Cellulose based polymers in development of amorphous solid dispersions
Rahul B Chavan,Sneha Rathi,Vaskuri G S Sainaga Jyothi,Nalini R Shastri
Solid State Pharmaceutical Research Group(SSPRG),Department of Pharmaceutics,National Institute of Pharmaceutical Education and Research,Hyderabad 500037,India
Keywords:Supersaturation HPMC Amorphous form Crystallization Polymers
ABSTRACT Cellulose derivatives have gained immense popularity as stabilizers for amorphous solid dispersion owing to their diverse physicochemical properties. More than 20 amorphous solid dispersion-based products that have been approved for marketing consist of cellulose derivatives as stabilizers, thus highlighting their importance in generation of amorphous solid dispersions.These polymers offer numerous advantages like drug solubilization,crystallization inhibition and improvement in release patterns of drugs.Exploring their potential and exploiting their chemistry and pH responsive behaviour have led to the synthesis of new derivatives that has broadened the scope of the use of cellulose derivatives in amorphous formulation development. The present review aims to provide an overview of different mechanisms by which these cellulose derivatives inhibit the crystallization of drugs in the solid state and from supersaturated solution. A summary of different categories of cellulose derivatives along with the newly explored polymers has been provided.A special segment on strengths,weaknesses,opportunities,and threats(SWOT)analysis and critical quality attributes (CQAs) which affect the performance of the cellulose based amorphous solid dispersion will aid the researchers in identifying the major challenges in the development of cellulose based solid dispersion and serve as a guide for further formulation development.?2018 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Polymers play a pivotal role in the stabilization of amorphous solid dispersions by retaining the drug in an amorphous form in the polymeric matrix during storage, thereby inhibiting crystallization of the drug in the matrix[1].Exposure to aqueous media results in rapid drug release from the amorphous solid dispersion, thus generating supersaturation. The rapid release of drug can be attributed to the elimination of energyrequired for disruption of the crystal lattice. The resultant supersaturated solution possesses the tendency to desupersaturate and attain the stable crystalline form. Stabilization polymers when used must be effective in inhibiting the crystallization of drug from the supersaturated solution for the duration of transport through the absorptive zones of the gastrointestinal (GI) tract. According to Fick’s law, absorption of a drug across the intestinal epithelial trans membrane is directly proportional to the achieved supersaturation ratio.Hence, polymer selection for development of amorphous solid dispersion is of paramount importance. The polymer must be compatible with the drug and promote drug-polymer interactions for effective stabilization of the produced system.
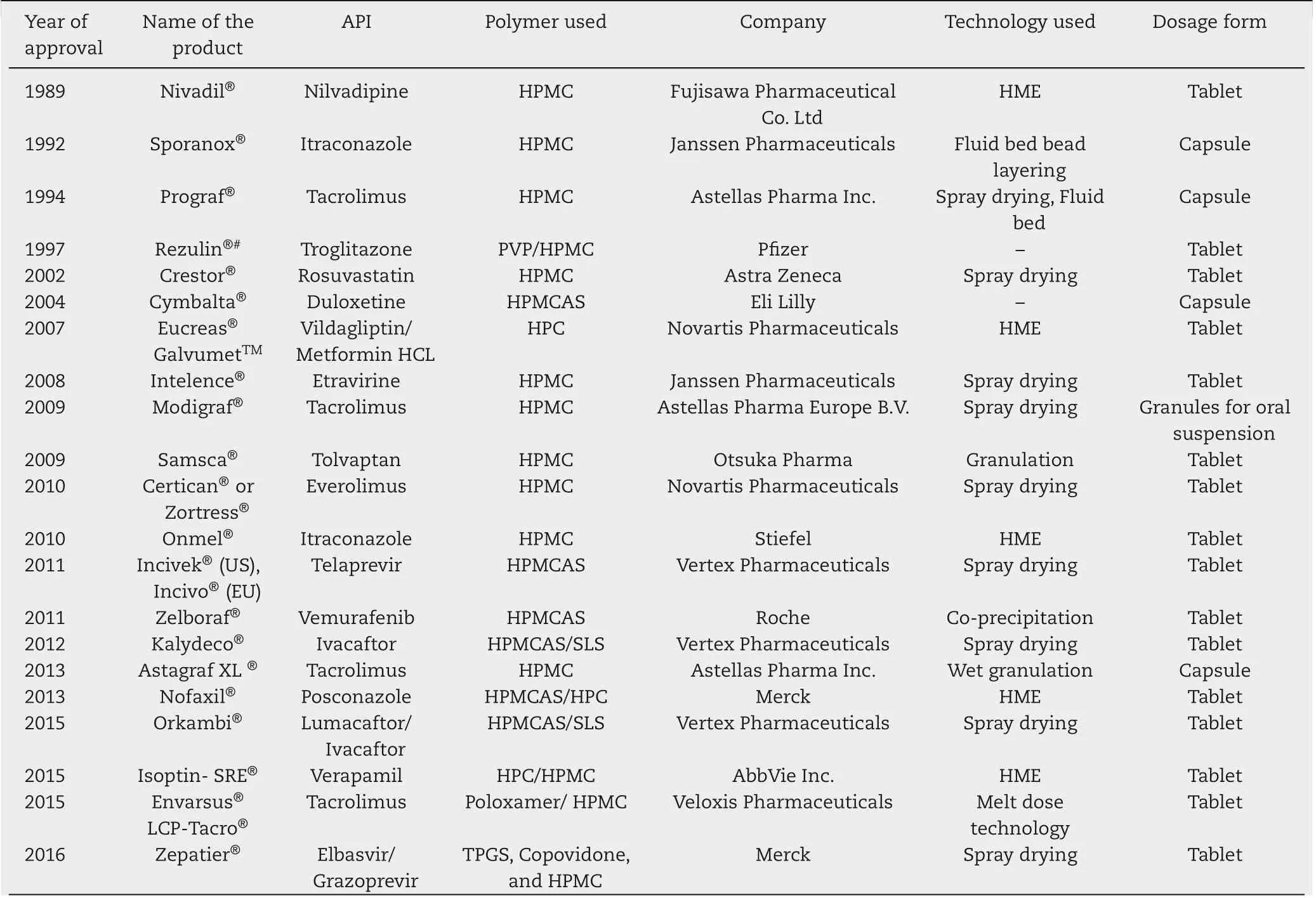
Table 1 - List of marketed amorphous solid dispersion products containing cellulose derivatives as stabilizers (#product withdrawn from market).
Cellulose derivatives have been predominantly used in the stabilization of amorphous form of drugs.The dominance of cellulose derivatives as stabilizers is evident from the number of amorphous solid dispersions [2], that have been approved by the regulatory authorities for marketing (Table 1).This remarkable popularity may be attributed to their high molecular weights and other physicochemical properties,as a result of which most cellulose derivatives cannot be absorbed from the GI tract.These properties render the cellulose derivatives safe as compared to their synthetic counterparts for many drug delivery applications.Along with the benign nature of the cellulose derivatives at physiological conditions,strong drugpolymer interactions and high glass transition temperatures(Tg) make them the best stabilizers for amorphous solid dispersion generation.The strengths,weaknesses,opportunities,and threats(SWOTs)of cellulose derivatives based amorphous solid dispersions are depicted in Fig.1.
Numerous articles shed light on the synthesis, characterization and application of cellulose derivatives in the development of amorphous solid dispersions, [3-7] however, an overview on the mechanism by which cellulose derivatives inhibit crystallization both in the solid state and in solution,post solubilization in the aqueous medium is lacking.Hence,a major objective of this review is to provide a comprehensive discussion on the role of cellulose derivatives in amorphous stabilization and maintenance of supersaturation. Additionally,a special segment has been provided on the preparation and characterization techniques along with the regulatory perspective,delineating the critical quality attributes(CQAs)that affect the performance of amorphous solid dispersions.
2. Background of cellulose-based polymers
Cellulose is the most abundant biopolymer in the world,followed closely by chitin.As the chief structural component of plants,cellulose is an almost inexhaustible polymeric raw material with a fascinating structure and properties.Cellulose has been in use as a raw material for over 150 years. This polysaccharide consists of a linear chain of a variable length of 1-4-linked β-D-anhydroglucopyranose units (Fig. 2). These repeating units are covalently linked via acetal functions between the equatorial-OH group of C4and the C1carbon atom.
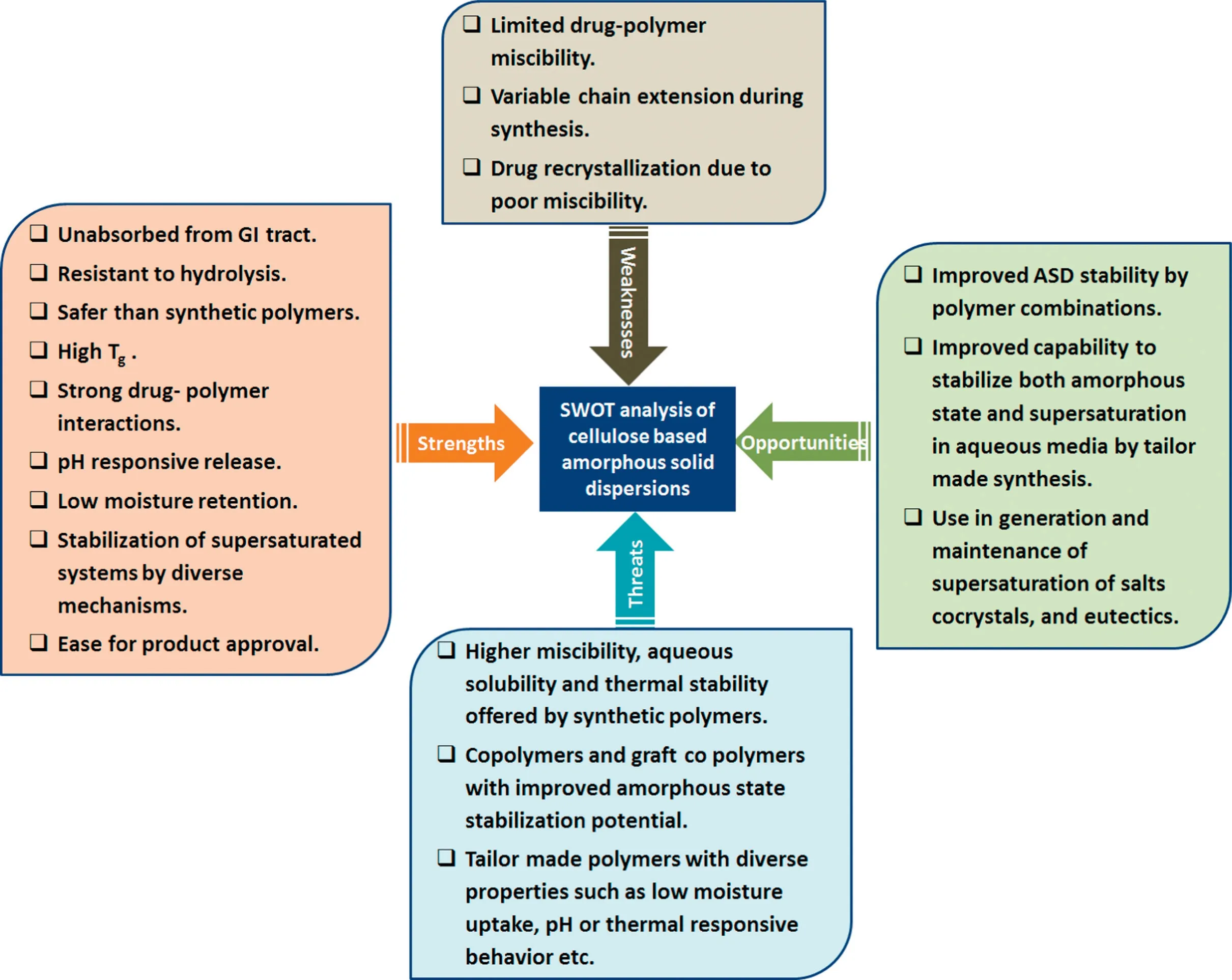
Fig.1-SWOTs analysis of cellulose derivatives based amorphous solid dispersions.
Cellulose is a highly hydrophilic polymer, having hydrophilic-lipophilic balance (HLB) number at 12.45. However, it is not soluble in water in its native form due to its strong intramolecular and intermolecular hydrogen bonding between the individual chains and a high degree of crystallinity (in the range of 40%-60%). Hence, cellulose is chemically modified to water-soluble cellulose ester or ether derivatives.In cellulose ethers,part of the hydrogen atoms of the three hydroxyl groups on the anhydroglucose repeating unit is replaced by alkyl or mixed alkyl groups (Fig. 2). Such modifications of cellulose via esterification or etherification of the hydroxyl groups are termed as"cellulosics’’.The versatile properties of cellulose ethers such as their aqueous solubility,enhanced viscosity,rheology and water retention ability have been utilized for diverse applications[8].They are well known for their use as suspension stabilizing agents, thickening agents, bonding agents, adhesives, coating compositions,film-forming agents,thermoplastic materials,finishing compositions,emulsion stabilizers,protective colloids and plastic sheets. These varied properties and applications of cellulose ethers have helped them to maintain a strong market presence.
Cellulose derivatives are generally categorized based on their pH-responsive behaviour and chemistry as pH responsive,hydrophilic and hydrophobic cellulose derivatives(Fig.3 and Table 2).Considering the extensive list of examples under all these categories, our discussion is restricted to the cellulose derivatives which find utility in the development of amorphous solid dispersion (Table 2). Summary of the properties,advantages and limitations of cellulose derivatives which are explored for amorphous solid dispersion development were summarized in Table 2.
2.1. Conventional cellulose esters and ethers
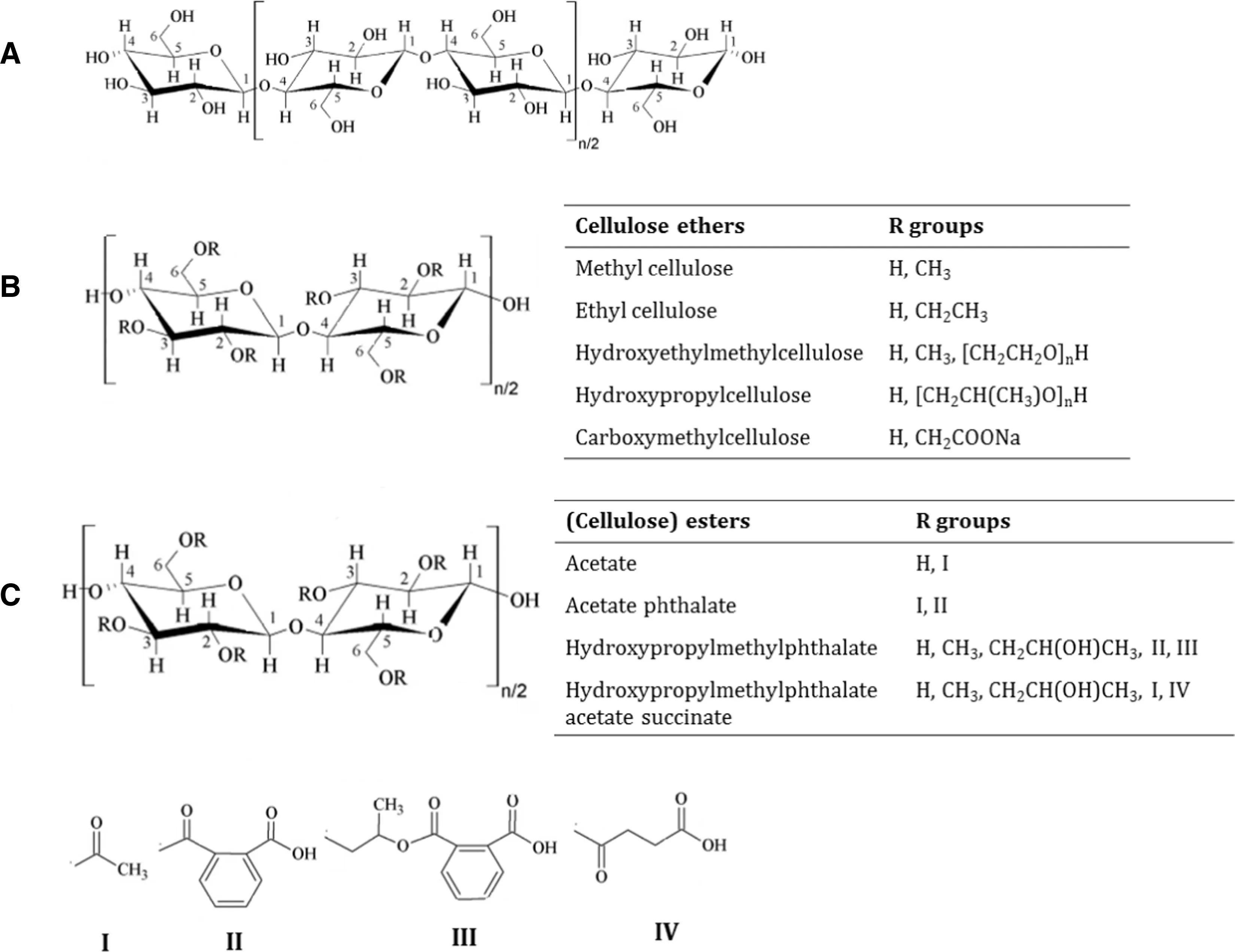
Fig.2-Chemical structure cellulose with two β-1,4 linked anhydroglucose units(A),cellulose ether derivatives(B)and cellulose ester derivatives(C).
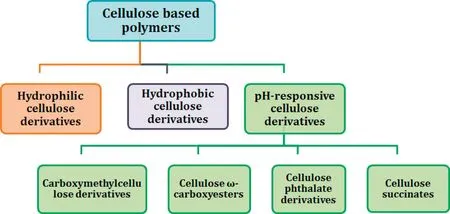
Fig.3-Classification of cellulose-based polymers.

Table 2-Cellulose derivatives explored in preparation of amorphous solid dispersions.
Alkylation of cellulose yields a class of polymers commonly termed as cellulose ethers. Methylcellulose (MC), ethylcellulose (EC), hydroxypropylcellulose (HPC), hydroxyethylcellulose(HEC),and hydroxypropylmethylcellulose(HPMC)are the most widely used cellulose ethers in pharmaceutical industry.Cellulose esters and ethers are of particular interest for developing amorphous solid dispersions because of their physicochemical properties such as high molecular weight and resistance to hydrolysis which prevents the absorption of most cellulose ethers and esters in the GI tract.Even under extreme circumstances, if a small degree of hydrolysis were to occur through chemical or lipase-catalyzed hydrolysis,the resulting by-products(cellulose,glucose and carboxylic acids)would be endogenous or dietary.Amongst the cellulose derivatives,cellulose ethers are relatively hydrolytically stable, and remain unmodified under GI conditions that proves advantageous in oral drug delivery systems[9,10].
HPMC is the most commonly explored cellulose derivative for generation of amorphous solid dispersion.This watersoluble cellulose ether is not pH-responsive and lacks very strong hydrogen bond donor and acceptor groups. Although HPMC lacks the ability to form strong intermolecular interactions,it has been effectively used in the formulation development of amorphous solid dispersions, which can be gauged from its presence in more than fifty percent of the marketed amorphous solid dispersion products(Table 1).
2.2. pH-responsive cellulose esters and ethers
In recent times, many cellulose derivatives have been explored for the development of amorphous solid dispersions with a special objective to devise pH-responsive release of drugs. Cellulose polymers containing carboxyl groups, remain unionized at the acidic pH, and prevent drug release in the gastric region. Rigid cellulosic polymer backbones,as in the case of cellulose phthalate and derivatives sterically hinder drug recrystallization and help in maintenance of supersaturation [10]. Polymers may be structurally modified to enable triggered drug release and enhance the affinity for drugs through specific interactions. Cellulose is inherently hydrophilic, and a low degree of substitution (DS)of nearly any substituent including non-polar ones, can disrupt the H-bonding and thereby impart water solubility. A typical polymer chain of this class contains an amphiphilic cellulose derivative with pendent carboxylic acids to achieve pH-triggered swelling and drug release.These polar pendent groups also help in the formation of energetically favourable specific molecular interactions. Various pH-responsive cellulose esters and ethers which have been explored for development of amorphous solid dispersions are discussed below along with the potential advantages over other cellulose ethers and esters.
2.2.1. Cellulose succinate
Eastman Chemical Company first reported the amphiphilic,water-dispersible cellulose succinate mixed esters. HPMCAS is the most extensively studied polymer under this category for formulation development as an amorphous solid dispersion matrix polymer. The capability of producing effective and stable amorphous solid dispersions is demonstrated by more than five HPMCAS containing products approved for marketing by the regulatory authorities. Similarly, research at Pfizer Inc. and Bend Research reported at least 1.5-fold solution concentration enhancement of ziprasidone hydrochloride by merely mixing drug with HPMCAS in solution[11]. In an another study, Kennedy et al., reported nearly 28-fold enhancement in the dissolution rate of a highly selective VR1 antagonist(AMG 517)from an amorphous solid dispersion with HPMCAS compared to crystalline drug,and a nearly 2-fold improvement compared to amorphous drug[12].HPMCAS has proved to be a highly effective polymer in a solid dispersion with nifedipine [3].Primarily,the amount of polymer required for generation of amorphous solid dispersion was less than that required for methacrylic acid-ethyl acrylate copolymer or PVP.Relatively less tendency to absorb moisture over PVP and PEG add to the advantages of using HPMCAS above the other two in the development of amorphous solid dispersion [12].Yin et al.,synthesized five HPMC esters using mono-substituted succinic anhydrides and evaluated these synthesized polymers for generation and maintenance of supersaturated solution of phenytoin for longer duration from spray dried dispersions. These new HPMC succinates showed superiority over HPMCAS for maintenance of supersaturation for longer duration mainly due to nucleation inhibition[13].
2.2.2. Carboxymethylcellulose derivatives
CMC is an anionic,water-soluble cellulose ether,which is usually manufactured in large quantities through etherification of activated alkali cellulose with chloroacetic acid[14].Allen and co-workers synthesized these CMC mixed esters with various DS (alkanoates),based on the feed ratios and reaction conditions. The commercially produced CMCAB polymers contain DS(CMC)of 0.29-0.35,DS(butyryl)of 1.37-1.64 and DS(Ac)of 0.30-0.55[10].For miscible,poorly water-soluble drug such as fexofenadine HCl,amorphous solid dispersion was generated using CMCAB polymer matrix using intimate mixing[15].This amorphous blend of fexofenadine HCl in CMCAB led to a sharp enhancement of solubility of drug along with sufficient solution stability(little or no crystallization over time).
2.2.3. Cellulose phthalate derivatives
Malm et al.,identified CAPhth as a pH-sensitive coating polymer[16].CAPhth is well-suited to this application as it is soluble in organic solvents and acts as a good film former. Due to its ionization at relatively low pH, CAPhth has been explored as a matrix for amorphous dispersions. In vivo testing conducted in Sprague Dawley rats demonstrated a significant two-fold enhancement in oral bioavailability from a CAPhth/itraconazole amorphous solid dispersion compared to Sporanox?, the marketed dosage form of itraconazole.Structural features of CAPhth, like rigid cellulosic polymer backbone, led to improved stabilization of the drug in amorphous solid dispersions as compared to those formulated using PVAP [17]. However, the cellulose phthalate derivatives have not yet been exploited to their full potential in development of stable amorphous solid dispersions due to challenges like limited miscibility with the drug molecules and thus,only a few reports have come to light so far[10].
2.2.4. Cellulose ω-carboxy esters
Cellulose alkyl ethers can be successfully esterified with ωcarboxyalkanoyl groups by reaction with monobenzyl adipoyl,suberoyl, and sebacoyl chlorides, and subsequent benzyl ester hydrogenolysis, to avoid cross linking. The resultant cellulose ester products are more hydrophobic than the starting ethers, and thus have good solubility in medium polarity solvents, and in polar aprotic solvents. The ethyl cellulose esters have broader organic solubility, ascribed to their higher DS(alkyl) and the relatively higher hydrophobicity of the ethyl group as compared to the methyl group[18].This organic solubility aids in the formation of amorphous solid dispersions from common solutions of drug and polymer, and is also predictive of broad miscibility with drug structures[19].The alkyl cellulose ω-carboxyalkanoates have shown favorable drug release patterns and stabilization of the drug against recrystallization after release from amorphous solid dispersion [19]. This chemistry promises to make available a very broad range of alkyl cellulose ω-carboxyalkanoates which can be tailored to achieve the desired outcome in developing amorphous solid dispersions. The promising nature of these renewable amphiphiles for solubility and bioavailability enhancement of otherwise poorly soluble drugs is underlined by successful formation of an amorphous solid dispersion of ritonavir[4].Similar results have been reported for other drugs(Table 3).
3. General mechanisms behind amorphous state stabilization and crystallization inhibition
In amorphous solid dispersion, drug is kinetically entrapped between the polymer chains in a high energy non-crystalline state leading to improved stability [20-23]. Extensive literature is available on the mechanisms of by which polymer stabilizes amorphous drug or glass solution of the drug in a glassy polymer matrix. Interested readers are encouraged to refer to the excellent reviews [20,24,25]. Some of the prominent mechanism by which polymers prevent crystallization of amorphous drug are described in Fig.4,which includes reduction of the drug molecular mobility, increasing the glass transition temperature, Tg(anti plasticization effect), and/or through molecular interaction with the drug [25]. Polymers may prevent the precipitation of the drug from supersaturated solution by increasing the viscosity of the medium which help in reduction of nucleation rate by reducing the molecular mobility. In addition, this impairs the crystal growth by affecting diffusion coefficient which is directly proportional to the crystal growth rate[26].Polymers may also get adsorbed ontothe interfacial layer of critical clusters and the reduce crystal growth rate.They alter molecule integration in crystal lattice by changing level of solvation at the crystal medium interface [27]. Similarly, few reports have proposed that reduction of nucleation rate by increasing cluster-medium interfacial energy and reduction in degree of supersaturation by enhancing the solubility of drug affects nucleation and crystal growth[28,29].

Table 3-Examples of cellulose-based polymers explored for amorphous solid dispersion development.

Table 3(continued)

Table 3(continued)
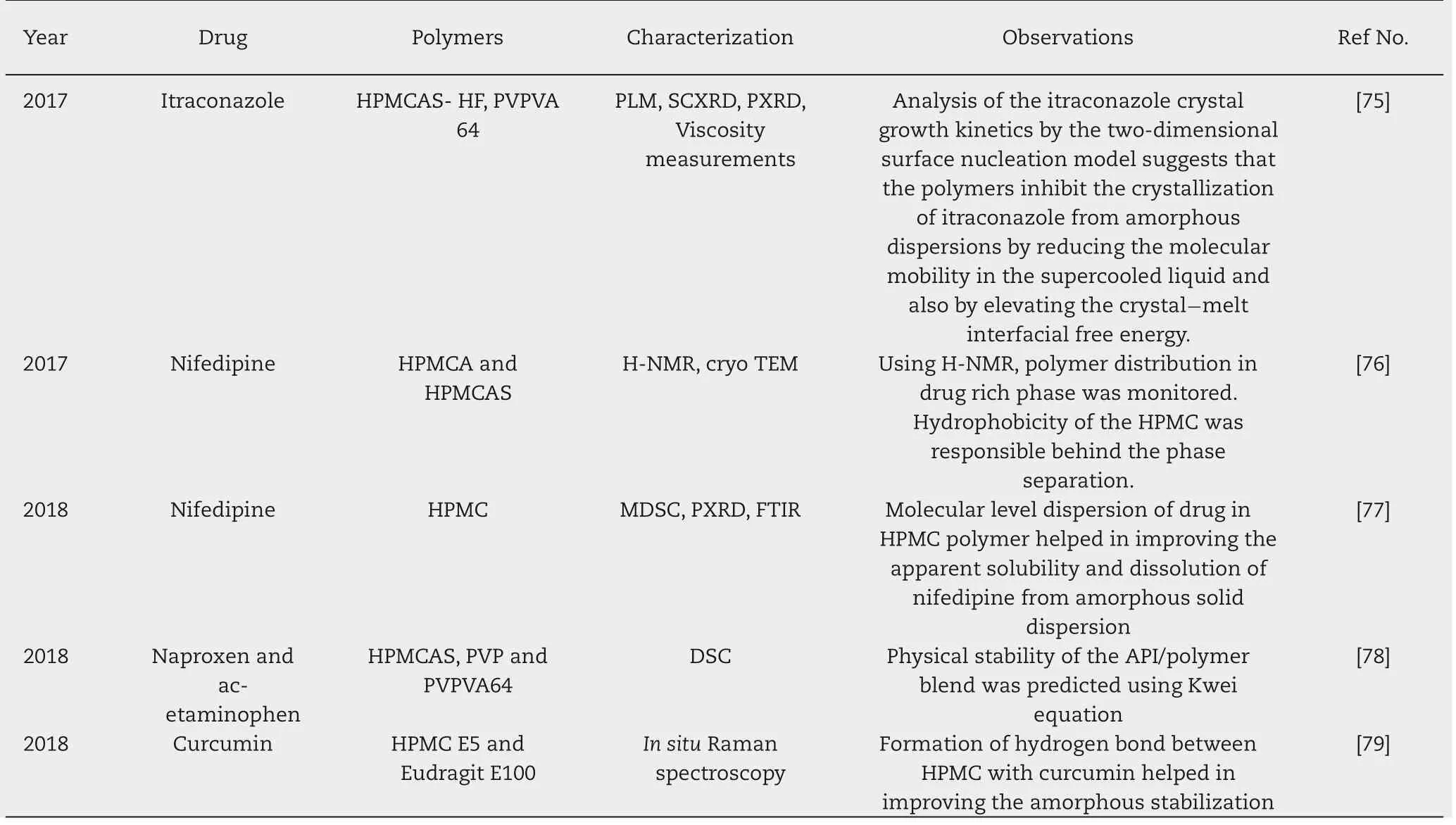
Table 3(continued)
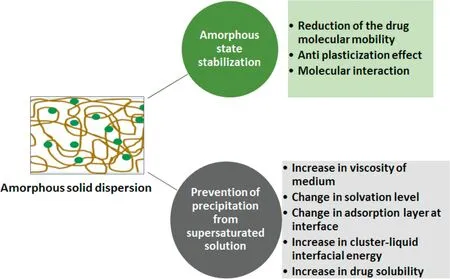
Fig.4-Mechanism behind amorphous state stabilization and prevention of precipitation from supersaturated solution by polymers.
4. Role of cellulose derived polymers in amorphous solid dispersion formation and stabilization
4.1. As precipitation inhibitors
Inhibition of crystallization/precipitation of the drug is crucial in drug delivery that are based on supersaturated delivery systems.The gravity of this problem increases with regards to the amorphous systems.Apart from solid-state stability issues,an efficient amorphous dispersion must also address the problems of drug precipitation in the solution state.Prevention of crystallization and maintenance of supersaturated solution for a biologically relevant time frame in GI tract is an essential requirement for sufficient permeation to occur thereby increasing the oral bioavailability. Well-crafted cellulosic polymers can not only can prevent crystallization in the solid state,but also prolong supersaturation after amorphous solid dispersions are dispersed in the aqueous medium of the GI tract.
HPMC is one of the most extensively explored cellulosic polymers as a precipitation inhibitor and has proven to be effective in stabilizing solid dispersion formulations of many drugs.A list of these solid dispersions is presented in Table 3,along with the methodology utilized for monitoring the precipitation inhibitory potential of the polymers.HPMC is a very versatile polymer and its extensive use can be attributed to the lack of drug specificity. An attempt to explain the mechanism by which HPMC stabilizes the supersaturated systems was made by Alonzo et al. They demonstrated that in presence of HPMC,nucleation and crystal growth rate of felodipine reduced significantly. However, the authors additionally emphasized that the impact of HPMC was more pronounced on nucleation than on crystal growth [5]. To obtain further insight into the mechanism,Kaoutar Abbou Oucherif et al.,used population balance modeling for quantitative analysis of the inhibitory effect of HPMC on nucleation and crystal growth rate.The authors’findings were similar to Alonzo et al.,that HPMC has more profound inhibitory activity against nucleation than crystal growth. Additionally, it was also observed that the polymer adsorbed on the crystal surface and slowed down the growth kinetics [30].In both studies,quantification of the inhibitory effect of HPMC on nucleation was carried out by calculating nucleation induction time in presence and absence of polymer.Findings from our lab when studying the impact of HPMC properties on nucleation and crystal growth inhibition of nifedipine from supersaturated solutions were in line with the previous reports.However,HPMC properties like viscosity, hydrogen bond donor/acceptor and drug solubility were found to influence/impact the inhibitory potential of polymer on nucleation or crystal growth[31].
HPMCAS,a pH-responsive derivative of HPMC is being explored,of late,to stabilize amorphous solid dispersions.This cellulosic polymer possesses four types of substituents which are semi-randomly substituted on the hydroxyl groups(bracketed values represent mass content): methoxy (12%-28%,w/w); hydroxypropyl (4%-23%,w/w); acetate (2%-16%,w/w);and succinate (4%-28%, w/w) [32]. Due to the succinate groups,HPMCAS have a pKa of about 5 [33]; therefore, below pH 4, 10% of the polymer ionizes while at least 50% are ionized at pH above 5. The pH-dependent solubility can be attributed to the ratio of succinate and acetyl groups.Thus,different grades of HPMCAS have different pH-dependent solubility (The L, M, and H grades dissolve at pH ≥5.5, 6.0 and 6.5, respectively). Presence of hydrophobic methoxy and acetate substituents make HPMCAS water-insoluble when unionized (about pH <5) and remains predominantly colloidal at intestinal pH (i.e. pH 6.0-7.5) [32]. As mentioned earlier,HPMCAS possesses many substituents(methoxy and acetate)which make it relatively hydrophobic.This ultimately results into poor solubility of polymer even when it is ionized in small intestinal pH and leads to the formation of colloidal polymer aggregates in aqueous solutions. The hydrophobic nature of the substituents combined with the colloidal nature of HPMCAS promote interactions between the polymer and insoluble drug molecules,resulting in the formation of amorphous drug/polymer nanostructures in solution.In addition,the negatively charged succinate groups keep these nanostructures stable,evading the large hydrophobic aggregates of the polymer and drug in solution.These drug/polymer nanostructures constitute a high-energy(high-solubility)form of amorphous drug that is stable for several hours to permit GI absorption.Few in vitro measurement studies reported that the nanostructured drug rapidly dissolves to produce a high free-drug concentration that is supersaturated relative to the solution generated by the crystalline drug.From a comparative analysis of the cellulosic polymers (Table 2),it can be inferred that HPMCAS has properties conducive to develop stable amorphous systems over its counterparts. Sachram et al., investigated the impact of change in polymer conformation as a function of pH on the rate of crystal growth using felodipine and HPMCAS.Effectiveness of the polymer was measured as the ratio of growth rate in the absence and presence of polymer.The study was performed at two different pH conditions such that the polymer remains unionized in one and ionized at the other.The outcome of the study revealed that the polymer was effective at both pH,but the effectiveness dropped by a factor of 1.8 when the polymer was unionized.Intramolecular interactions in the unionized polymer lead to coiling of the chains and are present as globules on the surface of the growing crystal,which increase the exposure of growth sites on the surface of the growing crystals to drug molecules,thus reducing the efficiency of the polymer.HPMCAS with moderate hydrophobicity are adsorbed with highest degree of surface coverage and inhibit crystal growth process significantly,while hydrophilic polymers like PVP and PAA may show little impact on crystal growth [34]. In addition, the polymer surface coverage can be correlated to the polymer effectiveness as a crystal growth inhibitor,using Kubota-Mullin model[35]. In a further study, Schram et al., demonstrated the effectiveness of HPMCAS in inhibiting the crystal growth steps,and reported a higher efficiency when the surface of the crystal was pre-poisoned with the polymer. In growth rate measurements, absence of the polymer from the supersaturated solution led to the development of a drug layer on the prepoisoned surface.In amorphous solid dispersions,where the drug is dispersed in the polymer matrix,the crystals formed were found to have smaller macroscopic features due to intimate interaction between the drug and the polymer[36].Additionally,Schram et al.,also demonstrated the impact of structural conformation of HPMCAS on the crystal growth inhibition of felodipine.At a pH higher than the pKa of the polymer,the polymer chains uncoiled and led to efficacious covering of growth sites on felodipine,thus resulting in efficient inhibition of crystal growth.However,the impact was lowered at the pH below the pKa of the polymer due to the coiling of its chains resulting in formation of compacts that were ineffectively adsorbed onto the surface of the drug, thus exposing the crystal growth sites for adsorption of more growth units[37].
Xie et al.,evaluated the impact of polymers on the crystallization propensity of celecoxib in pure amorphous form and also the impact on the dissolution rate from amorphous solid dispersions.PVP was found to be least effective,wherein the nucleation commenced within 60 min, whereas, HPMC and HPMCAS effectively inhibited the crystallization,as no nucleation or crystal growth was observed during the 6 h of the experiment.During the dissolution studies,dispersions with PVP showed tendency to undergo quick/immediate desupersaturation,while addition of cellulosic polymers reduced the rate of dissolution.The higher efficiency of the cellulosic polymers to inhibit the crystallization of the lipophilic molecule is due to their amphiphilic nature.PVP is relatively hydrophilic and thus interacts with the liquid phase as well.Meanwhile,the cellulosic polymers have greater ability to interact with the drug molecules and thereby effectively block the growth sites[38].Ueda et al.,tried to compare the inhibitory potential of two different grades of HPMCAS (LF and HF) on recrystallization of carbamazepine from supersaturated solution using nuclear magnetic resonance spectroscopy.The HF grade was more efficacious in inhibiting the recrystallization of carbamazepine than the LF grade,which is attributed to stronger intermolecular interactions between the drug and the polymer and higher suppression of molecular mobility of the former over the LF grade [39].HPMCAS is available in three substitution grades,namely L,M,H based on the content of the acetyl and succinoyl groups. Further each grade is available in two forms based on the particle sizes,fine (F) and granular (G).In an another interesting study,Ricarte et al.using light scattering demonstrated that HPMCAS enhanced the aqueous solubility of phenytoin and probucol through a formation of ~10 and ~100 nm sized structures in the media [40]. In a similar study,Huang et al.,stated that hydroxypropyl acetyl group is the most effective functional group for supersaturation maintenance,followed by the acetyl group,while the deprotonated succinyl group is the least effective functional group[41].
Numerous ω-Carboxyester derivatives of cellulose such as CAAdP and CASub are a novel class of cellulose ester derivatives that have been designed keeping in mind the prerequisites for a polymeric precipitation inhibitor for amorphous solid dispersions. CAAdp and CABSeb are effective in inhibiting crystallization of rifampin [6]. Ilevbare et al., studied the relative ability of structurally diverse cellulose ω-carboxyesters to stabilize lipophilic drugs (celecoxib,efavirenz,and ritonavir) in solution against nucleation[28] and crystal growth [42]. It has been speculated that the polymer disrupts the reorganization of a cluster of solute molecules into an ordered crystal structure and averts the crystal growth process on the surface of the generated drug crystal. Polymer-solute interactive forces were found to be a major factor behind crystal growth inhibition. The polymers with effective inhibitory potential possess an optimal level of hydrophobicity relative to that of drug molecules and the capability to undergo specific intermolecular interactions,mostly provided by a high number of ionized carboxylate groups (COO-) at pH values reflecting the intestinal environment. Li et al., demonstrated through a structure property study that both the hydrophobicity and carboxyl group content of the polymer significantly contributed in inhibiting the crystallization of ritonavir, specifically crystal growth inhibition,which was found to be highly sensitive to these parameters[43].
Ilevbare et al., investigated the reason behind crystal growth inhibition of ritonavir by novel cellulose adipate polymers at higher pHs[27].The reduced inhibitory effect of these polymers at low pH was not due to poor carrier adsorption on the ritonavir crystals for inhibiting their growth since a high amount of polymer got adsorbed on the crystals,but rather the polymer conformation at the solid-liquid interface that was found to be the critical factor in the crystal growth inhibition.At low pH,the poorly ionized polymers interacted poorly with the aqueous solvent and resulted into a more condensed conformation,making them less efficient in holistically covering the ritonavir crystal surface.In contrast,at high pH,the polymer chains got extended due to their higher degree of hydration and upon adsorption on the drug crystals,the extended polymer chains blocked more crystal growth sites and ultimately inhibited the crystallization process more effectively[27].
Ren, et al., demonstrated that interactions of polymer with drug played important role in prevention of crystallization from supersaturated solution. Author developed novel microscope-observation method for use following antisolvent recrystallization to evaluate polymers for their ability to inhibit drug crystallization [44]. Other examples of cellulose derivatives being used as stabilizers in amorphous solid dispersions are described in Table 3.
4.2. Role in amorphous state stabilization
Formulating poorly water-soluble drugs into amorphous form enhances the solubility.However,highly energetic,pure amorphous products are difficult to scale up as they may rapidly convert into a stable crystalline form by loss of free energy. Moreover, the tendency to recrystallize is exacerbated in presence of water. Zafirlukast (Accolate?, Astra Zeneca),quinapril hydrochloride(Accupril?,Pfizer)and cefuroxime axetil(Ceftin?,GlaxoSmithKline)are the very few commercially available pure amorphous drugs [45]. The amorphous form of the drug may be stabilized thermodynamically or kinetically by formulating as polymeric amorphous solid dispersions.However,amorphous solid dispersions face challenges of phase separation during their storage due to higher molecular mobility in the amorphous state and the tendency to recrystallize to a lower energy,stable crystalline form [46].The tendency of the drug to recrystallize must be carefully monitored and evaluated during all processing, storage and handling stages in the manufacturing of the drug product [47].If crystallization occurs, the enhancement in bioavailability will be negated.Cellulose derivatives are a prominent category of polymers which provide solutions to the above-mentioned challenges of amorphous systems. High Tgvalues limiting the drug mobility in amorphous solid dispersion, reduction in phase separation due to strong drug-polymer interactions and less moisture adsorbing tendency make cellulose derivatives superior to synthetic polymers like PVA and Eudragits.Numerous mechanisms have been proposed related to specific cellulose derivatives for stabilization of amorphous form of the drug through amorphous solid dispersions.An overview of the few reported studies is provided below.
Konno et al., compared the crystallization inhibitory potential of cellulose derivatives like HPMC and HPMCAS with PVP, on molecularly dispersed amorphous felodipine. It was reported that in the absence of moisture, all three polymers inhibited the nucleation of the drug with the same effectiveness in the amorphous solid dispersions,whereas HPMC and HPMCAS were found to be superior over PVP in crystallization inhibition of felodipine when exposed to atmospheric moisture [1]. Rumondor et al., demonstrated that under 75%RH storage condition,felodipine amorphous solid dispersion containing HPMCAS showed the least moisture adsorption and was resistant to moisture induced drug-polymer immiscibility and recrystallization as compared to PVP. In HPMCAS containing amorphous solid dispersion, crystal growth rate of felodipine was less sensitive to moisture as this polymer possesses numerous hydrogen bond acceptors and donors per repeating unit which can interact with absorbed moisture and resist the disruption of drug-carrier hydrogen bonding[48].
Cellulose phthalate derivatives are a prominent category of cellulose polymers which have shown benefits in the stabilization of amorphous solid dispersions via strong intermolecular interactions. For example, Nie et al., showed the potential of CAPhth in amorphous state stabilization of clofazamine through strong interactions using solid state spectroscopic techniques.Due to its ionization at relatively low pH,CAPhth has been explored as a matrix for amorphous dispersions[49].Liu et al.,group developed cellulose esters containing adipate as polymer matrices for amorphous solid dispersion. It was proposed that the presence of tetramethylene side chains in adipate groups accentuated the already hydrophobic nature of the cellulose esters, thus aiding in enhancing the miscibility of hydrophobic drugs in polymer,enabling slow drug release.Additionally, the polymer enhanced the miscibility and stability of amorphous solid dispersion through specific interactions between its terminal carboxyl group and hydrogen bond acceptors of the drug [19].Li et al.,in further studies demonstrated through structure property study that the strongly hydrophobic nature of these cellulose-ω-carboxyesters had contributed significantly to crystal growth inhibition in solid state,consequently improving the miscibility of polymer with a hydrophobic drug[42].
5. Regulatory overview of cellulose-based amorphous solid dispersion
Amorphous systems are inherently metastable or thermodynamically unstable due to lack of long-range order, unlike their crystalline counterparts. The amorphous systems have a propensity to revert back to their stable crystalline form,which will eventually hamper the performance of the amorphous solid dispersion. Moreover, the carriers used in amorphous solid dispersions can absorb moisture and catalyze the process of devitrification. Though a product may meet the specifications at the time of approval, it may fail at a later stage leading to batch recalls. Thus, it is essential that the target product is understood holistically to design a robust manufacturing process. It is thereby recommended to abide by the Quality by Design (QbD) framework which is strongly advocated by the US FDA.QbD is a structured and systematic framework that is based on the principle that quality should be built into the product rather than be tested at a terminal stage in the product development.The ICH guidelines Q8 R(2),Q9, and Q10 delineate the strategy adopted by QbD, which leads to a thorough understanding of the product and process. The Quality Target Product Profile (QTPP); dissolution and maintenance of supersaturation, amorphous/crystalline ratio, bioavailability (pharmacokinetic characteristics), sterility, shelf-life, assay and content uniformity should be considered during application of QbD for development of cellulose based amorphous solid dispersions. Post finalization of QTPP, Critical Quality Attributes (CQAs) can be derived from the QTPP,prior knowledge,and initial risk assessment.CQAs affecting the performance of the amorphous solid dispersions are described in the fish-bone diagram (Fig. 5). Among these CQAs, those related to polymers are found to play a critical role in the development and performance of amorphous solid dispersions.Hence,selection of the polymer is one of the most crucial steps in the formulation of amorphous solid dispersions, as it significantly impacts the efficacy of the product.Although cellulose based polymers and their derivatives are considered as inert substances, newly developed cellulose derivatives may raise concern regarding the safety and toxicity based on the manner by which they interact with the drug or biological surroundings. Therefore, USFDA has published a list in the code of federal regulations (CFR) for GRAS substances that are generally regarded as safe. Over the years, the agency has also maintained a list entitled inactive ingredient database (IID) for excipients that have been approved and incorporated in the marketed products. Both GRAS and IID information can be useful to the industry as an aid in developing cellulose based amorphous solid dispersions.In general,non-clinical and clinical studies are required to demonstrate the safety of new excipients before use.In this context,USFDA has recently published guidance for industry on the conduct of non-clinical studies for the safety evaluation of new pharmaceutical excipients. This guidance highlights the importance of performing risk-benefit assessment on the newly proposed excipients in the drug products while establishing the permissible and safety limit for the excipients.Cellulose derivatives like HPMC,HPMCAS,HPMCP,CMC,and CMCAB are considered to be relatively safe and their acceptable limits are available in IID. However, for the newly synthesized cellulose derivatives, it is mandatory to report the toxicity profile of the polymer including the maximum permissible limits, before using them in the pharmaceutical products.

Fig.5-Ishikawa diagram describing the CQAs affecting the performance of amorphous solid dispersion:OD/ID-outer diameter/inner diameter;A/C ratio-amorphous/crystalline ratio,temp.-temperature.
6. Conclusions
Use of cellulose derivatives as stabilizers in the development of amorphous solid dispersions of poorly soluble drugs has been a major area of interest for formulation scientists. The wide range of cellulosic polymers used as stabilizers in the marketed products validates their potential and assures that the cellulosic polymers and their derivatives are here to stay. Unique properties such as high molecular weight,hydrophilicity and hydrolytic stability make them ideal candidates for the development of polymeric dispersions.Significant efforts have been undertaken to understand the mechanism of crystallization inhibition in the solid and solution state at a molecular level. This review intends to guide the formulation scientist in appropriate selection of polymer for the development of amorphous solid dispersions by avoiding extensive screening experiments,thereby saving time.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the financial support from the Department of Pharmaceuticals(DoP),Ministry of Chemicals and Fertilizers,Govt.of India.
S upplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.09.003.
 Asian Journal of Pharmacentical Sciences2019年3期
Asian Journal of Pharmacentical Sciences2019年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Double-layered osmotic pump controlled release tablets of actarit:In vitro and in vivo evaluation
- Study of penetration mechanism of labrasol on rabbit cornea by Ussing chamber,RT-PCR assay,Western blot and immunohistochemistry
- In vitro/vivo assessment of praziquantel nanocrystals:Formulation,characterization,and pharmacokinetics in beagle dogs
- Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule(Avodart?):Effect of γ-cyclodextrin and solubilizers
- Percutaneous absorption and brain distribution facilitation of borneol on tetramethylpyrazine in a microemulsion-based transdermal therapeutic system
- Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis
