Expression and characterization of a bifunctional alginate lyase named Al163 from the Antarctic bacterium Pseudoalteromonas sp. NJ-21*
XIE Maisheng (謝買勝) , LI Jiang(李江) , , HE Peiqing (何培青) , LIN Xuezheng (林學(xué)政) ,
1 Key Laboratory of Marine Bioactive Substances, The First Institute of Oceanography, State Oceanic Administration (SOA),Qingdao 266061, China
2 Key Laboratory of Marine Natural Products of Qingdao, Qingdao 266061, China
Abstract In this study, an endolytic alginate lyase, named Al163, was identi fied, cloned, and characterized from the Antarctic bacterium Pseudoalteromonas sp. NJ-21. Comparative sequence analysis showed that the predicted amino acid sequence encoded by al163 belongs to the polysaccharide lyase 6 (PL-6) family and has a molecular mass of about 80 kDa. Recombinant enzyme was puri fied by Ni-Sepharose affinity chromatography. Recombinant Al163 exhibited maximum activity (258 U/mg) at pH 7.0 and 40° C, and thermal stability assays showed retention of almost 90% activity after incubation at 30° C for 30 min. Al163 activity was stimulated by Cd 2+, Ca 2+, Fe 3+, and Mn 2+, but inhibited by Cu 2+, Si 2+, Fe 2+, and Ni 2+. Thin-layer chromatographic analysis indicated that Al163 degraded sodium alginate, polyM, and polyG, generating disaccharides and trisaccharides as the final products. Only a few bacterial strains that produce a bifunctional alginate lyase have been reported. Our results indicate that recombinant Al163 exhibits broad substrate speci ficity and its products exhibit low degrees of polymerization. Both properties imply high potential for use of the enzyme in several industrial fields, including cosmetics and pharmaceuticals, based on the high demand for biologically active oligosaccharides.
Keyword: Pseudoalteromonas sp.; alginate lyase; PL-6 family
1 INTRODUCTION
Alginate is the main component of the cell wall of brown algae and is comprised of α-L-guluronic acid(G) and its C5 epimer β-D-mannuronic acid (M) as the basic framework (Garron and Cygler, 2010).Alginate is also a component of some bacterial bio films (Haug et al., 1967; Kim et al., 2011). Alginate has many potential applications in the food and pharmaceutical industries because of its high viscosity and gelling properties (Laurienzo, 2010). Alginate oligosaccharides are formed from alginate by alginate lyase through a β-elimination reaction, which produces unsaturated oligosaccharides with double bonds at the non-reducing end. Alginate oligosaccharides exhibit various physiological activities, including promotion of root growth in higher plants (Tomoda et al., 1994; Xu et al., 2003),acceleration of Bi fidobacterium sp. growth rate(Akiyama et al., 1992), enhancement of penicillin production in Penicillium chrysogenum (Ariyo et al.,1998), promotion of macrophage and keratinocyte proliferation and endothelial cell viscosity (Kawada et al., 1999; Iwamoto et al., 2003, 2005; Yamamoto et al., 2007), and lowering of blood pressure in humans.Therefore, the production of value-added oligosaccharides from alginate via alginate lyase is an attractive prospect, especially given the ability of this enzyme to directly degrade fronds of brown seaweed to release alginate oligosaccharides and convert elastic seaweed fronds into slurry-like homogenates(Inoue et al., 2014).
Alginate lyases are classi fied into two groups based on their substrate speci ficity (polyG lyase, EC4.2.2.11 and polyM lyase, EC4.2.2.3) (Zhu et al., 2015) or mode of action (endolytic or exolytic) (Wong et al.,2000). Because the oligosaccharide products of alginate lyase exhibit many types of bioactivity, a source from which highly-active enzyme can be produced affordably and on a large scale is needed.
Antarctica is too cold and dry for the survival of most organisms; however, there are multiple coldadapted microorganisms there that make essential contributions to nutrient recycling and organic matter mineralization using a special class of extracellular cold-adapted or cold-active enzymes. Enzymes from Antarctic microorganisms exhibit special properties,including high catalytic efficiency and excellent thermal stability at temperatures below 20° C when compared with those from other microorganisms.These enzymes have potential applications in industrial processes that require reactions at lower temperatures; therefore, the mechanisms associated with cold-adapted or cold-active enzymes have been a focus of study (Carrasco et al., 2012). However,studies of alginate lyases from Antarctic cold-adapted bacteria are scarce, and information on their sequence,structure, and function is required if their industrial potential is to be realized. It is anticipated that such enzymes with high activity and excellent thermal stability could be used to produce alginate-derived oligosaccharides.
In this study, we sequenced, cloned, and characterized an alginate lyase gene, al163, and puri fied and characterized the recombinant protein (Al163) from the Antarctic bacterium Pseudoalteromonas sp. NJ21.The biochemical properties of the recombinant Al163,including its speci fic activity and optimal reaction conditions, were determined, and the enzymatic products were characterized.
2 MATERIAL AND METHOD
2.1 Material
The bacterial strain NJ-21 was isolated from sediment samples from the Antarctic region(75° 45.9′E, 68° 5.65′S) and belongs to the genus Pseudoalteromonas. Escherichia coli strains DH5α and BL21 and vectors pUC57 and pET-30a(+) were purchased from GenScript (Nanjing, China). Sodium alginate, agar, agarose, chondroitin B, kanamycin,isopropyl β-D-1-thiogalactopyranoside (IPTG),dinitrosalicylic acid, and other reagents were purchased from Solarbio (Beijing, China). Tryptone and yeast extract were purchased from OXOID(Basingstoke, UK). PolyM and polyG were provided by the Ocean University of China.
2.2 Sequence analysis and gene cloning
Sequencing and annotation of the Pseudoalteromonas sp. NJ21 genome were performed by Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China)in our preliminary work (data not shown). The al163 sequence was ampli fied from the genomic DNA of Pseudoalteromonas sp. NJ21 by polymerase chain reaction (PCR) using primers al163-F (5′-ACTTGCCATGCCTATTTGCA-3′) and al163-R (5′-TCGTAGGTCACATTTGAGCTT-3′). The PCR products were puri fied and ligated into the pUC57 cloning vector. The al163 sequence was determined by GenScript, and the open reading frame was analyzed using DNAStar Lasergene (v7.1; DNASTAR, Madison, WI, USA).The al163 nucleotide sequence has been deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/)under accession number KF700697.
2.3 Construction of expression plasmid and puri fication of alginate lyase Al163 from E. coli
For al163 ligation into an expression vector, primer pair F 5′-CGCGGATCCACTTGCCATGCCTATTTGCA-3′ (including a BamH I restriction site, in bold letters) and R 5′-CCG CTCGAG TCGTAGGTCACATTTGA-GCTT-3′ (including an Xho I restriction site,in bold) was designed and synthesized with no stop codon and used to amplify the gene by PCR. The ampli fied fragment was inserted into the pET-30a(+)expression vector, which was used to transform E. coli BL21 (DE3) cells. Escherichia coli cells harboring the pET30a-Al163 vector were cultured at 37° C for 2–3 h in Lauria-Bertani medium. IPTG was added to a final concentration of 0.5 mmol/L and the cells were cultured at 16° C for an additional 20 h. Cells were pelleted by centrifugation at 12 000× g at 4° C for 20 min, washed with 50 mmol/L phosphate buffer (pH 7.5), resuspended in the same buffer, and then disrupted by sonication (Constant Systems Ltd., Daventry, UK).The disrupted cells were centrifuged at 12 000× g at 4° C for 15 min and dialyzed against 50 mmol/L phosphate buffer (pH 7.5) for 48 h. The dialyzed sample was freeze-dried, dissolved in binding buffer[50 mmol/L Tris-HCl (pH 8.0), 300 mmol/L NaCl, and 10 mmol/L imidazole], and loaded onto a Ni-Sepharose column (GE Healthcare, Uppsala, Sweden). The enzyme was eluted with a linear gradient of imidazole(20–500 mmol/L) in 50 mmol/L Tris-HCl (pH 8.0)and 300 mmol/L NaCl. Active fractions were desalted using an ultra filtration device with a 30-kDa cut-off(Merck Millipore, Darmstadt, Germany) and analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of the puri fied enzyme was determined using the Bradford method with bovine serum albumin as a standard (Bradford, 1976).
2.4 Determination of recombinant Al163 enzyme activity
The activity of alginate lyase Al163 was measured using the dinitrosalicylic acid method (Miller, 1959).Brie fly, puri fied Al163 (10 μ g) was dissolved in 0.1 mL 20 mmol/L sodium phosphate buffer (pH 7.0)and mixed with 0.9 mL 20 mmol/L sodium phosphate buffer (pH 7.0) containing 0.2% alginate, and the reaction system was incubated at 40° C for 15 min.Al163 activity was analyzed by measuring the mass of reducing sugar released by the reaction system.One unit of Al163 enzyme was de fined as the amount of enzyme required to produce 1 μg of reducing sugar per min.
2.5 Characterization of the biochemical properties of recombinant Al163
The optimal temperature for Al163 activity was determined in the reaction system from 0°C to 60° C in 20 mmol/L phosphate buffer (pH 7.0). Al163 thermal stability was investigated by incubating puri fied enzyme for 30 min at temperatures from 0°C to 60° C, followed by analysis of enzyme activity at 40° C. The optimal pH was evaluated by performing enzyme assays in different buffers (20 mmol/L):acetate buffer (pH 4–5), phosphate buffer (pH 6–8),glycine-NaOH buffer (pH 9–10), and Na2HPO4-NaOH buffer (pH 11). The pH stability of Al163 was determined according to the residual activity observed following incubation in the different buffers at different pH values (4.0–11.0) at 4° C for 3 h using the reaction system described above. To investigate the in fluence of various compounds on enzyme activity,10 μg puri fied Al163 was incubated in solutions containing 5 mmol/L of MnCl2, FeCl3, CaCl2, SDS,MgCl2, ZnCl2, KCl, NiCl2, CuCl2, or ethylenediaminetetraacetic acid (EDTA) in 1 mL of 20 mmol/L phosphate buffer (pH 7.0) containing 0.2% alginate at 40°C for 15 min. Enzyme activity was measured as described, with a reaction system lacking the compound used as a control. The in fluence of NaCl on enzyme activity was measured in buffers containing different concentrations of NaCl (0- 60 g/L) in standard enzyme activity assay conditions. An assay using a buffer lacking NaCl was used as a control.The highest activity was de fined as 100%.
2.6 Substrate speci ficity and kinetic parameters of recombinant Al163
To determine the substrate speci ficity of Al163,0.2% sodium alginate, polyM, polyG, agar, agarose,and chondroitin B were tested as substrates, and standard enzyme activity assays were performed as described in Section 2.4. The kinetic parameters of the recombinant Al163 with sodium alginate, polyM, and polyG as substrates were evaluated by measuring the initial rate of the reaction at different substrate concentrations (0.05- 0.5 mg/mL). Kmand Vmaxvalues were determined using double-reciprocal (Lineweaver-Burk) plots.
2.7 Analysis of the products of recombinant Al163
The activity of Al163 was measured using alginate,polyM, and polyG. The reaction system (1 mL),including 100 μg puri fied Al163 and 2 mg substrate,was incubated in phosphate buffer (pH 7.0) for 0–360 min at 40° C. The reaction was stopped by heating to 100° C for 5 min, followed by centrifugation at 12 000× g for 5 min to eliminate inactive enzyme.After centrifugation, 2 μL of the supernatant was loaded onto a thin-layer chromatography TLC-60 plate (Merck Millipore) and developed with a solvent comprising 1-butanol, ethanol, and water (2:1:1,v:v:v). After development, the plate was stained by spraying with a color-developing reagent (100 mL acetone, 2 g diphenylamine, 2 mL aniline, 10 mL 85%phosphoric acid, and 1 mL concentrated HCl) and dried at 110°C for 15 min in an oven.
3 RESULT
3.1 Cloning and sequence analysis of alginate lyase Al163
The Pseudoalteromonas sp. NJ-21 al163 gene encoding alginate lyase Al163 was successfully cloned and sequenced using speci fic primers based on the nucleotide sequence of al163. The al163 sequencecontained a 2 322-bp open reading frame (GenBank:KU360250) encoding a 773-amino-acid polypeptide that lacked a signal peptide. The translated Al163 sequence had a calculated molecular mass of 84.61 kDa and a pI value of 5.38. A BLAST search of the NCBI database (https://www.ncbi.nlm.nih.gov/)revealed the translated Al163 sequence shares 98%amino acid sequence identity with the putative polyM lyase from Paraglaciecola mesophila KMM241(GenBank: GAC22931) (Fig.1).

Table 1 Puri fication of recombinant Al163
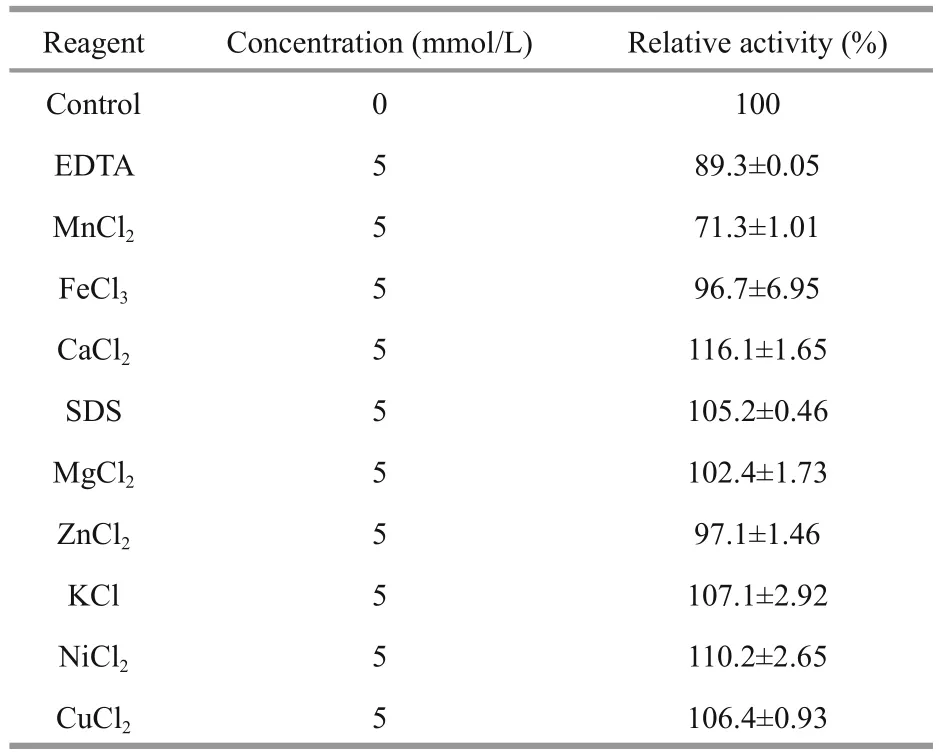
Table 2 In fluence of various compounds on Al163 activity
Sequence analysis revealed that residues 57 to 426 share high sequence identity with other members of the polysaccharide lyase 6 (PL-6) superfamily, and,interestingly, residues 57 to 458 also share high sequence identity with chondroitin B lyase, whereas residues 524 to 705 match a domain of unknown function (DUF4957). Multiple alignment of the Al163 N-terminal region with AlyGC from Glaciecola chathamensis S18K6T(GenBank: BAEM00000000.1)(Xu et al., 2017), KJ-2 alginate lyase from Stenotrophomonas maltophilia (GenBank:AFC88009.1) (Lee et al., 2012), AlyP from Pseudomonas sp. OS-ALG-9 (GenBank: BAA01182.1)(Maki et al., 1993), OalS6 from Shewanella sp. Kz7(GenBank: AHC69713.1) (Li et al., 2016), BBFL7 alginate lyase from Flavobacteria (GenBank:WP006795620.1), and chondroitin B lyase 1DBG_A from Pedobacter heparinus (GenBank: ACU03011.1)revealed a putative calcium-binding site and other residues potentially important for enzyme function(Fig.2).
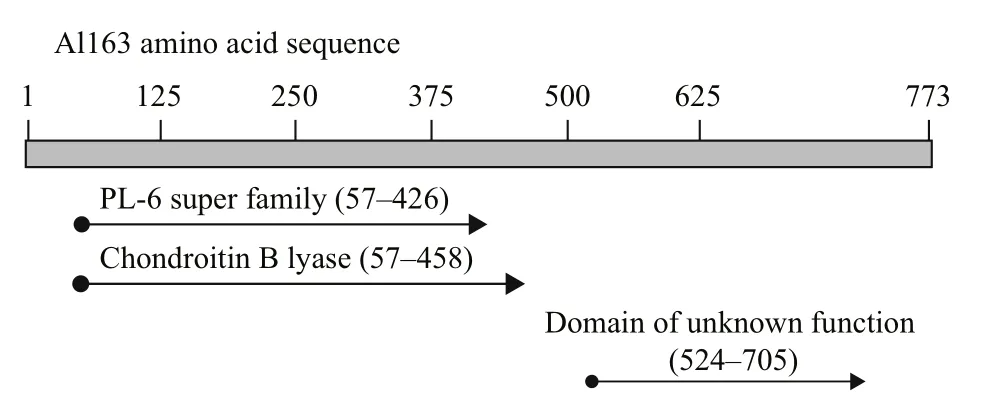
Fig.1 Structure of the translated Al163 amino acid sequence
3.2 Al163 expression, and puri fication of the Al163 protein
The al163 gene fragment encoding the mature Al163 enzyme was expressed in E. coli BL21 (DE3)cells as an intracellular protein in the presence or absence of IPTG induction, and total protein was analyzed by SDS-PAGE. Only one induced protein,which ran at 80 kDa, was observed (Fig.3). Puri fication details are summarized in Table 1. Overall, about 5.1-fold puri fication was achieved with 52.9% yield after a single step. The recombinant enzyme had a speci fic activity of 258 U/mg.
3.3 Biochemical characterization of recombinant Al163
The optimal temperature for recombinant Al163-catalyzed degradation of sodium alginate was 40° C(Fig.4a), and enzyme activity was similar between 30°C and 40° C. Thermal stability analysis showed>90% residual activity following incubation at temperatures ranging from 10°C to 30° C for 30 min,but the residual activity sharply declined at temperatures >40° C (Fig.4b). Enzyme activity was highest in sodium phosphate buffer at pH 7.0 (Fig.4c),and almost 20% of the activity remained after incubation at pH 4.0 for 3 h (Fig.4d).

Fig.2 Multiple sequence alignment of the N-terminal region of Al163 with other alginate lyases
The effects of various compounds on enzyme activity were tested (Table 2). Ca2+, SDS, Mg2+, K+,Ni2+, Cu2+, and Ca2+enhanced Al163 activity by up to 16.1% in the best case, while EDTA, Mn2+, Fe2+, and Zn2+repressed Al163 activity, with the largest decrease caused by Mn2+(71.3% residual activity compared with controls containing no additives). NaCl also increased Al163 activity (Fig.5), with the highest activity measured in the presence of 40 g/L NaCl,which was nearly 3-fold higher than that measured in the absence of NaCl. Furthermore, Al163 activity steadily increased from 0 to 40 g/L NaCl, suggesting that Al163 was activated by NaCl over a broad concentration range and can be considered a saltactivated alginate lyase. Many NaCl-activated enzymes have been isolated from marine organisms,in which the presence of NaCl in varying amounts is indispensable for survival.
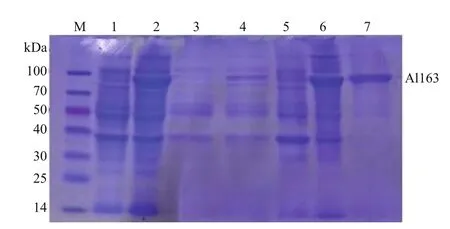
Fig.3 Puri fication of recombinant Al163
3.4 Substrate speci ficity and kinetic parameters of recombinant Al163
Alginate, polyM, polyG, agar, agarose, and chondroitin B were tested as potential substrates of the recombinant Al163 (Fig.6). The enzyme degraded alginate, polyM, and polyG, but showed no activity toward agar, agarose, or chondroitin B. The highest Al163 activity was observed toward polyG, almost 2-fold higher than that observed toward polyM or alginate.
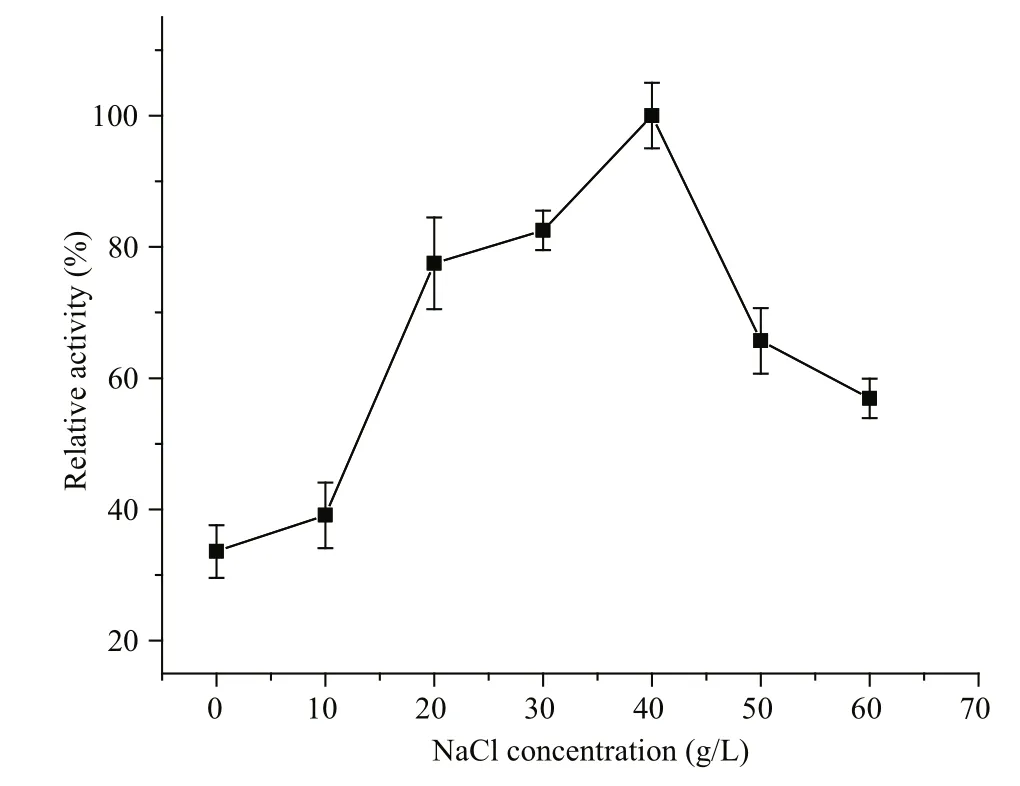
Fig.5 In fluence of NaCl concentration on Al163 activity

Fig.6 Substrate speci ficity of recombinant Al163
Kinetic parameters of the recombinant Al163 with alginate, polyM, and polyG were calculated from a series of initial rates ( V, U/mg) measured at various substrate concentrations and estimated using Lineweaver-Burk plots (Fig.7). The resulting Vmaxvalues for alginate, polyM, and polyG were 303 U/mg, 256 U/mg, and 625 U/mg, respectively, and the corresponding Kmvalues were 1.787 mg/mL,1.301 mg/mL, and 0.875 mg/mL. The Kmvalue for polyG was clearly lower than those for alginate and polyM, which was consistent with the results of substrate speci ficity analysis.
3.5 Analysis of the degradation products of substrates of recombinant Al163

Fig.7 Kinetic parameters of recombinant Al163
Degradation products of alginate, polyM, and polyG were investigated after incubation with recombinant Al163 for 0–360 min by thin-layer chromatography. Puri fied unsaturated dimeric,tetrameric, and heptameric agar oligosaccharides were used as standard markers (Fig.8). For all threesubstrates, the main degradation products were dimers and trimers. Small quantities of pentamers and heptamers were visible after only 15 min of incubation,while dimers began to appear after 2 h, suggesting Al163 initially hydrolyzed substrates into trimers,pentamers, and heptamers that were subsequently degraded into dimers and trimers. These results indicate that Al163 may hydrolyze substrates in an endolytic manner.
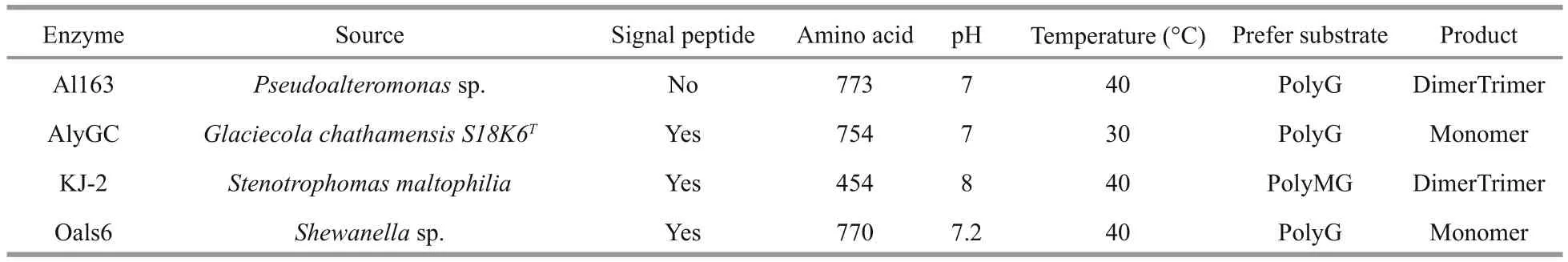
Table 3 Comparison of alginate lyases belonging to the PL-6 superfamily
4 DISCUSSION
Hundreds of alginate lyases have been obtained from multiple sources, including marine algae, marine mollusks, and a wide range of marine and terrestrial bacteria (Zhu and Yin, 2015). According to their amino acid sequence similarities, alginate lyases are classi fied into seven PL families (PL-5, -6, -7, -14,-15, -17, and -18) in the Carbohydrate-Active enZYmes (CAZy) database (Cantarel et al., 2009). In this study, alginate lyase Al163 from the Antarctic bacterium Pseudoalteromonas sp. NJ-21 was determined to belong to the PL-6 family, and was successfully expressed in E. coli BL21 (DE3) cells.Al163 shared >90% sequence identity with a putative polyM lyase from P. mesophila KMM241 (GenBank:GAC22931) and enzymes of unknown function from Pseudoalteromonas atlantica T6c (GenBank:ABG42142), and Paraglaciecola agarilytica(GenBank: WP008301990). Most endolytic bacterial alginate lyases have been assigned to the PL-5 and -7 families (Hashimoto et al., 2005; Yamasaki et al.,2005; Ogura et al., 2008), and there have been few reports of enzymes from the PL-6 family; only AlyGC, KJ-2, AlyP, and OalS6 have been characterized as alginate lyases belonging to the PL-6 superfamily(Table 3). Most PL-6 alginate lyases prefer polyG as the substrate and share similar optimal conditions for enzymatic degradation of polysaccharides.

Fig.8 Analysis of Al163 degradation products
Sequence alignment of Al163 with other alginate lyases revealed a putative calcium-binding site, and enzyme assays showed that Ca2+had the greatest effect on stimulating enzyme activity, supporting an important role for the putative calcium-binding site.The role of Ca2+in AlyGC-degradation of alginate was investigated recently, revealing a Ca2+ion is required for catalysis (Xu et al., 2017). Interestingly,based on amino acid sequence alignment and modeling analysis, Al163 shares high sequence similarity with chondroitin B lyase; however, Al163 did not exhibit chondroitin B lyase activity. Structurefunction relationship comparisons between Al163 and chondroitin B lyase will require analysis of enzyme structures.
Hundreds of alginate lyase sequences have been deposited in the NCBI database; however, few originate from organisms that inhabit extreme environments.The putative polyM lyase from the cold-adapted bacterium P. mesophila KMM241 (GenBank:GAC22931) (Qin et al., 2014) was predicted to be an alginate lyase, but the protein has not been puri fied and characterized. Studies of enzymes from extreme environments are important for developing more environmentally-friendly industrial catalysts.
Depolymerized alginates with low degrees of polymerization derived from enzymatic hydrolysis possess various biological activities (Wong et al.,2000; Hu et al., 2004; Zhang et al., 2004; An et al.,2009; Kobayashi et al., 2009; Zong et al., 2014), and numerous attempts have been made to produce valueadded materials, such as functional oligosaccharides and bioethanol, from alginate (Takeda et al., 2011;Wargacki et al., 2012; Enquist-Newman et al., 2014).There are many advantages in producing functional oligosaccharides using enzymatic methods rather than traditional approaches such as acid hydrolysis of alginate or supersonic schizolysis. The primary goal for developing enzymatic methods is the discovery of efficient and stable alginate lyases. Those described to date, including flalya (Inoue et al., 2014), A9m(Uchimura et al., 2010), and Algb (Wong et al., 2000),are gradually inactivated at temperatures >40° C. We found that the recombinant Al163 exhibited good stability at low temperatures (10–40° C) and maintained almost 20% residual activity even in extreme pH conditions (4.0 and 11.0). Additionally,recombinant Al163 was capable of degrading both polyM and polyG simultaneously, indicating bifunctionality. Previous studies reported that only a few bacterial strains produce bifunctional alginate lyases (Iwamoto et al., 2001). Bifunctional alginate lyases were found to assist in the recovery of pathogenic bacteria sensitive to antibiotics by degrading the alginate polysaccharides in biomembranes (Chung et al., 2007). Additionally, the recombinant Al163 exhibited broad substrate speci ficity, and its catalytic products exhibited low degrees of polymerization. Both properties suggest high potential for industrial use. Because Al163 was easily expressed in an E. coli-expression system,recombinant Al163 could provide a firm basis for future biotechnological studies of alginate lyases.Structural and functional studies of recombinant Al163, including those involving mutagenesis, are currently underway in our laboratory.
5 CONCLUSION
We cloned and characterized a novel PL-6 family endolytic alginate lyase (Al163) derived from the Antarctic bacterium Pseudoalteromonas sp. NJ-21.Recombinant Al163, which was puri fied by Ni-Sepharose affinity chromatography, had a molecular mass of 84.61 kDa. The puri fied enzyme showed maximum activity (258 U/mg) at pH 7.0 and 40° C.Substrate speci ficity experiments revealed its ability to degrade alginate, polyM, and polyG, with the highest affinity for polyG, indicating Al163 might be a polyG lyase (EC4.2.2.11). Al163 degraded alginate to oligosaccharide products with low degrees of polymerization (dimers and trimers), suggesting its potential for producing oligosaccharides with interesting, high-demand bioactivities.
6 DATA AVAILABILITY STATEMENT
Sequence data supporting the findings of this study have been deposited in GenBank under accession numbers KF700697 (16S rDNA sequence of Pseudoalteromonas sp. NJ21) and KU360250( Pseudoalteromonas sp. NJ21 al163 gene sequence).
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin?) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
