Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae)
Aksorn Chantawee, Mayura Soonwera
DThepaai lratnmde nt of Plant Production Technology, Faculty of Agricultural Technology, King Mongkut's Institute of Technology Ladkrabang, Bangkok,
1. Introduction
Mosquitoes (Culicidae: Diptera) are a serious worldwide threat for humans and animals. They are vectors of many serious pathogens and parasites including dengue, Zika virus, malaria and filariasis[1].Aedes aegypti(L.) (Ae. aegypti) that inhabits the tropical and subtropical zones carries arbovirus, and is generally known to be a vector of dengue and chikungunya.Ae. aegyptifemales are anthropophilic,humans are their preferred hosts and thus at risk of being attacked by them[2,3]. Studies have suggested that most females ofAe. aegyptimay spend all of their lives in or around the houses that they have emerged as an adult.Ae. aegyptitransmits dengue virus to susceptible humans[4]. It has been estimated recently that 3.9 billions of people in 128 countries are at risk of acquiring dengue and 390 million dengue infections occur every year, of which 294 millions clinically manifest the symptoms[5]. Severe dengue is a relatively rare but serious complication of dengue infection is manifested as plasma leaking, fluid accumulation,respiratory distress, severe bleeding or organ impairment[4,6].Therefore, it is of global public health concern to be able to control mosquitoes effectively[7]. Most mosquito control programs aim to control the larvae and pupae with larvicides and pupicides because adulticides may work well only for a temporary period[8-10].
Larviciding and pupiciding are common methods for reducing mosquito population and preventing dengue and chikungunya diseases. Larvicidal activity is very important in vector management because larvae that are in the growth stage are the easiest to kill. In particular, larvae control usually depends on extended application of organophosphates or other growth regulators such as diflubenzuron and methoprene[11]. Temephos is one of an organophosphate registered and produced commercially that has been extensively used for controllingAe. aegyptilarvae[12].
Today, synthetic chemical insecticides used for controlling mosquito vectors are being seriously questioned because of the irreversible damages they cause to the ecosystem and the various patterns of their mosquito resistance. In recent years, it has been suggested that plant essential oils (EOs) and their constituents can be good alternative larvicidal and pupicidal agents for mosquito control, mainly because their bioactive chemicals usually cause only inconsequential side effects on other organisms and the agricultural environment[12,13]. EOs from Apiaceae and Zingiberaceae plants have been reported to have potent repellent, larvicidal, pupicidal and adulticidal activities againstCulex pipiens, Culex quinquefasciatus, Ae. aegypti, andAnopheles stephensi[14-18].
In the present study, larvicidal and pupicidal activities of EOs from three Apiaceae species, namely,Anethum graveolensL. (An.graveolens),Foeniculum vulgareMill. (F. vulgare), andPimpinella anisumL. (P. anisum) as well as from a Zingiberaceae species,Alpinia galanga(L.) Willd (Al. galanga), were examined againstAe. aegypti(Linn.).
2. Materials and methods
2.1. Plant materials
Plant materials from fresh rhizomes ofAl. galanga, dried fruits ofAn. graveolens, dried fruits ofF. vulgare, and dried fruits ofP. anisumwere investigated in this study. The plants were identified positively by a herbal taxonomist at the Department of Plant Production Technology, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang (KMITL), Thailand. The rhizomes and fruits were cut into small pieces and distilled with water to obtain the EOs[10]. The plant materials were added with water at a ratio of 1:2(plant:water) and placed in a distillation column connected to a roundbottomed distillation flask[10]. The flask was heated to about 100 ℃and the distillation process began. It was stopped after 6 h. The EOs were dried with anhydrous sodium sulfate[10] and kept in a refrigerator at 4 ℃ until further use. Each EO was diluted to 1%, 5% and 10% in ethyl alcohol and kept in an airtight bottle at 4 ℃ for later uses[15].
2.2. Mosquito rearing
A number ofAe. aegypti(L.) were raised by the Department of Plant Production Technology, Faculty of Agricultural Technology,KMITL, Bangkok. The laboratory colony was kept under the following conditions: (29.5±2.0) ℃ with (75.5±2.0) relative humidity, and a photoperiod of 12-h light and 12-h dark (12L:12D). Eggs were hatched in plastic boxes (18 cm × 28 cm × 10 cm in size), each containing 1500 mL of tap water. The larvae were fed with fish food pellets (HIPRO?)until pupation occurred. Pupae were collected and transferred to an insect cage (30 cm × 30 cm × 30 cm) and adult mosquitoes were provided with 5% glucose on cotton wool. On day 5, blood meals were given to the female adults following an artificial membrane feeding method. For egg collection, after the females were fed with blood meals for 2-3 d and ready to spawn, moist filter papers were placed on the surface of the water in a cup where they could lay eggs on. For 7 d, the eggs were kept wet and then put on a pan to hatch. The early 4th instar larvae and pupal stages ofAe. aegyptiwere tested in this experiment.
2.3. Larvicidal and pupicidal bioassay
Larvicidal activity and pupicidal activity were each determined according to a test dipping assay by Soonwera and Phasomkusolsil[10].For each experimental treatment, 1 mL of a plant essential oil solution was added to 99 mL of distilled water in a 200 mL glass cup and shook lightly to ensure homogeneity. TenAe. aegyptiin an immature stage(early fourth instar larvae or pupae stage) were put into the glass cups containing 100 mL of EOs in prepared water mentioned above. For ten larvae susceptibility test, all larvae were exposed to EOs until pupation,and mortality was observed for 24 h[10]. For ten pupae susceptibility test, the pupae were exposed to EOs until some were grown into adults, and mortality was observed for 72 h. Thirty replicates for each concentration of essential oil were performed. For comparison, a commercial formulation of temephos was used as a positive control and ethyl alcohol served as a negative control. During the period of the experiment, the larvae were offered no food[10]. They were considered dead if at the end of a 24-h period, they did not swim or move even after getting prodded by a rod. The dead and moribund larvae that showed sluggishness or abnormal movement were recorded after 24 h.Also, the pupae were recorded at 72 h and considered dead if they did not swim or move even after getting prodded by a rod[10,19].
2.4. Morphological aberrations observed
A stereomicroscope was used to determine and categorize the morphological aberrations of the deadAe. aegypti, and a method described by Soonwera and Phasomkusolsil was used to categorize dead specimens[10].
Normal larvae (NL): This group represented the larvae that died after reaching the pre-pupal stage of development.
Deformed larvae (DL): This group represented the larvae that died abnormally. Dorsal splitting (arrow) of thoracic cuticle was observed in dying and dead larvae.
Pre-pupae and pupa that did not completely shed off its exoskeleton(PP): This group represented the larvae that died before they came out of their exoskeleton. Some specimens died when their heads were still enclosed in their exoskeleton.
White pupa (WP): This group represented the pupae that came out of their larval exoskeleton completely. The white cuticle made it known as“albino”.
Deformed pupae (DP): This group represented the pupae that died abnormally. In some cases, the dead pupa had an appearance of a tiny elephant and was designated “elephantoid”.
Dead normal brown pupae (BP): pupae in this group were brown and normal in appearance.
Adults attached to pupal case (PA): This group represented the adults that died when they were emerging from their exoskeleton; For example, their tarsi, legs, wing and abdomen were still enclosed in their exoskeleton.
Normal adult (NA): This group represented the adults that emerged completely from their exoskeleton with normal appearance.
2.5. Oviposition deterrent assay
The oviposition deterrent activity was conducted in a laboratory using the method of Reeganet al[20] and a dual-choice oviposition bioassay was performed on gravid females ofAe. aegypti. Fifteen gravid females(5 days old) ofAe. aegyptiwere introduced into an insect cage (30 cm× 30 cm × 30 cm) under room conditions of (29.5±2.0) ℃, (75.5±2.0) relative humidity and 12L:12D. The adults were provided with 5% glucose solution which was available at all time. Two 200-mL plastic cups for oviposition were filled with 99 mL distilled water,one for untreated cup and the other for treated cup. One milliliter of 1%, 5% and 10% of a plant essential oil solution and temephos was added to one cup to make up the preparation for a treatment, while the untreated cup was added with 1 mL ethyl alcohol. A support for oviposition was provided by placing a piece of filter paper (Whatman?No.1) on the inner surface of each plastic cup so that the lower half of it was submerged in the treated solution or untreated solution in order for the whole paper to get moistened while the upper half of it was above the solution where the mosquitoes would lay their eggs on. The untreated and treated cups were placed at alternate diagonally opposite locations for each replicate so as to nullify any effect of their locations on oviposition. After 3 d, the number of eggs laid in the treated and untreated cups were counted under a stereomicroscope[21].
2.6. Statistical analysis
The LT50and LC50values were calculated using probit analysis [10].The mortality rates that were the results of using different EOs at different concentrations were statistically analyzed by Duncan’s multiple range test to compare their different efficacies.
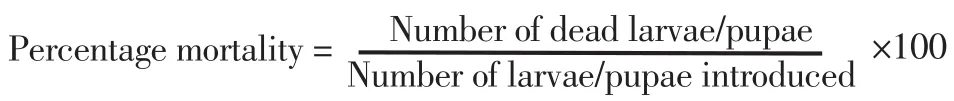
The oviposition activity index (OAI) was calculated using a formula used by Tikaret al[22]:
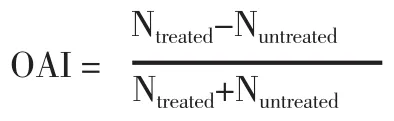
Where Ntreatedwas the number of eggs laid in the treated cups and Nuntreatedwas the number of eggs laid in the untreated cups. OAI was in the range of -1 and +1. Negative OAI values indicated that more eggs were laid in the untreated cup than in the treated cup and the treated solutions were a deterrent, whereas positive OAI values indicate that more eggs were laid in the treated cup than in the untreated cup,and that the treated solutions were attractive. Treatments of each concentration of EOs were replicated in six different cages. For oviposition deterrent assay, the percent effective repellency (ER) at each concentration was calculated by the following formula:
ER(%)=(Nuntreated-Ntreated)/Nuntreated×× 100
Where Nuntreatedwas the number of eggs found in the untreated, and Ntreatedwas the number of eggs found in the treated.
The mean numbers of eggs deposited in the treated and untreated cups were statistically analyzed by a pairedt-test and they were analyzed by at-test and one-way analysis of variance with SPSS software (version 23.0).
3. Results
3.1. Larvicidal and pupicidal activity
The outcomes of the larvicidal bioassay on the early fourth instars ofAe. aegyptitreated with the four EOs and the statistical data of mortality,LT50and LC50were shown in Table 1. All EOs at 10% concentration showed more toxicity than those at 1% and 5% concentrations. After 24 hours of exposure, it was found thatAn. graveolensEO at 1%concentration produced the mortality rate againstAe. aegyptiat 91%,while the oil achieved 100% mortality at 5% and 10% concentrations. The result showed that LT50decreased with increased concentration. Both 5%and 10% concentrations ofAn. graveolensexhibited LT50values of 0.8 againstAe. aegyptilarvae. Among the four EOs tested, the oil fromAn.graveolensexhibited the strongest larvicidal effect with the lowest lethal concentration, LC50value of -0.3% (Y=109.8×X+ 90.71, χ2=77.2),followed by the essential oil fromF. vulgarewith LC50of 0.5% (Y=95.9×X+91.89, χ2=155.9),P. anisumwith LC50of 0.6% (Y=362.3×X+69.34,χ2=173.0 ) andAl. galangawith LC50of 7.8% (Y=931.9×X+11.63,χ2=289.0). On the other hand, temephos at 1% concentration (positive control) showed 94.3% mortality at 24 h with LT50value of 255.7 h.Larvicidal activity of four EOs showed a positive relationship between mortality rates and exposure periods which were significant (Figure 1). The results of the pupicidal activity againstAe. aegyptipupae were shown in Table 2.An. graveolensoil at 10% concentration showed more toxicity than the oil at 1% but the same toxicity to the oil at 5%.At 1% concentration, no EOs produced any larvae mortality during the observation period. As for the results for 5% concentration,An.graveolensoil produced the highest mortality againstAe. aegyptipupae with 100% mortality at 72 h and LT50value of 10.3 h, followed byF. vulgarewith 94.0% mortality at 72 h and LT50of 14.6 h. At 10%concentration,An. graveolensshowed the highest pupicidal activity againstAe. aegyptipupae with 100% mortality at 72 h, LT50of 6.7 h and LC50of 2.9% (Y=1066×X+9.836, χ2=0.02), followed by the essential oil fromF. vulgarewith 99.7% mortality, LT50of 7.5 h and LC50of 3.5% (Y=1062×X+7.769, χ2=63.9),P. anisumwith 98.3%mortality and LC50of 3.84% (Y=1052×X+7.67, χ2=106.0) andAl. galangawith 92.0% mortality and LC50of 6.3% (Y=1028×X-12.71, χ2=317.0). Pupicidal activity of four EOs showed a positive relationship between mortality rates and exposure periods which were significant (Figure 2). The low value of LC50forAn. graveolensoil demonstrated its good larvicidal and pupicidal activity againstAe.aegypti.In contrast, temephos (positive control) showed only 4.8%mortality at 72 h with LT50of 98.7 h. Not surprisingly, ethyl alcohol,the negative control, did not produce any mortality of pupae during the observation period. Therefore, cultivated in the negative control,all larvae were active and exhibited normal movement. Conversely,cultivated in the treatments, larvae were observed to have restless movements. After 1 h of treatment, all treated larvae started to have tremor and convulsion, and dead larvae started to settle to the bottom of the cup.
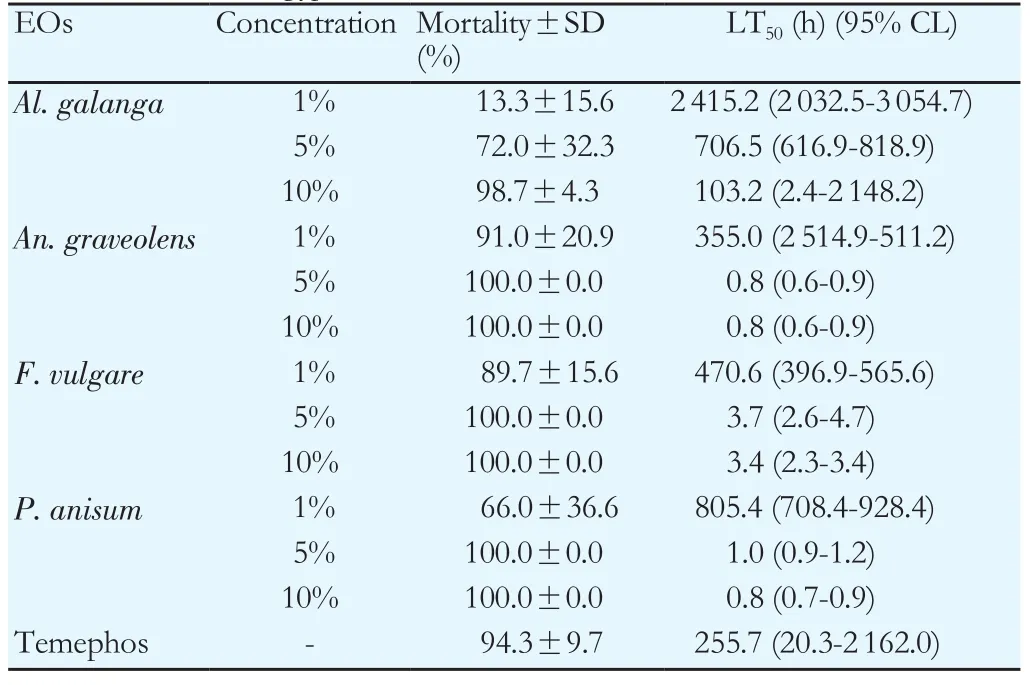
Table 1 Effect of herbal EOs at three concentrations (1%, 5% and 10%) against 4th instar larvae of Ae. aegypti at 24 h.
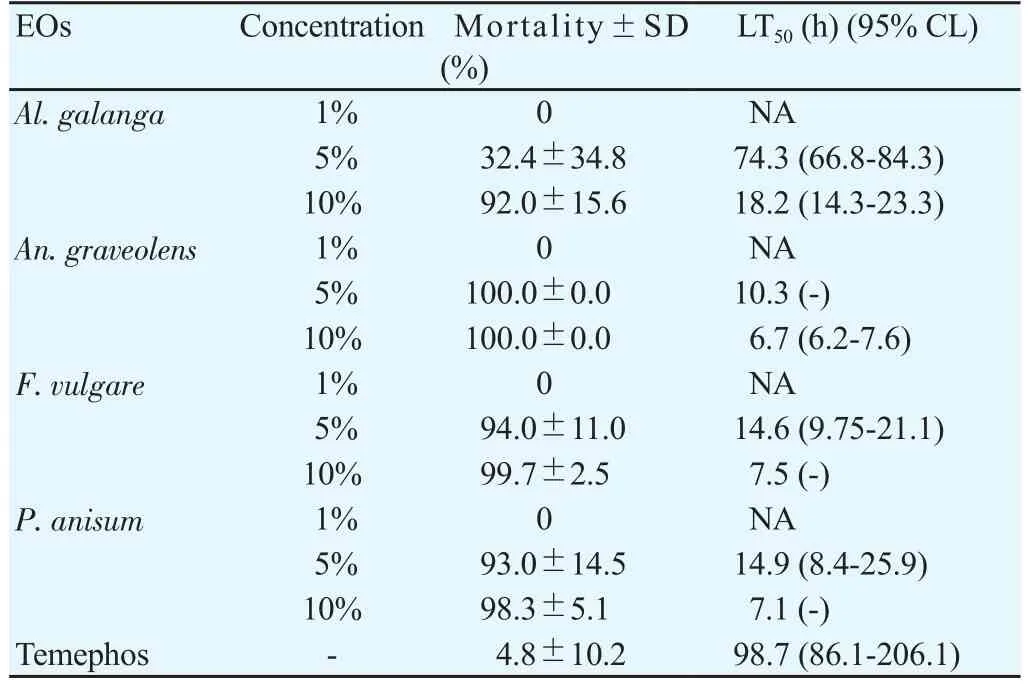
Table 2 Effect of herbal EOs at three concentrations (1%, 5% and 10%) against pupae of Ae. aegypti at 72 h.
3.2. Morphological aberrations
The mortality and morphological aberrations of larvae ofAe.aegyptiwere observed after 24 h of exposure to 1%, 5% and 10%concentrations of EOs which were shown in Table 3. At these concentrations, all EOs caused morphological aberrations at the time of death of the larvae. The results showed the morphological aberrational changes ofAe. aegyptilarvae from NL to DL and deformed PP. The death of larvae at the highest mortality was usually as NL. One percent concentration ofAl. galanga, An. graveolens, F.vulgareandP. anisumoils caused no morphological change (NL) at 11.6%, 84.0%, 47.7% and 56.0% NL mortality rate (Figure 3A). They caused some changes at the time of death at 1.0%, 5.3%, 39.0%, and 6.0% DL mortality rate (Figure 3B-3D). The absence of underlying epithelium in the dead larvae from EO treatment might indicate that lectin larvicidal activity was probably due to damage in theAe.aegyptimidgut. The deformations were such as damaged anal papillae,distorted body, darken body and shrunken cuticle. They also caused abnormal PP with deformed cephalothorax and posterior abdominal segment at 0.7%, 1.6%, 3.0% and 4.0% PP mortality rate (Figure 3E).At the concentrations of 5% and 10%, all EOs caused the greatest NL mortality rate.
All of the EOs caused some morphological aberration in the specimens during pupation after 72 hours of exposure as shown in Table 4. The main characteristic of death from EOs was DP: some abnormal pupae died with enlarged cephalothorax and wing pads were not appressed to the body; their head and body also turned black (Figure 3G-3I). Ten percent concentration ofAl. galanga, An.graveolens, F. vulgareandP. anisumcaused major changes almost found at the time of death at 83.0%, 75.3%, 99.0% and 97.7% DP mortality rate, respectively. However, some pupae were found dead as normal BP, their cephalothorax and abdomen had normal brown color(Figure 3F). Ten percent concentration ofAl. galanga, An. graveolensandP. anisumoils caused no morphological change (BP) at 5.7%,24.7% and 0.3% BP mortality rate. Some adults died while they were emerging (PA). Their tarsi, legs, wings, and abdomen were still attached to the pupal exoskeleton (Figure 3J-3K). They also caused partially emerged, tarsi-deformed adult (PA) at 3.3% (Al. galanga), 0.7% (F.vulgare) and 0.3% (P. anisum) PA mortality rate.
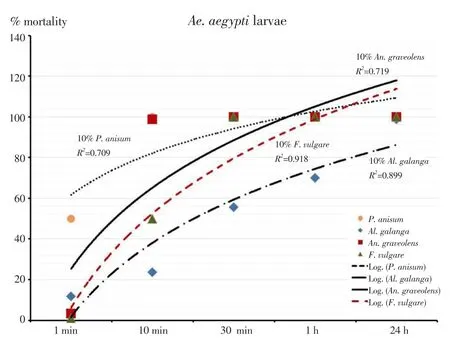
Figure 1. Relationship of mortality rate and exposure periods of larvicidal activity of essential oils against Ae. aegypti larvae expressed as regression.
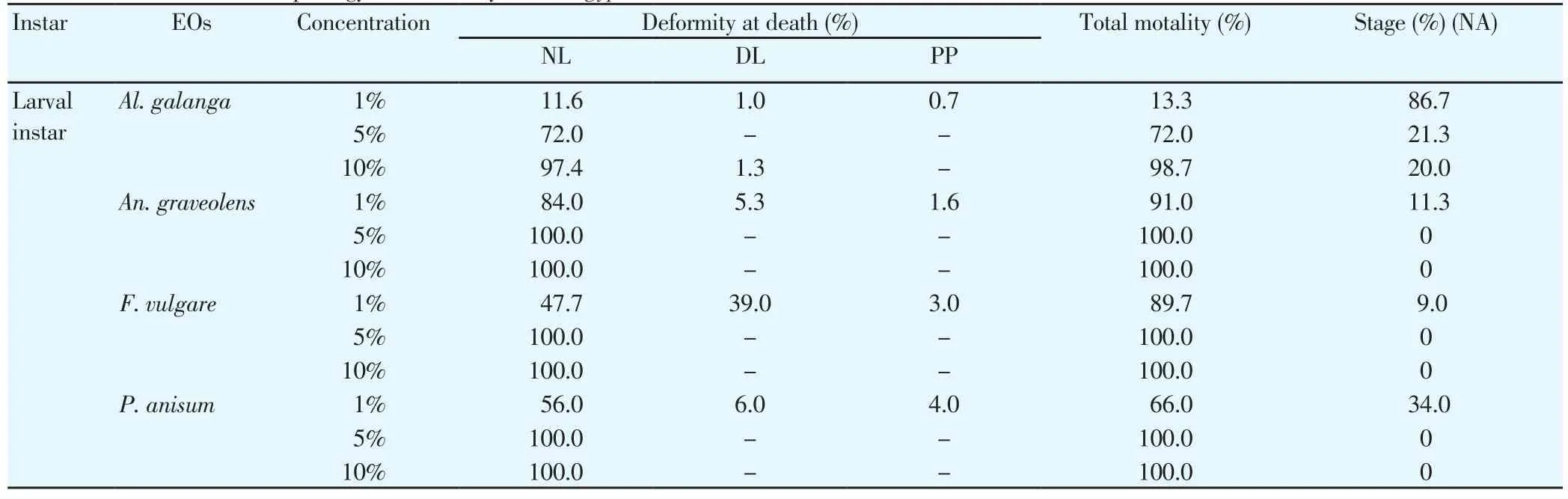
Table 3 The effect of four EOs on morphology and mortality of Ae. aegypti larvae.
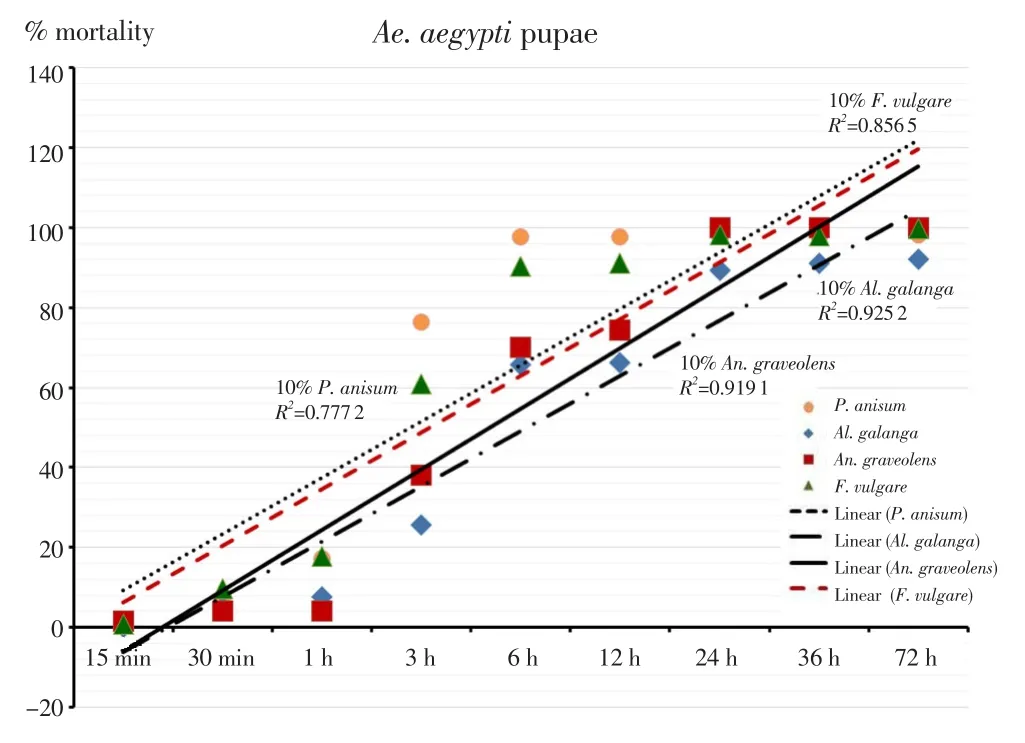
Figure 2. Relationship of mortality rate and exposure periods of pupicidal activity of essential oils against Ae. aegypti pupae expressed as linear regression.
3.3. Oviposition deterrent activity assay
The resulted obtained from the oviposition deterrent assay of four EOs at all three concentrations againstAe. aegyptiwere shown in Table 5. The results showed that all concentrations of all EOs were able to reduce the number of deposited eggs by gravidAe. aegypticompared to the number of eggs from the gravid females treated with the ethyl alcohol. All EOs at three concentrations tested were observed to repel mosquitoes from oviposition and repellency of four EOs increased with the increase of concentration. The range of the mean number of eggs laid in the cups with the four EOs at three different concentrations 1%, 5% and 10% were 6.8-162.4. In addition, there was also a marked difference in the amount of the eggs laid.An. graveolensexhibited the most effective repellency activity against gravid female mosquitoes. The mean number of laid eggs in the cups with 1%, 5%and 10% ofAn. graveolensoil were 145.6, 22.6 and 6.8 eggs per cup,respectively, while the untreated cups gave a mean number of 392.0,385.4 and 355.8 eggs per cup. A pairedt-test confirmed that these results were significantly different (P<0.05). The percentage of ER caused byAn. graveolensagainst oviposition were 62.9%, 94.1% and 98.1% for 1%, 5% and 10% concentrations, respectively. The range of OAI ofAn. graveolensat three concentrations was from -0.5 to-1.0. The results showed that gravid femalesAe. aegyptipreferred to lay eggs in the untreated cups rather than in the treated cups,thus it was demonstratedAn. graveolensoil had potential to repel mosquito females for laying eggs. The present results indicated that the oviposition deterrent activity depended on concentrations as 10%An. graveolensoil exhibited the strongest deterrent effect. On the other hand, temephos provided a mean number of 249.8 eggs laid per cup, while the untreated cups gave a mean number of 319.8 eggs per cup. The OAI value of temephos was -0.1; there was no significant difference in the number of eggs laid in the treated and untreated cups in this case. Therefore, temephos showed a lowest oviposition deterrent activity againstAe. aegyptifemales than those of plant EOs.

Figure 3. Morphological aberration of larvae Ae. aegypti after treatment with essential oils.
4. Discussion
EOs derived from plants have a good potential for controlling mosquitoes in their larval and pupal stages. In the present study, 10%An. graveolensoil recorded the highest larvicidal and pupicidal activities of 100% mortality rate againstAe. aegyptiimmature stages.
Anethum graveolensL., commonly known as dill, is a medicinal plant with tiny yellow flowers that belongs to the plant family Apiaceae[23].Leaves, stems and fruits of dill are widely used in various applications in the food industry, especially for their unique taste and spicy aroma[24].Extract ofAn. graveolensobtained from the seeds have antibacterial,antispasmodic, antioxidant, antimicrobial properties[25]. The EO ofAn.graveolensexhibited a larvicidal activity among many biological activities.EO of bulk dill (pure, not in a formulation) at different concentrations(10-100 ppm) was evaluated againstAnopheles stephensi[26]. Meanwhile,larvicidal activity was observed at the concentration of 20 ppm and increased with increasing concentration ofAn. graveolens. Lethal concentrations at 50% and 90% ofAn. graveolensEO were found to be 38.8 and 65 ppm, respectively, against the 3rd and 4th instar larvae ofAnopheles stephensi[27]. The EO ofAn. graveolensat a concentration of 0.1 mg/mL has also shown a strong larvicidal activity against Asian tiger mosquito,Aedes albopictus(90% mortality)[28]. In another report,An. graveolensEO has also shown an effect againstCulex pipiensadult(LC50=0.495) and larvae (LC50=16.996)[29]. Moreover,An. graveolensEO is also toxic larvicides to other insect pests.An. graveolensseed essential oil was found from continuous exposure and fumigant toxicity bioassays to be toxic toPeriplanata americanaL.,Musca domesticaL.andTribolium castaneum.The mortality againstPeriplanata americanaranged from 25% to 100% during the first 3 h in a contact toxicity bioassay and during the first 12 h in a fumigant toxicity bioassay. In case ofMusca domesticaL., mortality ranged from 33.3% to 70.0%during the first 3 h and from 58.3% to 100.0% during the first 24 h forTribolium castaneum[23]. In a previous report, the EO ofAn. graveolenswas assessed for insecticidal activity againstCallosobruchus maculatesL. adults through a fumigant bioassay with LC50value at 12.75 μ/L air[30]. In another study, the toxicity ofAn. graveolens(leaves) plant extract at 5 and 10 mg/mL concentrations against 2-day-old ( first instar)and 6-day-old (third instar) larvae ofSpodoptera liturawas investigated[31].Many researchers have determined the chemical composition of essential oil of the fruits and seeds ofAn. graveolenswhich was extracted by steam distillation and hydrodistillation[32,33]. The constituents were found to be carvone, limonene,α-phellandrene, dichloromethane, αα-terpinene,p-cymene, α- and β-pinene, γ-terpinene, cumin aldehyde, neral, transanethole, thymol, carvacrol, myristicin, apiol, and carotol constituents.However, the EO extracted by steam distillation contained higher amounts of limonene and carvone than the oil extracted by hydrodistillation. From the literature, carvacrol,α-pinene, and β-pinene were found to inhibit the activity ofAedes albopictusacetylcholinesterase with LC50values of 0.057,0.062, and 0.190 mg/mL, respectively[28]. The insecticidal property ofAn.graveolens(that contains 59% phellandrene, its most abundant compound)has been evaluated against larvae of the 3rd and early 4th instars ofCulex pipienswith LC50value of 52.74 mg/L[34]. Rochaet al.[35] reported morphological changes in the anal papillae ofAe. aegyptilarvae after they were in contact with some EOs and the major chemical constituents ofAn. graveolens: (+)-limonene and (-)-limonene. After contact with these two compounds, an accumulation of dark pigmentation was observed all over the chest and at the base of the anal papillae. Structural damages to the larvae exposed to (-)-limonene include destruction of the gut and extrusion of hemolymphatic contentetc, while damages to the larvae exposed to (+)-limonene were such as a darkening at the base of the anal papillae extended to the apex region. The present results confirm the previously reported results, revealing similar morphological changes such as changes in head and abdomen pigmentation, distorted body,darkened body and anal papillae and shrunk cuticle. The larvicidal activity of EOs may be according to diverse mechanisms. Mortality may occur at different development stages. Owing to contact effect, mode of action of EOs may act on digestive or neurological enzymes and interact with the insect’s integument. Several studies have reported tremors and paralysis of larvae in their assays as well as dying larvae staying at the bottom of the containers[26,36]. In another study, it was found that several EOs blocked the effects on chemosensory receptors at the mouth parts stimulated by glucose and inositol[37]. The EOs and their constituents disrupted the endocrinological balance of the insects. They induced neurotoxicity via various mechanisms hence disrupting the normal process of morphogenesis. The damages on the muscles caused by the oils might affect the larvae’s movement for respiration or feeding and the adults’ development and flying ability[38].
Using for modifying the oviposition behavior of mosquitoes,oviposition deterrents and attractants play an important role in mosquito control programs. Oviposition site selected by gravid females is a critical factor that determines the proliferation and population density of the species as well as its dispersion in different geographical areas[39,40]. As female mosquitoes approach an oviposition site, they use a site-specific olfactory cue as a short-range signal for determining its quality. Volatile chemical emanated from an oviposition site is sensed and evaluated by the olfactory receptors located on the antennae, palps,labrum, and tarsi[41,42]. It has been observed that gravid females of many species of mosquitoes preferred an oviposition site over some others. This preference may be due to the presence of oviposition pheromones or oviposition attractants or repellents at the site[15,43]. In this study,An. graveolensoil exhibited the highest oviposition deterrent activity against femaleAe. aegypti. The strongest activity was produced by the highest concentration ofAn. graveolensoil tested. It might produce the maximum effective repellency against oviposition by acting as a chemical signal that was detected by the sensory receptors on the antenna of the mosquitoes[44]. Warikooet al.[45] have reported that pureAn. graveolensoil deterred oviposition completely and boasted 75% effective repellency. The EO derived from dried fruits ofAn. graveolensexhibited a repellent activity against the adults ofAe.aegypti[46].An. graveolensoil’s insecticidal, oviposition deterrent,egg hatching and developmental inhibitory activities were determined against pulse beetleCallosobruchus chinensis[47]. Another study showed thatAn. graveolensoil reducedTribolium castaneum’s oviposition potential and lengthened its developmental period in comparison with the control group. The oviposition potential ofTribolium castaneumdecreased significantly when it was fumigated withAn. graveolensoil[48].
The ability of gravid mosquitoes to perceive the presence of organic acids and hence detect unsuitable oviposition sites might have been acquired through evolutionary adaptation. This ability helps mosquitoes to avoid ovipositioning in unsuitable breeding sites that contain harmful toxic compounds[43]. EOs affect mosquito’s nervous system; it can affect adult mosquitoes’ ability to find the right host to feed on or to find the right oviposition site[38] by possible interference with nerve impulse transmission to the brain, hence changing the mosquito’ response to internal and external stimuli.
In Thailand,An. graveolensis a cultivated crop in the Northeastern region. Its aerial part (dill weed) is a popular seasoning agent[33].An.graveolensfruits in Thailand have already been used as aromatic plants and spices for food preservation and in medicine, alternative medicine and natural therapy for a long time. In this study,An. graveolensoil showed strong larvicidal, pupicidal and oviposition deterrent effects,thus, the good potential ofAn. graveolensoil for controllingAe.aegyptihas been verified.An. graveolensoil can be used in places where mosquitoes usually breed for controlling larvae and pupae population and for preventing egg-laying by femaleAe. aegyptiadults.If the oviposition activity ofAe. aegyptiis inhibited, its life cycle will be disrupted and its population growth will be reduced.
In conclusion, our results clearly show thatAn. graveolensoil can be applied as larvicide, pupicide and oviposition deterrent for controllingAe. aegyptipopulation, and warrant further studies for field application.We believe that this study has demonstrated the usefulness ofAn.graveolensinsecticidal properties againstAe. aegypti.An. graveolenshas a full potential to be used as an inexpensive, safe and efficient larvicide on its own as well as a supplement to other larvicides.
Conflict of interest statement
The authors confirm that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
This study was sponsored in part by the National Research Council of Thailand (NRCT), (Grant no. GRAD6006 KMITL) and by the Faculty of Agricultural Technology, KMITL (Grant no. 01-04-001).The authors wish to extend our thanks to the plant taxonomist and entomologist of the Faculty of Agricultural Technology, KMITL, for their help in identifying the species of the four herbs and the mosquito.We also wish to express our gratitude to Mr. Pratana Kangsadal, the KMITL proofreader, for reviewing and giving comments on the manuscript.
[1] Benelli G, Lo Iacono A, Canale A, Mehlhorn H. Mosquito vectors and the spread of cancer: An overlooked connection?Parasitoly Res2016; 115(6):2131-2137.
[2] Elumalai D, Hemavathi M, Hemalatha P, Deepaa CV, Kaleena PK.Larvicidal activity of catechin isolated fromLeucas asperaagainstAedes aegypti, Anopheles stephensi, andCulex quinquefasciatus(Diptera: Culicidae).Parasitol Res2016; 115(3): 1203-1212.
[3] Yu KX, Wong CL, Ahmad R, Jantan I. Mosquitocidal and oviposition repellent activities of the extracts of seaweedBryopsis pennataonAedes aegyptiandAedes albopictus.Molecules2015; 20(8): 14082-14102.
[4] Zuharah WF, Fadzly N, Ali Y, Zakaria R, Juperi S, Asyraf M, et al. Larvicidal efficacy screening of anacardaciae crude extracts on the dengue hemorrhagic vector,Aedes aegypti.Trop Biomed2014; 31(2): 297-204.
[5] Santos LMM, Nascimento JS, Santos MaG, Marriel NB, Bezerra-Silva PC,Rocha SKL, et al. Fatty acid-rich volatile oil fromSyagrus coronataseeds has larvicidal and oviposition-deterrent activities againstAedes aegypti.Physiol Mol Plant Pathol2017; 100: 35-40.
[6] World Health Organization.Dengue and severe dengue[Online]. Available from: http://apps.who.int/iris/bitstream/handle/10665/204161/Fact_Sheet_WHD_2014_EN_1629.pdf;jsessionid=50A6965D286F096AC854B491B57A0968?sequence=1[Accessed on 21th December 2017].
[7] Hazra DK, Samanta A, Karmakar R, Sen K, Bakshi P. Mosquito vector management knowledge, attitude, practices and future of user & environment friendly new generation botanical mosquitocide formulations: A review.Int J Chem Sci2017; 5(3): 32-37.
[8] World Health Organization.Dengue vaccine safety update[Online]. Available from: http://www.who.int/vaccine_safety/committee/topics/dengue/Dec_2017/en/ [Accessed 21th December 2017].
[9] Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils againstAnopheles stephensi, Aedes aegyptiandCulex quinquefasciatus. Bioresour Technol2005;96(16): 1749-1757.
[10] Soonwera M, Phasomkusolsil S. Effect ofCymbopogon citratus(lemongrass)andSyzygium aromaticum(clove) oils on the morphology and mortality ofAedes aegyptiandAnopheles diruslarvae.Parasitol Res2016; 115(4): 1691-1703.
[11] Conti B, Flamini G, Cioni PL, Ceccarini L, Macchia M, Benelli G.Mosquitocidal essential oils: Are they safe against non-target aquatic organisms?Parasitol Res2014; 113(1): 251-259.
[12] Perumalsamy H, Kim NJ, Ahn YJ. Larvicidal activity of compounds isolated fromAsarum heterotropoidesagainstCulex pipiens pallens, Aedes aegypti,andOchlerotatus togoi(Diptera: Culicidae).J Med Entomol2009; 46(6):1420-1423.
[13] Perumalsamy H, Jang MJ, Kim JR, Kadarkarai M, Ahn YJ. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified inMillettia pinnataseed toward three mosquito species.Parasit Vectors2015; 8(1): 237.
[14] Phukerd U, Soonwera M. Larvicidal and pupicidal activities of essential oils from Zingiberaceae plants againstAedes aegypti(Linn.) andCulex quinquefasciatussay mosquitoes. Southeast Asian J Trop Med Public Health2013; 44(5): 761-771.
[15] Soonwera M, Phasomkusolsil S. Adulticidal, larvicidal, pupicidal and oviposition deterrent activities of essential oil fromZanthoxylum limonellaAlston (Rutaceae) againstAedes aegypti(L.) andCulex quinquefasciatus(Say).Asian Pac J Trop Biomed2017; 7(11): 967-978.
[16] Badgujar SB, Patel VV, Bandivdekar AH.Foeniculum vulgareMill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology.Biomed Res Int2014; 2014: 842674.
[17] Ferioli F, Giambanelli E, D’antuono LF. Fennel (Foeniculum vulgareMill. subsp.piperitum) florets, a traditional culinary spice in Italy: Evaluation of phenolics and volatiles in local populations, and comparison with the composition of other plant parts.J Sci Food Agric2017; 97(15): 5369-5380.
[18] Sihoglu Tepe A, Tepe B. Traditional use, biological activity potential and toxicity ofPimpinellaspecies.Industrial Crops and Products2015; 69: 153-166.
[19] Kumar S, Wahab N, Warikoo R. Bioefficacy ofMentha piperitaessential oil against dengue fever mosquitoAedes aegyptiL.Asian Pac J Trop Biomed2011; 1(2): 85-88.
[20] Reegan AD, Gandhi MR, Paulraj MG, Ignacimuthu S. Ovicidal and oviposition deterrent activities of medicinal plant extracts againstAedes aegyptiL. andCulex quinquefasciatusSay mosquitoes (Diptera:Culicidae).Osong Public Health Res Perspect2015; 6(1): 64-69.
[21] Cheah SX, Tay JW, Chan LK, Jaal Z. Larvicidal, oviposition, and ovicidal effects ofArtemisia annua(Asterales:Asteraceae) againstAedes aegypti,Anopheles sinensis, andCulex quinquefasciatus(Diptera: Culicidae).Parasitoly Res2013; 112(9): 3275-3282.
[22] Tikar SN, Yadav R, Mendki MJ, Rao AN, Sukumaran D, Parashar BD.Oviposition deterrent activity of three mosquito repellents diethyl phenyl acetamide (DEPA), diethyl m toluamide (DEET), and diethyl benzamide(DEB) onAedes aegypti, Aedes albopictus, andCulex quinquefasciatus.Parasitoly Res2014; 113(1): 101-106.
[23] Babri RA, Khokhar I, Mahmood Z, Mahmud S. Chemical composition and insecticidal activity of the essential oil ofAnethum graveolensL. seeds.Sci Int (Lahore)2012; 24(4): 453-455.
[24] Amanpour A, Kelebek H , Selli S. Aroma constituents of shade-dried aerial parts of Iranian dill (Anethum graveolensL.) and savory (Satureja sahendicaBornm.) by solvent-assisted flavor evaporation technique.J Food Meas Charact2017; 11(3): 1430-1439.
[25] Singh S, Das S, Singh G, Perotti M, Schuff C, Catalan C. Comparative studies of chemical composition, antioxidant and antimicrobial potentials of essential oils and oleoresins obtained from seeds and leaves ofAnethum graveolensL.Toxicol Open Access2017; 3(119): 2-9.
[26] Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world.Annu Rev Entomol2006; 51:45-66.
[27] Osanloo M, Sereshti H, Sedaghat MM, Amani A. Nanoemulsion of dill essential oil as a green and potent larvicide againstAnopheles stephensi.Environ Sci Pollut Res Int2017; 25(7): 6466-6473.
[28] Seo SM, Jung CS, Kang J, Lee HR, Kim SW, Hyun J, et al. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents againstAedes albopictusand formulation development.J Agric Food Chem2015; 63(45): 9977-9986.
[29] El Zayyat EA, Soliman MI, Elleboudy NA, Ofaa SE. Bioefficacy of some Egyptian aromatic plants onCulex pipiens(Diptera: Culicidae) adults and larvae.J Arthropod Borne Dis2017; 11(1): 147-155.
[30] Ebadollahi A, Nouri-Ganbalani G, Hoseini SA, Sadeghi GR. Insecticidal activity of essential oils of five aromatic plants againstCallosobruchus maculatusF. (Coleoptera: Bruchidae) under laboratory conditions.J Essent Oil Bear Pl2012; 15(2): 256-262.
[31] Bhatt P, Thodsare N, Srivastava R. Toxicity of some bioactive medicinal plant extracts to Asian army worm.Spodoptera litura. J Appl Nat Sci2014;6(1): 139-143.
[32] Chahal K, Monika AK, Bhardwaj U, Kaur R. Chemistry and biological activities ofAnethum graveolensL.(dill) essential oil: A review.J Pharmacogn Phytochem2017; 6(2): 295-306.
[33] Ruangamnart A, Buranaphalin S, Temsiririrkkul R, Chuakul W,Pratuangdejkul J. Chemical compositions and antibacterial activity of essential oil from dill fruits (Anethum graveolensL) cultivated in Thailand.Mahidol Univ J Pharm Sci2015; 42(3): 135-143.
[34] Evergetis E, Michaelakis A, Haroutounian SA. Exploitation of Apiaceae family essential oils as potent biopesticides and rich source of phellandrenes.Ind Crops Prod2013; 41(1): 365-370.
[35] Rocha DK, Matosc O, Novoa MT, Figueiredo AC, Delgado M, Moiteiro C.Larvicidal activity againstAedes aegyptiofFoeniculum vulgareessential oils from Portugal and Cape Verde.Nat Prod Commun2015; 10(4): 677-682.
[36] Mendes LA, Martins GF, Valbon WR, Da Silva De Souza T, Menini L,Ferreira A, et al. Larvicidal effect of essential oils from Brazilian cultivars of guava onAedes aegyptiL.Ind Crops Prod2017; 108(2017): 684-689.
[37] Chauhan N, Malik A, Sharma S, Dhiman RC. Larvicidal potential of essential oils againstMusca domesticaandAnopheles stephensi.Parasitol Res2016; 115(6): 2223-2231.
[38] Fallatah SA, Khater EI. Potential of medicinal plants in mosquito control.J Egypt Soc Parasitol2010; 40(1): 1-26.
[39] Tikar SN, Yadav R, Mendki MJ, Rao AN, Sukumaran D, Parashar BD.Oviposition deterrent activity of three mosquito repellents diethyl phenyl acetamide (DEPA), diethyl m toluamide (DEET), and diethyl benzamide(DEB) onAedes aegypti, Aedes albopictus, andCulex quinquefasciatus.Parasitol Res2014; 113(1): 101-106.
[40] Tawatsin A, Asavadachanukorn P, Thavara U, Wongsinkongman P,Bansidhi J, Boonruad T, et al. Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects againstAedes aegypti(Diptera: Culicidae).Southeast Asian J Trop Med Public Health2006; 37(5): 915-931.
[41] Choo YM, Buss GK, Tan K, Leal WS. Multitasking roles of mosquito labrum in oviposition and blood feeding.Front Physiol2015; 6(306): 1-11.
[42] Day JF. Mosquito oviposition behavior and vector control.Insects2016;7(4): 65.
[43] Hwang YS, Kramer WL, Mulla MS. Oviposition attractants and repellents of mosquitoes.J Chem Ecol1980; 6(1): 71-80.
[44] Rajkumar S, Jebanesan A. Larvicidal and oviposition activity ofCassia obtusifoliaLinn (Family: Leguminosae) leaf extract against malarial vector,Anopheles stephensiListon (Diptera: Culicidae).Parasitol Res2009; 104(2):337-340.
[45] Warikoo R, Wahab N, Kumar S. Oviposition-altering and ovicidal potentials of five essential oils against female adults of the dengue vector,Aedes aegyptiL.Parasitol Res2011; 109(4): 1125-1131.
[46] Choochote W, Chaithong U, Kamsuk K, Jitpakdi A, Tippawangkosol P,Tuetun B, et al. Repellent activity of selected essential oils againstAedes aegypti. Fitoterapia2007; 78(5): 359-364.
[47] Chaubey MK. Fumigant toxicity of essential oils from some common spices against pulse beetle,Callosobruchus chinensis(Coleoptera: Bruchidae).J Oleo Sci2008; 57(3): 171-179.
[48] Chaubey MK. Insecticidal activity ofTrachyspermum ammi(Umbelliferae),Anethum graveolens(Umbelliferae) andNigella sativa(Ranunculaceae)essential oils against stored-product beetleTribolium castaneumHerbst(Coleoptera: Tenebrionidae).Afr J Agric Res2007; 2(11): 596-600.
 Asian Pacific Journal of Tropical Biomedicine2018年4期
Asian Pacific Journal of Tropical Biomedicine2018年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Proteomic approach in human health and disease: Preventive and cure studies
- Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne
- Physicochemical properties, antioxidant and anti-inflammatory activities of coumarin-carbonodithioate hybrids
- Anti-inflammatory and antinociceptive activities of Rhipicephalus microplus saliva
- Comparison of antioxidant capacity and α-glucosidase inhibitory activity between bitter melon (Momordica charanti) fruit and leaf extract
