Evaluation of physicochemical properties as supporting information on quality control of raw materials and veterinary pharmaceutical formulations
Sr Silv Anlto,Mrll Mtos Coriro Borgs,Hnn Lijoto Olivir,Anrss Ris Vint,Euro Cost Figuiro,Mron Augusto Ll Olivir,Bárr Julin Pinhiro Borgs,Mrlo Antonio Olivir,Wrly Souz Borgs,Kyllr Bstos Borgs,*
aDepartamento de Ciências Naturais,Universidade Federal de S?o Jo?o del-Rei,Campus Dom Bosco,Pra?a Dom Helvécio 74,Fábricas,36301-160 S?o Jo?o del-Rei,Minas Gerais,Brazil
bDepartamento de Química,Universidade Federal do Espírito Santo,Avenida Fernando Ferrari,514,Goiabeiras,29075-910 Vitória,Espírito Santo,Brazil
cLaboratório de Análise de Toxicantes e Fármacos,Faculdade de Ciências Farmacêuticas,Universidade Federal de Alfenas,Rua Gabriel Monteiro da Silva 700,Centro,37130-000 Alfenas,Minas Gerais,Brazil
dDepartamento de Química,Universidade Federal de Juiz de Fora,Rua José Louren?o Kelmer,s/n,36036-330 Juiz de Fora,Minas Gerais,Brazil
eCentro de Ciências da Saúde,Universidade Federal do Espírito Santo,Avenida Marechal Campos,1468,Maruípe,29.043-900 Vitóri,Espírito Santo,Brazil
fCentro Universitário Norte do Espírito Santo,Universidade Federal do Espírito Santo,Br 101 Norte,Km 67,S?o Mateus,Espírito Santo,Brazil
1.Introduction
During the development of pharmaceutical products,it is crucial to know the physicochemical properties of drugs.Pharmaceutical laws oblige drug manufacturers to assess the compatibility of active substances,excipients,and medicinal products with established standards.However,characterization of the active pharmaceutical ingredients(APIs)improves the quality parameters of all raw materials used during the manufacturing process of pharmaceuticals,as well as those in the final products[1].
For adequate investigation of APIs,it is necessary to use appropriate instrumental analysis techniques,and recent reports have shown a high interest in the use of thermogravimetry analysis(TGA)[2,3],Fourier transform infrared spectroscopy(FTIR)[4,5],near-infrared spectroscopy[6],and Raman spectroscopy[7].
Fluoroquinolones are broad-spectrum antibiotics with potent activity against pathogens that have clinical relevance in human and veterinary medicine.They are mainly employed in prevention of and therapy for diseases such as infections of the urinary,gastrointestinal,and respiratory tract,sexually transmitted diseases,skin infections,and chronic osteomyelitis[8].
This class of agents is characterized by a favorable pharmacokinetic pro file,high tissue penetration,and rapid bactericidal action.Currently,the most frequently used fluoroquinolone is enrofloxacin(EFX,Fig.S1).Its antimicrobial properties constitute an advantage for use in poultry,notably for treating mycoplasma infections,colibacillosis,and in animals on pastures.EFX is also used in aquaculture,both as a prophylactic and as a chemotherapeutic agent.In the aquatic environment,it is employed against common bacterial pathogens,namelyYersinia ruckeri,Vibrio anguillarum,Renibacterium salmoninarum,andAeromonas salmonicida.There are reports that ciprofloxacin(CFX,Fig.S1),a primary metabolite of EFX,has a greater microbial efficacy[9].In fact,CFX has become the first fluoroquinolone widely available since the second half of the 1980s.This drug is efficient against numerous gram-negative and gram-positive pathogens,and has been used for treating a variety of bacterial infections[10],both in humans and poultry.One of its disadvantages is the food-drug interaction with divalent and trivalent atoms,such as Ca2+and Al3+,respectively[11].The oral absorption of CFX can be significantly reduced by concomitant administration of food containing milk,for example.Hence,CFX interaction with food may result in changes both in the rate and the extent of absorption and can potentially lead to sub-therapeutic concentrations of the drug and even treatment failure.
Lidocaine(LID,Fig.S1),the most commonly used local anesthetic,is also used as a diluent in injectable formulations.LID contributes to relieving pain related to surgical,dental,and gynecological procedures,both in humans and in animals,despite having no antimicrobial or antiparasitic activity[12].Thus,it can be administered in association with other drugs,such as antiparasitics,anti-inflammatories,and antibiotics.For example,the use of LID in conjunction with fluoroquinolones has been reported previously∶CFX for human prostate surgery[13]and EFX for surgical procedures in birds,for insertion of transmitters for the purpose of species migration research [14].Therefore,the simultaneous analysis of LID,CFX,and EFX in pharmaceutical formulations and other matrices is highly desirable.
Several bioanalytical methods used for measuring concentrations of CFX have been reported,including capillary electrophoresis[15],spectrophotometry[16,17],high performance liquid chromatography(HPLC)with ultraviolet(UV)detection[18–23],and fluorescence detection[24–31].Recently,HPLC methods coupled with mass spectrometry for the determination of CFX in human plasma have also been published.However,there have been few reports of methods for simultaneous determination of EFX and CFX,and mostly in biological fluids and animal tissue[32–41].No method for simultaneous analysis of these compounds in raw materials and veterinary pharmaceutical formulations has been reported.In addition,many HPLC procedures for analysis of LID in pharmaceutical preparations and biological fluids have been published[42–44],as have the use of electrochemical methods for the direct quantification of LID and its impurities in pharmaceutical samples[42–46].
Hence,the objectives of this study were to∶(i)show that FTIR,thermogravimetry(TG),and scanning electronic microscopy(SEM)can be useful for evaluation of the APIs(bulk drugs),i.e.LID,CFX,and EFX,supplied as raw materials;(ii)develop and validate an HPLC method for simultaneous determination of LID,CFX,and EFX in bulk drugs and veterinary pharmaceutical formulations;and(iii)apply this method in commercial tablets of EFX;and injectables of CFX and LID.Despite the existence of a wide range of studiesfordetermining LID,CFX,and EFX,simultaneous determination of these drugs in raw materials and veterinary pharmaceutical formulations,aimed at application in quality control,are still lacking.Similarly,there is a lack of physicochemical studies of raw materials as a coadjutant in quality control described in literature.Finally,the availability of new methods with multidetection ability is very important,because this strategy can simplify the routine,improve the sensitivity and selectivity,and decrease operational costs.
2.Experimental
2.1.Standards and samples
All reference standards of LID,CFX,and EFX from United States Pharmacopeia(USP)were acquired from Sigma Aldrich?(St Louis,MO,USA).Samples of CFX and EFX as bulk drugs were obtained from Hebei Veyong(Shanghai,China)and LID was obtained from Henrifarma(S?o Paulo,SP,Brazil).Samples of finished product of Enrotrat?200mg(Ourofino?,Ribeir?o Preto,SP,Brazil),Lidovet?injectable 2%(Bravet?,Rio de Janeiro,RJ,Brazil),and Ciprodez?injectable 10%(Biovet,Vargem Grande Paulista,SP,Brazil)were purchased from commercial sources in the local market.
2.2.Solvents and chemicals
Acetonitrile and methanol(HPLC grade)and triethylamine were obtained from J.T.Baker?(Mexico City,MX,Mexico).Water was distilled and purified using Millipore Milli-Q Plus system(Bedford,MA,USA).Analytical grade phosphoric acid(H3PO4,85%)was purchased from Merck?(Darmstadt,Germany).All other chemicals were of analytical grade with the highest purity available.
2.3.Instruments for characterization of raw materials
Analysis by FTIR was carried out using Fourier Transform Spectrometer(Bomem Hartmann&Braun,MB series,Quebec,Canada),operating between 4000 and 400 cm–1,with a resolution of 4 cm–1,using the KBr pellet method.TGA was conducted in a termobalance(2950 Thermal Analysis Instrument,TA Instrument,New Castle,DE,USA)with a heating rate of 10°C/min,under a flow rate of nitrogen at 50 mL/min,25–600 °C.The SEM images were obtained at magnifications of 200×and 500×using a microscope TM3000 Hitachi Analytical Table Top(Tarrytown,NY,USA)with an acceleration of tension at 5 kV,employing carbon tape to fix raw materials in the carrier.
2.4.Instrument for chromatographic separation
The Agilent(Agilent Technologies,Palo Alto,CA,USA)chromatographic system used to develop and validate this method consisted of an Agilent LC 1260 quaternary pump(G1311 B),a thermostat,model 1290(G1330B),an automatic injector,model 1260 Hip ALS(G1367E),a column oven,model 1290 TCC(G1316C),and a diode array detector(DAD),model 1260 VL+(G1315C).An Agilent OpenLAB Chromatography Data System?was used to control the HPLC system and was used for data acquisition.Separation was performed on a Gemini C18column(250mm × 4.6mm,5μm)from Phenomenex?(Torrance,CA,USA).The analyses were performed at the Laboratório de Separa??es,Departamento de Ciências Naturais,Universidade Federal de S?o Jo?o del-Rei(UFSJ).
2.5.Analytical conditions
The mobile phase consisted of a mixture of 10mM of phosphoric acid(pH 3.29)∶acetonitrile(85.7∶14.3,v/v).UV detection was performed at 210 and 280 nm.All chromatographic procedures were conducted at 25°C.A flow rate of 1.5 mL/min was used,and the injection volume was 10 μL for standards and samples.
2.6.Preparation of reference solutions and mobile phase
Working solutions of LID,CFX,and EFX used during the method validation step were prepared daily by diluting the stock solution with methanol to concentrations of 48,52,56,60,64,68,and 72 μg/mL for CFX;96,104,112,120,128,136,and 144 μg/mL for EFX and 144,156,168,180,192,204,and 216 μg/mL for LID.
The aqueous solution was prepared by diluting 2.5 mL concentrated phosphoric acid in 500mL of purified water.The pH adjustment(pH 3.29)was performed with solutions of 1.0,0.1,and 0.01 M triethylamine.
2.7.Validation of the method
The parameters were evaluated following the International Conference on Harmonization[47].The method was validated for analysis of bulk drugs from Hebei Veyong?(CFX and EFX)and Henrifarma?(LID),and samples of finished products∶Lidovet?injectable 2%,Ciprodez?injectable 10%,and Enrotrat?200mg tablets.The following parameters were studied∶selectivity,linearity,limit of detection(LOD),limit of quantification(LOQ),precision,and accuracy.
Samples were fortified and analyzed to evaluate the selectivity of the method.The linearity of the assay method was determined by constructing three calibration graphs using seven concentration levels ranging from 80%to 120%of the assay analytes concentration of LID(144,156,168,180,192,204,and 216μg/mL),CFX(48,52,56,60,64,68,and 72μg/mL),and EFX(96,104,112,120,128,136,and 144μg/mL).Three replicate injections of the standard solutions were performed,and the peak areas of the chromatograms were plotted against the concentrations of analytes to obtain the respective calibration curves.The data were then subjected to regression analysis by the least-squares method in order to calculate the calibration model and correlation coefficient(r)value.
The LOD and LOQ values were calculated directly using the calibration curve.The LOD and LOQ were calculated from the slope and the standard deviation(SD)of the intercept of the mean of the three calibration curves determined by a linear regression model[47].
The precision of the method was determined by repeatability and intermediate precision studies.Repeatability was determined by analyzing samples at three different concentrations of LID(156,180,and 204μg/mL),CFX(52,60,and 68μg/mL),and EFX(104,120,and 136 μg/mL)on the same day and under the same experimental conditions(intraday).The intermediate precision of the method was assessed by performing the analysis on two different days(interday).The accuracy was evaluated by applying the proposed method to the analysis of an in-house mixture of the placebo with known amounts of analytes.In order to carry out the test,the pharmaceutical solutions were prepared at the same concentration levels as precision test,and submitted for analysis under previously determined conditions so as to obtain the band areas of each reference chemical substance,at each concentration level.Precision and accuracy results obtained were expressed in terms of RSD(%)and relative error percentage(RE,%),respectively.
2.8.Pharmaceutical formulation and sample preparation
The Enrotrat?200mg tablet samples were milled using a mortar and pestle.Twenty tablets were weighed separately,triturated and dissolved in methanol and diluted to a concentration of 120 μg/mL for EFX analysis.The mixture was then sonicated for 10min and allowed to rest for 10 min.The samples of Lidovet?injectable 2%and Ciprodez?injectable 10%were diluted with methanol at 60 and 180 μg/mL.All analyses were performed in real triplicates and filtered through a Millipore Millex nylon membrane with a 0.45 μm pore size(Merck,Darmstadt,Germany).
3.Results and discussion
There have been some reports of studies combining analytical techniques,such as thermogravimetry and spectroscopyin stability tests[48],polymorphism[49],and quality control of drugs[2,5,6,50–52].Wesolowski et al.[5]evaluated the quality of 27 medicinal products and the composition of marketed pharmaceutical preparations using differential scanning calorimetry,FTIR,and Raman spectroscopy.In order to assess the utility of TG,FTIR and SEM as potential techniques for identification of the constituents of the raw materials,LID,CFX,and EFX were chosen,because methods for the simultaneous determination of these drugs in raw materials and veterinary pharmaceutical formulations are currently lacking.The results obtained by the physicochemical characterization of raw materials are represented by FTIR spectra(Fig.1),TGA(Fig.2),and images of morphological structures(Fig.3).
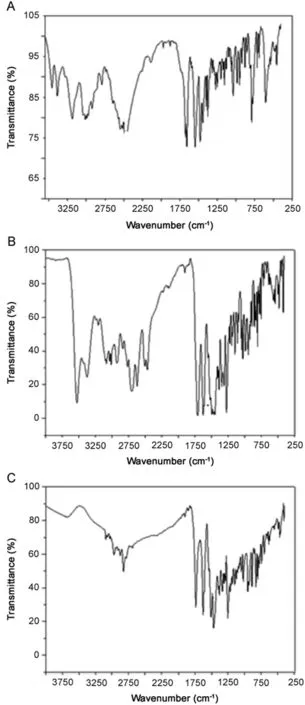
Fig.1.FTIR spectra of(A)lidocaine (LID),(B)ciprofloxacin (CFX),and(C)enrofloxacin(EFX).
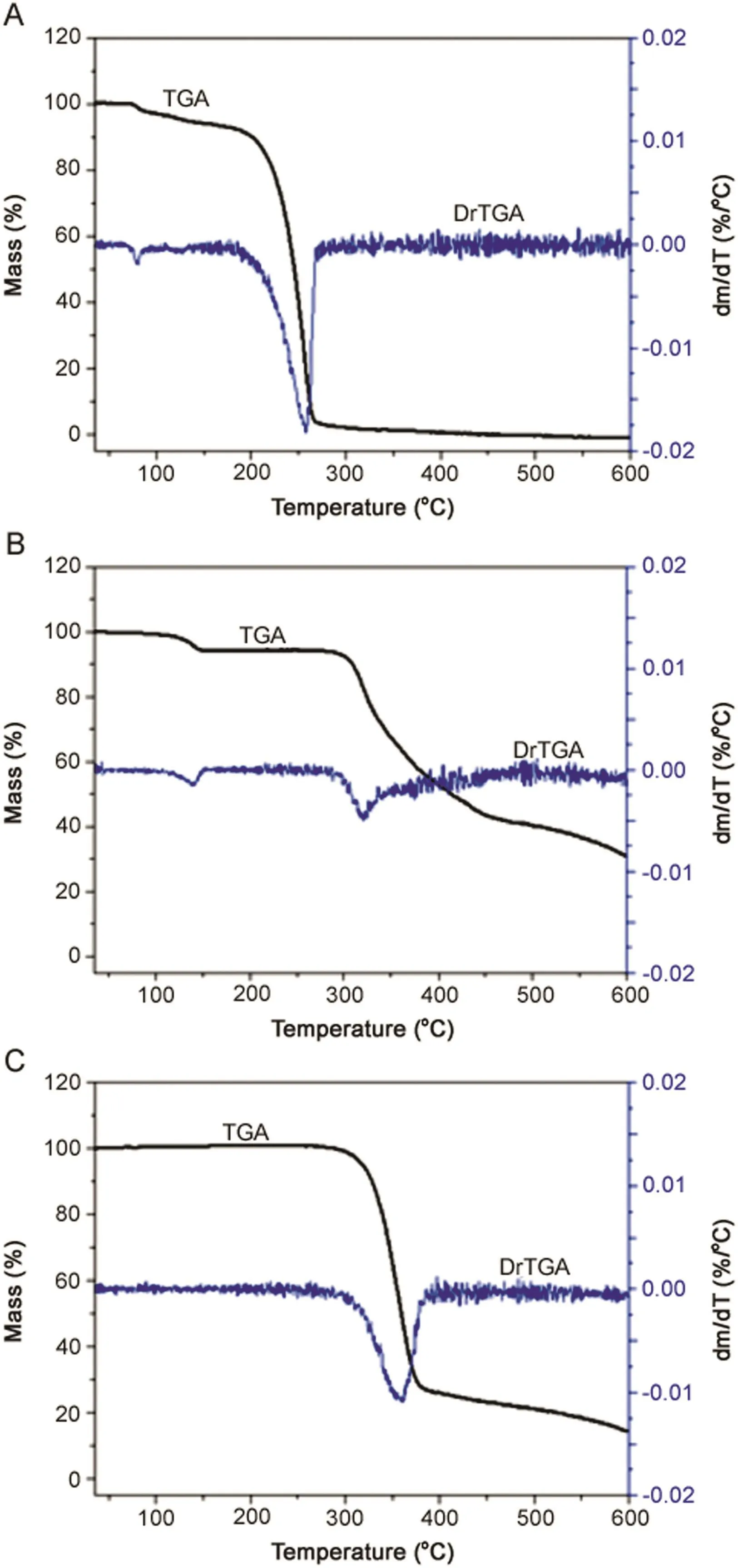
Fig.2.Thermogravimetric curve(TGA)and derivative thermogravimetric curve(DrTGA)of(A)lidocaine(LID),(B)ciprofloxacin(CFX),and(C)enrofloxacin(EFX).
3.1.Characterization of CFX,EFX,and LID raw materials
3.1.1.FTIR
The FTIR spectra offered valuable information about the bulk pharmaceuticals(APIs).As can be seen in Fig.1,the spectra of raw material were different,and can be used for identification of APIs from different suppliers.Fig.1A presents the FTIR spectrum of LID∶3500cm–1(OH stretching and bonding intermolecular H),3000cm–1(aromatic CH stretch and alkene),1750cm–1(CO stretch acid group),1600cm–1(NH bending present in quinolones),1500cm–1(CO stretch carbonyl group),1250cm–1(OH bending),and 1050cm–1(stretching the group CF).The FTIR spectrum of CFX shown in Fig.1B presents the following as main bands∶low intensity band at 3600cm–1(OH stretching),3000cm–1(aromatic CH stretch and alkene),1750cm–1(CO stretch acid group),1600cm–1(NH bending present in quinolones),1250cm–1(OH bending),and 1000cm–1(stretching the group CF).The FTIR spectrum presented in Fig.1C for EFX demonstrates the following as main bands∶3400cm–1(stretch NH2),1650cm–1(stretching C?O primary amide),1550cm–1(stretching C?C aromatic ring),1480 and 1450cm–1(stretch C-N),and 800cm–1( flexing outside the aromatic ring plane).
3.1.2.TGA
TGA of LID,CFX,and EFX in nitrogen presented two,two,and one thermal decomposition stages,respectively.Fig.2A shows two thermal events for LID.The first event(between 100 and 150°C)exhibits a small mass loss(<10%),due to evaporation of volatile compounds.The second event(approximately 300°C)indicates the decomposition process of the drug,demonstrating rapid weight loss(around 55%).CFX presented two thermal events as can be seen in Fig.2B.The first thermal event(up to 100°C)has a small weight loss(<10%)due to water evaporation.The second thermal event(approximately 350°C)indicates the drug decomposition process,which causes a rapid weight loss(around 85%).The thermogram of Fig.2C for EFX shows only a thermal event.In the single thermal event(about 350°C),the drug decomposition process causes rapid weight loss(around 70%).
3.1.3.SEM
These figures were obtained at magnifications of 200×and 500×for each raw material.Figs.3A and B show that LID has a morphological structure that is highly heterogeneous as it is possible to observe some larger,some smaller,and even some intermediate parts.It is also possible to observe that the LID particles are much larger than CFX and EFX particles.Figs.3C and D show that the morphological structure of CFX is very homogeneous and takes the form of small needles,unlike the others compounds.Finally,in Figs.3E and F the morphological structure of EFX is shown;it is heterogeneous and it is possible to observe some larger and other smaller parts.There is a large difference in the particle size of CFX and EFX.
3.2.HPLC method development
Due to the need for a method allowing simultaneous determination of CFX and EFX in the presence of the analgesic LID,we have developed and validated a reverse HPLC method.Analytical conditions were selected after testing the influence of different parameters,such as different columns,mobile phase composition, flow rate,temperature,and other chromatographic conditions.The chromatograms presented in Fig.S2 show that the increase in temperature caused loss of resolution and asymmetry.The optimized chromatographic conditions,such as mobile phase,column,wavelength, flow rate,injection volume,temperature and elution mode,are described in Table 1.Moreover,the chromatographic parameters,such as asymmetric factor,resolution,retention factor,separation factor,and theoretical plates,presented satisfactory results(Table 2).Fig.4 shows typical chromatograms of LID,CFX,and EFX under optimized conditions at 210 and 280 nm,in which LID does not show absorbance at 210nm.
In the present study,some validation parameters,such as selectivity,linearity,LOD,LOQ,precision,and accuracy,were evaluated.Tables 3 and 4 show that the results were satisfactory.The application of the developed method showed satisfactory results.In order to optimize the separation of all analytes,three HPLC columns∶Gemini C18column(250mm × 4.6mm,5μm)from Phenomenex?,Gemini C8column(250mm × 4.6mm,5μm)from Phenomenex?,and an Agilent Poroshcell 120 EC-C18column(100mm×3.0mm,2.7μm),and several mobile phase compositions(phosphoric acid and monopotassium phosphate buffer solutions at different pHs,and different acetonitrile and methanol percentages)were evaluated.Experiments carried out using different mobile phases showed that the chromatographic signals corresponding to the three analytes were better resolved using the Gemini C18column.
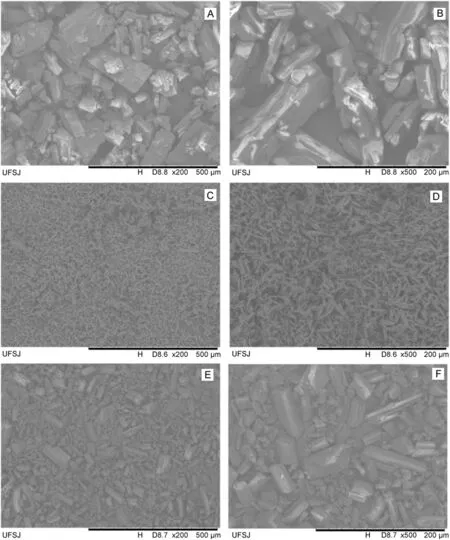
Fig.3.Scanning electron microscopy(SEM)images of lidocaine(LID)at magnifications of 200×(A)and 500×(B),ciprofloxacin(CFX)at magnifications of 200×(C)and 500×(D),and enro floxacin(EFX)at magnifications of 200×(E)and 500×(F).
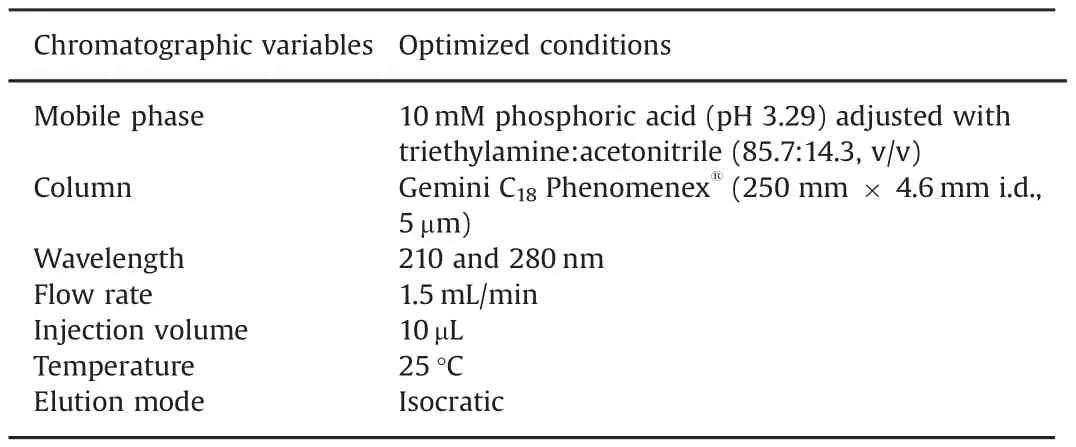
Table 1 HPLC conditions for determination of lidocaine(LID),ciprofloxacin(CFX),and enrofloxacin(EFX)in raw material and pharmaceutical veterinary formulations.

Table 2 Chromatographic parameters for lidocaine(LID),ciprofloxacin(CFX),and enrofloxacin(EFX)under optimized conditions.
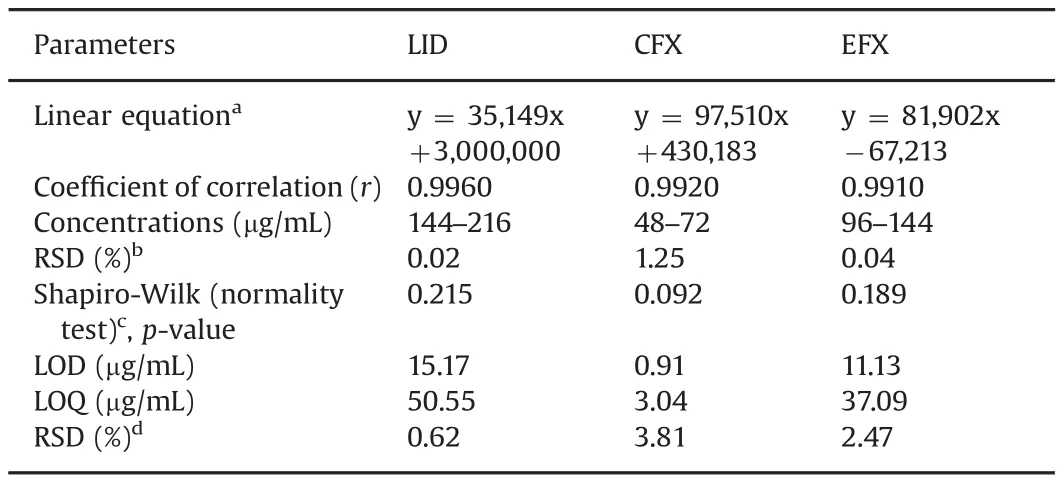
Table 3 Linearity,limit of detection,and limit of quantification of the proposed method.
CFX and EFX are amphoteric,with pKa1values between 5.5 and 6.0 and pKa2between 7.7 and 8.5,respectively[53].LID has a pKa value of 7.9[54].As they have functional ionisable groups,the pH of the mobile phase is a key factor in their separation.In this way,acid pH has been used to protonate the amino groups and the residual silanol groups of the stationary phase,in such a way that peak asymmetry could be reduced.
To achieve the best chromatographic separation of the analytes,different mixtures of methanol and/or acetonitrile with 10 mM of phosphoric acid with the pH adjusted between 3 and 5 with 1,0.1,and 0.01 M triethylamine,were evaluated as the mobile phase.The tests showed that 10 mM of phosphoric acid(pH 3.29)provided a better separation,resulting in narrow and symmetrical peaks with good resolution.The analytical conditions were selected as adequate once all analytes showed baseline separation within 12 min.After careful evaluation of the electronic spectrum pro file in the DAD system,the wavelength was set at 210nm for LID and at 280 nm for CFX and EFX for quantitative analytical purposes.Therefore,a simple,rapid,low cost,and efficient HPLC method for separation of LID,CFX,and EFX was developed.
3.3.HPLC method validation
The calibration curves were prepared by plotting peak areas of LID,CFX,and EFX against the analyte concentrations,and they were linear in the range of 144–216μg/mL,48–72μg/mL,and 96–144μg/mL,respectively.Peak areas and concentrations were subjected to least-squares linear regression analysis to calculate the calibration equation andrvalue.Allrvalues were≥0.99,showing acceptable linearity for all analytes,since the normality test(Shapiro-Wilk)performed in residues presented no statistically significant result(p-value>0.05).The developed method presented LOD and LOQ in the concentration range of μg/mL,which permitted correct determination of the concentration of the studied drugs.The LOD and LOQ were found to be between 0.91 and 15.17 and between 3.04 and 50.55μg/mL,respectively,for all analytes.The precision and accuracy of the method were evaluated by calculating RSD%and RE%,respectively,for six determinations of CFX(52,60,and 68μg/mL),EFX(104,120,and 136μg/mL),and LID(156,180,and 204μg/mL)over the course of 2 days and under the same experimental conditions.Six replicates were carried out for each concentration(n=6)in the intraday test.These results confirmed the precision and accuracy of the method within the desired range.The results were satisfactory,as all values were less than 3.0%.The validation procedure was repeated the next day in order to evaluate the efficiency of the method on different days(interday).All the studied compounds showed good results with low RE%.
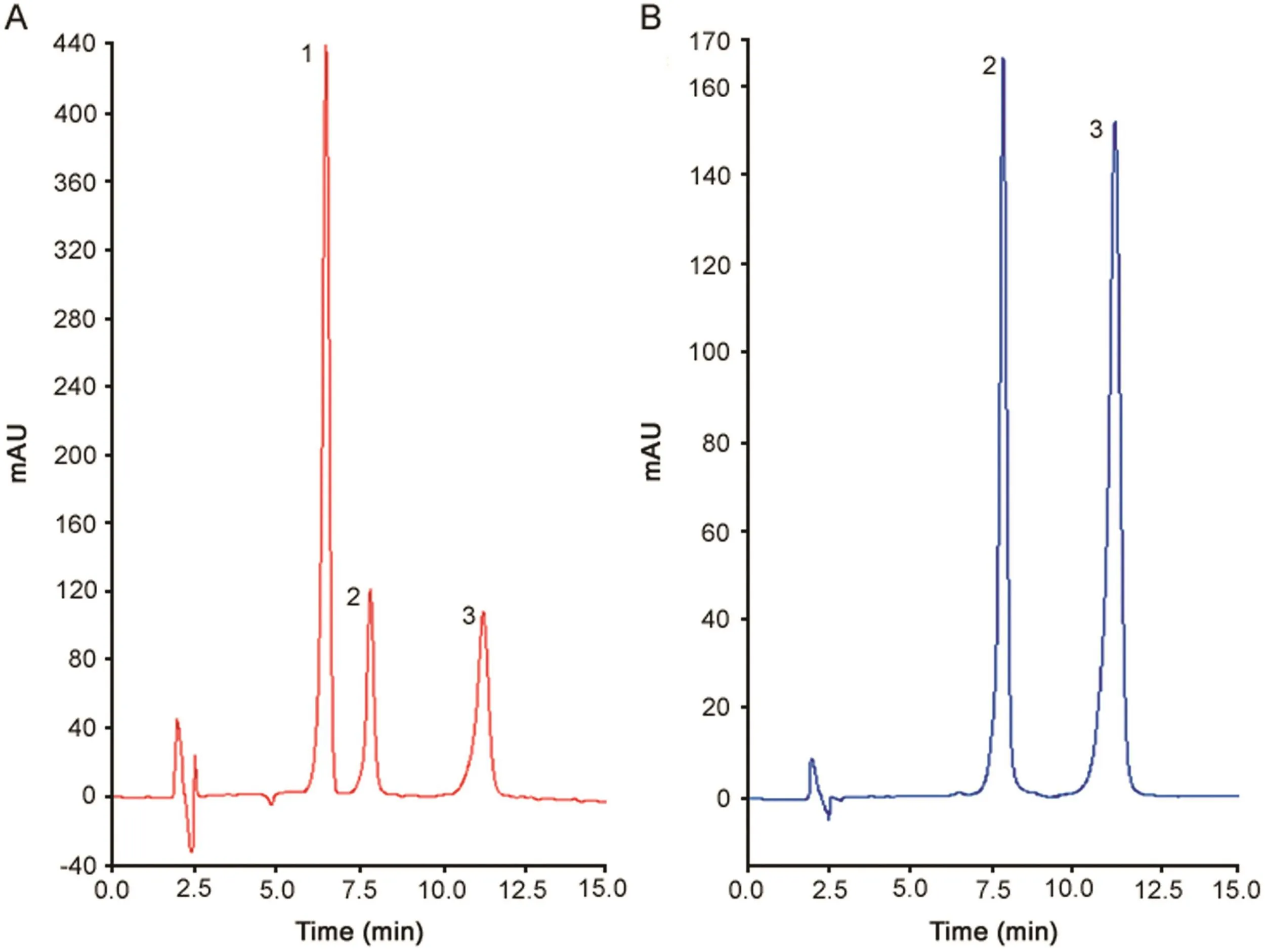
Fig.4.Chromatogram referring to the optimized method for analysis of(1)Lidocaine(LID),(2)ciprofloxacin(CFX),and(3)enrofloxacin(EFX).Conditions∶Gemini C18(250 mm × 4.6 mm i.d.,5.0 μm)Phenomenex? column,mobile phase consisting of 10 mM of phosphoric acid(pH 3.29)∶acetonitrile(85.7∶14.3,v/v)at a flow rate of 1.5 mL/min,detection at 210 and 280 nm using a DAD,temperature at 25 °C,injection volume of 10 μL,and isocratic mode.

Table 4 Precision and accuracy for the simultaneous determination of lidocaine(LID),ciprofloxacin(CFX)and enro floxacin(EFX).
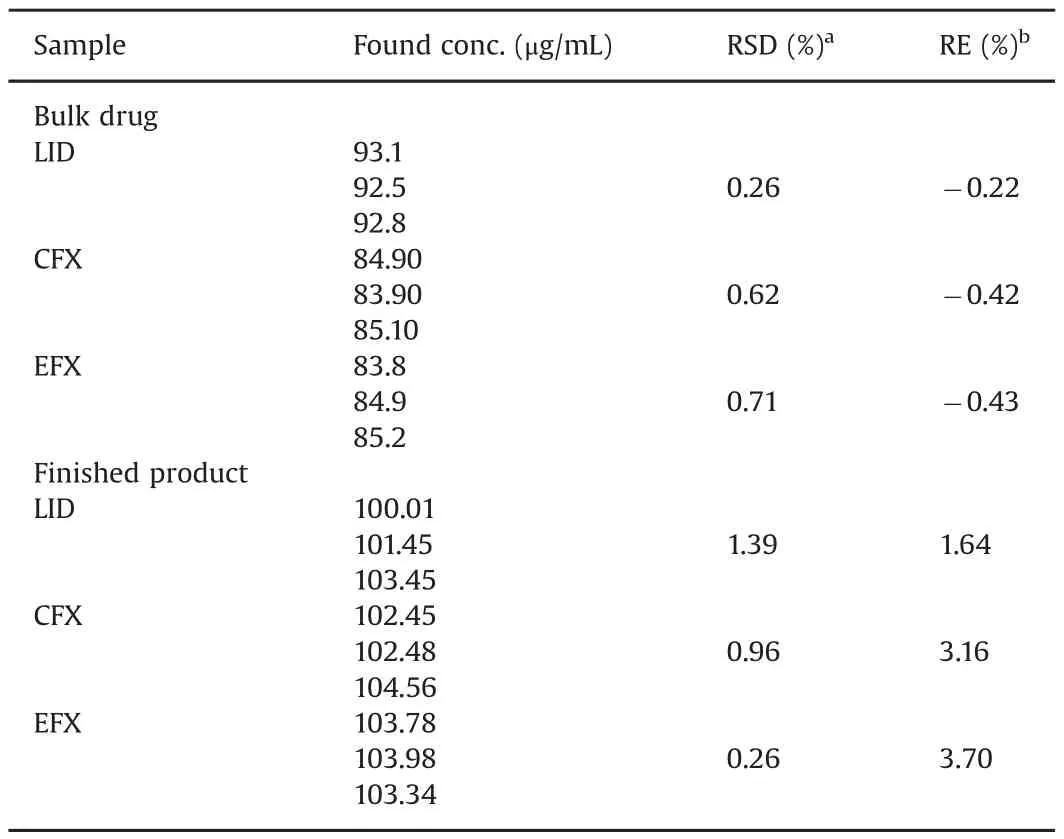
Table 5 Determination of lidocaine(LID),ciprofloxacin(CFX)and enrofloxacin(EFX)in bulk drugs and finished products.
3.4.Application of the method in drug bulk and finished products
The applicability of the proposed method was evaluated by determination of LID,CFX,and EFX in drug bulk and finished products,which were Enrotrat?200mg(Ourofino?,Ribeir?o Preto,SP,Brazil),Lidovet?injectable 2%(Bravet?,Rio de Janeiro,RJ,Brazil),and Ciprodez?injectable 10%.All results are presented in Table 5.All analyses showed RSD(%)lower than 5%,which proves the efficiency of the developed method.In addition,bulk drugs showed values around 92.8%for LID and 84.6%for CFX and EFX.This approach allows testing of raw material and can help raw material suppliers to produce finished products with the correct dosage and to adjust the dosage appropriately.Finally,all finished products that were analyzed are in accordance with their specifications.
4.Conclusions
The physicochemical proprieties obtained by FTIR,TGA,and SEM for LID,CFX,and EFX raw materials showed the presence of bands that are characteristic of the presence of the organic groups that comprise their molecular structures,a significance difference in the thermal events related to mass loss,and large differences between their morphological structures,respectively.These findings are useful for the evaluation,differentiation and quality assurance of raw materials.In this study,a HPLC-DAD method has been developed that allows simultaneous determination of raw materials and veterinary pharmaceutical formulations containing LID,CFX,and EFX.This method was proved to be simple,rapid,low cost,and efficient.The obtained results were considered satisfactory since the baseline separation for all analytes occurred in less than 12min under isocratic conditions.Temperature and pH conditions can markedly influence the elution of analytes during the optimization process.The method developed was proved to be useful for evaluation of raw materials and finished products,in addition to providing an analytical method for simultaneous determination of EFX,CFX,and LID,which can also be extended to other matrices and applications.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank the Brazilian agencies CNPq(Conselho Nacional de Desenvolvimento Científico e Tecnológico),CAPES(Coordena??o de Aperfei?oamento de Pessoal de Nível Superior),FAPES(Funda??o de Amparo à Pesquisa e Inova??o do Espírito Santo)and FAPEMIG(Funda??o de Amparo à Pesquisa do Estado de Minas Gerais)for financial support.This work is a collaborative research project with members of Rede Mineira de Química(RQ-MG)supported by FAPEMIG(Project∶REDE-113/10;Project∶CEX-RED-0010–14).
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi∶10.1016/j.jpha.2018.01.001.
[1]ICH,International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use,〈http∶//www.ich.org/home.htlm〉(Accessed 16 September 2016).
[2]A.P.B.Gomes,L.P.Correia,M.O.da Silva Simoes,et al.,Development of thermogravimetric method for quantitative determination of ketoconazole,J.Therm.Anal.Calorim.91(2008)317–321.
[3]M.Lappalainen,M.Karppinen,Techniques of differential scanning calorimetry for quantification of low contents of amorphous phases,J.Therm.Anal.Calorim.102(2010)171–180.
[4]C.Tanase,L.Odochian,N.Apostolescu,TG-FTIR analysis applied to the study of thermal behaviour of some edible mushrooms,J.Therm.Anal.Calorim.103(2011)1079–1085.
[5]M.Wesolowski,P.Szynkaruk,E.Makurat,DSC and IR as supporting tools for identification of methylxanthines in solid dosage forms of drugs,J.Therm.Anal.Calorim.109(2012)807–815.
[6]G.J.Vergote,Ch Vervaet,J.P.Remon,et al.,Near-infrared FT-Raman spectroscopy as a rapid analytical tool for the determination of diltiazem hydrochloride in tablets,Eur.J.Pharm.Sci.16(2002)63–67.
[7]Y.Roggo,K.Degardin,P.Margot,Identification of pharmaceutical tablets by Raman spectroscopy and chemometrics,Talanta 81(2010)988–995.
[8]P.C.Appelbaum,P.A.Hunter,The fluoroquinolone antibacterials∶past,present and future perspectives,Int.J.Antimicrob.Agents 16(2000)5–15.
[9]N.Gorla,E.Chiostri,L.Ugnia,et al.,HPLC residues of enrofloxacin and ciprofloxacin in eggs of laying hens,Int.J.Antimicrob.Agents 8(1997)253–256.
[10]D.A.Talan,K.G.Naber,J.Palou,et al.,Extended-release ciprofloxacin(Cipro XR)for treatment of urinary tract infections,Int.J.Antimicrob.Agents 23(Suppl.)(2004)S54–S66.
[11]K.Pápai,M.Budai,K.Ludányi,et al.,In vitro food–drug interaction study∶which milk component has a decreasing effect on the bioavailability of ciprofloxacin?J.Pharm.Biomed.Anal.52(2010)37–42.
[12]D.Smith,Determination and temperature effects of lidocaine(Lignocaine)hydrochloride,epinephrine,methylparaben,2,6-dimethylaniline,and p-hydroxybenzoic acid in USP Lidocaine Injection by ion-pair reversed-phase high pressure liquid chromatography,J.Chromatogr.Sci.19(1981)253–258.
[13]D.E.Leocádio,T.L.Frenkl,B.S.Stein,Office based transurethral needle ablation of the prostate with analgesia and local anesthesia,J.Urol.178(2007)2052–2054.
[14]J.-P.L.Savard,L.Lesage,S.G.Gilliland,et al.,Molting,staging,and wintering locations of common eiders breeding in the Gyrfalcon Archipelago,Ungava Bay,Arctic 64(2011)197–206.
[15]M.I.Pascual-Reguera,G.P.Parras,A.M.Díaz,Solid-phase UV spectrophotometric method for determination of ciprofloxacin,Microchem.J.77(2004)79–84.
[16]K.H.Bannefeld,H.Stass,G.Blaschke,Capillary electrophoresis with laser-induced fluorescence detection,an adequate alternative to high-performance liquid chromatography,for the determination of ciprofloxacin and its metabolite desethyleneciprofloxacin in human plasma,J.Chromatogr.B 692(1997)453–459.
[17]S.Mostafa,M.El-Sadek,E.A.Alla,Spectrophotometric determination of ciprofloxacin,enrofloxacin and pefloxacin through charge transfer complex formation,J.Pharm.Biomed.Anal.27(2002)133–142.
[18]L.Pou-Clave,F.Campos-Barreda,C.Pascual-Mostaza,Determination of ciprofloxacin in human serum by liquid chromatography,J.Chromatogr.B 563(1991)211–215.
[19]G.Mack,Improved high-performance liquid chromatographic determination of ciprofloxacin and its metabolites in human specimens,J.Chromatogr.B 582(1992)263–267.
[20]G.J.Krol,G.W.Beck,T.Benham,HPLC analysis of ciprofloxacin and ciprofloxacin metabolites in body fluids,J.Pharm.Biomed.Anal.14(1995)181–190.
[21]M.T.Maya,N.J.Gon?alves,N.B.Silva,et al.,Simple high-performance liquid chromatographic assay for the determination of ciprofloxacin in human plasma with ultraviolet detection,J.Chromatrogr.B 755(2001)305–309.
[22]R.M.Pellegrino,F.Segonoli,C.Cagini,Simultaneous determination of ciprofloxacin and the active metabolite of prulifloxacin in aqueous human humor by high-performance liquid chromatography,J.Pharm.Biomed.Anal.47(2008)567–574.
[23]S.Watabe,Y.Yokoyama,K.Nakazawa,et al.,Simultaneous measurement of pazufloxacin,ciprofloxacin,and levo floxacin in human serum by high-performance liquid chromatography with fluorescence detection,J.Chromatogr.B 878(2010)1555–1561.
[24]S.Zhai,M.R.Korrapati,X.Wei,et al.,Simultaneous determination of theophylline,enoxacin and ciprofloxacin in human plasma and saliva by highperformance liquid chromatography,J.Chromatogr.B 669(1995)372–376.
[25]B.B.Ba,D.Ducint,M.Fourtillan,et al.,Fully automated high-performance liquid chromatography of ciprofloxacin with direct injection of plasma and Mueller–Hinton broth for pharmacokinetic/pharmacodynamic studies,J.Chromatogr.B 714(1998)317–324.
[26]H.Scholl,K.Schmidt,B.Weber,Sensitive and selective determination of picogram amounts of ciprofloxacin and its metabolites in biological samples using high-performance liquid chromatography and photothermal post-column derivatization,J.Chromatogr.416(1987)321–330.
[27]A.Misuno,T.Uematsu,M.Nakashima,Simultaneous determination of ofloxacin,nor floxacin and ciprofloxacin in human hair by high-performance liquid chromatography and fluorescence detection,J.Chromatogr.B 653(1994)187–193.
[28]A.Zotou,N.Miltiadou,Sensitive LC determination of ciprofloxacin in pharmaceutical preparations and biological fluids with fluorescence detection,J.Pharm.Biomed.Anal.28(2002)559–568.
[29]S.Imre,M.T.Dogaru,C.E.Vari,et al.,Validation of an HPLC method for the determination of ciprofloxacin in human plasma,J.Pharm.Biomed.Anal.33(2003)125–130.
[30]O.R.Idowu,J.O.Peggins,Simple,rapid determination of enrofloxacin and ciprofloxacin in bovine milk and plasma by high-performance liquid chromatography with fluorescence detection,J.Pharm.Biomed.Anal.35(2004)143–153.
[31]Z.Bybíralová,M.Nobilis,J.Zoulova,et al.,High-performance liquid chromatographic determination of ciprofloxacin in plasma samples,J.Pharm.Biomed.Anal.37(2005)851–858.
[32]K.L.Tyczkowska,K.M.Hedeen,D.P.Aucoin,et al.,High-performance liquid chromatographic method for the simultaneous determination of enrofloxacin and its primary metabolite ciprofloxacin in canine serum and prostatic tissue,J.Chromatogr.493(1989)337–346.
[33]V.Hormazabal,A.Rogstad,I.Steffenak,et al.,Rapid assay for monitoring residues of enrofloxacin and sara floxacin in fish tissues by high performance liquid chromatography,J.Liq.Chromatogr.14(1991)1605–1614.
[34]K.Flammer,D.P.Aucoin,D.A.Whitt,Intramuscular and oral disposition of enrofloxacin in African grey parrots following single and multiple doses,J.Vet.Pharmacol.Ther.14(1991)359–366.
[35]J.A.Tarbin,D.J.Tyler,G.Shearer,Analysis of enrofloxacin and its metabolite ciprofloxacin in bovine and porcine muscle by high‐performance liquid chromatography following cation exchange clean‐up,Food Addit.Contam.9(1992)345–350.
[36]M.Horie,K.Saito,N.Nose,et al.,Simultaneous determination of benofloxacin,dano floxacin,enrofloxacin and o floxacin in chicken tissues by highperformance liquid chromatography,J.Chromatogr.B 653(1994)69–76.
[37]K.L.Tyczkowska,R.D.Voyksner,K.L.Anderson,et al.,Simultaneous determination of enrofloxacin and its primary metabolite ciprofloxacin in bovine milk and plasma by ion-pairing liquid chromatography,J.Chromatogr.B 658(1994)341–348.
[38]G.Carlucci,Analysis of fluoroquinolones in biological fluids by high-performance liquid chromatography,J.Chromatogr.A 812(1998)343–367.
[39]L.Escuder-Gilabert,S.Sagrado,R.M.Villanueva-Camafiasand,et al.,Analysis of pharmaceutical preparations containing local anesthetics by micellar liquid chromatography and spectrophotometric detection,Chromatographia 49(1999)85–90.
[40]J.Manceau,M.Gicquel,M.Laurentine,et al.,Simultaneous determination of enrofloxacin and ciprofloxacin in animal biological fluids by high-performance liquid chromatography∶application in pharmacokinetic studies in pig and rabbit,J.Chromatogr.B 726(1999)175–184.
[41]A.Posyniak,J.Zmudzki,S.Semeniuk,et al.,Determination of fluoroquinolone residues in animal tissues by liquid chromatography,Biomed.Chromatogr.13(1999)279–285.
[42]Z.Fija?ek,E.Baczynski,A.Piwońska,et al.,Determination of local anaesthetics and their impurities in pharmaceutical preparations using HPLC method with amperometric detection,J.Pharm.Biomed.Anal.37(2005)913–918.
[43]M.A.A.Mohammad,LC determination of lidocaine and prilocaine containing potential risky impurities and application to pharmaceuticals,Chromatographia 70(2009)563–568.
[44]M.Abdel-Rehim,M.Bielenstein,Y.Askemark,et al.,High-performance liquid chromatography–tandem electrospray mass spectrometry for the determination of lidocaine and its metabolites in human plasma and urine,J.Chromatogr.B 741(2000)175–188.
[45]British Pharmacopoeia,Medicines and Healthcare Products Regulatory Agency,London,UK,2011.
[46]European Pharmacopoeia,European Directorate for the Quality of Medicines&Healthcare(EDQM),Strasbourg,France,2011.
[47]International Conference on Harmonization(ICH),Validation of Analytical Procedures∶Methodology,Technical Requirements for the Registration of Pharmaceuticals for Human Use,Geneva,Switzerland,1996.
[48]S.Y.Lin,S.L.Wang,Advances in simultaneous DSC–FTIR microspectroscopy for rapid solid-state chemical stability studies∶some dipeptide drugs as examples,Adv.Drug Deliv.Rev.64(2012)461–478.
[49]S.Y.Lin,An overview of famotidine polymorphs∶solid-state characteristics,thermodynamics,polymorphic transformation and quality control,Pharm.Res.31(2014)1619–1631.
[50]L.C.S.Cides,A.A.S.Araújo,M.Santos-Filho,et al.,Thermal behaviour,compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions,J.Therm.Anal.Calorim.84(2006)441–445.
[51]F.A.Aguiar,C.M.de Gaitani,K.B.Borges,Capillary electrophoresis method for the determination of isradipine enantiomers∶stability studies and pharmaceutical formulation analysis,Electrophoresis 32(2011)2673–2682.
[52]F.A.de S.Ribeiro,C.R.T.Tarley,K.B.Borges,et al.,Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract ofCucurbita pepoL,Sens.Actuators B Chem.185(2013)743–754.
[53]J.Barbosa,D.Barron,J.Cano,et al.,Evaluation of electrophoretic method versus chromatographic,potentiometric and absorptiometric methodologies for determining pKa values of quinolones in hydroorganic mixtures,J.Pharm.Biomed.Anal.24(2001)1087–1098.
[54]H.A.McLure,A.P.Rubin,Review of local anaesthetic agents,Minerva.Anestesiol.71(2005)59–74.
 Journal of Pharmaceutical Analysis2018年3期
Journal of Pharmaceutical Analysis2018年3期
- Journal of Pharmaceutical Analysis的其它文章
- JPA Prize in 2016
- Sensitive and rapid determination of amantadine without derivatization in human plasma by LC–MS/MS for a bioequivalence study
- Physics,chemistry,and Hirshfeld surface analyses of gamma-irradiated thalidomide to evaluate behavior under sterilization doses
- Enrichment and immobilization of macromolecular analytes on a porous membrane utilizing permeation drag
- Enhancing the dissolution of phenylbutazone using Syloid?based mesoporous silicas for oral equine applications
- Identi fication of three kinds of Plumeria flowers by DNA barcoding and HPLC specific chromatogram
