Physicochemical properties, antioxidant and anti-inflammatory activities of coumarin-carbonodithioate hybrids
Suresh S. Kumbar, Kallappa M. Hosamani?, Arun K. Shettar
1Department of Studies in Chemistry, Karnatak University, Pavate Nagar, Dharwad 580003, Karnataka, India
2P G Department of Studies in Biotechnology and Microbiology, Karnatak University, Pavate Nagar, Dharwad 580003, Karnataka, India
1. Introduction
Many organic reactions are carried out in microwave (MW) irradiators for chemical transformations, which has a great importance[1]. This technology has played a significant role in the development, such as enhanced reaction rates and good yields with high purity[2,3]. In these conscious days of deteriorating environment, eco-friendly reactions are successfully carried out by MW irradiators to obtain most important bioorganic molecules such as coumarin containing heterocycles[4].
Design of drug development work aims for small active molecules,which leads to ingestible capsules or tablets. If the tablet dissolves in the digestive tract, the active contents of the drug are absorbed as small molecules, and chemical composition of the drug often helps to easily enter into the cell membranes and transport to almost any region in the body[5,6]. From these above information, in 1997, Christopher Lipinski,a medicinal chemist and co-workers[7], examined the physicochemical parameters of over 2000 drugs and concluded that a compound, if it matches the following criterion, is more liable to be a drug candidate and easily retained in the body:
?A molecular weight of the chemical compound should fall inside 500 Daltons.
?Lipophilicity of the compound, expressed as a quantity logP is below 5 (given by logP < 5).
?The number of functionalities in the compound that can give hydrogen atoms to hydrogen bonds is less than 5.
?The number of functionalities that can approve hydrogen atoms to form hydrogen bonds should be below 10.
?Molar refractivity should be between 40-130.
Today, the rule of five (RO5) is widely used by medicinal chemists worldwide to assess not only the absorption of compounds but also specific drug-similarity[8]. Hence, with these highlights in mind,we designed coumarin compounds and analyzed them for their physicochemical properties set by RO5, drug-likeness, toxicity prediction with LD50value, and bioactivity scores. It was found that none of the derived conjugates go against the rule and they are accomplished within the frame of RO5.
Naturally derived products are greatly employed as medicines;amongst them coumarins possess vital and remarkable biological activities. Along with the natural sources of coumarins, there are various synthetic routes that have been developed with enhanced biomedicinal properties[9]. In particular, coumarin compounds play an important role in drug discovery due to its unique structure. They have a special attribution, which permits their derivatives to easily bind by weak bond interactions with different receptors in enzymes and in organisms, and display more pharmacological properties as medicinal drugs. The various properties of natural and synthetic coumarins depend on their chemical structures. Coumarins are the class of oxygen-containing heterocycles. Across the globe, a lot of researchers have designed and synthesized coumarin containing heterocycles for the treatment of various diseases[10]. Thus, a particular potentiality with binding capacity and their superior physiological feature that coumarin containing drugs possess in the eradication of diseases such as cancer,HIV, Tuberculosis,etc., have become an extreme highlight[11,12].
The reactive oxygen species (ROS) plays a vital part in the normal physiological process. In eukaryotic cells, these ROS are generated along with a result of aerobic metabolism. In cell physiology, the concentrations of ROS at low-to-high make an impact over activity,viz, directive of cellular signal transduction pathways, resistance against pathogens and cell development[13,14]. Simultaneously an extra production of unstable, highly ROS is regarded as the main donor to cellular and metabolic variations. In many degenerative diseases,the oxidative stress has to play the main role in the pathogenesisi.e.inflammatory, cancer, diabetes; tumor growth and Alzheimer’s disease are contributed by increased cell oxidation[15]. In modern drug development system, maintaining the balance between antioxidant defense system and ROS formation is believed to be a crucial concept for healthy biological systems[16]. Consequently, this is of urgency to invent new derivations with less priced, chemically derived antioxidants for formulations in food and pharmaceutical areas. Most of the coumarin compounds are of plant origin, and chemically derived molecules, which have essential components and can be used to regulate oxidation and in stress-related chronic diseases such as cardiovascular diseases and diabetes. The potential properties of coumarins are directly related to their chemical nature to eliminate free radicals[17] by undergoing oxidation, producing toxic compounds, which generate effects on infective micro-organisms[18].As a normal prophylactic response of damaged tissue, inflammation is a common symptom and has impact on some chronic diseases[19].Inflammation is the denaturation of proteins at tissue level, which is known fact that leads to inflammatory and arthritic diseases[20]. Under inflammatory effects, some chemical mediators are released[21]. Various heterocyclic compounds containing coumarins have been used to treat inflammatory disorders since last two decades. From the literature, it is evident that most of the drugs containing coumarins are helpful for the treatment of the inflammatory disorder[22,23].
In continuation of our earlier research work on coumarincarbonodithioates[24] synthesis, we extended work forin-vitrobiological evaluation of their immune-modulatory potential. Here we described a novel multi-purpose tool for performing nucleophilic condensation reactions, leading to coumarin-carbonodithioates through thioether linkage, with a short reaction time, acutely non-toxic, easy to handle and easy work up without using any purification techniques.
2. Materials and methods
The substituted 4-bromomethyl coumarin (a-e) was synthesized using cyclization reaction of phenols and 4-Bromo ethyl acetoacetate[11].Further condensation of substituted coumarins (a-e) with potassium O-ethyl/methyl carbonodithioate[25] (1) in ethanol solvent afforded coumarin (1a-1j) conjugates under both MW and conventional method(Figure 1). It was determined that MW synthesis is evidenced as exceedingly fast method which comes out as better percentage of yields than the conventional method. The improvement was the speed of reaction, being 35-40 times quicker compared to other method.
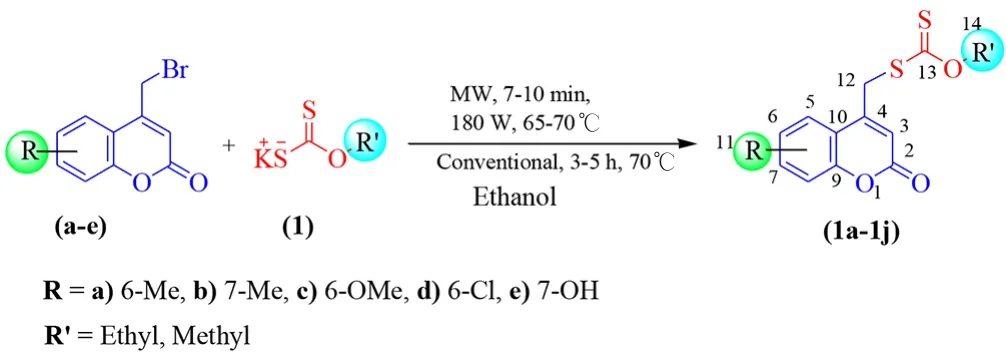
Figure 1. Syntheses of coumarin-carbonodithioate derivatives.
2.1. Representative procedure for synthesis of (1a-1j)
MW method: A mixture of substituted coumarins (0.01 mol) and potassium-O-ethyl dithiocarbonate (0.01 mol) with 5 mL of dry ethanol in the 10mL vial was put into MW irradiator at 60-70 ℃ for 6-10 min and cooled. The reaction progress was monitored by thin layer chromatography. The reaction mass was quenched onto the ice;after filtered, the solid obtained was washed with water.
Conventional method: A mixture of substituted coumarins (0.01 mol)and potassium-O-ethyl dithiocarbonate (0.01 mol) with 5 mL of dry ethanol in 10 mL RB flask was refluxed at 70 ℃ for 3-6 h and cooled.The product formation was confirmed by thin layer chromatography and followed the procedure as prescribed in MW method.
The spectroscopic data of title compounds were confirmed. For compound (1a), in IR spectrum the stretching band at 1712 cm-1assigned for -C=O, the band at 1044 cm-1assigned to the thiocarbonyl group, stretching frequency of S-OEt group observed at 942 cm-1. Further proton-NMR spectra, one triplet corresponding to –C7-H and –C8-H coumarin resonated at 7.33-7.37 ppm (J= 6.2 Hz). Adjacent to this, –C5-H appeared at δ7.22 ppm as a singlet,one more singlet corresponding to –C3-H appeared at δ6.54 ppm.Remaining all protons resonated at their expected values.13C NMR furnished extra support for the compound 1a. The thiocarbonyl and the lactone carbonyl carbons resonated at δ211.86 and 160.66 ppm respectively. The remaining carbons have shown signals in their expected values. Finally, mass spectrum provided an extra supporting data to the architecture of compound 1a {m/zat 294 [M+]}. The remaining coumarin derivatives gave satisfactory results, which were correlated with their structures.
2.2. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability assay
The radical scavenging property of coumarin (1a-1j) molecules was examined by DPPH free radical-scavenging ability assay, and DPPH radical was used as a reagent[26]. One hundred μL of a stock solution in ethanol (60 μM) was thoroughly stirred with 100 μL of the sample solution in 0.5% DMSO (at different w/v). The resultant solution was placed for incubation at RT for half an hour in the dark and then measured absorbance at 517 nm using UV-VIS spectrophotometer. The reference standard used was ascorbic acid. The DPPH scavenging activity of all coumarin compounds was examined using the following equation:% inhibition = (Ac-At)/Ac×100
Acis the absorbance of control solution, and Atis the absorbance of all derivative samples. Experiment was carried out in triplicate. The indication of higher free radical properties was based on lower absorbance of the compound mixtures.
2.3. Evaluation of in vitro anti-inflammatory activity
The anti-inflammatory property of coumarin (1a-1j) compounds was investigated by protein denaturation method[27]. Diclofenac sodium,a powerful non-steroidal drug, was used as a standard compound. A mixture of 2 mL of varied concentrations of coumarin conjugates (500 μg/mL) with diclofenac sodium and 2.8 mL of pH 6.4 phosphate buffer solution was stirred thoroughly with 2 mL of egg albumin and placed in incubation at (27±1) ℃ for 15 min. Denaturation was brought about by maintaining the final solution at 70 ℃ for 10 min,followed by cooling and measuring its absorbance at 660 nm using double distilled-water as a blank and was calculated in triplicate. The %inhibition is examined by using the following expression:
% inhibition = (At-Ac)/Ac×100
Where Atis the absorbance of sample solution and Acis the absorbance of control.
2.4. Physicochemical properties
The theoretical calculation of adsorption, distribution, metablism,and excretion - toxicity properties for synthesized compounds was done and compared with RO5. This was expressed as octanol/water partition coefficient, also called as logP. Besides, other theoretical calculations were carried out such as topological polar surface area,number of hydrogen bond acceptors (n-ON) and number of hydrogen bond donors (n-OHNH).
2.5. Statistical analysis
The result of all compounds was expressed as mean ± SD. To examine the variation and level of statistical significances, oneway ANOVA was used between groups.P< 0.05 was considered statistically significant difference.
3. Results
3.1. In vitro antioxidant assay
Here the different concentrations of coumarin compounds were subjected to DPPH free radical scavenging technique. The antioxidant capacity of all the molecules was compared with standard antioxidant.The results revealed that 1b, 1c, 1e, 1f, and 1j among the compounds were highly active. Similarly, the compounds 1a, 1d, 1h and 1i showed good activity, whereas the only compound 1g found least active compared with standard ascorbic acid. The results were demonstrated in Table 1.
3.2. In vitro anti-inflammatory assay
Obtained results revealed a concentration-dependent inhibition by determining anti-inflammatory activity of coumarin derivatives concentration and standard drug diclofenac sodium at 100 μg/mL.The results of the coumarin compounds were comparable to that of diclofenac sodium. A significant difference in the thermally induced inhibition of denaturation of protein was found in 1b, 1f, 1h, and 1i bearing methyl and methoxy derivatives and these were highly active.Similarly, 1a, 1c, 1d, 1e, 1g, and 1j derivatives showed moderate inhibitory activity. The results were given in Table 2. From Table 2,it could be concluded that synthetic compounds showed effective inhibition of the protein denaturation. With this, evidence-based studies were required to regulate the components behind its anti-inflammatory actions and its mechanisms.

Table 1 In-vitro antioxidant screening results by DPPH free radical scavenging assay.
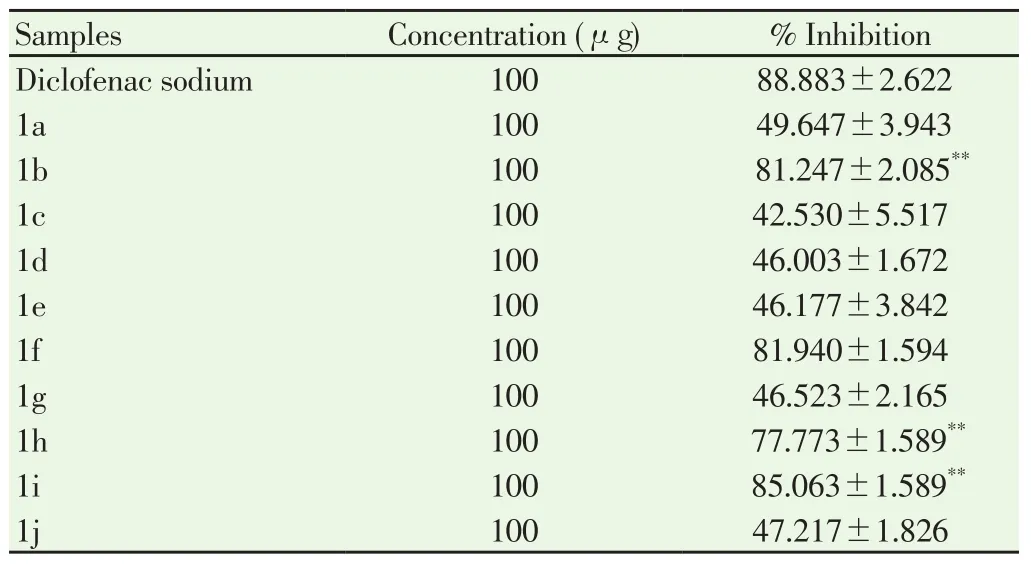
Table 2 In-vitro anti-inflammatory activity results by protein denaturation method.
3.3. Drug-likeness, bioactivity score and toxicity prediction
It was observed that these compounds were in agreement with RO5, indicating more ‘drug-like’ nature. These calculations for all compounds were summarized in Table 3. It was found that the all coumarin-carbonodithioates indicating more drug-like nature and bioactive computability.
The predicted LD50with 650-1500 mg/kg for all the compoundswas summarized in Table 4. As claimed by the developer’s limits,all the synthesized compounds come under the category of class 4 toxicity except hydroxyl substituted compounds which were of class 5 toxicity category and there were no toxic fragments present. This toxicity prediction study revealed that coumarin compounds could act as the lead compounds for further detailed investigations. Based on the investigation ofin-silicotoxicology, all the compounds have shown median LD50values ranging from 625 to 1500 mg/kg. Compounds 1b,1d and 1i showed LD50values of 625 mg/kg while only one compound 1 g showed 1000mg/kg. Furthermore, compounds 1a, 1c, 1f and 1h have shown LD50values of 1250 mg/kg and remaining derivatives 1e and 1j LD50values of 1500 mg/kg. Almost all the targeted compounds belonged to toxicity class of 4 and none of them showed toxicity fragments. The predicted results of all the compounds were tabulated in Table 4.
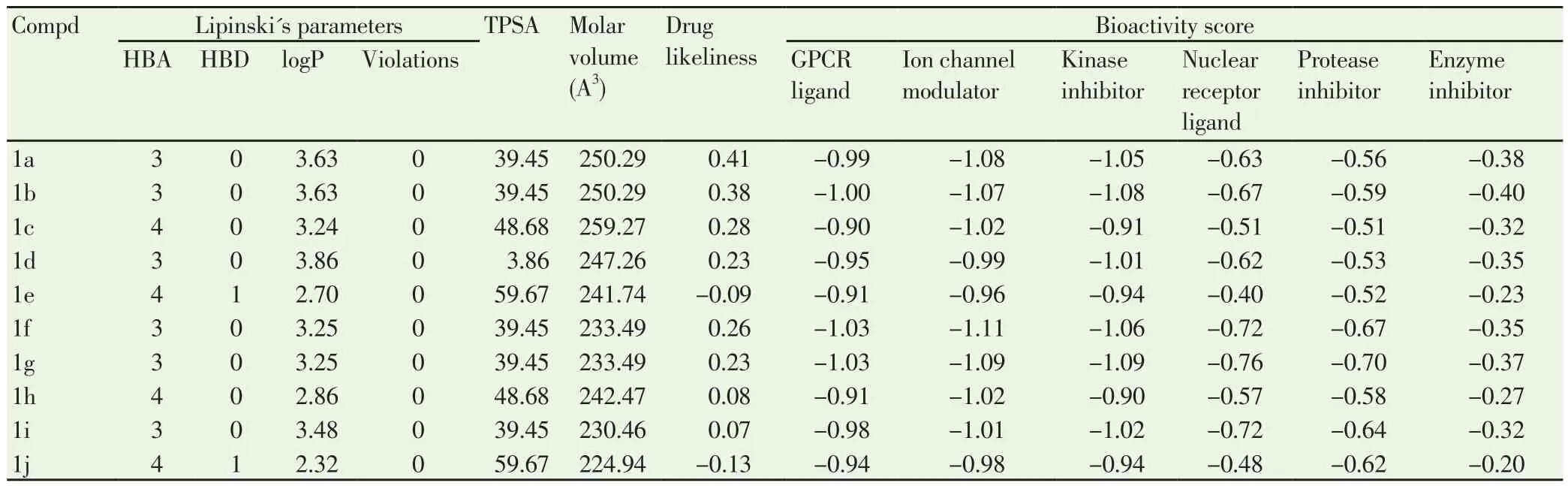
Table 3 Drug-likeness property (RO5) of compounds (1a-1j).
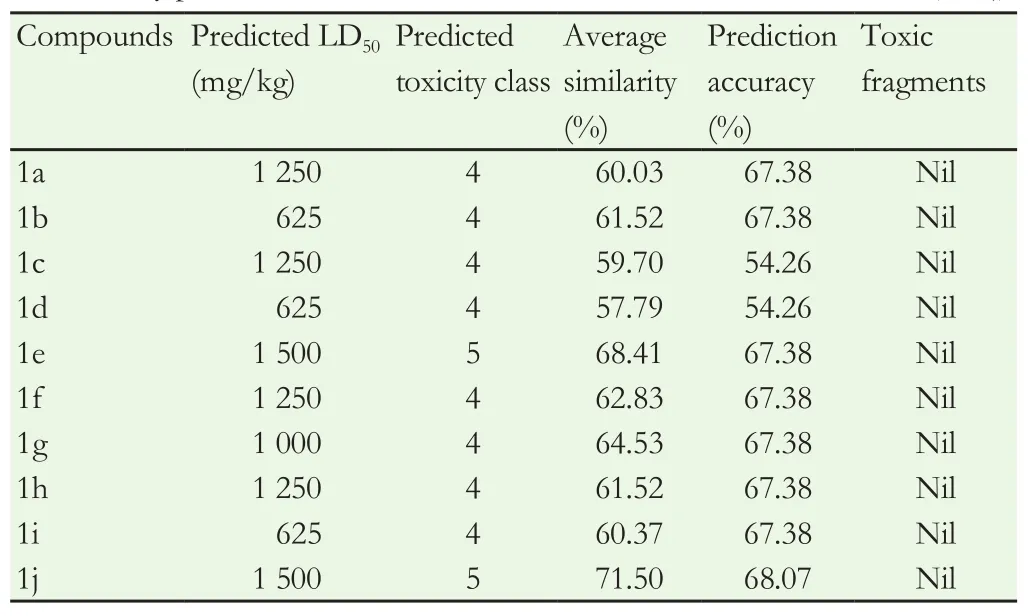
Table 4 Oral toxicity prediction results of coumarin-carbonodithioate derivatives (1a-1j).
4. Discussion
Natural antioxidants make an impact over the enhancement in the antioxidant capacity of blood plasma and help in the prevention of many diseases. Coumarins are also best-known to have several biological properties such as anti-inflammatory, anti-tumor, and antioxidant activities. Thus, in the present study, the coumarin-based biomolecules are synthesized using MW irradiation technique, which would help in the exploration of novel drugs in synthetic organic chemistry. In summary, a series of new coumarin (1a-1j) compounds were found under MW conditions with a short reaction time, nontoxic, easy to handle and simple work up without any purification techniques.
DPPH free radical scavenging assay is an easy method for screening thein-vitroantioxidant properties of coumarin compounds. In the present study, antioxidant activity results revealed that the compounds with substituted coumarins were prime candidates to exhibit excellent activity. Compounds 1b, 1c, and 1e showed higher activity, while the remaining derivatives were moderately active. A significant difference was observed among antioxidant activities of all synthesized compounds and the methyl substituted coumarins came out as a superior total antioxidant capacity, with significantly higher results in all performed.
For many chronic diseases, inflammation is a common symptom.Presently in the market, non-steroidal anti-inflammatory drugs are used for inflammatory diseases but are having side effects of an ulcer, and many others[28]. Drugs which are designed particularly are in demand for management of inflammation due to their fewer side effects and cost-effective. Thus, in the present study simple/viable protein denaturation method was used to screen the anti-inflammatory activity of the coumarin-carbonodithioates. All the derived biomolecules were subjected to anti-inflammatory assay with diclofenac sodium as a reference drug. In comparison to the reference drug, -chloro substituted coumarins showed higher inhibitory activity followed by methyl substitutions whereas remaining derivatives exhibited moderate activity. However, based on this promising observation, it is immature to arrive at the conclusion on a structure-activity aspect of these molecules and further evaluation is needed for their clinical use.
The toxicity prediction study reveals that coumarin compounds can act as the lead compounds for further investigations and potent applications of pharmacological interest. From overall findings,these studies suggest that all the potentialities are because of oxygenated coumarin heterocycle and later enhanced by condensing carbonodithioates. In conclusion, the coumarin compounds can be utilized as antioxidants to inhibit the occurrence and approach of many diseases. However, the further detailed study of coumarins is needed for the exploration of antioxidant and anti-inflammatory drugs.
Conflict of interest statement
The authors declare that there is no conflict of interest.
[1] Loupy A.Microwaves in organic synthesis. Weinheim: Wiley-VCH; 2002.
[2] Kumar KTM, Gupta VK, Sharma A. A green, catalyst-free, solvent-free, high yielding one step synthesis of functionalized benzo[f]furo[3,2-c]chromen-4-(5H)-ones and furo[3,2-c]quinolin-4-(5H)-ones.RSC Adv2015; 5(20): 17087-17095.
[3] Cheng XM, Liu XW. Microwave-enhanced one-pot synthesis of diversified 3-acyl-5-hydroxy benzofurans.J Comb Chem2007; 9(6): 906-908.
[4] Ameta SC. Punjabi PB, Ameta R, Ameta C.Microwave-assisted organic synthesis: A green chemical approach. London: Apple academic press; 2014.
[5] Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs.Exp Dermatol2000; 9(3): 165-169.
[6] Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD.Molecular properties that influence the oral bioavailability of drug candidates.J Med Chem2002; 45(12): 2615-2623.
[7] Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.Adv Drug Delivery Rev1997; 23: 3-25.
[8] Leeson P. Drug discovery: Chemical beauty contest.Nature2012; 481(7382):455-456.
[9] Mazzone G, Galano A, Juan R, Idaboyc A. Coumarin–chalcone hybrids as peroxyl radical scavengers: Kinetics and mechanisms.J Chem Inf Model2016;56(4): 662-670.
[10] O’Kennedy R, Thornes RD.Coumarins–biology, applications and mode of action. UK: John Wiley & Sons Ltd.; 1997.
[11] Reddy DS, Hosamani KM, Devarajegowda HC, Kurjogi MM. A facile synthesis and evaluation of new biomolecule-based coumarin–thiazoline hybrids as potent anti-tubercular agents with cytotoxicity, DNA cleavage,and X-ray studies.RSC Adv2015; 5: 64566-64581.
[12] Reddy DS, Hosamani KM, Devarajegowda HC. Design, synthesis of benzocoumarin-pyrimidine hybrids as novel class of antitubercular agents,their DNA cleavage and X-ray studies.Eur J Med Chem2015; 101: 705-715.
[13] Mittler R. Oxidative stress, antioxidants, and stress tolerance.Trends Plant Sci2002; 7: 405-410.
[14] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease.Int J Biochem Cell Biol2007; 39: 44-84.
[15] Arun KS. Katrahalli K, Kaliwal BB, Vedamurthy AB. Evaluation ofin vitroantioxidant and anti-inflammatory activities of Ximenia americana extracts.Asian Pac J Trop Dis2015; 5(11): 918-923.
[16] Tiwari AK. Imbalance in antioxidant defense and human diseases: Multiple approach of natural antioxidant therapy.Curr Sci2001; 81: 1179-1187.
[17] Jorge EG, Rayar AM, Barigye SJ, Rodriguez MEJ, Veitía MSI.Development of anin Silicomodel of DPPH? free radical scavenging capacity: Prediction of antioxidant activity of coumarin type compounds.Int J Mol Sci2016; 17(6): 881.
[18] Vermerris W, Nicholson R.Phenolic compound biochemistry. Amsterdam:Springer; 2006.
[19] Ashley NT, Weil ZM, Nelson RJ. Inflammation: Mechanisms, costs, and natural variation.Annu Rev Ecol Evol Syst2012; 43: 385-406.
[20] Williams LA, O’Connar A, Latore L, Dennis O, Ringer S, Whittaker JA,et al. Thein vitroanti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of antiinflammatory compounds, without the use of animals, in the early stages of the drug discovery process.West Indian Med J2008; 57: 327-331.
[21] Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation.Cytokine Growth Factor Rev2002; 13: 413-421.
[22] Wang YT, Yan W, Chen QL, Huang WY, Yang Z, Li X, et al. Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus.Biomed Pharmacother2017; 87: 583-588.
[23] Abraham GG, David M, Aparicio S, Hidemi AM, Abraham GR, Luis FR, et al. Synthesis of 3-carboxylated coumarins by Knoevenagel condensation and exploratory anti-inflammatory activity evaluation byin vivomodel.Am J Org Chem2016; 6(1): 17-28.
[24] Suresh SK, Kallappa MH, Arun KS, Srinivas DJ. Environmentally benign synthesis, computational investigation, and mechanistic studies of novel coumarin-carbonodithioate frameworks as anticancer drugs: An approach to microwave synthesis.Eur J Pharm Med Res2017; 4(11): 389-400.
[25] Brian SF, Antony JH, Peter Smith WG, Austin RT.Textbook of practical organic chemistry. 5th Edition. England: Longman Scientific & Technical;1989.
[26] Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds.Trends Plant Sci1997; 2: 152-159.
[27] Padmanabhan P, Jangle SN. Evaluation ofin-vitroanti-inflammatory activity of herbal preparation, a combination of four medicinal plants.Int J Basic Appl Med Sci2002; 2(1): 109-116.
[28] Voravuthikunchai S, Lortheeranuwat A, Jeeju W, Sririrak T, Phongpaichit S, Supawita T. Effective medicinal plants against enterohaemorrhagicEscherichia coli0157:H7.J Ethnopharmacol2004; 94: 49-54.
 Asian Pacific Journal of Tropical Biomedicine2018年4期
Asian Pacific Journal of Tropical Biomedicine2018年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Proteomic approach in human health and disease: Preventive and cure studies
- Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae)
- Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne
- Anti-inflammatory and antinociceptive activities of Rhipicephalus microplus saliva
- Comparison of antioxidant capacity and α-glucosidase inhibitory activity between bitter melon (Momordica charanti) fruit and leaf extract
