Peripheral biomarkers for pediatric brain tumors:current advancements and future challenges
Markus J. Bookland, Antonina Kolmakova
1Division of Neurosurgery, Connecticut Children’s Medical Center, Hartford, CT 06106, USA.
2Department of Pediatrics, University of Connecticut Health Center, Farmington, CT 06106, USA.
Abstract Circulating biomarkers - nucleic acids, proteins, and metabolites - have been used in several adult oncologic processes to affect early detection, measure response to treatment, and offer prognostic information. The identification and validation of biomarkers for pediatric brain tumors, however, has been meager by comparison.Early detection and serial screening of pediatric brain tumors has the potential to improve outcomes by allowing for rapid therapeutic interventions and more targeted therapies. This is particular resonant for pediatric brain tumors where treatment success is heavily dependent on early surgical intervention. This highlights the need for biomarker development in pediatric neuro-oncology. The authors reviewed current circulating biomarker targets in various biofluid reservoirs and discuss the current barriers to biomarker development in pediatric neuro-oncology patients.
Keywords: Pediatric, brain tumor, biomarker, miRNA, biofluid, overview
INTRODUCTION
Pediatric brain tumors are the leading, oncologic cause of death in children under 10 in North America.Roughly 4,600 children are diagnosed with brain tumors every year in the United States, and maximal, safe surgical resection remains the primary treatment modality[1-3]. Five-year survivals can vary by as much as 70% with some of the most common pediatric brain tumors, depending on a surgeon’s ability to achieve complete safe resection of the mass[4-8]. This makes early diagnosis and intervention, potentially, critical to ensuring favorable outcomes, as surgical resection is generally more successful when tumors are smaller,confined to a single location, and less entwined with critical neural structures.
Despite a general awareness of the importance of establishing a prompt diagnosis for pediatric brain tumor care, delay in time to diagnosis is common with pediatric brain tumor patients, as children have a unique capacity to tolerate intracranial volume and pressure changes with minimal outward symptoms[9].Additionally, the immature nature of the nervous system in infants makes clinical screening for brain tumor-related symptoms challenging. Widespread radiographic screening of children, be it with computed tomography or magnetic resonance imaging, is impractical due to the risks of radiation exposure to the developing central nervous system, neurotoxic effects of early anesthetic exposure, and prohibitive diagnostic costs[10-12].
As such, a minimally invasive, reliable, and cheaply implemented screening tool has become a great clinical need for physicians and researchers seeking to improve early tumor detection, develop minimally invasive methods for molecular stratification of tumors, and allow for reliable post-treatment screening in pediatric brain tumor populations. Numerous permutations exist for researchers seeking to discover and validate novel biomarkers, as potential tumor markers need to be examined in different biofluid reservoirs and with different analytic techniques. Each variation in biomarker sampling and analysis exposes different advantages and limitations that researchers and clinicians must be cognizant of as they search for accurate and realistically deployable brain tumor biomarker assays. The authors will review here the history of biomarker development for pediatric brain tumors to date, the limitations and advantages of current target biofuids, promising biomarkers for the most prevalent pediatric brain tumors, and the major limitations of biomarker development in pediatric populations.
Historical biomarkers
Attempts to acquire objective evidence of a tumor from extra-tumor biofuids date back at least 100 years,beginning with cerebrospinal fuid (CSF) cytology. Tumor cytology is the oldest and most well-understood method for detecting and differentiating pediatric brain tumors via a biofluid[13]. The use of cytology to diagnose tumors in a minimally invasive manner dates back to the 19th century[14]. The process for acquiring CSF samples for cytologic analysis has the benefit of being relatively simple to perform and widely available in most regions of the world. In order to acquire a CSF sample, a lumbar puncture is performed with a sterile 20-22-gauge needle at or below the L2/L3 interlaminar space. Ventricular CSF samples have been used, as well, for CSF cytology; but the sensitivity of samples acquired from cerebral ventricular punctures is significantly lower than samples drawn from the lumbar cistern, likely due to sedimentation of tumor cells into the dependent lumbar cistern[15]. CSF samples are then fixed and processed for light microscopic evaluation or flow cytometry. CSF cytology can provide pathologic diagnoses in many oncologic cases.Immunohistochemical assays are also possible, making the cytologic analysis of CSF somewhat comparable to tissue biopsy in the range of qualitative information it can provide. However, CSF cytology has a low sensitivity (45%), unless performed repeatedly on the same patient, making it an inaccurate and impractical brain tumor screening modality by itself[16,17].
Arguably, modern pediatric intracranial tumor serum biomarker assay development began in the 1960s and 70s in the field of germ cell tumor diagnostics. Research with immunoperoxidase staining of testis germ cell tumors revealed excesses in alfa-fetoprotein (AFP) and beta human chorionic gonadotropin (βHCG)production within the tissues of some tumor types[18]. Immunostaining and radioimmunoassay techniques quickly proliferated for these compounds; and by the mid-1970s, pathologists were able to utilize AFP, βHCG,as well as other proteins, to distinguish endodermal sinus tumors, choriocarcinomas, dysgerminomas, and teratomas on immunohistochemical review and via serum-based tests.In 1979, Jeffrey Allen, MD and colleagues at Memorial Sloan-Kettering applied serum and CSF AFP/βHCG radioimmunoassays for use in a group of 6 pediatric patients with biopsy proven intracranial germ cell tumors. This study was not powered to allow for statistical discrimination of the results, but it demonstrated,within individual cases, high correlations between serum AFP and CSF βHCG levels and the presence of intracranial germ cell tumors. Furthermore, those patients with elevated AFP or βHCG assay levels had complete normalization of their serum and CSF levels concurrent with successful tumor radiographic response to therapy[19]. This work has been expanded upon numerous times since, and the correlations found in Allenet al.[19]’s paper have proven to be robust within larger cohorts. AFP and βHCG serum and CSF assays have now become routine components of intracranial germ cell tumor diagnostics, in some cases obviating the need for tissue sampling entirely[20-23].
The story of AFP/βHCG serum and CSF assays is a rare success story in the realm of clinical oncologic biomarkers for pediatric brain tumor patients, and one that has, frustratingly, not been matched since. No other intracranial pediatric tumor has a widely accepted diagnostic peripheral biomarker capable - in select circumstances - of replacing tissue biopsy in the clinical evaluation algorithm[23]. Even for intracranial germ cell tumors, AFP/βHCG serum and CSF assays can provide false negatives, and research continues as we seek ever more reliable biofuid-based biomarkers.
CSF biomarkers
The most studied and fruitful biofluid target for pediatric oncologic biomarkers is CSF. A normally lowprotein, relatively acellular biofuid[24], CSF is a natural choice for mining biomarker data for central nervous system (CNS) pathologies as it lies in direct contact with the whole of the CNS’s pial and ependymal surfaces.Cells, both pathologic and normal, shed proteins and nucleic acids, be they naked or within extracellular vesicles, into the extracellular spaces. These compounds and vesicles will accumulate within the local biofuid compartments. In the case of the CNS, intravascular spaces, parenchymal interstitial spaces, and the intraventricular/subarachnoid spaces represent the major extracellular compartments where biomarkers can collect. With diffusion into the intravascular spaces being limited by the blood brain barrier (BBB)[25], the CSF and interstitial spaces, theoretically, should be the most rich in cytologic, genetic, and protein markers of tumor growth[16]. Most CSF based biomarker studies have focused on detecting: (1) tumor cells; (2) cellfree tumor DNA; (3) non-coding RNA; or (4) tumor-related metabolites. The authors have already discussed CSF cytology (i.e., detecting tumor cells) in the previous section, so we will only focus on the last three biomarker sources going forward.
Cell-free DNA
Circulating, cell-free tumor DNA (cfDNA) analysis employs several techniques to identify specific genetic sequences or screen for arrays of genetic sequences within biofluids. Methods for detection of genomic alterations in cfDNA are mainly PCR-based assays: real-time PCR, methylation-specific PCR, droplet digital PCR, and next-generation sequencing (NGS). The latter two techniques have particularly improved the sensitivity of cfDNA detection compared to older PCR-based assays[26]. While not specifically confined to use in the CSF, it has been most successful at correlating with brain tumor pathologies when examined in the CSF, due to the fact that non-CSF biofuids typically contain a very small percentage (≤ 1%) of brain tumor genetic material in a sea of normal tissue genetic material[27]. Within CSF, cfDNA has been shown to detect the presence of a CNS neoplasm at rates significantly higher than CSF cytologic analysis[28], as well as identify clinically relevant mutations, even when present at strikingly low frequencies (1:1 × 107), through the use of NGS techniques like duplex sequencing[29].
CSF cfDNA has been shown in several studies of adult intracranial tumor patients to exceed CSF cytologic detection levels when specific mutations are screened for (EGFR for glioblastoma, KRAS for non-small cell lung cancer, BRAF p. V600E for melanoma,etc.)[27,28,30,31]. Among pediatric brain tumor patients,this biomarker has been only sparsely examined and almost exclusively in CSF. Despite cfDNA’s limited utilization, to date, in pediatric brain tumor biomarker development, the authors feel that it bears special discussion, due to its potential as a low-risk diagnostic for midline gliomas.
Midline gliomas represent a one of the most difficult groups of pediatric primary brain tumors to treat.Characterized by a H3K27M histone mutation[32], these tumors are generally aggressive and often not safely surgically accessible[33], making it difficult for clinicians to acquire biopsies. This limits the amount of molecular information available to direct therapy and inform prognosis, and it elevates the clinical need for a safe, reliable method of extracting molecular information from these tumor patients.
Using cfDNA isolated from CSF samples from pediatric brain tumor patients, researchers have made significant progress towards the detection of diffuse midline glioma histone mutations important for prognostication. A study conducted by Huanget al.[34]that reviewed CSF samples from 6 pediatric patients with diffuse midline gliomas found that the H3F3A mutation could be isolated or detected in up to 83% of patients, with a specificity of 100%. This methodology has, subsequently, been confirmed in a larger cohort of 48 patients in a multi-institutional study. In that study by Panditharatnaet al.[35], the authors found that the H3 p. K27M mutations in CSF strongly correlated with the presence of a diffuse midline glioma with the same H3K27M mutation. The amount of detectable genetic material also correlated with the response of that tumor to radiation therapy. The accuracy of the H3 p. K27M cfDNA was 87% in this study. Interestingly,Panditharatna’s group also looked at the presence of the H3 p. K27M mutant genetic material in matched serum samples, as well. They reported comparable sensitivity and sensitivity results with serum as compared to CSF, though with markedly lower quantities of detectable cfDNA[35].
CSF-based cfDNA does have important limitations that have yet to be overcome. While sensitivity for intracranial tumor detection with CSF-based cfDNA is superior to CSF cytology in general, its sensitivity for primary CNS tumors seems to be much less than for extra-CNS neoplasm. In a study of 53 adult patients with intracranial tumors, 62.5% of patients with metastatic CNS tumors had detectable tumor-specific mutant cfDNA in the CSF, while only 50% of patients with primary CNS neoplasms had detectable cfDNA in the CSF[27]. cfDNA detection in the CSF may be limited by anatomic factors, such as tumor location relative to CSF cisterns. A study of 35 pediatric and adult patients with primary brain tumors found that 86% of tumors abutting a CSF cistern had detectable cfDNA in the CSF, but none of the tumors with entirely parenchymal locations had detectable CSF cfDNA[36]. Tumor biology may also limit detection as some authors have noted low to absent rates of cfDNA detection even for some ependymomas with extensive surface contact with CSF[37].
Non-coding RNA
Non-coding RNA, predominantly miRNA, are small (18-25 nucleotides) RNA that function to regulate mRNA pre- and post-translationally. miRNA form from a precursor RNA (pri-miRNA) that is cleaved by the RNase III nuclear enzyme Drosha into a 60-70 nucleotide intermediate known as pre-miRNA, which is then exported from the nucleus via Exportin-5. Once in the cytoplasm, pre-miRNA is cleaved again by Dicer, another RNase III enzyme, into miRNA, which then complexes with a transactivation-responsive RNA binding protein and Argonaute to form an RNA-induced silencing complex[38]. This miRNA-enzyme complex is the active unit at the end of miRNA biogenesis, and it is capable of recognizing complementary mRNA and (1) inhibiting translation initiation via cap-40S association; (2) inhibiting translation initiation via 40S-AUG-60S association; (3) inhibiting elongation; (4) causing premature ribosome drop-off; (5) causing cotranslational protein degradation; (6) causing sequestration of mRNA in P-bodies; (7) causing increased mRNA degradation; (8) increasing mRNA cleavage; or (9) effecting transcriptional inhibition by interaction with promoter sequences or increased methylation of promoters[39].
There are currently > 1,900 known human miRNA (miRbase.org) regulating at least 30% of human genes[40].Table 1 lists some of the more prominent miRNA implicated in pediatric brain tumor biology[41-43]. These molecules are secreted into the extracellular spaces, predominantly in microvesicles, apoptotic bodies,and exosomes. Within these vesicles, the miRNA are protected from degradation by endogenous RNases,making them an unusually stable transcript compared to other forms of RNA. Furthermore, research has shown that extracellular vesicles from tumor patients tend to be particularly enriched with tumor-related miRNA[44,45], and these extracellular vesicles can induced oncogenic protein expression changes in adjacent,normal cells[46].
A myriad of miRNA have been detected in the CSF of adult brain tumor patients, most prominently miR-21,miR-15b, miR-125b, and miR-223[16,44,48,49]. These markers individually or in combination with other miRNA have demonstrated high levels of specificity and sensitivity for gliomas and medulloblastomas[47,49]. However,there are no published articles at the time of this review analyzing the miRNA in the CSF of pediatric glioma, embryonal, or ependymal tumor patients in isolation from adult populations. The composition and clinical applicability of miRNA in the CSF of pediatric brain tumor patients remains a significant knowledge gap, at present, in the field of pediatric neuro-oncology.
Tumor metabolites and proteins
Aberrations in tumor metabolism and cell signaling pathways relative to normal tissues tends to lead to an accumulation of well-characterized small molecules that can be used to predict the presence of a malignancy;and in certain instances, even predict the molecular signature of that malignancy[51]. Dyscrasias have been identified in all of the major metabolic cycles employed by cancer cells, including glucose metabolism, the pentose phosphate pathway, amino acid metabolism, and fatty acid metabolism, as well as many proliferative signaling pathways, such as the PI3K/AKT and WNT/β-catenin pathways[8,16]. Lactate, isocitrate, citrate, and D-2-hydroxyglutarate are metabolites known to accumulate in gliomas utilizing aerobic glycolysis as their dominant ATP source[52]. D-2-hydroxyglutarate, in particular, has been shown to be elevated in the CSF of patients with IDH1 mutant gliomas, and its detection in the CSF may be a surrogate for this prognostically important mutation[53].
As with CSF-based miRNA, very little research has been done examining the use of CSF metabolites to diagnose, track, and molecularly subtype pediatric brain tumors. What work has been done revolves around monoamine metabolism in pediatric brain tumors. In 1987, Bostrom and Mirkin looked at the concentrations of homovanillic acid (HVA), hydroxymethoxyphenylethyleneglycol (MHPG), and vanillylmandelic acid in the CSF of adult and pediatric patients with leukemia, glial, neuroectodermal, or retinoblastoma tumor histories. In their study, MHPG and VMA were significantly elevated among patients with primary CNS tumors or neuroblastoma cranial metastases, suggesting that these metabolites increased in response to disruption of the BBB or mass effect within the CNS[54]. This work was followed in 2014 by a study by Varelaet al.[55]of 22 pediatric patients with posterior fossa astrocytomas, medulloblastomas,and one case of an ependymoma. Ventricular CSF samples and serum samples were acquired and levels of MHPG, HVA, and 5-hydroxyindoleacetic acid were measured. The authors found that MHPG levels were significantly higher (19.4%) in astrocytoma patients versus medulloblastoma patients. However, none of the measured monoamine metabolites significantly varied from age-matched controls in this small study[55].
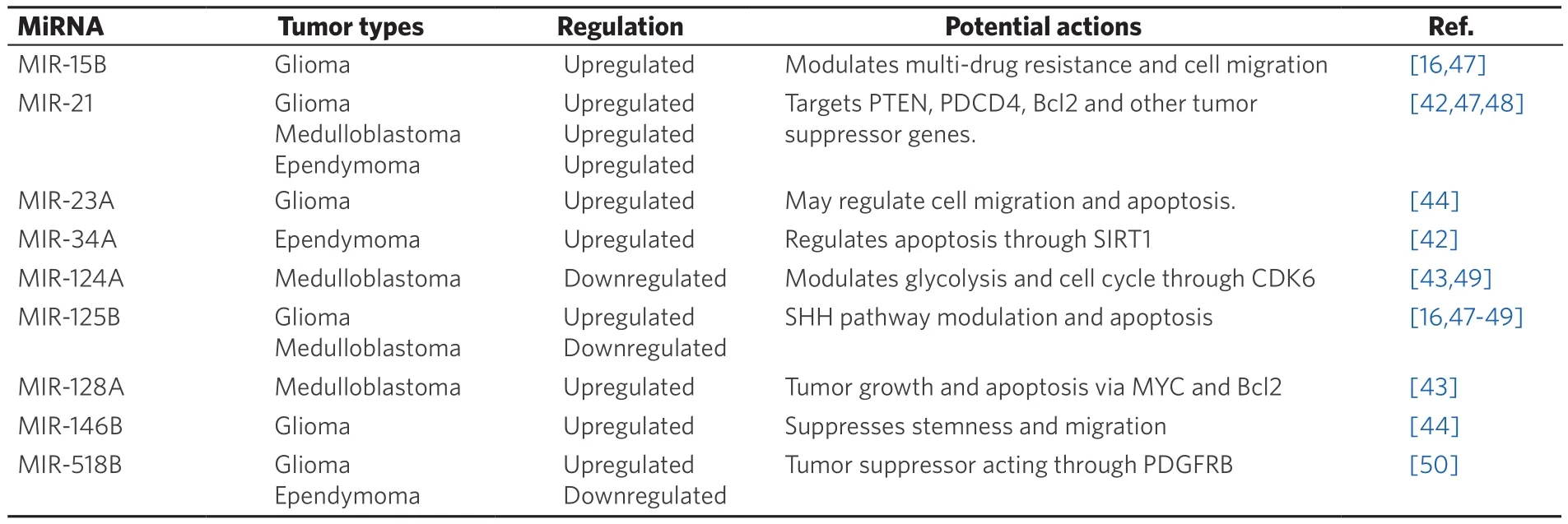
Table 1. Summary of commonly implicated miRNA alterations in pediatric brain tumors
Like tumor metabolite CSF biomarkers, most protein biomarkers investigated in CSF are part of welldefined aberrant tumor cell signaling pathways. One of the most well studied family of proteins infuencing proliferation and differentiation in pediatric brain tumors is the insulin-like growth factor (IGF) family of mitogens, binding proteins, and receptors. The IGF mitogens are primarily synthesized in the liver and circulate in conjunction with any of 7 IGF binding proteins (IGFBP) to promote CNS development, among other functions. They have been implicated in the proliferation and formation of medulloblastomas and ependymomas inin vitroand murine models[56,57], and IGF-1 levels have been shown to correlate with an individual’s propensity for developing cancer[58].
In a study of 35 CSF samples from pediatric patients, mostly medulloblastoma patients, IGFBP-3 levels were found to be significantly elevated in the CSF of medulloblastoma patients compared to control or ependymoma patients. This finding was comparable to mRNA levels for IGFBP-3 in the tumors themselves.The study did have several limitations that tempered the confidence that could be assigned to the results. The levels of IGFBP were overall highly correlated with total CSF protein levels, as has been the case with other CSF-based protein biomarkers for brain tumors[59,60], suggesting that IGFBP may just be an indicator of BBB disruption, rather than a specifically oncologic biomarker. The authors did attempt to normalize IGFBP-3 levels to total CSF protein levels, and the significance of the finding remained. Another issue with the study was that correlation with tumor treatment could not be assessed, as follow-up CSF samples in all patients were taken within 2 weeks of surgical resection, potentially contaminating the samples with tumor cell slough and physiologic changes related to general anesthesia and surgery[61]. Even so, this study suggests that the IGF signaling pathway may be leveraged in the future to identify and track disease in pediatric patients with medulloblastoma.
Another paper by Desiderioet al.[62]examined changes in the CSF proteome of 14 pediatric brain tumor patients and 5 controls from the time of surgical resection and at 6 days post-surgery via top down liquid chromatography-mass spectrometry. The authors found a non-significant trend towards decreased LVV-h7 and VV-h7 hemorphins in the CSF of pediatric patients with brain tumors. In Desiderioet al.[62]’s cohort,the LVV-h7 and VV-h7 protein suppression reversed in the same patients if they underwent a gross total resection of their tumor and had no metastatic tumor burden. These findings have yet to be verified in a follow-up study, and the results included CSF samples within the window of surgical contamination[62].
A similar whole-CSF proteome approach was utilized by Saratsiset al.[63]to search for biomarkers predictive of diffuse intrinsic pontine gliomas. In this study, 55% of patients with diffuse intrinsic pontine gliomas demonstrated elevated levels of cyclophilin A in their CSF, while no patients with alternate supratentorial gliomas or control patients had meaningfully detectable levels of the same protein. Those patients with elevated cyclophilin A levels also showed a greater tendency towards radiographic evidence of necrosis and rapid tumor progression, suggesting that cyclophilin A may be indicative of more aggressive forms of diffuse intrinsic pontine glioma[63].
Lastly, a study of 33 pediatric medulloblastoma patients and 25 age matched controls in a multi-centered study of prostaglandin D2 synthase (PGD2S), a highly abundant glycoprotein found in CSF, found a 6-fold reduction in PGD2S levels in medulloblastoma patients compared to controls. This was a highly significant finding (P< 0.00001). The authors theorized that this may be related to a host response to the tumor, as it has been described in other brain tumor patients and in some demyelinating diseases. PGD2S CSF levels remained suppressed after tumor patients went into remission, so it is unlikely that this protein would be useful for longitudinal screening of pediatric brain tumor patients[64].
Limitations to CSF-based biomarkers
While relatively minimally invasive, accessing the lumbar cisterns in order to collect CSF samples is still a more difficult affair than phlebotomy, saliva collection, or urine sampling. The procedures involved in CSF sampling - lumbar puncture or ventricular puncture - require skilled, advanced practice medical personnel with knowledge of basic anatomy and sterile technique. In the case of pediatric patients, sedation is sometimes required in order to perform a lumbar puncture, and it is mandatory for ventricular access procedures. These technical difficulties currently limit the availability of patient CSF as a routine diagnostic medium and will likely frustrate attempts to use CSF-based biomarker assays for screening large populations of pediatric patients. This limits the availability of patient CSF as a routine diagnostic medium.
As has been stated earlier, CSF may also be an inappropriate source of biomarker molecules for more parenchymal based tumors, where tumor cells do not make direct contact with ependymal or pial surfaces and are, therefore, less likely to release genetic material and small molecules into CSF. In reality, 70% of pediatric brain tumors will arise in or near the 4th cerebral ventricle, making this potential limitation applicable to a minority of cases[65].
In the same vein, though, the tendency of pediatric brain tumors to arise near the 4th ventricle in the posterior fossa makes lumbar puncture more perilous for patients, as drainage of CSF from the lumbar cistern, rarely, can create a pressure gradient across the infratentorial compartment in the setting of a large mass lesion. These pressure gradients, in severe cases, can lead to brainstem and cerebellar herniation through the foramen magnum, causing significant neural injury and even death[66]. For this reason, routine lumbar punctures for the purposes of oncologic screening may not be deemed safe in many pediatric brain tumor patients.
Serum biomarkers
While CSF can be problematic to acquire, more peripheral biofuids, such as serum, urine, and saliva, can and are easily acquired from pediatric patients in ambulatory settings every day. In this sense, they are far more practical sources for pediatric brain tumor biomarker discovery than CSF; but discoveries in these media must be approached cautiously, as biomarkers in these biofuids are subject to dilution and filtration at multiple points between synthesis and their collection sites, sometimes greatly compromising the fidelity of the observed biomarker concentration[67-69].
That being said, serum remains the most successful source of clinically relevant biomarkers in practice today. Examples of serum biomarkers in clinical use include prostate specific antigen, AFP, βHCG, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and carcinoembryonic antigen[70,71]. As is the case in adult oncology, most research into pediatric brain tumor peripherally circulating biomarkers has focused on protein biomarkers, similar to those just listed, but miRNA are also beginning to show promise as potentially specific and easily measured diagnostics for detecting, diagnosing, and tracking pediatric brain tumors. Due to their relatively rapid turnover in the peripheral blood stream, DNA and RNA based biomarkers are particularly well suited for gauging tumor response to therapy[72].
Non-coding RNA
As with CSF, serum miRNA and other forms of non-coding RNA have been targeted for potential diagnostic and screening purposes in neuro-oncology. miR-21, miR-125b, miR-128, miR-15b, and miR-182 have all been implicated in adult glioma studies of miRNA signatures within patient sera[73]. Serum miR-21 and miR-15b,in particular, have been shown to strongly predict glial tumors in adult patient populations, with combined sensitivities and specificities of 100% in a series of 112 patients with gliomas, non-small cell lung cancer,and controls[74]. Numerous studies examining serum miRNA signatures in gastric cancer, hepatocellular carcinoma, lung cancer, and breast cancer have also demonstrated the potentially powerful predictive value of serum miRNA in screening, risk-stratifying, and tracking cancer patients[75-79].
In the realm of pediatric neuro-oncology, however, serum miRNA have only begun to be leveraged for tumor detection and screening. The first study to examine the potential utility of serum miRNA in detecting and screening pediatric brain tumors was done by this author and their colleagues (Booklandet al.[41]) in a small cohort of pediatric juvenile pilocytic astrocytoma, ependymoma, and control patients. The authors identified four miRNA (miR-21, miR-15b, miR-23a, and miR-146b) that accurately predicted the presence,tumor nodule size, and response to therapy of juvenile pilocytic astrocytoma patients with a sensitivity of86% and specificity of 100%. In particular, levels of miR-15b, miR-21, and miR-23a correlated strongly with tumor nodule volume (miR-15b, R2= 0.86; miR-21 R2= 0.92; miR-23a, R2= 0.86) and returned to normal levels within 24 h of gross total tumor resection. Interestingly, patients with tumors situated more deeply within the brain parenchyma demonstrated higher ratios of miRNA to tumor nodule volume than those more peripherally situated[41]. These results further support the notion that oncologic miRNA serum levels may be affected by tumor anatomic location, just has been speculated with CSF biomarkers [Figure 1].
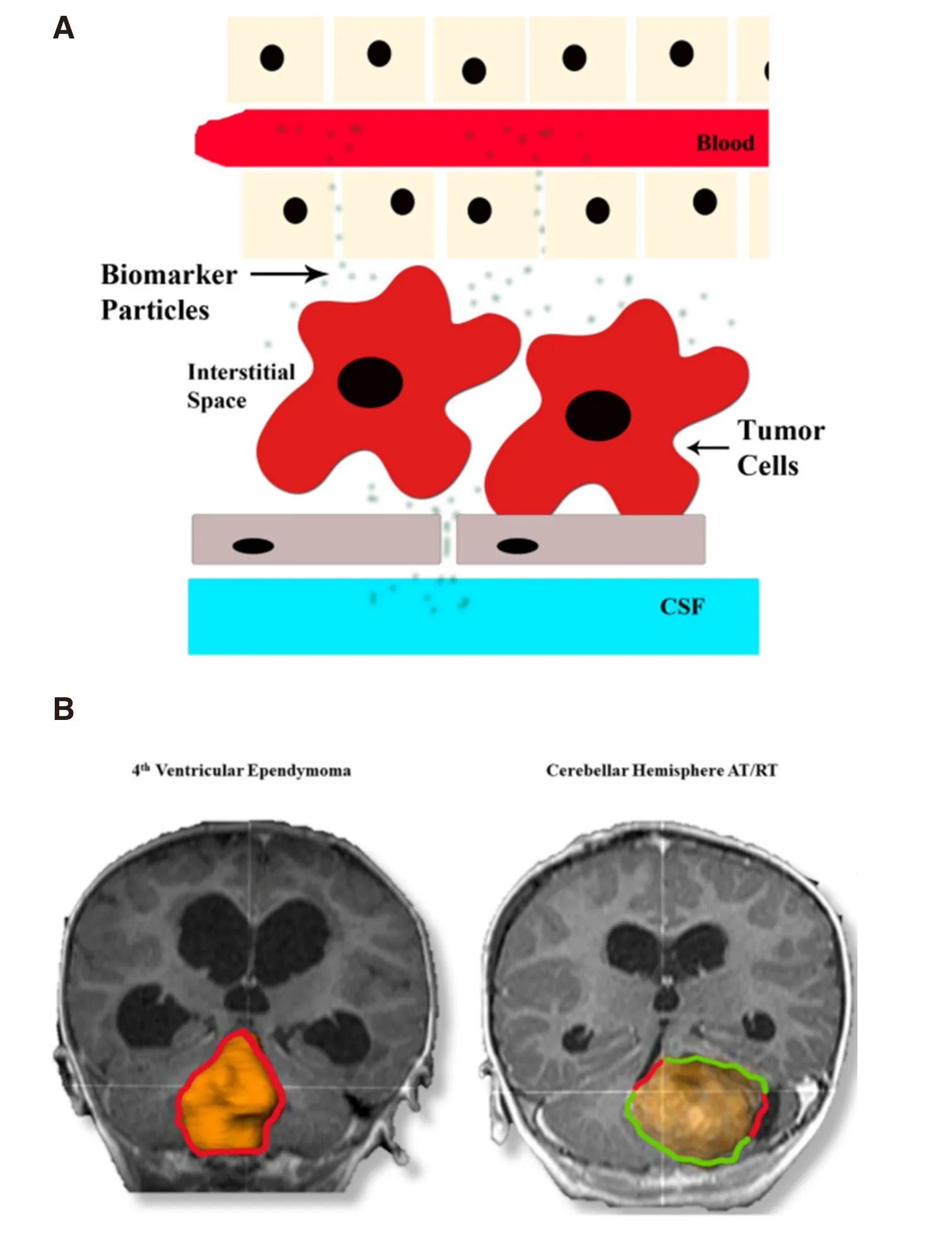
Figure 1. A: Schematic of diffusion of tumor nucleic acids, proteins, metabolites, and microvesicles into various extracellular compartments; B: Representative MRi images of an intraventricular (left) and mainly parenchymal (right) tumor with cerebrospinal fluid(CSF) contact points denoted in red and parenchymal contact points denoted in green
This pilot study evaluating the potential utility of serum miRNA as a biomarker for pediatric brain tumors is encouraging, but this initial work also highlighted significant barriers to serum miRNA development,including: (1) normalization of miRNA profiles; (2) low miRNA yields compared to tissue or CSF sources;(3) and a poor understanding of relative contributions tumor and host tissues make to the final serum miRNA signatures[41,71]. One significant example of these challenges can be demonstrated in observing the effect that general anesthesia has on select serum miRNA levels. miR-125b, which has been implicated as an oncomir in gliomas and medulloblastomas[80,81], is suppressed in humans and rodents after exposure to general anesthetic agents[41,82]. Yet, few miRNA biomarker studies have controlled for the effects of anesthesia miRNA levels. Indeed, controlling for anesthesia in pediatric clinical trials is an ethically and technically complicated matter that adds a layer of complexity to serum miRNA interpretation of which clinical researchers must be cognizant. This example highlights the importance of developing a thorough understanding of the interplay between oncologically-relevant miRNA and the normal functions they regulate so that confounding host and external forces acting on miRNA of interest can be controlled.
Protein/small molecule
As mentioned previously, protein and small molecule serum biomarkers have been extensively studied,and they represent, perhaps, the most significant cohort of potential biomarkers for pediatric brain tumors.An exhaustive list of all proteins and small molecules implicated in pediatric brain tumor diagnostics and prognostics is beyond the scope of this brief review, but we will touch on some of the more widely reported biomarkers in the literature.
Osteopontin is an extracellular matrix protein expressed in a wide variety of tissues and involved in mineralization, immune modulation, cell migration, and anti-apoptosis[83]. It has been shown to be overexpressed in atypical teratoid/rhabdoid tumors (AT/RT), as well as several other CNS tumors. In a series of 39 pediatric patients with AT/RT, medulloblastoma, epilepsy, or hydrocephalus, serum and CSF levels of osteopontin were shown to be significantly elevated in AT/RT patients versus medulloblastoma patients (~2:1 in serum) and versus control patients (~4:1 in serum). Serum osteopontin levels also correlated with AT/RT response to therapy, and higher serum levels were associated with a more malignant disease course[84].This work raises the possibility that osteopontin may be a highly specific and reliable biomarker for riskstratifying AT/RT patients and gauging their response to therapy.
Another protein involved in a variety of carcinogenic signaling pathways, including proliferation,immunomodulation, migration, anti-apoptosis, and chemotherapy resistance, is metallothionein. Different isomers of metallothionein have been implicatedin vitroin inducing chemoresistance in neuroblastoma,melanoma, rhabdomyosarcoma, and medulloblastoma cell lines[85-87]. A group of researchers, using differential pulse voltammetry to measure serum metallothionein levels, attempted to exploit metallothionein’s association with oncogenesis to see if it could be reliably detected at supernormal levels in pediatric solid tumor patients,including in 10 medulloblastoma patients and 4 ependymoma patients. All 38 pediatric solid tumor patients in this study had serum levels of metallothionein roughly 6-8-fold higher than adult controls (P< 0.05)[88]. This work strongly suggests that serum metallothionein may be useful as a non-specific oncologic biomarker in pediatric patients, though additional work is needed to confirm the results with age-matched controls.
A larger study of 106 pediatric patients with brain tumors, including gliomas, medulloblastomas, and ependymomas, studied the relationship between serum levels of the pro-angiogenic growth factors and pediatric brain tumors. In this study, serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) levels were measured at presentation and following surgical resection or debulking of the tumor. While the authors of the study found no statistically significant changes in bFGF levels between groups, serum VEGF was 16.4% higher (P= 0.05) in pediatric brain tumor patients compared to controls.Interestingly, subset analysis of only the glioma patients showed that serum VEGF levels were 16.4% lower(P< 0.05) than controls[89]. This is a curious result given glioblastoma and other high-grade glial tumors are known to overexpress VEGF[90]. The authors also found no statistically significant difference in serum VEGF or bFGF levels in any of the post-surgical patients, regardless of the degree of tumor resection achieved[89].These results are, in the end, difficult to reconcile; and this may refect the extremely diverse collection of pediatric brain tumors included.
Behrendset al.[91]in a study of 40 pediatric cancer patients, including 10 brain tumor patients, employed a less conventional method to identify humoral targets for biomarker development. In contrast to other biomarker development projects that rely on a priori knowledge of cell signaling proteins known to be abnormal in the disease process of interest, Behrend’s group used SEREX technology to identify potential humoral targets, in some cases finding cancer antigens with no described function. The group utilized autologous sera and serially screened these sera against autologous cDNA expression libraries in 4 pediatric medulloblastoma patients. From this, the group identified 15 antigens. Humoral responses to these 15 antigens were then tested in the 40 pediatric cancer patients, as well as in 40 pediatric controls. Antibodies were found to 5 of the 15 antigens exclusively in pediatric cancer patients. The authors noted, though, that the humoral responses to these antigens was not uniform. Only 2-3 out of 5 medulloblastomas had detectable antibodies to any one antigen, and the humoral responses varied over time depending on response of the tumor to therapy. The authors also point out that humoral responses may change, as well, due to mutations in some tumors that support immune evasion[91]. Taken in balance, SEREX screening for humoral biomarker targets is an innovative technique for identifying novel pediatric brain tumor biomarkers, but clinical application is likely to be limited by the marked variability in humoral response across patients.
Limitations to serum biomarkers in pediatric brain tumor patients
While familiar and easily accessible, serum sources for biomarkers do have significant drawbacks. As a peripheral biofuid, the milieu of serum proteins and genetic material is affected by every organ system in the human body. Even large amounts of oncologic biomarkers can easily be diluted out by normal serum components[35]. Additionally, it is difficult to know what sources - host or tumor - are driving the targeted biomarker concentration levels. Studies have shown that even epigenetic factors, such as a change in diet,can significantly alter measured levels of some serum biomarkers for adult cancers[38]. As with CSF, the location and biology of pediatric brain tumors also seems to play a part in serum levels of some presumptive biomarkers, with certain tumor-related proteins being undetectable outside of the BBB[61,63].
Urine
Urine is a filtrate of serum via the glomeruli, and it represents a particularly easy and well-studied biofuid to obtain from children. Excreted oncologic biomarkers such as metanephrine and VMA have been employed clinically for decades as routine components in the work-up of several catecholamine producing solid tumors, including pheochromocytomas and neuroblastomas in both children and adults[92].
A paper by Pricola Fehnelet al.[93]in 2016 examined the use of urinary bFGF, MMP13 and TIMP3 levels as biomarkers for juvenile pilocytic astrocytomas. The study followed 21 astroctyoma, 17 medulloblastoma,and 21 control pediatric patients with serial urine samples pre-tumor treatment and post-tumor treatment.The authors also examined bFGF and TIMP3 levels in primary juvenile pilocytic astrocytoma cell line conditioned media. They found a significant elevation in bFGF and TIMP3 urine concentrations among the astrocytoma patients, with a combined sensitivity and specificity of 100% and 95%, respectively. Conditioned media from cultured primary astrocytoma cells also showed relative enrichment of bFGF and TIMP3,concordant with the findings in patient urine samples. Among 9 patient samples where pre- and posttreatment imaging was available, bFGF and TIMP3 levels also significantly correlated with tumor volume changes on imaging[93].
Another, earlier project from the same group looked at VEGF, MMP2, and MMP9 in urine and CSF samples from a cohort of 28 brain tumor patients (n= 11 pediatric,n= 17 adult) and 23 control patients. Urinary concentrations of VEGF, MMP2 and MMP9 were all significantly increased within the tumor patient cohort;and urinary VEGF and MMP2, in particular, had excellent sensitivity and specificities for the presence of a brain tumor. VEGF had 95.2% sensitivity and 89.5% specificity, while MMP2 demonstrated 82.1% sensitivity and 95.7% specificity. Both of these proteins were undetectable in the urine of 5 patients for whom follow-up imaging demonstrated complete resolution of their tumors[94].
Both of these studies are very encouraging for the prospect of developing a clinically deployable and accurate laboratory assay for pediatric brain tumors. However, as with all biomarker sources, urine has special limitations as a diagnostic media that have still not completely been addressed in either of the aforementioned studies. Nolenet al.[69]laid out in an extensive analysis of over 200 potential urinary biomarkers the effects of normalization methods, population variability, and temporal variability on measured biomarker concentrations. The authors of this study found that, while urine total protein had the smallest impact on biomarker variability, none of the normalization methods tested (urine creatinine,urine albumin, urine B2M) was clearly superior to the other. Additionally, the authors observed significant intra- and inter-day variability in urine biomarker concentrations; in most cases, the coefficients of variation exceeded 50%[69]. These findings should prompt caution when interpreting urinary biomarker data, as robust differences in biomarker concentrations between diseased and normal states will be needed to consistently overcome such high levels of biomarker variability. Even so, the work by Pricola Fehnelet al.[93]suggests that such high levels discrimination may indeed exist for some pediatric brain tumor urinary biomarkers.
Challenges of developing biomarkers from pediatric populations
Compared to the adult neuro-oncology world, the development of circulating biomarker candidates for pediatric brain tumor patients is still in its infancy. This is partially due to a significant statistical barrier to research within pediatric brain tumor patient populations. There are an estimated 4,600 new pediatric brain tumor diagnoses each year in the United States of America[1]. This compares to an average of 22,172 new adult primary brain tumor diagnoses per year[95]. As these statistics demonstrate, pediatric brain tumor cases are relatively rare, and individual institutions often do not have enough volume to organize large scale studies on their own. Multi-centered trials and novel screening assays are needed in order to create study populations with enough power to generate meaningful conclusions.
Another issue is the lack of comparative data across biofluids and pediatric brain tumor clinical stages for individual biomarker candidates. As mentioned in this review article, there exist significant theoretical and practical limitations to each biofuid currently targeted for biomarker discovery. Additionally, there is mounting evidence that tumor stage and location relative to the biofuid of interest can alter biomarker yields[36,41,96]. A large study examining one or more biomarkers in multiple biofuids serially across early and late stages of brain tumor therapy will be needed to answer the question of which biofluid is most suitable for diagnosing and screening which pediatric brain tumors. This is a daunting ask given the difficulties researchers face accruing and maintaining large population pediatric brain tumor studies. A study by Pageset al.[97]is currently underway examining the cfDNA in the CSF, serum, and urine of 192 pediatric brain tumor patients, but results are not available as of the production of this manuscript. It is hoped that this study and others to come like it may help to define the effect of biofuid choice on biomarker sensitivity and specificity for pediatric brain tumors.
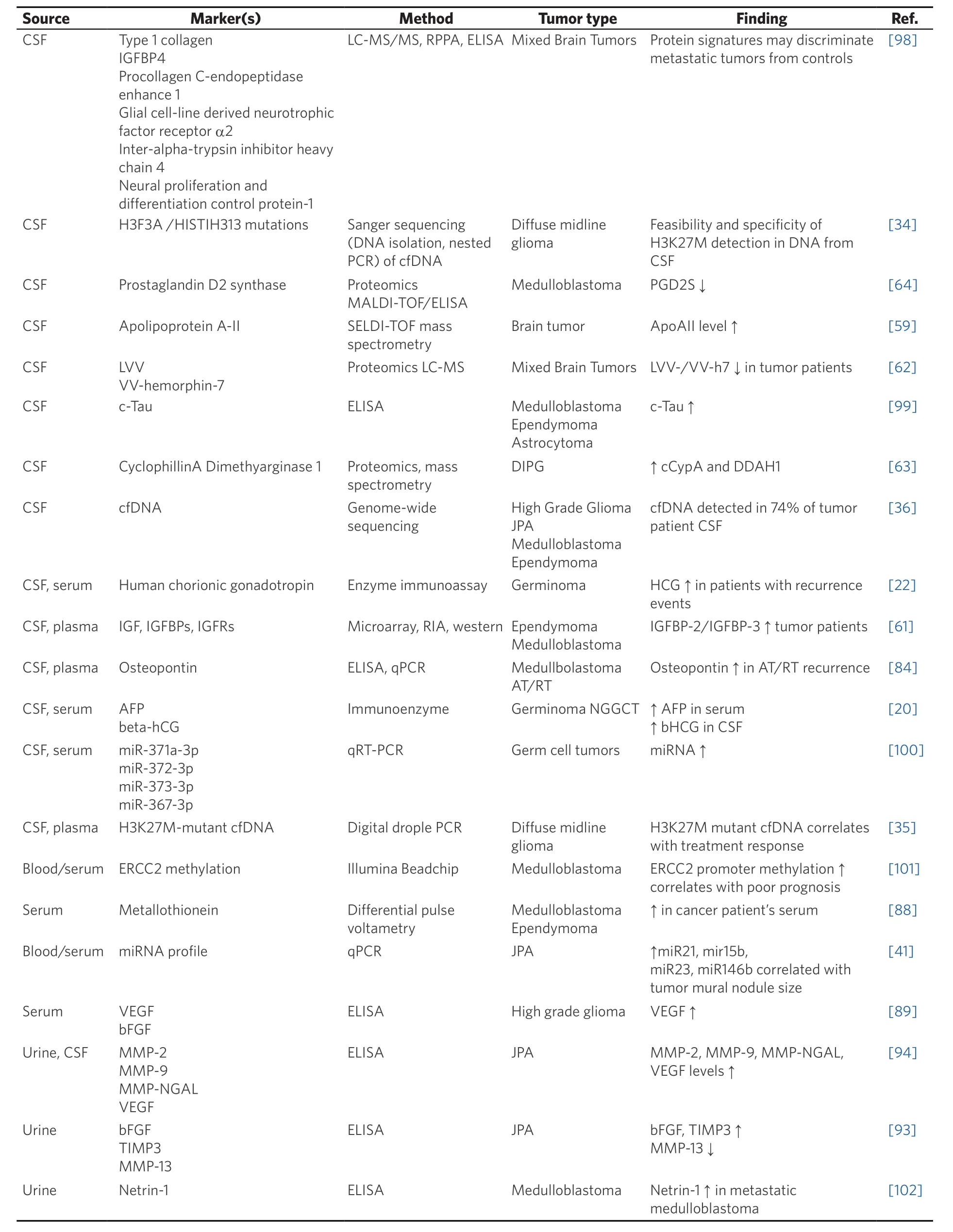
Table 2. Summary of select works identifying circulating biomarkers for pediatric brain tumors
CONCLUSION
Pediatric brain tumors remain the most significant oncologic cause of death among children, and pediatric brain tumor diagnosis and longitudinal screening is heavily reliant on MRI - a very limited and expensive screening tool. The creation of reliable and easily deployed biomarker-based assays could dramatically improve pediatric brain tumor detection. Early detection could, in turn, improve current care by allowing for early surgery. Patient therapy could also be tailored based on biomarker-directed tumor risk stratification.
While there are currently many circulating biomarker candidates in CSF, serum, and urine, all the studies to date are hobbled by small cohort size and often mixed populations of tumor types [Table 2]. Follow-up multi-centered studies have failed to materialize for most biomarkers, leaving the validity of virtually all of these candidate pediatric brain tumor biomarkers in doubt. Hopefully, with continued interest, time, and collaboration, these current research needs will be met.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the review and performed data collection:
Bookland MJ
Performed data acquisition, as well as provided administrative, technical, and material support: Kolmakova A
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conficts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年4期
Journal of Cancer Metastasis and Treatment2019年4期
- Journal of Cancer Metastasis and Treatment的其它文章
- AUTHOR INSTRUCTIONS
- Cancer stem cells in liver metastasis from colon adenocarcinoma express components of the reninangiotensin system
- Breast cancer, metastasis, and the microenvironment: disabling the tumor cell-tostroma communication network
- Sensitive and specific detection of circuIating tumor ceIIs promotes precision medicine for cancer
- Targeting autophagy with small molecules for cancer therapy
- Breast cancer metastasis to the stomach
