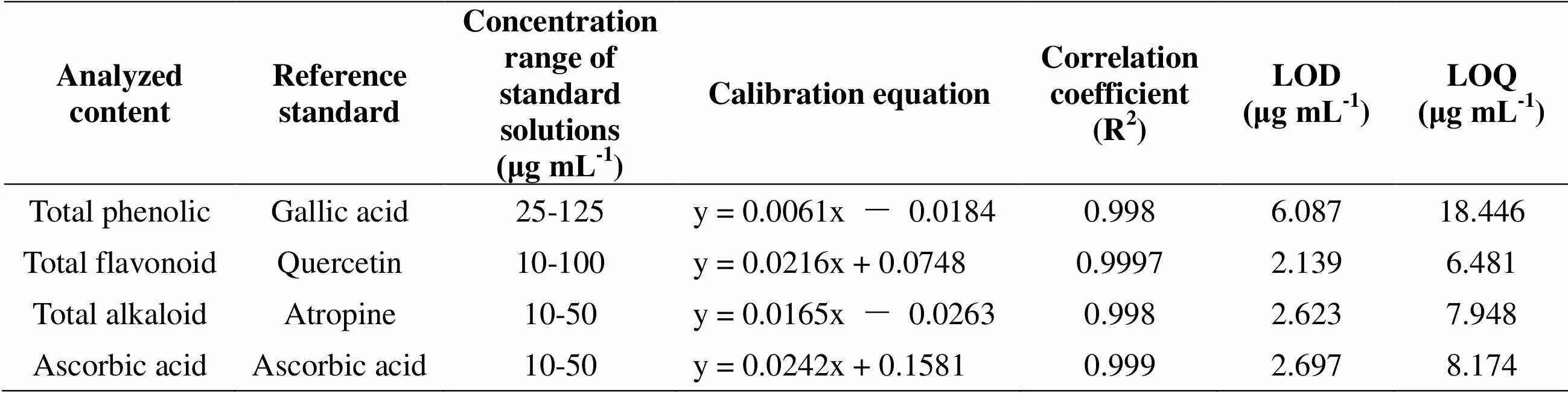
Table 2 Analytical characteristics of the method validation for different spectrophotometric methods
LOD: Limit of detection; LOQ: Limit of quantitation.
In the current study, total alkaloid yield of commercialL dried aerial parts ranged in the limit of 0.266-1.009 mg AtE/g DW, while Maiza-Benabdesselam. reported total isoquinoline alkaloid yield of methanolic extract of two different fumaria species includingL. andL., were 4.26 and 5.21 mg AtE/g DW, respectively [22]. According to a research carried out by Rathi, the concentration of protopine in the hydroalcoholic (ethanol 80%) extract ofL. was reported 0.2 mg/g DW, whereas in this study, using the same solvent for the extraction, total alkaloid yield was found 1.009 mg AtE/g DW [23]. According to Wagner and Bladt, total alkaloid yield inwhich expressed as protoberberine type alkaloid was reported 5-10 mg/g DW [24]. In the present study, ascorbic acid yield of commercialLiosel. dried aerial parts ranged in the limit of 0.106-0.737 mg ascorbic acid/g DW. As comparison, in a study done by Kanaujia. ascorbic acid yield inwas reported in the range of 0.3-0.65 mg ascorbic acid/g DW [25]. It can be concluded thatL. is rich in total phenol, total flavonoid, total alkaloid and ascorbic acid yields and can be used as an antioxidant plant. In the present study, regarding different extracting methods, oxalic, maleic, citric, succinic and fumaric acid yields in dried commercial sample ofL. ranged in the limits of 0.0703-0.2551, 0.0352-0.0905, 0.5818-2.3596, 0.685-12.8921 and 0.6529-1.3372 mg organic acid/g DW, respectively. Citric, succinic and fumaric acids were found in all the extracts. The obtained results showed different levels of organic acid yields in two different samples ofL. which were extracted by the same method (aqueous macerated for 24 hrs). The natural flora sample extract had more oxalic, maleic and citric acid yields than commercial one, whereas the commercial sample extract showed more succinic and fumaric acid yields than natural flora one. Among five different extracts of the commercial sample, the water-boiled extract were showed the highest yields of oxalic, citric and succinic acids. Also, the highestmaleic and fumaric acid yields were found for the aqueous macerated (for 24 hrs) extract. The results also indicated that among different aqueous macerated extracts of the commercial sample, by increasing the maceration time the yields of oxalic, maleic, fumaric, succinic, and ascorbic acids and total phenol and total flavonoid would decrease.
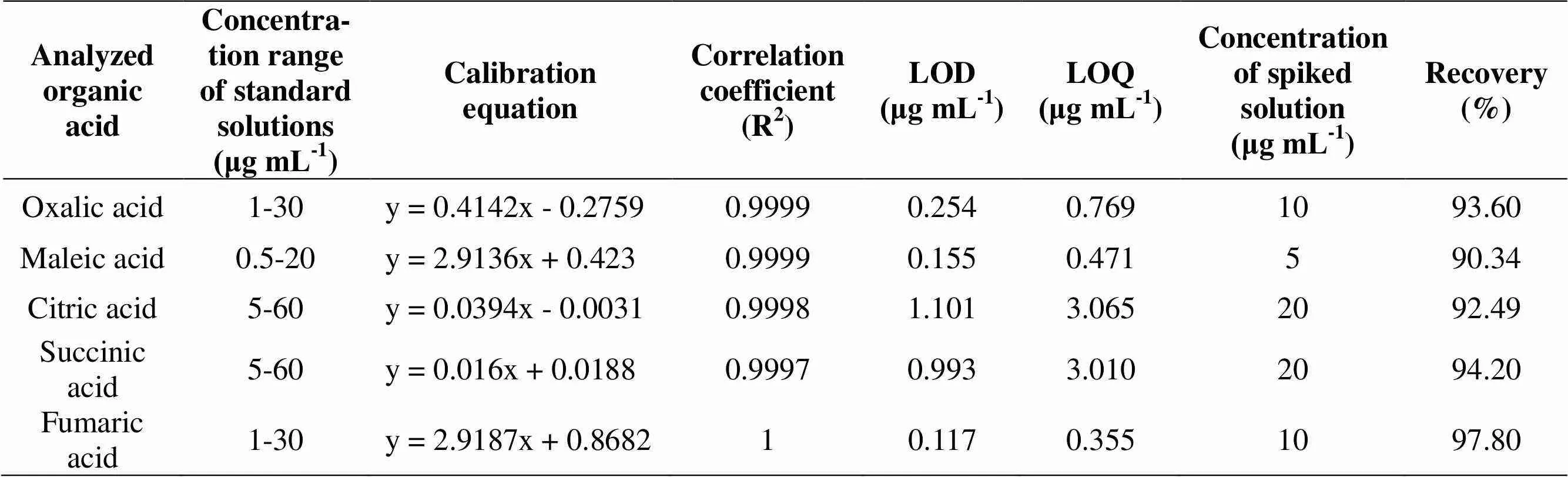
Table 3Analytical characteristics of the method validation for HPLC method
LOD: Limit of detection; LOQ: Limit of quantitation.

Table 4Yields of total phenol, flavonoid, alkaloid and ascorbic acid (mg/g DW) of Fumaria vaillantii L.
Total phenol: all< 0.001, 12, 3, 4, 5 or 6,< 0.001, 5 vs 6,< 0.001; total flavonoid: all< 0.001, 12, 3, 4, 5 or 6,< 0.001; total alkaloid: all< 0.001, 21, 3, 4, 5 or 6,< 0.001, 5 vs 6,< 0.001; ascorbic acid: all< 0.001, 21, 3, 4, 5 or 6,< 0.001, 5 or 6,0.023.
1: Commercial sample boiled in double distilled water for 15 mins; 2: commercial sample macerated in ethanol 80% for 24 hrs; 3: commercial sample macerated in double distilled water for 72 hrs; 4: commercial sample macerated in double distilled water for 48 hrs; 5: commercial sample macerated in double distilled water for 24 hrs; 6: natural flora sample macerated in double distilled water for 24 hrs. DW: Dried weight (of the plant); GaE: Gallic acid equivalent;QE: Quercetin equivalent; AtE: Atropine equivalent.
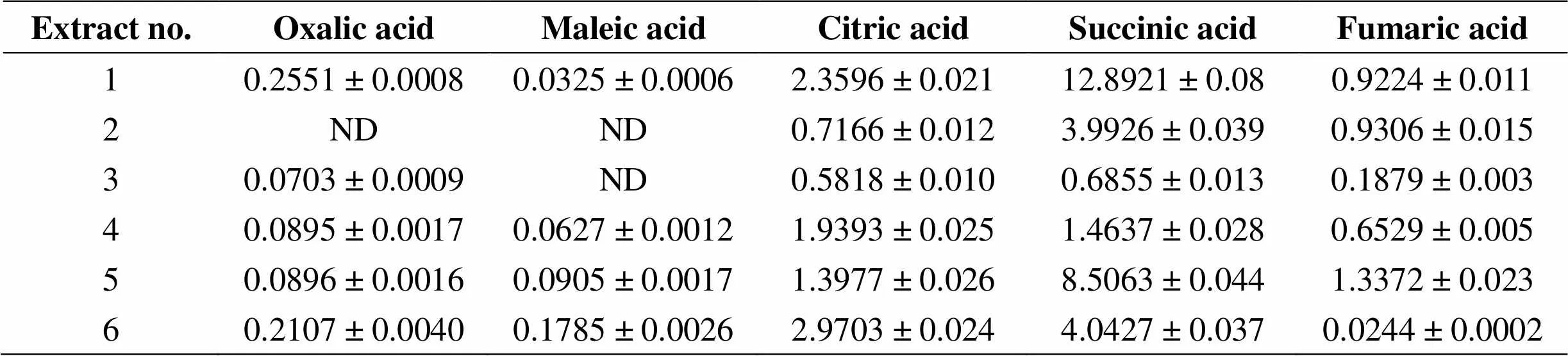
Table 5Yields of organic acid (mg/g) DW of Fumaria vaillantii L. aerial parts
Oxalic acid: all< 0.001, 12, 3, 4, 5 or 6,< 0.001, 5 vs 6,< 0.001; maleic acid: all< 0.001, 61, 2, 3, 4 or 5,< 0.001; citric acid: all< 0.001, 61, 2, 3, 4 or 5,< 0.001; succinic acid: all< 0.001, 12, 3, 4, 5 or 6,< 0.001, 5 vs 6,< 0.001; fumaric acid: all< 0.001, 51, 2, 3, 4 or 6,< 0.001.
1: Commercial sample boiled in double distilled water for 15 mins; 2: commercial sample macerated in ethanol 80% for 24 hrs; 3: commercial sample macerated in double distilled water for 72 hrs; 4: commercial sample macerated in double distilled water for 48 hrs; 5: commercial sample macerated in double distilled water for 24 hrs; 6: natural flora sample macerated in double distilled water for 24 hrs. DW: Dried weight (of the plant); ND: Not detected.
Fumitory is a major source of fumaric acid, which due to its anti-inflammatory properties is widely used for the treatment of skin disorders such as psoriasis and itching. This study aimed to achieve a fumitory extract containing the highest possible yield of fumaric acid. Considering the extractive values, among different extraction methods, macerating in double-distilled water for 24 hrs resulted in an extract with the highest fumaric acid yield.
According to Guerrant, short-chain acids such as pyruvic, succinic, lactic, fumaric, formic, acetic and propionic acids were identified and determined in anaerobic bacteria in the United States using a HPLC system equipped with a cation-exchange column and UV detector at the wavelength of 210 nm. Furthermore, GC-MS method was used to confirm the presence of fumaric acid in the cultivated extract [27]. Stein. determined the short-chain fatty acids such as formic, acetic, propionic and n-butyric acids in biological materials in Germany using HPLC system equipped with UV detector at 214 nm [28]. The concentrations of low molecular weight organic acids such as lactic, formic, acetic, propionic, n-butyric and iso-butyric acids in seawater samples were determined by Albert and Martens in the United States of America, using HPLC method [29]. Organic acids including citric, coumaric, ferulic, fumaric, malic, 3-hydroxybenzoic, protocatechuic and caffeic acids (and its methylester) were investigated by Sou?ek. in Czech Republic in seven fumaria species:andusing GC-MS technique. The presence of fumaric, malic, citric and caffeic acids were exhibited in the aerial parts ofL[6]. Lian. carried out an investigation on simultaneous determination of organic acids including oxalic, fumaric, maleic and succinic acids in pharmaceutical compounds in China, using ion-suppression RP-HPLC system equipped with UV detector at 210 nm [30]. According to a study done by Khalighi-Sigaroodi, fumaric acidyield inL. was reported as 9.3 mg fumaric acid/g DW in Iran, using HPLC method [31]. In the present study, fumaric acid yield of commercialL. was in range of 0.1879-1.3372 mg fumaric acid/g DW, which is lower than the value reported in the previous study (with different species of fumaria). According to a research conducted by Jaberian. in Iran, fumaric acid yields of aqueous, methanolic-aqueous and methanolic extracts ofLwere determined by using GC-MS method. The methanolic-aqueous extract showed higher fumaric acid yield (1.966 mg fumaric acid/g DW) in comparison with aqueous (1.49 mg fumaric acid/g DW) and methanolic (0.875 mg fumaric acid/g DW) extracts [1], whereas in our study, the highest fumaric acid yield were found for the aqueous macerated (for 24 hrs) extract with commercialL., and then it was decreased in 80% of ethanol, water-boiled, aqueous macerated (for 48 hrs) and aqueous macerated (for 72 hrs) extracts, respectively. Vrancheva. identified six organic acids including citric, iso-citric, malic, succinic, glyceric and threonic acids in Bulgaria in five species of fumaria:L.Boiss.Jord.Knaf.andVelen. using GC-MS method [32]. In the present study, it was revealed that among the identified organic acids, succinic acid was the dominant organic acid in all the extracts except the aqueous macerated (for 48 hrs) extract.
Conclusion
Based on the results of the current study, it can be concluded thathigh total phenol and flavonoid yield in boiled extract and high levels of total alkaloid and ascorbic acid in 80% of ethanol macerated extract. Moreover, among different aqueous macerated extracts of the commercial sample, as the maceration time increased, the yields of total phenolic, total flavonoid, ascorbic, oxalic, maleic, succinic and fumaric acids would decrease.
1. Suau R, Cabezudo B, Rico R,. Direct determination of alkaloid contents in Fumaria species by GC‐MS. Phytochem Anal 2002, 13: 363-367.
2. Mozaffarian V. A dictionary of Iranian plant names: Latin, English, Persian. Farhang Mo'aser, 1996.
3. Guna G. Pharmacological activity of Fumaria indica-A review. J phytopharmacol 2017, 6: 352-355.
4. Gilani AH, Bashir S, Janbaz KH,. Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J Ethnopharmacol 2005, 96: 585-589.
5. Mortazavi SR, Nassiri-Asl M, Farahani-Nick Z,. Protective effects ofextract on carbon tetrachloride-induced heaptotoxicity in rats. Pharmacolonline 2007, 3: 385-393.
6. Sou?ek J, Guedon D, Adam T,. Alkaloids and organic acids content of eight Fumaria species. Phytochem Anal 1999, 10: 6-11.
7. Cerutti P. Oxidant stress and carcinogenesis. Eur J Clin Invest 1991, 21: 1-5.
8. Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev 1994, 52: 253-265.
9. Riaz T, Abbasi M, Rehman A,. Fumaria indica: A valuable natural source of antioxidants for protection against oxidative stress. J Pharm Sci Innov 2012, 1: 16-21.
10. Moghaddam M, Khaleghi Miran SN, Mehdizadeh L. Total phenolic content and antioxidant activity ofextract at three phenological stages assayed by various methods. Int J Hortic Sci Technol 2018, 5: 105-114.
11. Pandey V, Dasgupta B, Bhattacharya S,. Chemistry and pharmacology of the major alkaloid of Fumaria indica (Haussk) Pugsley. Curr Sci 1971, 40: 455-457.
12. Mehmood MH, Al-Rehaily AJ, Mothana RA,. Species and tissue-specificity of prokinetic, laxative and spasmodic effects of Fumaria parviflora. BMC Complement Altern Med 2012, 12: 16.
13. Kumar Singh G, Rai G, Sunder Chatterjee S,. Anti-aggressive, brain neurotransmitters and receptor binding study of Fumaria indica in rodents. Curr Psychopharmacol 2012, 1: 195-202.
14. Singh GK, Rai G, Chatterjee SS,. Beneficial effects of Fumaria indica on chronic stress-induced neurobehavioral and biochemical perturbations in rats. Chin Med 2012, 3: 49.
15. Shakya A, Singh GK, Chatterjee SS,. Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J Intercult Ethnopharmacol 2014, 3: 173.
16. Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2:251-86.
17. Herrmann KM. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell 1995, 7: 907.
18. Kelebek H, Selli S, Gubbuk H,. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem 2015, 173: 912-919.
19. Soyer Y, Koca N, Karadeniz F. Organic acid profile of Turkish white grapes and grape juices. J Food Compost Anal 2003, 16: 629-636.
20. Shui G, Leong LP. Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A 2002, 977: 89-96.
21. Ivanov I, Vrancheva R, Marchev A,. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol App Sci 2014, 3: 296-306.
22. Maiza-Benabdesselam F, Khentache S, Bougoffa K,. Antioxidant activities of alkaloid extracts of two Algerian species of Fumaria: Fumaria capreolata and Fumaria bastardii. Rec Nat Prod 2007, 1: 28.
23. Rathi A, Srivastava AK, Shirwaikar A,. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine 2008, 15: 470-477.
24. Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. Springer Science & Business Media. 1996.
25. Ravikanth K, Kanaujia A, Thakur D,. Nutritional constituents of the plants fumaria indica and caesalpinia bonducella. Int J Adv Pharm Biol Chem 2014, 3: 698-702.
26. Orhan IE, ?ener B, Musharraf SG. Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp Pathol 2012, 64: 205-209.
27. Guerrant G, Lambert M, Moss CW. Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol 1982, 16: 355-360.
28. Stein J, Kulemeier J, Lembcke B,. Simple and rapid method for determination of short-chain fatty acids in biological materials by high-performance liquid chromatography with ultraviolet detection. J Chromatogr 1992, 576: 53-61.
29. Albert DB, Martens CS. Determination of low-molecular-weight organic acid concentrations in seawater and pore-water samples via HPLC. Marine Chemistry 1997, 56: 27-37.
30. Lian H, Mao L, Ye X,. Simultaneous determination of oxalic, fumaric, maleic and succinic acids in tartaric and malic acids for pharmaceutical use by ion-suppression reversed-phase high performance liquid chromatography. J Pharm Biomed Anal 1999, 19: 621-625.
31. Khalighi-Sigaroodi F, Yazdani D, Taghizadeh M,. Quantitative determination of an effective component of Fumaria parviflora Lam. J Med Plants 2005, 4:62-71.
32. Vrancheva RZ, Ivanov IG, Aneva IY,. GC-MS based metabolite profiling of five Bulgarian Fumaria species. J BioSci Biotechnol 2014, 3: 195-201.
:
This work was supported by a grant from Persian Medicine and Pharmacy Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran numbers 94-03-96-30194 & 95-03-96-32757.
:
ROS, Reactive oxygen species; HPLC, High performance liquid chromatography; ANOVA, Analysis of variance; AtE, Atropine equivalent; GaE, Gallic acid equivalent; QE, Quercetin equivalent; DW, Dried weight.
:
The authors declare that they have no conflict of interest.
:
Fahimeh Mohajerani, Zeinab Pourjabbar, Fatemeh Zamani Mazdeh,. Quantitation of phytochemical constituents ofL. with different extract methods. Traditional Medicine Research 2019, 4 (5): 237-245.
Cui-Hong Zhu, Nuo-Xi Pi.
:27 February 2019,
12 May 2019,
:18 August 2019.
10.12032/TMR20190905134
Mannan Hajimahmoodi,Drug and Food Control Department, Faculty of Pharmacy, Tehran University of Medical Sciences, 21 Dameshgh St., Vali-e Asr Ave., Tehran 1416753955, Iran.Email: hajimah@sina.tums.ac.ir.
 Traditional Medicine Research2019年5期
Traditional Medicine Research2019年5期
- Traditional Medicine Research的其它文章
- Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity
- Prescribing Chinese patent medicines without traditional Chinese medicine training is now banned in China
- Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A
- Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements
- Plant distribution and pharmacological activity of flavonoids
- Antitumor applications of nano-traditional Chinese medicine
