In vitro/vivo assessment of praziquantel nanocrystals:Formulation,characterization,and pharmacokinetics in beagle dogs
Ruyi Yang,Tao Zhang,Jiang Yu,Yan Liu,Yingli Wang,Zhonggui He
Wuya College of Innovation,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
Keywords:Praziquantel Nanocrystals Microcrystals Dissolution Pharmacokinetics
ABSTRACT To investigate the impact of particle size on in vitro/vivo performance of praziquantel(PZQ),nanocrystals(NCs)and microcrystals(MCs)of PZQ were prepared using the methods of wet milling and jet milling, respectively. PZQ NCs and MCs were characterized with dynamic light scattering,laser particle size analyzer,transmission electron microscopy,differential scanning calorimetry,X-ray powder diffraction and fourier transform infrared spectroscopy.The average diameters of PZQ NCs and MCs were 364.4 nm and 3.7 μm, respectively. No change in crystalline form was observed. Dissolution tests were performed in two different media,where the cumulative dissolution and dissolution rate of NCs were significantly improved in comparison with those of MCs and KANGQING? in non-sink condition.Similarly,oral bioavailability of PZQ NCs in beagle dogs was 1.68(P <0.05)and 1.83 fold(P <0.01)higher than that of MCs and KANGQING?.Considering the advantages of in vitro/vivo performance and facile preparation,PZQ NCs may have a great application in the treatment of schistosomiasis.?2018 Published by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Schistosomiasis caused by trematode flatworms of the genus Schistosoma is an infectious disease that brings millions of people in trouble of lives[1].At present,praziquantel(PZQ)is still the best effective drug against adult schistosome worms[2]. PZQ, as a heterocyclic pyrazinoisoquinoline, is poorly soluble in water. The commercially available formulation is KANGQING?with low bioavailability,which limits its clinical application.The long-term use may increase the risk of drug resistance and further lead to therapeutic failures[3,4].
Over the last decades, nanomedicine has been widely investigated to improve the in vivo performance of hydrophobic biologically active molecules [5]. A series of novel nanoformulations such as nanoparticles [6,7], solid dispersions [8],nanoemulsions [9],nanogels [10],and nanocrystals [11] were applied to increase the solubility and dissolution rate thereby enhancing the bioavailability. These nanosystems exhibited several advantages including their ease of preparation and general applicability. Nanocrystals are nanoparticles of pure drug with a mean particle size below 1 μm (typically in the range of 200-500 nm), and they can be prepared in both water and non-water media as colloidal nanosuspensions stabilized by surfactants or polymers [12]. Several methods for preparation of drug nanocrystals have been widely used including “top-down” processes, “bottom-up” techniques and CO2-assisted in-situ nanoamorphization methods [13].So far, many water-insoluble drugs such as fenofibrate [14],itraconazole [15], and pranlukast [16], were formulated into nanocrystals with an enhancement on both solubility and bioavailability.What’s more,nanocrystals have shown a good prospect in drug delivery for its high drug loadings, lower solvent related adverse reactions, various administration routes and huge commercial value[17,18].
Recently, some researches revealed that praziquantel nanosuspensions had an important effect on energetic metabolism than conventional PZQ [19]. For the aims of further study and possible industrialization, we successfully constructed praziquantel nanocrystals (PZQ NCs) by screening the prescription of PZQ through a “top-down” method.Praziquantel microcrystals(PZQ MCs)were also prepared as a reference.The characterizations of NCs and MCs were carried out by microscope, dynamic laser scanning, Transmission electron microscopy and Differential scanning calorimetry,showing reduced particle size and identical rod-like morphology of crystalline form. Furthermore, dissolution tests and pharmacokinetic study were executed to evaluate the consistence in vitro and in vivo.
2. Materials and methods
2.1. Materials
Praziquantel (PZQ,>99%) was purchased from Shanghai Bangcheng chemical Co., Ltd. (Shanghai, China). Diazepam was obtained from National Institute of Control of Pharmaceutical and Biological Products(Beijing,China).KANGQING?was purchased from Shenyang Hongqi Co., Ltd. (Shenyang,China). Soluplus and poloxamer 407 (F127) were donated by BASF Co., Ltd. (Shanghai, China). Sorbital and mannitol were bought from Tianjin Bodi Chemical Holding Co.,Ltd.(Tianjin,China). Formic acid was obtained from Sigma-Aldrich Co.,Ltd.(Shanghai,China).Acetonitrile and methanol were of the chromatographic grade. Distilled water was filtered before used.
2.2. HPLC analysis
The content of PZQ in dissolution tests was determined by HPLC with a UV detector (Hitachi, Tokyo, Japan). The sample separation was analyzed on a BDS HYPERSIL C18 column(5 μm, 250 mm×4.6 mm, Dikma, Beijing, China) with mobile phase acetonitrile and water in proportion of 60:40 (v/v) at a flow rate of 1 ml/min at 30°C. 20 μl of the samples were measured at 263 nm.The linearity of the method was within the range of 10-1600 μg/ml(R2=1).
2.3. Preparation of PZQ nanocrystals(NCs)
2.3.1. Wet milling method for PZQ NCs preparation
A PM planetary ball mill(Nanjing Chishun Science&Technology Co., Ltd., Nanjing, China) was used to prepare PZQ NCs.Zirconium dioxide beads with the diameter of 0.5 mm were applied to crush the crude drug. Briefly, primary suspension was prepared by dispersing PZQ (5%, w/v) uniformly into 20 ml of aqueous solution containing Soluplus (1.25%, w/v)and F127 (0.25%, w/v). Then the mixture was transferred to ball mill with 74 g beads for milling simultaneously. The PZQ NCs were obtained after 160 min milling at the speed of 35 HZ.
2.3.2. Lyophilization
PZQ NCs were lyophilized in order to keep stable and to study further. Mannitol (3%, w/v) and sorbitol (3%, w/v) acted as cryoprotective agents. Firstly, 1 ml of sample was frozen in a vial at -80°C for 3 h in an ultra-low-freezers (SANYO, Tokyo,Japan), and then freeze-dried by an FDU-1100 EYELA freeze dryer (Rikakikai, Tokyo, Japan). The inlet temperature stayed at-40°C for 3 h followed by being raised to-20°C for another 15 h. Finally, the temperature came back to 25°C and stayed for another 6 h.
2.4. Preparation of PZQ microcrystals(MCs)
An air jet mill system was equipped with a screw air compressor (RICH-RC75A, Shanghai Rich Machine Manufacture Co.,Ltd.,Shanghai,China),an air precision filter(C-001,Shanghai Jialong Purification Equipment Co., Ltd., Shanghai, China),a refrigerant dryer (AD-1HF, Shanghai Jialong Purification Equipment Co.,Ltd.,Shanghai,China)and a sample injection.A balanced amount of crude drug was inhaled into the sample injection at a steady rate. PZQ was ground under 0.5 Mpa of the air pressure.
2.5. Characterization
2.5.1. Particle size,particle size distribution and zeta potential
The average hydrodynamic particle size,particle distribution index (PDI) and zeta potential of PZQ NCs were determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 instrument (Malvern, UK). PZQ NCs were diluted 300 times with pure water before determined. The diameters of PZQ MCs were evaluated by BT-9300S laser particle size analyzer (Bettersize Co., Ltd., Dandong, China). Each sample was measured in triplicate.
2.5.2. Observation by microscopy
The appearance of PZQ NCs was observed by an optical microscope (YS2-H,NIKON,Japan).One drop of sample (diluted three times with purified water)was dripped on a glass slide,subsequently covered with a glass coverslip.The sample was observed under the 40-fold magnification condition.
2.5.3. Transmission electron microscopy(TEM)
The shape and specific morphology of PZQ NCs were examined by TEM (JEM-2000, JEOL, Japan). Approximately 10 μl of diluted PZQ NCs(0.5 mg/ml)was placed on a 200-mesh carbon membrane covered copper grid.After staying for 30 s,the excess was removed by a piece of filter paper and air-dried.Then the sample was negatively stained with phosphotungstic acid 1%(w/v)for 30 s.
2.5.4. Differential scanning calorimetry(DSC)
Crystalline form was determined by DSC with a STARe system(Mettler-Toledo,Switzerland).Samples were heated from 30°C to 200°C at a rate of 10°C/min under anitrogen atmosphere.
2.5.5. X-ray powder diffraction(XRPD)
XPRD were performed to investigate the crystallinity of the samples using a D/Max 2700 PC X-ray diffractometer (Hao Yuan Co.Ltd,China) with Cu-Ka radiation.The scanning rate was 0.3°/min and the scanning increment was 0.03° over the range 3°-50°
2.5.6. Fourier transform infrared spectroscopy(FT-IR)
An FT-IR spectrometer (Bruker Corporation, Switzerland)was applied to analyze the interaction between PZQ and excipients.After mixing with potassium bromide,samples of blank excipients, PZQ, physical mixtures, PZQ MCs and PZQ NCs separately were scanned over the range 4000 to 400 cm-1.
2.6. Physical stability
Physical stability of PZQ NCs was assessed at 4°C and 25°C.Samples were collected at 0, 5, 10, 15 and 20 d for light microscopy observation and particle size analysis.
2.7. Dissolution test
Two media,0.1 M HCl solution with 0.2%SDS as well as water,were employed in dissolution test. The test was conducted in 900 ml medium at 37°C using a ZRS-8 G dissolution device(Tianjin Tianda Tianfa,Tianjin,China)according to the paddle method of Chinese Pharmacopeia Apparatus Method II. The paddle speed was set at 50 rpm. PZQ NCs, PZQ MCs and KANGQING?(200 mg of equivalent PZQ) were put into each medium. 5 ml of media was sucked out at the time of 5, 10,15, 20, 30, 45, 60 min and then replaced with fresh media of the same volume. After filtering and discarding 2 ml of the primary filtrate, samples were analyzed by HPLC. Each experiment was repeated three times.
In addition, to investigate the effects of excipients on dissolution,physical mixtures were as reference.
2.8. Pharmacokinetics study
2.8.1. Animals and blood sampling
Nine healthy male beagle dogs (10-12 kg) were chosen as experiment animals.Being complied with the“Guidelines for the Care and Use of Laboratory Animals”,all the experiments were approved by the Animal Ethics Committee of Shenyang Pharmaceutical University. Before administration, the dogs fasted overnight and had water freely. Three formulations,including PZQ NCs, PZQ MCs and KANGQING?, were administered. PZQ NCs were filled into capsules, while PZQ MCs were put into capsules with quantities of excipients in the prescription of NCs. The 3×3 cross-over design was applied in the experiment with a washout period lasted for one week. Briefly, beagle dogs were randomly divided into three groups accordingly. Each group was orally administered at a single dose of 200 mg. Before blood collection, a detaining needle was used. Approximately 3 ml of blood samples were transferred into a pre-heparinized tube at 0 (pre-dose), 0.25,0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 36 h after dosing, and then centrifuged at 3500 rpm for 10 min to obtain the plasma.Finally,plasma samples were stored at-80°C until analysis.
A protein precipitation method was employed to treat the plasma samples. Briefly, samples were thawed at room temperature. Then 50 μl of plasma mixed with 50 μl of an internal standard solution(diazepam,50 ng/ml),and vortexed for 1 min.Then 400 μl of acetonitrile was added and vortexed for another 3 min. Each sample was then centrifuged at 13 000 rpm for 15 min. The final supernatant was injected into the LC-MS/MS system for analysis.
2.8.2. Plasma sample preparation and LC-MS/MS analysis
The concentrations of PZQ in plasma were detected by LCMS/MS (Waters Co.,Ltd.,Milford,MA,USA).Chromatography conditions were as follows: ACE UltraCore Super C18 column(50 mm×2.1 mm, 2.5 μm), column temperature 30°C, a flow rate of 0.2 ml/min,a mobile phase consisting of water containing 0.025%formic acid and acetonitrile at a ratio of 75:25(v/v).The retention time of PZQ and diazepam was 0.84 min and 0.96 min, respectively. Multiple reaction monitoring (MRM)was applied to detect the fragment of PZQ(m/z 313→174)and diazepam (m/z 285→154). The collision energy was 35 V for PZQ and 30 V for diazepam. The standard curve was linear(R2>0.99)over the range 10-10 000 ng/ml.
2.8.3. Statistical analysis
All results were expressed as mean±SD.Student’s t-test was used to analyze the statistics and P <0.05 was considered as a significant difference. The pharmacokinetics data were obtained by DAS software.
3. Results and discussion
3.1. Formulation optimization
The purpose of this research was to improve the oral bioavailability of PZQ by decreasing the particle size.Herein,PZQ NCs were prepared by a wet milling method while MCs with an air jet pulverization process,respectively.
PZQ NCs were optimized based on particle size by selecting right stabilizers and milling time.When particle size reduced,free energy markedly increased, and thereby resulted in thermodynamic instability of NCs. To minimize the energy,NCs tended to agglomerate and/or aggregate [20].Depending on the electrostatic barriers, steric barriers and/or hybridcombinations, stabilizers can furnish activation energy to improve the physical stability in virtue of preventing from agglomeration and/or aggregation [21]. On the other hand,milling time can also influenced particle size and a longer milling time generally obtained a smaller particle.Nevertheless, no more reduction in particle size occurred along with the milling time due to reaching the limit.
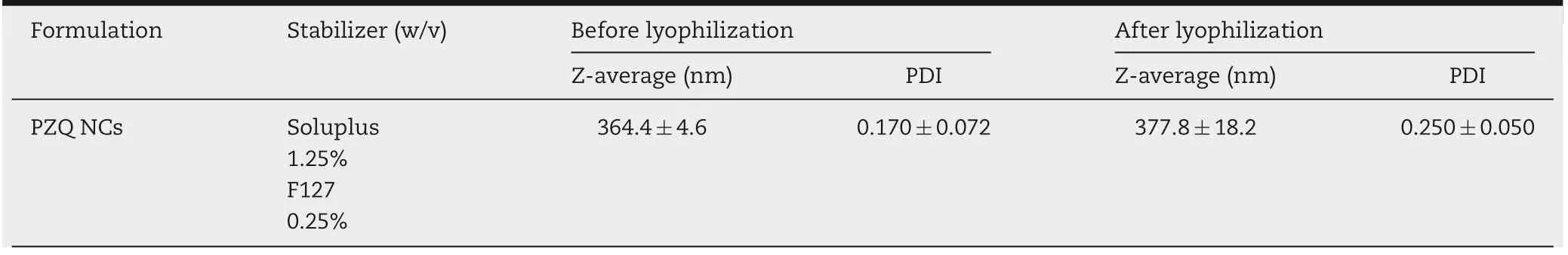
Table 1-Z-averages,PDIs and zeta potential of PZQ NCs(mean±SD,n=3).
The size and PDI of PZQ NCs were determined by DLS.However,some NCs tend to agglomerate or aggregate without suitable stabilizers [22]. To observe the possible agglomeration or aggregation,microscopy was used as a supplementary means to observe the selected NCs by DLS.It helped us to find the best nanocrystals among the formulations.After screening,the combination of Soluplus(1.25%,w/v)and F127(0.25%,w/v)was set as the best proportion of stabilizer and optimized milling time was 160 min. The parameters of representative formulations were shown in Table 1.The particle size of PZQ NCs was 364.4±4.6 nm and PDI was 0.170±0.072, indicating a uniform colloid system. Zeta potential of PZQ NCs was-11.3 mV which showed a great steric hindrance and energy barrier by F127 and Soluplus [14]. The powder was easy to re-disperse with little change in particle size(377.8±18.2 nm)and PDI (0.250±0.050) after lyophilization. Meanwhile, PZQ MCs were prepared by micronization with a jet mill system[23,24].The D10,D50,D90of PZQ MCs were 1.0,3.7 and 14.1 μm,respectively. The span was 3.5,which indicated that the size distribution was narrow [25]. The particle size distribution curve of PZQ MCs was shown in Fig.S1.
3.2. Transmission electron microscopy(TEM)
As shown in Fig.1,PZQ NCs had a rod-like morphology with a particle size ranging from 300 to 400 nm. The particle size was consistent with that obtained by ZS-90 nanoparticle size analyzer.
3.3. Differential scanning calorimeter(DSC)
DSC profiles of blank excipients, PZQ raw drug, physical mixtures, PZQ MCs and NCs are shown in Fig. 2. PZQ exhibited a sharp endothermic peak at 140°C, while none of the excipients showed a melting peak at the same position. The endothermal peaks of PZQ were found in PZQ NCs, MCs and physical mixtures,but their intensities were reduced owing to the presence of the excipients.Particularly for NCs,the peak positions shifted to lower values. The intensities of NCs and MCs were weaker than that of physical mixtures as a result of the micronization/nanonization processes increasing the free energy of the system and reducing the lattice energy[26].

Fig.1-TEM image of PZQ NCs.

Fig.2-DSC patterns of(a)Blank excipients(Soluplus,F127,mannitol and sorbitol);(b)PZQ raw drug;(c)Physical mixtures;(d)PZQ MCs;(e)PZQ NCs.
3.4. X-ray powder diffraction(XRPD)
XRPD diffractograms for blank excipients, PZQ raw drug,physical mixtures, PZQ MCs and PZQ NCs were shown in Fig. 3. The bulk PZQ exhibited a series of sharp and strong crystal diffraction peaks at 4.01°, 7.5°, 14.74°, 15.35°, 16.39°,18.48° and 20.06° [27]. The other samples showed the same drug diffraction peaks except blank excipients, indicating that the drug crystals stayed the same.The results of the DSC test were confirmed.
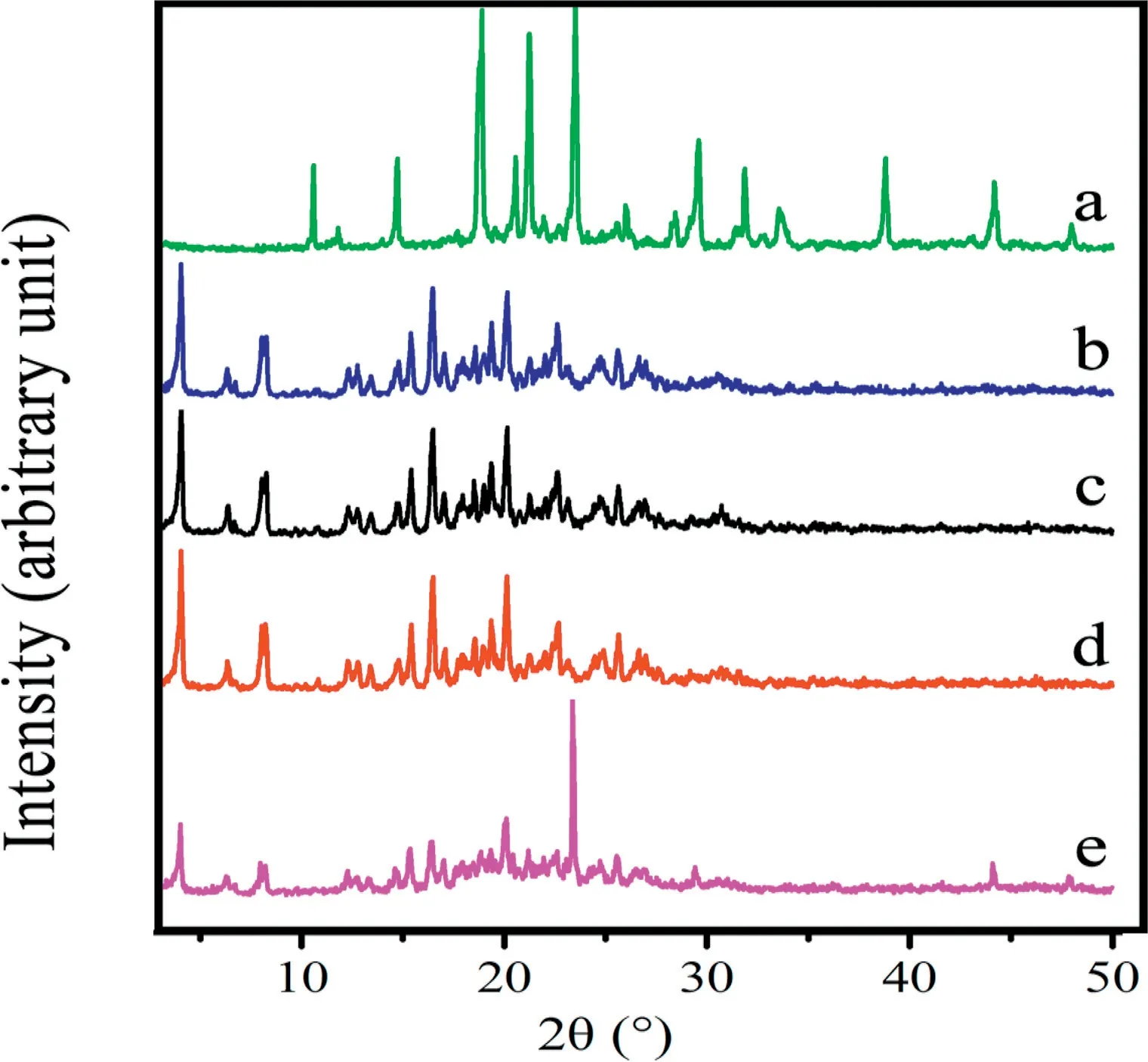
Fig.3-XRPD patterns of(a)Blank excipients(Soluplus,F127,mannitol and sorbitol);(b)PZQ raw drug;(c)Physical mixtures;(d)PZQ MCs;(e)PZQ NCs.
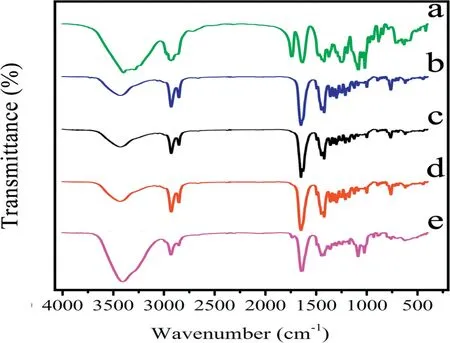
Fig.4-FT-IR spectra of(a)Blank excipients(Soluplus,F127,mannitol and sorbitol);(b)PZQ raw drug;(c)Physical mixtures;(d)PZQ MCs;(e)PZQ NCs.
3.5. Fourier transform infrared spectroscopy(FT-IR)
The absorption spectrum was shown in Fig. 4. The main absorption band of PZQ was observed at 1649 cm-1which corresponded to C=O group. The bands at 2929 cm-1and 2853 cm-1are associated with CH vibrations of the symmetric and asymmetric CH3and CH2axial deformations. All the characteristic peaks of PZQ did not markedly shift as shown in the spectra, indicating that the chemical structure of PZQ stayed the same in NCs and MCs.
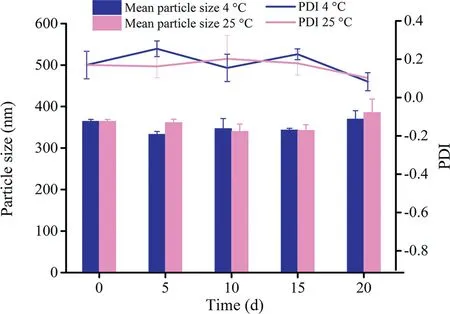
Fig.5-Physical stability of PZQ NCs stored at 4°C and 25°C(mean±SD,n=3).
3.6. Physical stability study
As shown in Fig. 5, the diameter of PZQ NCs did not significantly change after 20 d at both 4°C and 25°C.Although little aggregation was observed on the 21th day at 25°C,it was easy to re-disperse into the nanosuspensions with a gentle shake.Above all,PZQ NCs exhibited good physical stability.
3.7. Dissolution test
As for BCS class Ⅱdrugs, water solubility was the critical factor for their oral bioavailability, and a lot of dissolution tests had been carried out to foretell the in vivo pharmacokinetic results and the relationship between in vitro and in vivo performance [28]. Media in the dissolution test were chosen depending on Chinese Pharmacopoeia and equilibrium solubility assay. Briefly, the standard dissolution medium of PZQ tablets prescribed by Chinese Pharmacopoeia was 0.1 M HCl solution containing 0.2%SDS.The results(Table S1)of equilibrium solubility assay in different pH conditions and different concentrations of SDS solution indicated that solubility of PZQ was pH-independent, and 0.1 M HCl solution containing 0.2%SDS was the sink condition.Non-sink conditions usually have a better dissolution-discriminative capability than sink conditions for hydrophobic drugs [29], and therefore water was chosen as the medium of non-sink condition considering the convenience.
The dissolution results were shown in Fig.6.From Fig.6A we can see that cumulative dissolution of all the formulations were nearly 100% in the sink medium, whereas the dissolution rates were different. PZQ NCs completely dissolved within 5 min, showing the highest dissolution rate.There were no obvious difference between PZQ MCs and KANGQING?. Fig. 6B showed that cumulative dissolution of PZQ NCs,MCs and KANGQING?in water were 99.14%,13.06%and 73.43%, respectively. PZQ NCs dissolved faster and more than MCs and KANGQING?. To find out the underlying reasons,we investigated the effects of excipients on dissolution.A dissolution study of physical mixtures (PMs), which were composed of MCs and quantities of excipients in the prescription of NCs, was carried out. The results in Fig. 6 indicated that no change happened in 0.1 M HCl solution containing 0.2% SDS, while the dissolution of PMs in water was 63.17%.More dissolution under non-sink condition may be ascribed to the addition of stabilizers[30].
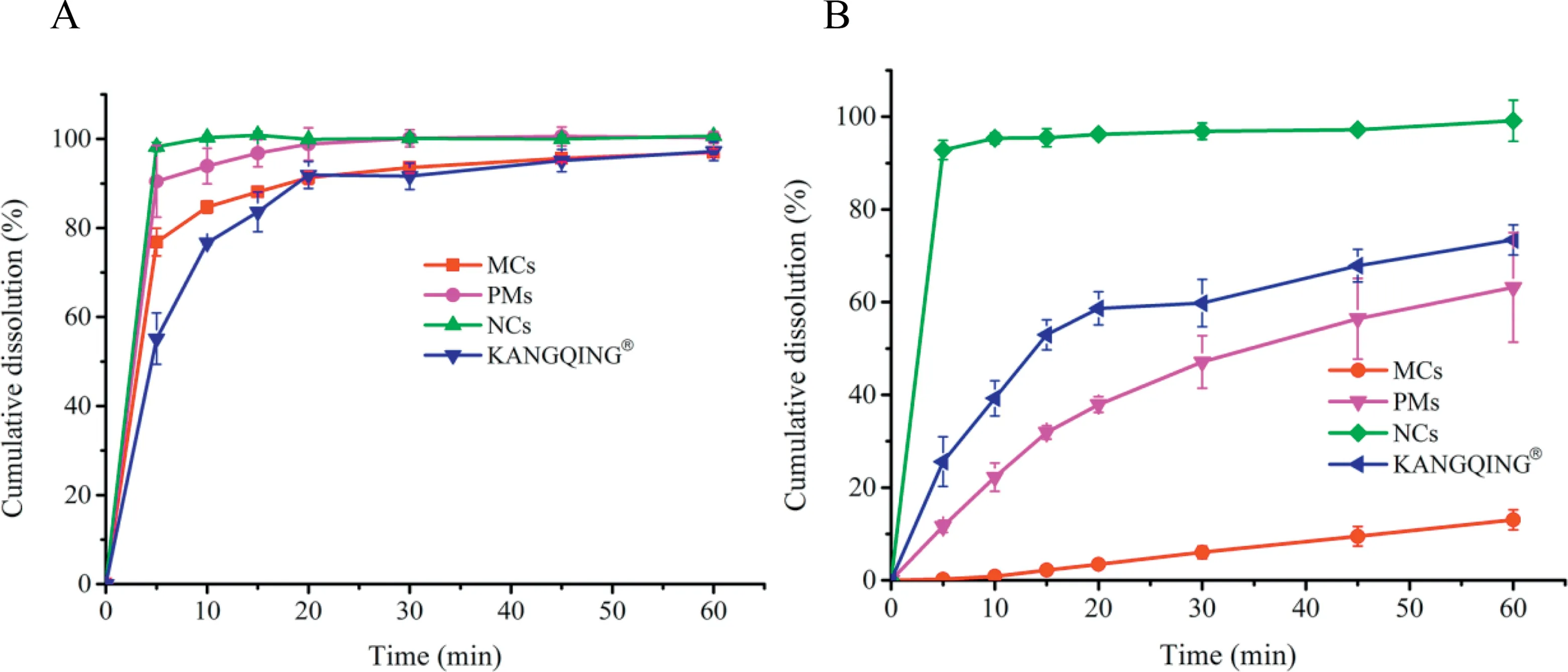
Fig.6-Dissolution profiles of PZQ NCs,PZQ MCs,PMs and KANGQING? in(A)0.1 M HCl solution with 0.2%SDS and(B)water(mean±SD,n=3).
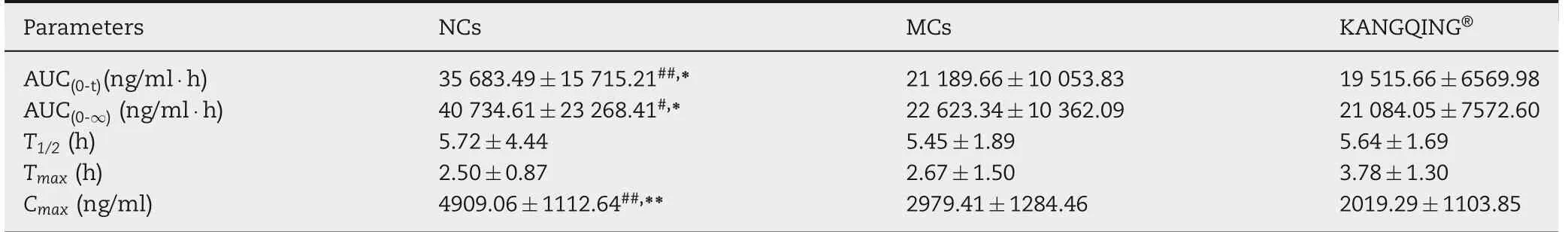
Table 2-Pharmacokinetic parameters of PZQ after oral administration of NCs,MCs and KANGQING?to beagles(mean±SD,n=9).
The better dissolution performance of PZQ NCs under nonsink condition agreed to the viewpoint that supersaturation condition could distinguish the quality of preparations [31].The results of dissolution test also revealed that the increased dissolving ability of PZQ NCs under non-sink condition might be attributed to reduced particle size and increased area wetting based on Noyes-Whitney equation. Stabilizers may also play a role in improving the dissolution. In conclusion,the dissolution results under supersaturation condition had considerable reference value for in vivo absorption.
3.8. Pharmacokinetics study in beagle dogs
PZQ NCs, PZQ MCs and KANGQING?were given orally to beagle dogs at a single dose of 16.67 mg/kg. According to the results of dissolution test, excipients improved dissolution of PZQ to some extent. To find out the impact of excipients on pharmacokinetics, PZQ MCs were administered to beagle dogs with quantity of excipients in the prescription of NCs.Blood samples were analyzed by LC-MS/MS. The plasma concentration versus time profiles were illustrated in Fig. 7 and the pharmacokinetic parameters were presented in Table 2.PZQ NCs performed a significant increase on AUC and Cmaxcompared with those of MCs and KANGQING?.The AUC0-36hvalue of PZQ NCs was about 1.68-fold and 1.83-fold higher than that of MCs(P <0.05)and KANGQING?(P <0.01),respectively. For Cmax, PZQ NCs was about 1.65-fold higher than PZQ MCs (P <0.01) and 2.43-fold higher than KANGQING?(P <0.01).
These results suggested that PZQ NCs presented an improved oral bioavailability compared with MCs and KANGQING?,which was corresponding with the results of the dissolution test under non-sink conditions.We could draw a conclusion that in vitro dissolution experiments of PZQ could commendably forecast the in vivo pharmacokinetics. The enhanced oral bioavailability was mainly caused by reduction of particle size, which may assist in increasing dissolution and adhesion of PZQ NCs in the gastrointestinal tract.
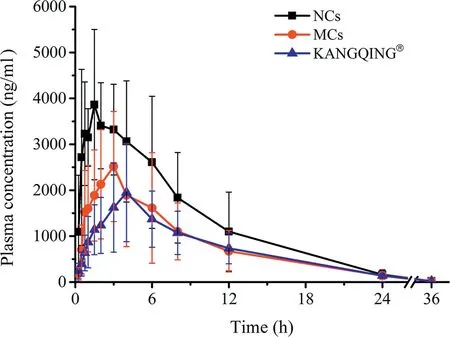
Fig.7-Mean concentration-time curves of PZQ NCs,PZQ MCs and KANGQING? after oral administration in beagle dogs(mean±SD,n=9).
4. Conclusion
In this work, PZQ NCs and MCs were prepared successfully by wet milling and jet milling methods,respectively.DLS and TEM experiments demonstrated that the average diameters of PZQ NCs and MCs were 364.4 nm and 3.7 μm, respectively.The characterization of DSC, XRPD and FT-IR proved that both NCs and MCs kept the original structural state of crystals. Compared with commercially available tablets and PZQ MCs, PZQ NCs showed increased dissolution rate and oral bioavailability. Additionally, there was no significant difference between MCs and tablets. The results revealed that the formulation of PZQ NCs especially at size about 360 nm is a great potential for clinical application in improving the oral absorption of PZQ and other related drugs.
Conflict of interest
The authors declare that there is no conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.06.001.
 Asian Journal of Pharmacentical Sciences2019年3期
Asian Journal of Pharmacentical Sciences2019年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Double-layered osmotic pump controlled release tablets of actarit:In vitro and in vivo evaluation
- Study of penetration mechanism of labrasol on rabbit cornea by Ussing chamber,RT-PCR assay,Western blot and immunohistochemistry
- Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule(Avodart?):Effect of γ-cyclodextrin and solubilizers
- Percutaneous absorption and brain distribution facilitation of borneol on tetramethylpyrazine in a microemulsion-based transdermal therapeutic system
- Effects of granulation process variables on the physical properties of dosage forms by combination of experimental design and principal component analysis
- MSNCs and MgO-MSNCs as drug delivery systems to control the adsorption kinetics and release rate of indometacin
