Ustekinumab: “Real-world” outcomes and potential predictors of nonresponse in treatment-refractory Crohn's disease
Peter Hoffmann, Johannes Krisam, Cyrill Wehling, Petra Kloeters-Plachky, Yvonne Leopold, Nina Belling,Annika Gauss
Abstract BACKGROUND Ustekinumab was approved in Europe for the treatment of adults with moderate to severe Crohn's disease (CD) in 2016, and there is an urgent need for data on its everyday use.AIM To obtain data on the daily use of ustekinumab.METHODS This is a retrospective monocentric study. Patients with moderate to severe CD who began ustekinumab therapy at the inflammatory bowel diseases outpatient clinic of the Heidelberg University Hospital between December 2016 and March 2018 were selected based on electronic patient files. The primary study endpoint was combined steroid-free clinical remission or steroid-free clinical response at 24± 6 wk of ustekinumab therapy. Secondary study endpoints were: achievement of mucosal healing, sonographic and magnetic resonance imaging response,biochemical response, the need for intestinal surgery within 24 ± 6 wk after treatment initiation, the occurrence of adverse events, treatment discontinuation due to nonresponse or adverse events, improvement of extraintestinal manifestations, clinical response at 48 ± 6 wk of therapy, and association of response with nucleotid oligodimerisation domain 2 mutations.RESULTS Fifty-seven patients with CD (5.3% anti-tumour necrosis factor α na?ve, 63.2%having undergone at least one intestinal surgery) were included in the study.Twenty patients (35.1%) achieved steroid-free clinical remission, 6 (10.5%)steroid-free clinical response and 31 (54.4%) were non-responders. Treatment discontinuation due to adverse events occurred in two patients (3.5%). Male sex,the presence of extraintestinal manifestations and the use of steroids at baseline were predictors of nonresponse to ustekinumab therapy.CONCLUSION In a “real-world” treatment-refractory cohort of patients with CD, ustekinumab appeared efficacious and safe.
Key words: Eeal-world; Ustekinumab; Crohn's disease distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/
INTRODUCTION
Contemporary long-term treatment options for Crohn's disease (CD) include immunomodulators like thiopurines and methotrexate or biologicals like tumour necrosis factor α (TNF-α) inhibitors, the integrin inhibitor vedolizumab and surgery.Despite the rising number of approved medications, a considerable proportion of patients with CD remain insufficiently treated.
The most recently approved medical treatment among therapeutic options for CD is ustekinumab. This agent is an immunoglobulin G1 monoclonal antibody directed against the p40 subunit of interleukin (IL)-12 and IL-23. By binding p40, the activity of the IL-23 and IL-23 receptors, which are found on T cells, natural killer cells, and antigen-presenting cells, is blocked[1]. IL-12 and IL-23 are necessary for the differentiation, survival and expansion of Th1 and Th2 cells[2,3]. IL-12 is needed for the differentiation of Th1 cells, which produce TNF-α and interferon gamma[2,4]. IL-23 is necessary for the survival and expansion of Th17 cells[5]and Th22 cells[6]. The activation of T cells may progress into a condition of chronic immunological response,lacking negative feedback regulation.
In the UNITI-1 trial, 741 patients with CD who had failed previous anti-TNF-α therapy were included, while the UNITI-2 trial comprised 628 patients with CD with prior failure of conventional CD therapy[7]. The primary endpoint of both trials was clinical response at week 6. The UNITI-1 and UNITI-2 induction studies showed 34.3% to 55.5% clinical remission rates at week 6 in the ustekinumab groups,compared with 21.5% to 28.7% in the placebo groups (P < 0.003).
Meanwhile, long-term efficacy data through week 92 and safety data through week 96 from IM-UNITI have been reported[8]: Rates of adverse events, serious adverse events, and serious infections in the ustekinumab group and the placebo group were similar.
A retrospective “real-world” multicentric cohort study from Canada, including 167 patients with CD who were treated with subcutaneous ustekinumab, revealed clinical response rates of 38.9%, 60.3%, and 59.5%, as well as remission rates of 15.0%, 25.2%,and 27.9% after 3, 6, and 12 mo, respectively[9].
As ustekinumab has been available for CD clinical routines for just over two years,“real-world” data on ustekinumab in the treatment of CD are still scarce. The goals of the present study were (1) to gather more “real-world” data on the performance of ustekinumab in the therapy of patients with CD; and (2) to discover variables that may influence therapy outcomes. Besides clinical routine parameters, the three main CD-associated nucleotid oligodimerisation domain 2 (NOD2) mutations rs2066844,rs2066845, and rs20566847 were analysed in the included patients to search for a potential association with response to ustekinumab therapy. To date, NOD2 represents the most important genetic predictor of disease course in CD[10]. Several studies have demonstrated an increased carrier rate of these three mutations of the NOD2 gene in patients with CD compared with healthy controls[11,12]. Recently,associations of mutations in the NOD2 gene and specific phenotypes, as well as lower anti-TNF-α trough levels, were shown in patients with CD[13]. Nothing is known about potential associations between NOD2 mutations and the efficacy of ustekinumab in CD. Therefore, we decided to incorporate this aspect into our study.
MATERIALS AND METHODS
Study design and data extraction
This is an uncontrolled, retrospective monocentric study including outpatients with moderate to severe CD at a German university hospital serving as a tertiary referral centre for the treatment of inflammatory bowel diseases (IBD). The study was approved by the local Ethics Committee (Alte Glockengie?erei 11/1, 69115 Heidelberg; protocol number: S-520/2018). For NOD2 genotyping, written informed consent was required for participation in our prospective IBD registry (protocol number: S-238/2017).
Inclusion criteria were: Age ≥ 18 years; ascertained diagnosis of moderately to severely active CD; complete treatment with ustekinumab at our IBD outpatient clinic during the first 6 mo; initiation of ustekinumab therapy between December 1, 2016 and March 31, 2018; and a documented follow-up of at least 24 ± 6 wk from start of ustekinumab therapy. Exclusion criteria were: Age < 18 years, diagnoses of ulcerative colitis and indeterminate colitis, incomplete treatment documentation and a follow-up of less than 24 ± 6 wk from start of ustekinumab therapy. The follow-up ended for all patients on December 31, 2018. This time point was defined as the cut-off time point for data acquisition. For efficacy analyses, patients who had to discontinue ustekinumab therapy due to adverse events prior to week 24 were considered to be non-responders.
All data were retrieved from entirely computerised medical records. Demographic and clinical parameters of all eligible patients were entered into a Microsoft Excel spreadsheet.
Definitions
The Montreal classification for CD[14]was used to categorise disease phenotypes. The Harvey-Bradshaw-Index (HBI) was routinely determined at every patient's visit at the IBD outpatient clinic[15]. Steroid-free clinical remission was defined as an HBI of ≤ 3 points without the use of any steroid preparation [budesonide, prednis (ol) one, or methylprednisolone]. Steroid-free clinical response was defined as an HBI reduction of ≥ 3 points without the use of any steroid preparation. Nonresponse was considered if (1) the absolute HBI score was > 3 points with an HBI reduction of < 3 points from baseline, or if (2) steroid treatment was initiated, or if (3) ustekinumab therapy was discontinued prior to week 24 due to an inadequate treatment response. In addition,patients who discontinued ustekinumab therapy for other reasons, like adverse events, were defined as non-responders.
Mucosal healing (MH) was defined as an absence of ulcers in all endoscopically visualised bowel segments. Sonographic treatment response was defined as an absence of bowel wall thickening (wall thickness ≤ 3 mm). Magnetic resonance imaging (MRI) response was defined as improved or absent signs of inflammation including contrast enhancement and bowel wall thickness. Biochemical response was defined as any reduction of faecal calprotectin (FC) or plasma C-reactive protein(CRP) concentrations in comparison to baseline.
The follow-up time was defined as the number of complete months after week 24 until December 31, 2018, or until discontinuation of ustekinumab treatment.
Treatment schedule
All patients initially received a single intravenous ustekinumab dose (weight rangebased dosing of approximately 6 mg/kg in categories of 260 mg, 390 mg or 520 mg),followed by subcutaneous administration of ustekinumab 90 mg at week 8 and every 8 or 12 wk thereafter.
At our IBD treatment facility, patients receiving ustekinumab therapy are routinely examined by an experienced physician at ca. Six and twelve weeks after treatment initiation, followed by visits at intervals of three months. Whether intervals of 8 or 12 wk were chosen for maintenance therapy depended on individual patient treatment responses. The decision to shorten the ustekinumab dosing interval to 8 wk was made by the treating physician based on patients' symptoms ca. Four weeks after the first subcutaneous dose, or later in cases of secondary decrease of response. The decision to discontinue ustekinumab therapy due to inadequate response or adverse events was consistently made by a senior gastroenterologist.
Study endpoints
The primary study endpoint was a combined endpoint of steroid-free clinical remission or steroid-free clinical response at 24 ± 6 wk of ustekinumab therapy.Secondary study endpoints were achievement of MH, sonographic and MRI response,biochemical response, the need for intestinal surgery within 24 ± 6 wk of ustekinumab therapy, the occurrence of adverse events, discontinuation of ustekinumab therapy due to nonresponse or due to adverse events, improvement of extraintestinal manifestations, response at 48 ± 6 wk of ustekinumab therapy and association of response with NOD2 mutations.
Data collection
Further information retrieved from electronic patient charts included gender; age at data acquisition and at first diagnosis of CD; disease duration; disease location;disease behaviour; prior CD-related intestinal surgery; family history for IBD;presence of extraintestinal manifestations; smoking status; body mass index (BMI);history of anti-TNF-α, anti-integrin, or immunomodulator treatment; number of prior biological therapies; reason for ustekinumab treatment initiation; history of IBDrelated hospitalisation(s) within 12 mo of start of ustekinumab therapy; previous and concomitant medications; suspected side effects of ustekinumab therapy; blood and stool biochemical markers measured prior to and after start of ustekinumab therapy;and endoscopic, MRI and sonographic findings.
As the patients did not consistently visit the IBD outpatient clinic after exactly 6, 12,24, 36 and 48 wk of ustekinumab therapy, all visits at 6 ± 2, 12 ± 3, 24 ± 6, 36 ± 6 and 48± 6 wk of ustekinumab therapy were included in the analyses.
Stool samples were mailed or delivered directly to the IBD outpatient clinic by the patients. Baseline evaluations included results of stool samples, colonoscopy, MRI,and ultrasound, collected up to 12 wk prior to start of ustekinumab therapy. FC concentrations measured at 6 ± 2, 12 ± 3, 24 ± 6, 36 ± 6, and 48 ± 6 wk of ustekinumab therapy, and colonoscopy, MRI, and ultrasound findings from 24 ± 6, 36 ± 6 and 48 ± 6 wk of ustekinumab were included.
FC concentrations of < 30 μg/g and > 1800 μg/g were rated as 30 μg/g and 1800 μg/g, respectively. Plasma CRP concentrations < 2 mg/L were rated as 1 mg/L.
DNA extraction and NOD2 genotyping
To investigate a potential association with response to ustekinumab therapy, patients'blood samples were analysed for the three main CD-associated NOD2 mutations,rs2066844, rs2066845, and rs2066847. Genomic DNA was isolated from EDTAanticoagulated peripheral venous blood using a QIAamp DNA Blood Midi kit according to the manufacturer's protocol (Qiagen GmbH, Hilden, Germany). The genotypes of three reported IBD mutations of the NOD2 gene (rs2066844, rs2066845,and rs2066847) (Table 1) were screened by a LightCycler?480 Instrument I (Roche Diagnostics International AG, Rotkreuz, Switzerland). Polymerase chain reaction(PCR) was performed with the LightCycler?fast start DNA Master HybProbe according to the manufacturer's protocol (Roche Diagnostics International AG,Rotkreuz, Switzerland). PCR was performed as follows: denaturation at 95 °C for 10 min, followed by 45 cycles of 10 s at 95°C, 10 s at 60°C, and 15 s at 72 °C. The primers were designed and synthesised by TIB MOLBIOL GmbH (Berlin, Germany) according to the dbSNP database of NCBI (https://www.ncbi.nlm.nih.gov/projects/SNP).Primers and PCR conditions are specified in Table 1.
Statistical analysis
Descriptive statistics were calculated as percentages for discrete variables and presented as medians with ranges, or as means, if the results were normally distributed. To identify potential predictors of response to therapy, the mann-whitney test was used for ordinal and continuous variables, and chi-squared tests for categorical variables. As a multivariable analysis, factors that were univariately associated with the outcome with a P value of 0.1 or less were included in a logistic regression model with variable selection. The model with the best Bayes information criterion (BIC) was selected as the optimal model. Odds ratio (OR) estimates for the selected variables were reported together with 95% confidence intervals. The area under the curve (AUC) of the optimal model was calculated together with a 95%confidence interval in order to quantify the ability of the model to predict response to therapy. Due to the exploratory nature of the trial, P values are to be interpreted in a descriptive manner, and thus, no adjustment for multiple testing was performed. P values below 0.05 were regarded as statistically significant. The statistical analyses were performed using IBM SPSS Statistics 25 (Chicago, IL, United States). In order to determine the optimal multivariable logistic regression model, R version 3.4.2(http://r-project.org) together with R package “bestglm” was used[16].

Table 1 Primers for polymerase chain reaction
RESULTS
Demographics and clinical characteristics
Between December 1, 2016 and March 31, 2018, 68 patients with moderate to severe CD began ustekinumab therapy at our IBD outpatient clinic. Eleven of these 68 patients were excluded from the study as they received parts of their treatment at other treatment facilities.
In total, 57 patients met the inclusion criteria and were included in the study. All patient demographics and clinical baseline characteristics and their concomitant medications are presented in Table 2. Thirty-five patients (61.4%) reached the end of the follow-up period on December 31, 2018 while still on ustekinumab therapy. Two patients (3.5%) were lost to follow-up at week 24 and three months of follow-up. The median follow-up period after the first 24 wk of ustekinumab therapy was 8 mo(range: 2-18 mo).
Among the 57 included patients, 53 (94.7%) had been treated with anti-TNF-α prior to the start of ustekinumab therapy. 22.8% of the patients had received three biologic therapies before, and 63.2% had undergone at least one CD-related intestinal surgery(Table 2). The mean HBI at start of ustekinumab therapy was 6.6 points. There were several patients with NOD2 mutations, mostly heterozygous, as shown in Table 2.
Response to ustekinumab therapy
Twenty patients (35.1%) achieved steroid-free clinical remission, 6 (10.5%) steroid-free clinical response and 31 (54.4%) were non-responders to ustekinumab therapy. This means that 26 of the patients (45.6%) achieved the combined primary study endpoint of steroid-free clinical remission or response.
Forty-eight of the 57 included patients (84.2%) remained on ustekinumab at 24 ± 6 wk after the start of treatment.
For 24 patients (42.1%), data was available at 48 ± 6 wk from start of ustekinumab therapy. In this subgroup, 7 patients (12.3%) were still on concomitant steroid therapy, while one patient (1.8%) was on concomitant immunomodulator therapy.
Several statistically significant differences were found between the characteristics of responders versus those of non-responders to ustekinumab therapy (Table 3). The occurrence of extraintestinal manifestations and steroid use at baseline were negatively associated with response. Also, a higher HBI at treatment initiation was a negative predictor for response (Table 3). Male sex was nearly significantly associated with nonresponse (P = 0.05; Table 3). In contrast, the presence of NOD2 mutations was not associated with therapy outcome. The factors with a P value below 0.1-extraintestinal manifestations, steroid use at baseline, HBI at treatment initiation and
sex were included in the multivariable logistic regression model. All those factors remained in the optimal model, the odds ratios and P values for which are shown in Table 4. Except for baseline HBI with a P value of 0.071, the other three factors were statistically significantly associated with response, thus confirming the results of the univariate analysis. With an AUC of 91.07%, the model showed excellent predictive qualities.
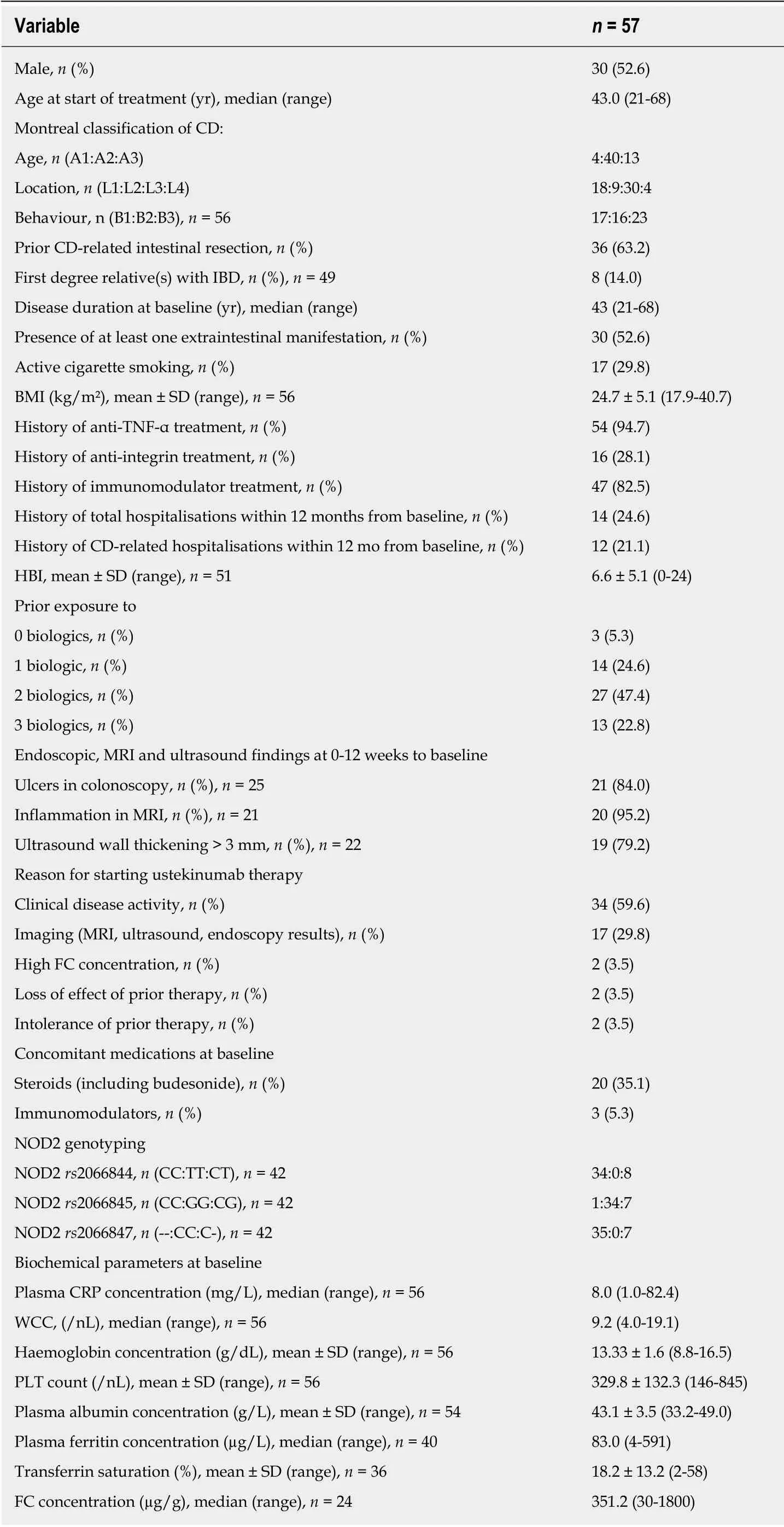
Table 2 Baseline characteristics
Secondary endpoints
Forty-eight among the 57 included patients (84.2%) continued to be on ustekinumab therapy at 24 ± 6 wk. Nine patients (15.8%) stopped ustekinumab therapy due to the following reasons: One was diagnosed with a neuroendocrine carcinoma of the small bowel with hepatic metastases at week 12, one stopped at week 6 at the patient's own request, two developed side effects (one allergic reaction with nausea, dizziness,difficulty in breathing; one strong joint pain after intravenous ustekinumab); and five discontinued therapy due to nonresponse.
The two cases of discontinuation of ustekinumab therapy due to adverse events were added in the efficacy analysis in the lack of response group.
At 24 ± 6 wk, 6 colonoscopies were available: all of them belonged to the nonresponse group, and none of them showed MH (Table 5). Ultrasound imaging at 24 ± 6 wk (n = 13) revealed a wall thickening of > 3 mm in 37.5% of the responders compared to 60% of the non-responders. Seven MRIs were performed, 6 of them in the nonresponse group, showing improvement in 50%. One patient underwent ileocecal resection in week 24 of ustekinumab therapy due to subileus. The biochemical parameters at 24 ± 6 wk did not differ significantly between the response and the nonresponse group (Table 5). The only parameter which was significantly reduced in the response group is the presence of extraintestinal manifestations (4.2%vs 28.6%). There was no association between the presence of NOD2 mutations and response to ustekinumab therapy.
Secondary loss of response is a major concern for all biologic therapies. Follow-up data of 28 patients who continued on ustekinumab therapy at 48 ± 6 wk were available in our study cohort. At 48 ± 6 wk, the rate of steroid-free clinical remission and response was still 64.3%. Among the patients with CD who had achieved steroidfree clinical remission or response at 24 ± 6 wk from treatment initiation, 16 (87.5%)remained in steroid-free clinical remission or response at 48 ± 6 wk. In Table 6,adverse events at week 6, 12, 18, 24, 36, and 48 of ustekinumab therapy are listed separately. The rate of adverse events under ustekinumab therapy varied between 52.7% and 64.3%, while the rate of infections varied between 0% and 21.4% across the set of time points.
DISCUSSION
As ustekinumab has been in clinical use for CD outside study conditions for only two and a half years so far, published “real-world” experience is scarce. The acquisition and application of these data are crucial to the treatment of patients with CD, because patients in randomised controlled trials are well chosen and not representative of IBD patients in general[17].
Our present study shows a clear benefit from ustekinumab treatment at 24 ± 6 wk of therapy in “real-world” treatment-refractory patients with CD among whom only three patients had not failed anti-TNF-α therapy.
The UNITI-1 and UNITI-2 induction trials revealed clinical remission rates of 34.3%to 55.5% at week 6 of therapy[7]. In comparison, the outcome data we collected for 57 patients at 24 ± 6 wk of ustekinumab treatment are similar, with 20 (35.1%) of the patients achieving steroid-free clinical remission and 6 (10.5%) steroid-free clinical response, whereas 31 patients (54.4%) did not respond to ustekinumab therapy.
The primary endpoint in IM-UNITI of clinical remission at week 44 was reached by 48.8% to 53.1% of the patients[7]. During our follow-up at 48 ± 6 wk (n = 28), we found that 64.3% of the patients had achieved steroid-free clinical response or remission, and 53.6% were in steroid-free clinical remission, which is in line with the results from the IM-UNITI trial.
Due to the retrospective nature of our study, colonoscopy or imaging results were only available in a small percentage of patients. Patients who were asymptomatic were usually not willing to undergo colonoscopy, especially if decreasing FC concentrations indicated a response. Therefore, endoscopy results at 24 ± 6 wk were only available for non-responders.
We also analysed potential predictors of response to ustekinumab therapy. The occurrence of extraintestinal manifestations, the use of steroids at treatment initiation,and male sex proved to be independent negative predictors of response.
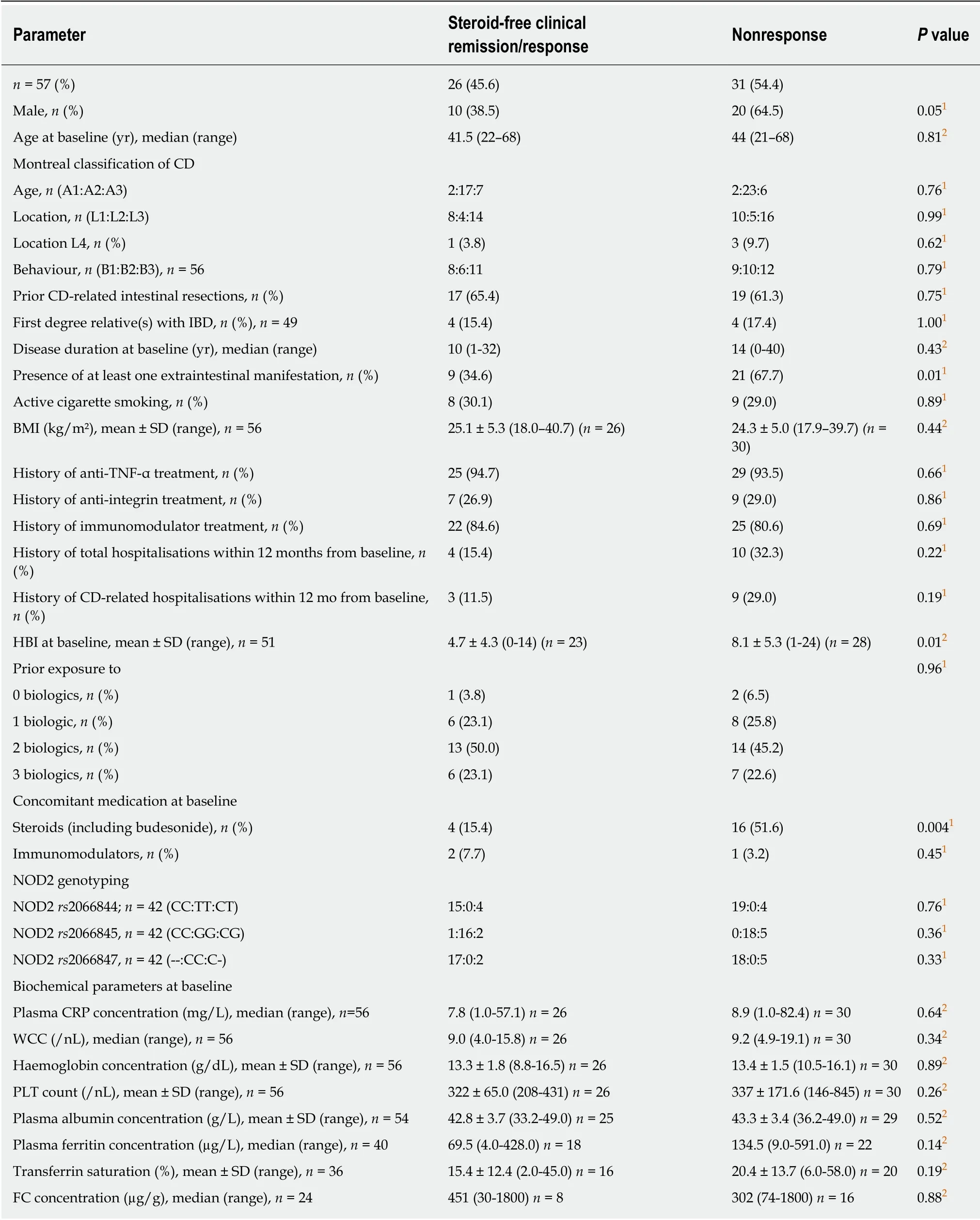
Table 3 Comparison of baseline characteristics between the subgroups of patients with steroid-free clinical response versus nonresponders to ustekinumab therapy
In a previous study on vedolizumab therapy in patients with CD, the achievement of clinical remission was found to be associated with the absence of extraintestinal manifestations and no hospitalisation within one year prior to treatment initiation[18].In comparison, our data have confirmed the association between the absence of extraintestinal manifestations and the achievement of steroid-free clinical response or remission under ustekinumab therapy. However, no association was established between response and the history of IBD-related hospitalisations within the first 12 mo after baseline.
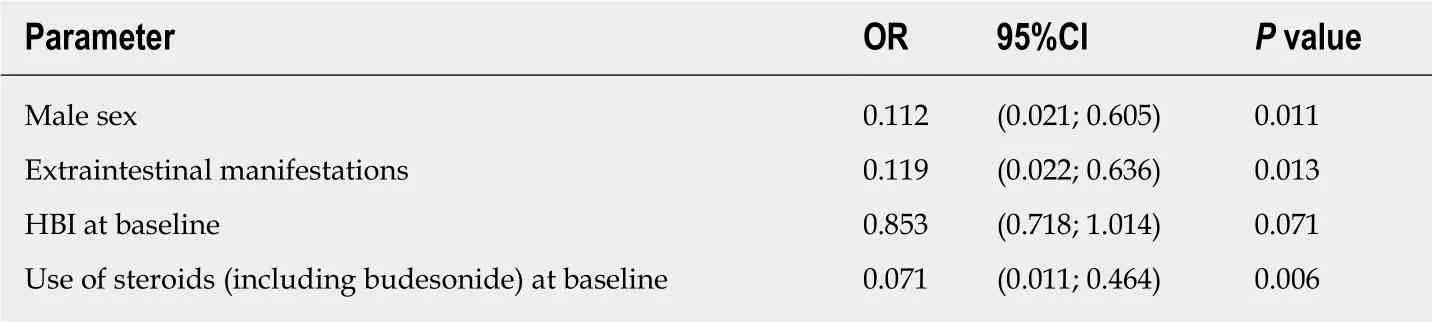
Table 4 Characteristics entering the multivariable logistic regression model
In the multivariable logistic regression model, male sex was a predictor of nonresponse to ustekinumab therapy. Recently, some sex differences in patients with CD were revealed. Thus, an association between male sex and upper gastrointestinal tract involvement has been identified in patients with CD of the Swiss IBD Cohort Study Group[19]. Perhaps in the future, a greater focus should be placed on potential genderrelated differences in IBD treatment.
One of the secondary endpoints of the study was the occurrence of adverse events under ustekinumab therapy. In the UNITI-1, UNITI-2- and IM-UNITI trials, the prevalence of any adverse event was 50.0% to 81.7 %, and infections of any kind occurred in 14.6% to 48.1% of the patients7. These findings are similar to ours. There were two cases of discontinuation of ustekinumab therapy due to adverse events-one due to joint pain and one due to an allergic reaction. In the UNITI trials, serious adverse events were observed in 1.2% to 5.3% of patients. There was one serious adverse event in our study: one male patient died of a neuroendocrine carcinoma of the small bowel with hepatic metastases which was diagnosed while he was on ustekinumab therapy; he had received multiple biologics and immunomodulators,also in combination, for a very refractory, long-term disease course of the small bowel.
Due to the location of his malignancy, it was suspected by the treating physicians that its development was related to high-grade chronic inflammation rather than immunosuppressive medications.
A limitation of our study is the lack of imaging and colonoscopy results, which is mainly due to the invasiveness of a colonoscopy and the retrospective nature of the study. Two of the clear strengths of our study are the strict endpoint of steroid-free clinical remission or steroid-free clinical response, and the fact that our data set of HBI documentation was nearly complete.
Another strength of the present study is that the efficacy of ustekinumab was evaluated relatively late at 24 ± 6 wk from application of the first ustekinumab dose.In contrast, efficacy analyses were already performed at week 6 in the induction studies UNITI-1 and UNITI-2. There are some advantages for later time points to assess the efficacy of a drug: Late responders are also detected; there may be less interference with other contemporaneous induction therapies like steroids; and finally, there will be less chance for placebo effects due to a longer time period.
All but three of the included patients had previously failed anti-TNF-α treatment.The percentage of concomitant steroid use at the start of ustekinumab therapy (53.1%)was also large, underlining the disease severity in our study cohort.
In conclusion, our data strongly suggest that ustekinumab is effective in treatmentrefractory, moderate to severe CD under “real-world” conditions. In particular, more data is needed on the long-term safety of this drug and on potential predictors of response.
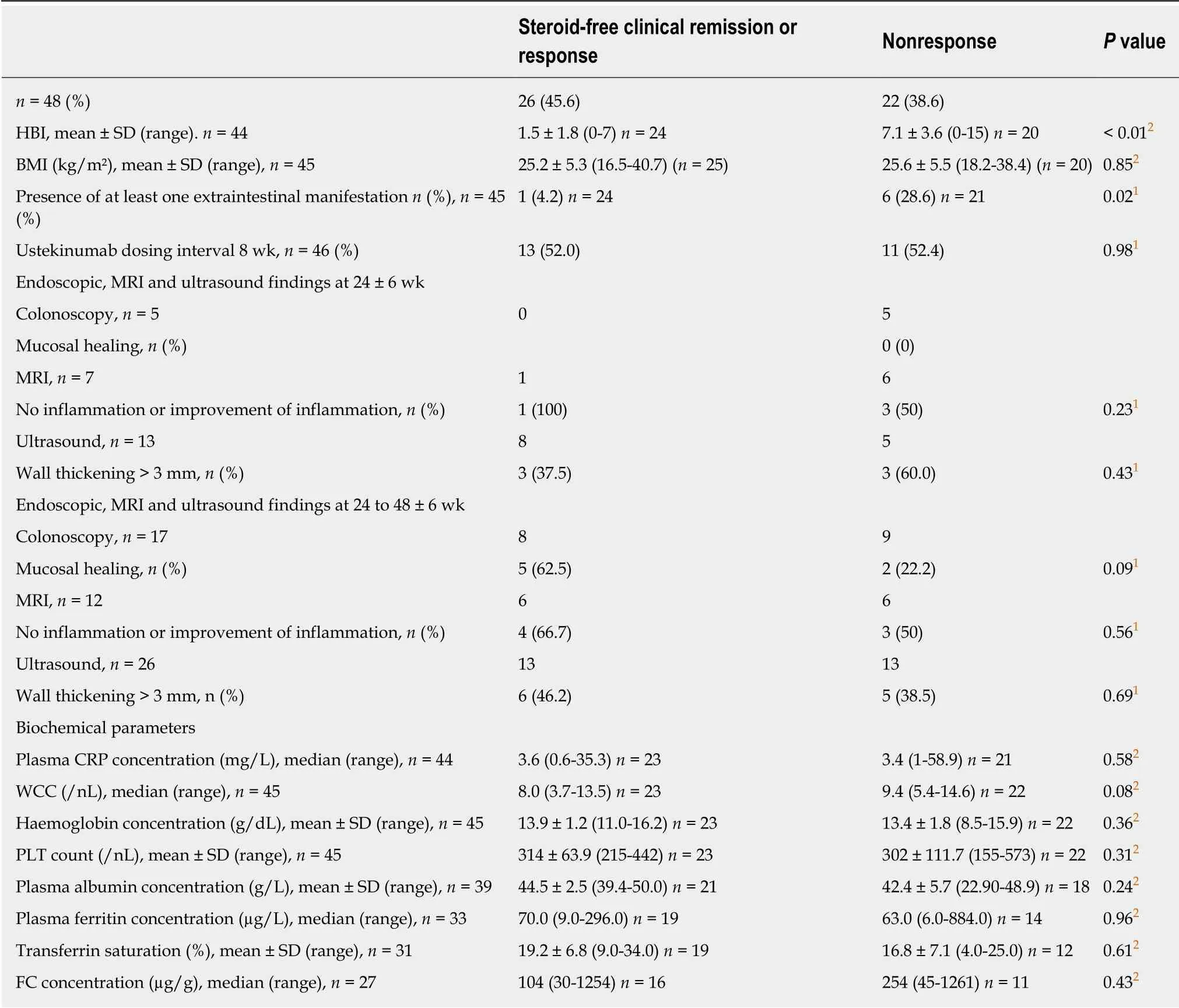
Table 5 Comparison between parameters determined at 24 ± 6 wk from start of ustekinumab therapy between steroid-free clinical responders and non-responders
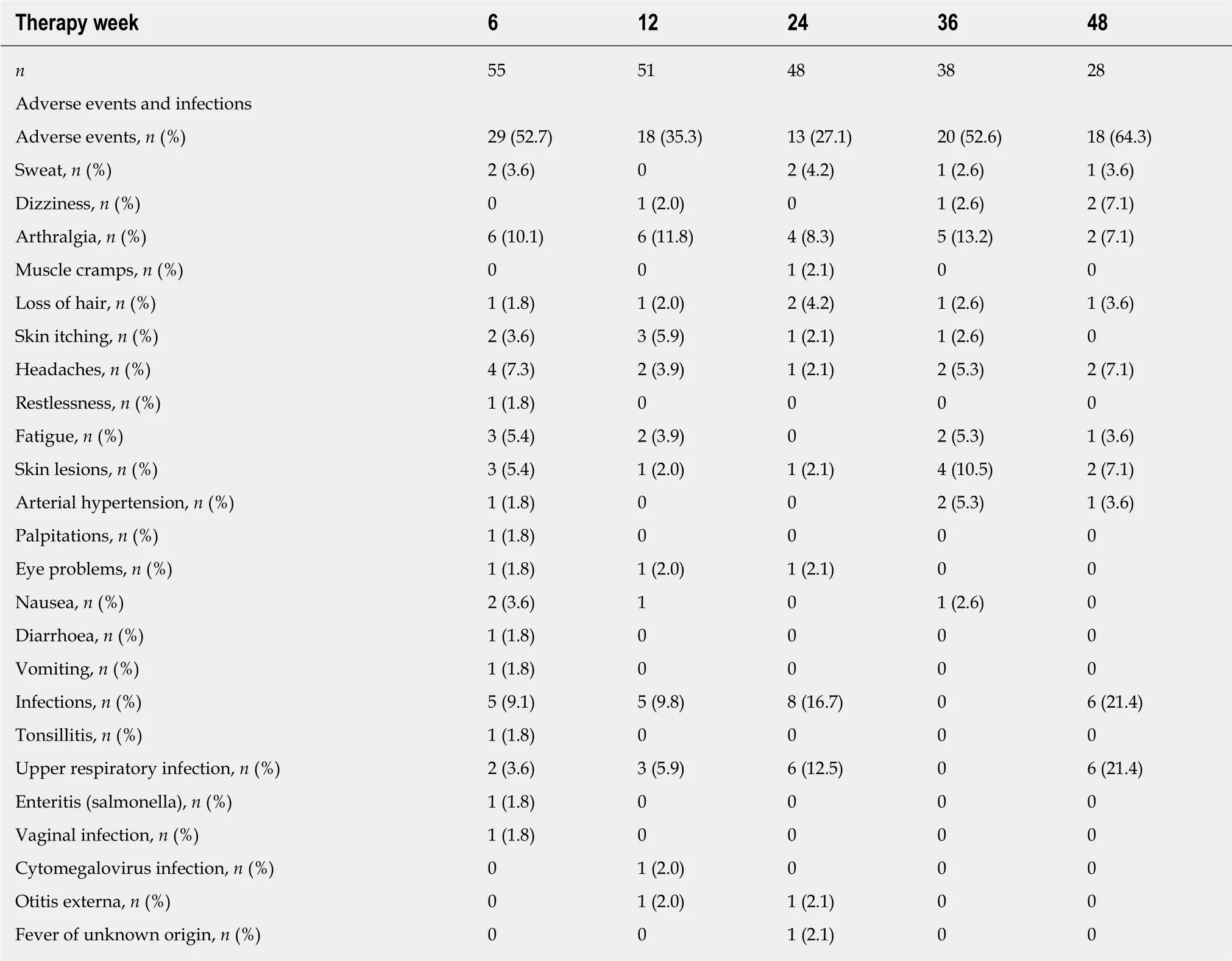
Table 6 Adverse events and infections in the study cohort listed according to the time of their occurrence
ARTICLE HIGHLIGHTS
Research background
Ustekinumab is a relatively new therapy for Crohn's disease (CD) which blocks the activity of interleukin-12 and interleukin-23.
Research motivation
“Real world” data on ustekinumab in CD are scarce.
Research objectives
This study investigated the response and remission rates in a German cohort of patients with CD receiving treatment with ustekinumab.
Research methods
This is a retrospective analysis of response and remission rates at 24 ± 6 wk of ustekinumab therapy in patients with CD who began therapy between December 2016 and March 2018.
Research results
Fifty-seven patients were included in the study. Twenty patients (35.1%) achieved remission and 6 (10.5%) achieved clinical response. Male sex, extraintestinal manifestations, and the use of steroids at baseline were predictors of nonresponse to ustekinumab therapy.
Research conclusions
In a “real-world” treatment-refractory cohort of patients with CD, ustekinumab appeared efficacious and safe.
Research perspectives
The identified predictors of nonresponse to ustekinumab therapy, comprising male sex,extraintestinal manifestations, and the use of steroids at baseline, should be verified in a prospective study.
 World Journal of Gastroenterology2019年31期
World Journal of Gastroenterology2019年31期
- World Journal of Gastroenterology的其它文章
- node dissection for low rectal cancer: ls it necessary?
- Layered enhancement at magnetic resonance enterography in inflammatory bowel disease: A meta-analysis
- Small bowel capsule endoscopy and treat-to-target in Crohn's disease: A systematic review
- Systematic review and meta-analysis of esophageal cancer in Africa:Epidemiology, risk factors, management and outcomes
- Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer
- lmpact of pediatric inflammatory bowel disease diagnosis on exercise and sports participation: Patient and parent perspectives
