Parametric study and effect of calcination and carbonation conditions on the CO2 capture performance of lithium orthosilicate sorbent
Nurul Azrin Zubbri,Abdul Rahman Mohamed ,*,Maedeh Mohammadi
1 Low Carbon Economy(LCE)Research Group,School of Chemical Engineering,Engineering Campus,Universiti Sains Malaysia,Nibong Tebal 14300,Malaysia
2 Faculty of Chemical Engineering,Babol Noushirvani University of Technology,Babol 47148,Iran
1.Introduction
Greenhouse effect occurs concurrently with the presence of greenhouse gases(GHGs)in the atmosphere,which has led to global warming due to the absorption and reflection of the solar and atmospheric energy.These phenomena have contributed to the rise of the Earth's temperature[1].Consequently,global warming has affected the average temperature of the Earth,as previously reported in the International Agency Energy(IEA)Report of 2013.In a period of a hundred years,the world's temperature had increased by 0.74%and forecasted to rise up to another 6.4%by the end of the 21st century[2].
Generally,the easiest way to capture CO2from post-combustion emission gases is via post-combustion capture.This technology applies two methods in capturing CO2,namely,the wet sorbent[3–5]and the dry sorbent[6,7].A selective CO2sorption process using dry sorbent basically involves solid–gas interaction and a cyclic regeneration process[8].In order to treat large volumes of CO2from various sources,it is important to enhance the CO2sorption capacity and selectivity performance.Therefore,one of the solutions to this problem was by introducing a functional group,mainly the amine group(high affinities towards CO2),onto the surface of the dry sorbent.Amine group's incorporation onto the solid sorbent is also expected to improve the polarisation properties and CO2capture performance[1,9,10].
Various solid sorbents have been investigated and proposed in the literature.Lithium-containing materials were found to be among the potential sorbents that are able to capture CO2at higher capacity[11–16].Lithium orthosilicate is one of the promising materials for CO2capture compared to other lithium-containing materials,such as lithium zirconate(Li2ZrO3),lithium ferrite(LiFeO2),lithium nitrite(LiNiO2),and lithium trioxide(Li2TiO3),due to its high CO2sorption capacity,faster adsorption rate,and better cyclic regeneration properties[17,18].
Several routes have been developed to produce Li4SiO4sorbent,such as via solid state reactions[11,19,20],spray drying method[21],sol–gel[22],and precipitation[4,23].Traditional solid state reaction and precipitation method usually require high temperature processes.This condition could create problems,such as volatilization,and difficulty to control the structure and composition[24].A solid state reaction of a mechanical mixture of fumed silica and lithium nitrate was performed through gluconic acid treatment coupled with a carbon coating process[25].However,the sol–gel method would be more appropriate because it offers products with nanometre in particle size,high purity,and good chemical homogeneity.Nano-sized materials are able to provide enough surface areas for the chemisorption reaction with CO2,which will increase the efficiency of CO2sorption into the sorbent.Additionally,the sol–gel method was found to be able to produce relatively high purity(94%)Li4SiO4,with Brunauer–Emmett–Teller(BET)surface area of 1–14 m2·g?1[26].Moreover,pure nanocrystalline Li4SiO4,with BET surface area of 7 m2·g?1and 4–12 nm in particle size were successfully synthesised by combining the sol gel method with the reverse microemulsion technique[23].
2.Materials and Methods
2.1.Synthesis of lithium orthosilicate
In this study,Li4SiO4sorbents were prepared from raw chemicals,namely,lithium nitrate(LiNO3)and tetraethylorthosilicate(SiC8H20O4).Other chemicals were also used in the synthesising process,which include ammonium fluoride(NH4F),ammonia solution(NH4OH),ethanol(C2H5OH),and deionised water(H2O).All gases utilised in the experiments were supplied by Golden Air Sdn.Bhd.The properties of all chemicals and gases used are listed in Table 1.
The Li4SiO4sorbent used in this study was prepared via the sol gel method.Two separate solutions were prepared(alkoxide and catalyst solution).The alkoxide solution contained 11 ml of ethanol and 5 ml of tetraethylorthosilicate,while the catalyst solution contained 11 ml of ethanol,7 ml of deionised water,and 0.371 ml of either ammonium fluoride(NH4F)or ammonium hydroxide(NH4OH).Meanwhile,the catalyst stock solution was prepared by mixing 1.852 g of NH4F with 22.78 ml of NH4OH.Details of the resulting sorbent's compositions are shown in Table 2.
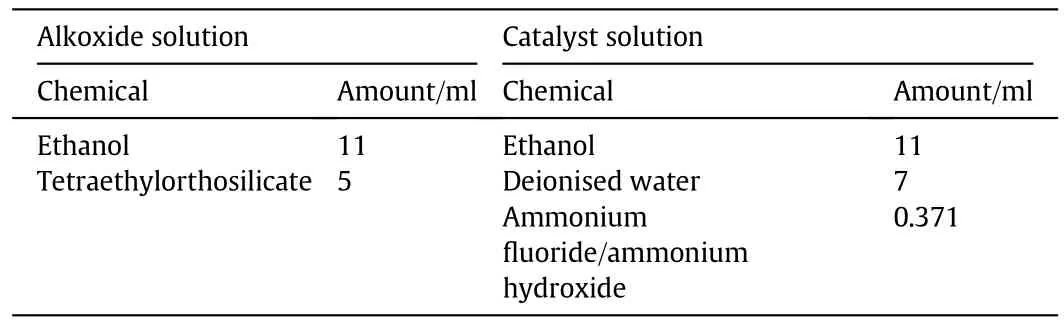
Table 2 Composition of lithium orthosilicate sorbent
Then,the prepared mixtures were mixed together using a hot plate stirrer for 15 min untilthey formed a heavy,cloudy colloid sol.Next,the sol was transferred to a water bath and continuously stirred(water temperature:70°C)until it slowly transformed into gel form.The gel was then dried in the oven at 100°C overnight,followed by calcination in a muf fle furnace.The temperature increase and decrease ramping rates were set at 5 °C·h?1and 10 °C·h?1,respectively.Finally,the resulting sorbent was ground completely in an agate mortar to break down any agglomeration that might have occurred during the calcination process.The synthesising procedure for the sorbent is simplified in the flowchart in Fig.1.
One-factor-at-a-time(OFAT)method was used when conducting the parametric study where only one parameter was varied at each run.The range of each parameter was set according to the literature.The experiments were continuously conducted until all factors have been studied.In this study, five factors were chosen to study the effects of each parameter on the sorbent's CO2sorption performance and properties.The sorbent was prepared according to the factors studied,which consisted of different Li:Si molar ratio,calcination temperature,and calcination time.The process study conditions,including carbonation and calcination temperatures,were also studied after the three parameters of the sorbent preparation were optimised.The list of parameters studied in this experiment is shown in Table 3.
2.2.CO2 sorption study
TGA analysis is a technique in which the mass change of the sorbent was measured as a function of temperature.In each experiment,approximately 20 mg of the prepared sorbent was placed in the sample pan.The analysis was initiated with argon flow at room temperature(25 °C)with a ramping rate of 10 °C·min?1.The temperature was increased to 800 °C with gas flow rate of 50 ml·min?1.The experiment was isothermally held at 800°C for 10 min in order for the argon gas to purge all residues and moistures,thus providing extra pores for the adsorption to take place.After 10 min of purging,the temperature was lowered down from 800°C to the desired carbonation temperature,while the feed gas was switched to CO2with a flow rate of 100 ml·min?1.The experiment was held at this condition for 30 min to let the CO2sorption process to occur.
2.3.CO2 cyclic study
CO2sorption–desorption study was conducted to examine the durability of the sorbent samples throughout the multiple cycles of adsorption–desorption.Subsequent to the CO2adsorption process,desorption process was conducted at a higher temperature(800°C)where the reversible reaction could occur.The adsorption–desorption loop was repeated 10 times(cycle)for every sorbent sample tested.
2.4.Characterisation of sorbent
SEM analysis was performed using a Philips XL30S model of the Scanning Electron Microscope(SEM)at the accelerating voltage of 5.0 kV to obtain the sorbent's surface morphology.SEM scanned the morphology of the sorbent using the focused beam of electrons.When the electrons interact with the electrons ator near the sorbent's surface,the image ofthe surface and composition can be detected.The morphology of the sorbent was scanned by controlling the magnification of the SEM in order to obtain clearer images.
XRD is a non-destructive analytical technique to identify and quantitatively determine various crystalline phases.This technique can offer valuable information regarding the crystal structure,crystallinity of the sorbent,crystallite size,and chemical analysis.XRD can also determine the amorphous contents of the samples.A Siemens Diffractometer D5000 with CuKαradiation was used for this purpose.The generator voltage and current were set at 40 kV and 40 mA,respectively.The range of 2θ diffraction angle(10°–90°)was used at a scan rate of 0.05(°)·s?1.The sorbent was packed on the sample stage so that it could be scanned by the X-ray.
2.5.Theoretical CO2 sorption capacity of Li4SiO4
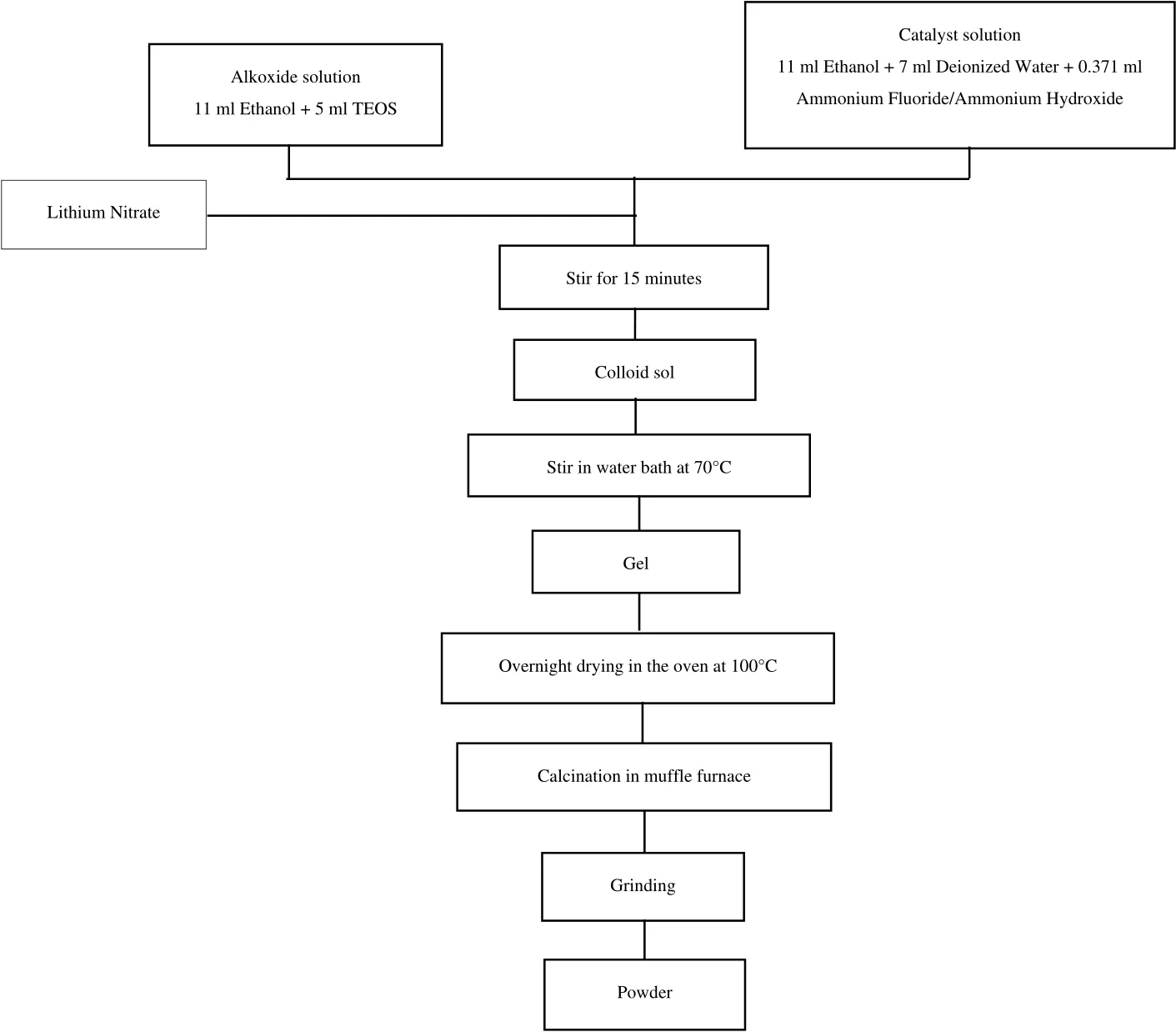
Fig.1.Overall experimental setup for the preparation of Li4SiO4 sorbent.
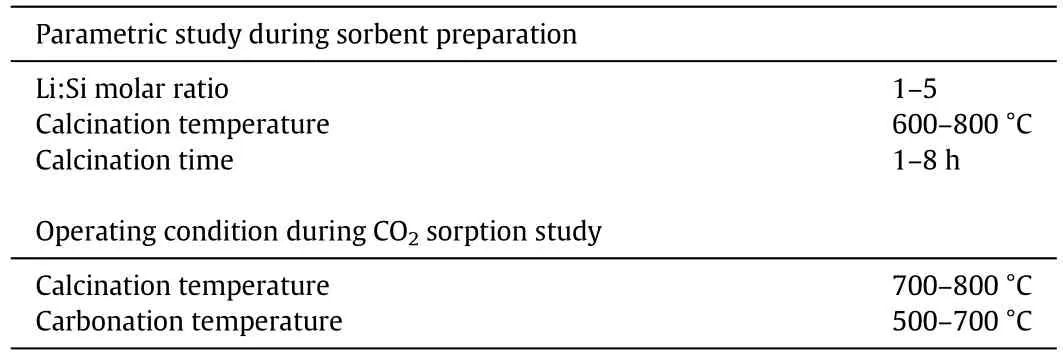
Table 3 List of synthesis parameters studied
Two different values were used in this experiment,which were the theoretical value and experimental value.Theoretical value was calculated based on the stoichiometry reaction,which in this case was the theoretical amount of CO2sorption capacity given by the sorbent when the limiting reactant has completely reacted.On the other hand,the experimental value involved the amount of CO2sorption capacity,which was given by the sorbentprepared in this study.The experimental value of the CO2sorption capacity was compared with the theoretical value in order to measure the efficiency of the sorbent's performance and presented in the form of yield percentage(%).The theoretical value of CO2sorption capacity for Li4SiO4sorbent is shown in the calculation and chemisorption reaction in Eq.(1)[27].

Theoretical CO2sorption capacity=
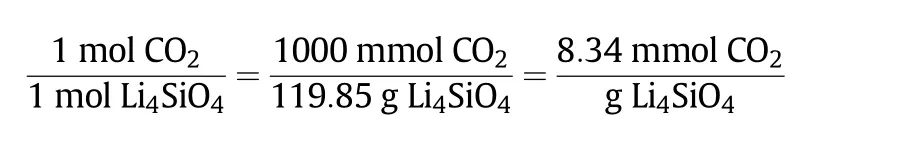
The calculated theoretical value of CO2sorption capacity for Li4SiO4was 8.34 mmol CO2·(g sorbent)?1.Therefore,the experimental value of the synthesised sorbent has to match the theoretical value or at least be lower than that.
3.Results and Discussion
3.1.Effect of Li:Si molar ratio
Li4SiO4sorbent was synthesised using LiNO3and SiC8H20O4as precursors,with different molar ratios.TEOS was fixed at 1 mol,while the concentration of LiNO3was varied from 1 to 5 mol.The Li:Si molar ratio of 1:1 had produced a sorbent sample with the highest CO2sorption capacity of 7.72 mmol CO2·(g sorbent)?1,while the lowest CO2sorption capacity(2.46 mmol CO2·(g sorbent)?1)was obtained by the sample with Li:Si=5:1.The excellent CO2sorption performance of the Li:Si=1:1 sample is suggested to relate to the stoichiometric ratio of the reaction between LiNO3and SiC8H20O4.The following equation further elaborates the stoichiometric ratio of these two reactants.

According to Eqs.(2a),(2b),(2c),1-mol LiNO3is supposed to be enough to react with 1-mol SiC8H20O4to produce 1-mol Li4SiO4.Experimentally,Li:Si=1:1 followed the stoichiometric relationship between LiNO3and SiC8H20O4.Then,the CO2sorption capacity of Li:Si=1:1 was compared to the theoreticalCO2sorption capacity,thus presented in the form of yield percentage,as shown in Eq.(3).
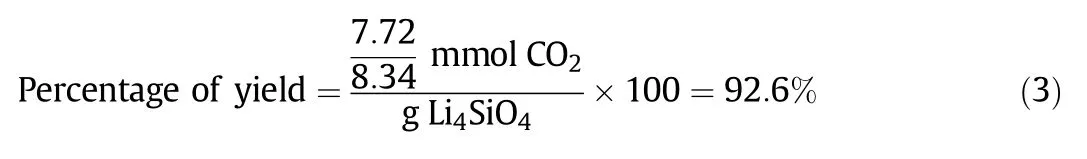
Ninety-three percent of the theoretical yield signified that some of the active sites(7%)did not or had only partially reacted with CO2.The reaction failed to produce 100%yield due to the presence of some unreacted precursors at the end of the process.XRD analysis was conducted to confirm the side products that were present in the sorbent,as depicted in Fig.2.
According to the XRD pattern for the Li:Si=1:1 sample,the main diffraction peaks due to Li4SiO4(●)can be found at 2θ =22.5°,23.0°,24.0°,28.2°,34.0°,35.0°,38.2°,48.4°,and 60.7°.However,a small quantity of Li2SiO3phase(Δ)was observed for this sample at 2θ=18.0°,27.0°,and 33.2°.The presence of Li2SiO3phase had resulted from the incomplete reaction between LiNO3and SiC8H20O4.Different compounds of lithium silicate have different sorption properties towards CO2.Li2SiO3did not seem to have good sorption properties towards CO2,while Li4SiO4showed excellent behaviour as a potential CO2sorbent.
Referring to the XRD pattern for the Li:Si=5:1 sample,Li4SiO4species as the main phase was detected.However,another phase of Li2Si2O5was also observed in the sorbent,which was also suspected to have poor properties for CO2sorption.Even though the peaks for the Li4SiO4phase observed in the Li:Si=1:1 and Li:Si=5:1 samples were almost similar,the Li:Si=5:1 sample did not possess an excellent CO2sorption performance compared with the Li:Si=1:1 sample,where it can only capture 2.46 mmol CO2·(g sorbent)?1.As previously explained,a decreasing trend was observed for the CO2sorption capacity with the increase of the Li:Si molar ratio,which signified that higher lithium content used to prepare Li4SiO4was not favourable for producing a sorbent with high CO2sorption ability.The excess lithium in the Li:Si=5:1 sample did not take part in the reaction with CO2,but acted as a barrier that prevented a direct contact between the gas and the active sites of the sorbent.
3.2.Effect of temperature(CT)and duration(Ct)of calcination process
Calcination is imperative in a sol–gel preparation method as a stabilisation process so that the synthesised materials are able to avoid structural changes.Hydroxyl group,as well as physisorbed and chemisorbed water are among the main residues that need to be eliminated during this process.The calcination temperature in this study was varied at 600,700,and 800°C.
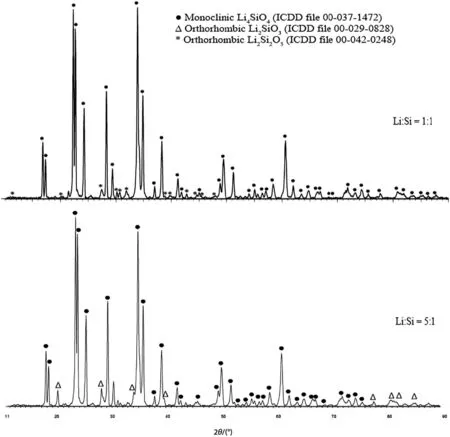
Fig.2.XRD pattern for Li:Si=1:1 and Li:Si=5:1.
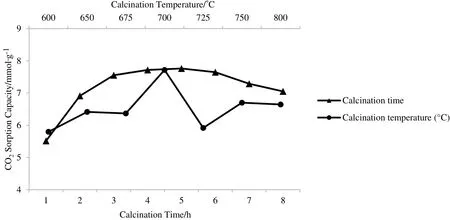
Fig.3.CO2 sorption profiles of sorbent at different calcination temperature and time.
CO2sorption profile shown in Fig.3 depicts that the sorbent sample with CT=700°C has the highest CO2sorption capacity among the other samples,with 7.72 mmol CO2·(g sorbent)?1,which was 93%of the theoretical value.At this calcination temperature,the reaction between LiNO3and SiO2was almost fully completed.It is suggested that some of the impurities that were present in sorbent sample with CT=600°C could have prevented the sorbent from capturing more CO2(5.80 mmol CO2·(g sorbent)?1).This might be due to the low calcination temperature,which was unable to provide enough energy to remove the undesired residues.Additionally,residue compounds(Li2SiO3and SiO2)could be present in the sorbent due to the incomplete reaction between Li and Si compounds at low calcination temperature(CT=600°C).These undesired products can be eliminated by increasing the calcination temperature.Apparently,the Li2SiO3content in sorbent sample with CT=800°C had also increased when the calcination temperature was increased from 700 to 800°C.This might be due to the Li atoms moving out of the structure,which was caused by the sublimation effect of LiNO3at higher temperature.Therefore,this could have led to insufficient Li compound to react with Si compound to produce Li4SiO4[28].High calcination temperature could produce agglomerated Li4SiO4and affect the sizes of the particles,as seen in the SEM images shown in Fig.4(a),(b),and(c).Consequently,it will reduce the surface areas of these particles,which could automatically limit the area for chemisorption reaction.
As shown in Fig.4,the morphology of the sorbent sample calcined at CT=700 °C was almost similar to the CT=600 °C sample.However,the CT=800°C sample showed a much more compact surface morphology,where the particles were agglomerated and the primary particle size was larger than the other two sorbent samples.This might be due to the higher calcination temperature,which led to the sintering effect on the particles.However,CT=600°C and CT=700°C samples showed almost a similar morphology based on the SEMimages.However,their CO2sorption abilities were different,whereby the CT=600°C sample had captured 25%less CO2than the CT=700°C sample.This could be due to incomplete reactions between the precursors at low calcination temperature,which produced impure Li4SiO4.
After the calcination temperature has been optimised,the duration of the calcination process was varied.The temperature was fixed at 700°C,while the calcination time was varied from 1 to 8 h.The results are depicted in Fig.3.The sorbent sample that was calcined for Ct=5 h had produced the highest CO2sorption capacity of 7.76 mmol CO2·(g sorbent)?1.The CO2sorption capacity had increased when the calcination time was increased from Ct=1 h to Ct=3 h.Next,calcination time was slowly increased until Ct=5 h and then,gradually decreased at Ct=7 h and Ct=8 h.The longer the sorbent is calcined,the higher the opportunity to remove impurities from the sorbent.In this study,CO2sorption capacity had remained constant for sorbent samples that were put through 5 to 6 h of calcination.The sorption capacity had slightly decreased when the calcination time was increased to longer than 6 h due to the growth of particle size and the agglomeration of particles.With the increased of the calcination time,the agglomerated particles became larger,which increased the crystallite size.This condition had simultaneously decreased the surface area of the sorbent.Low surface area had provided fewer sites for the chemisorption reaction between Li4SiO4and CO2gas.
3.3.Optimisation of operating conditions during CO2 sorption study
3.3.1.Calcination temperature,Tcalcination
The calcination and carbonation temperatures were controlled during the CO2sorption study to find a suitable condition that can result in the highest CO2sorption capacity.The experiments were performed using a thermogravimetric analyser(TGA).During each run,the two steps involved were the calcination and carbonation steps.Calcination is the process of heating up the sorbent to a desired temperature under an inert gas(Argon),while carbonation is the process where the sorbent reacts with CO2to form different products.Inert gas was used during the calcination process to ensure that no solid–gas reaction can occur.The effect of calcination was investigated by employing different calcination temperatures(Tcalcination=700,750,and 800 °C),while the carbonation temperature was fixed at 700 °C.Fig.5 shows the results of CO2sorption capacity at different calcination temperatures.
Based on Fig.5,the mass of the sorbent samples had decreased during the calcination step.The mass loss was due to the dehydration of the sorbents and the evaporation of the remaining ethanol.Some sorbents have shown large mass losses,which was suspected to be the result of the polycondensation of silicon compounds,the decomposition of lithium nitrate,and the complicated reaction between lithium nitrate and silicon compounds.The mass of the samples remained constant at certain temperatures after some times,which signified that most of the unwanted compounds have been removed from the sorbent.
It was observed that after undergoing CO2sorption,the sorbent calcined at Tcalcination=800°C had become solidified and lostits powder form.It can be seen from the TGA profile where its mass loss was lesser than the other two samples during the calcination step due to the sintering process at high temperature that agglomerated the powders.Sintered particle structures often lead to insufficient surface areas for dehydration of the sorbent and evaporation of the remaining ethanol.Therefore,the calcination temperature during sorption study was reduced to Tcalcination=700°C(which resulted in CO2sorption capacity of 5.51 mmol CO2·(g sorbent)?1to overcome the sintering problem.Another CO2sorption test was conducted at an intermediate temperature of Tcalcination=750°C and the CO2sorption capacity of the sorbent was also low,with 5.82 mmol CO2·(g sorbent)?1,which was close to the result obtained by the sample with Tcalcination=700°C.As can be seen from the results,the sorbent sample at Tcalcination=750 °C(5.82 mmol CO2·(g sorbent)?1)has shown lower CO2sorption capacity compared to the sample at Tcalcination=800 °C(7.95 mmol CO2·(g sorbent)?1),while maintaining its powder form and producing a less sintered structure after undergoing CO2sorption.This can be related to the insufficient heat to remove the impurities,which appeared during the storing period.
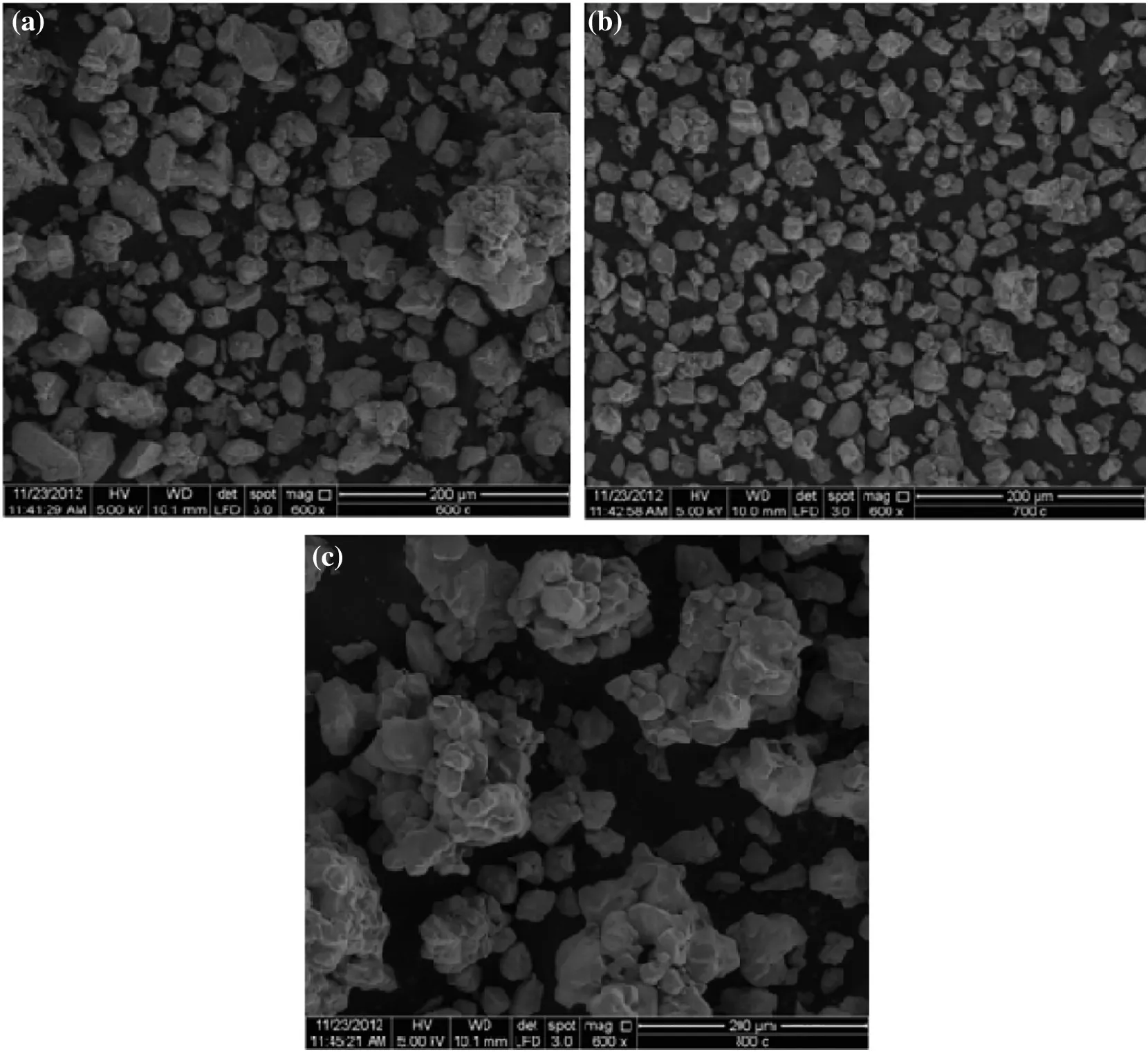
Fig.4.SEM images of sorbents(a)CT=600 °C,(b)CT=700 °C and(c)CT=800 °C(600× magnification).
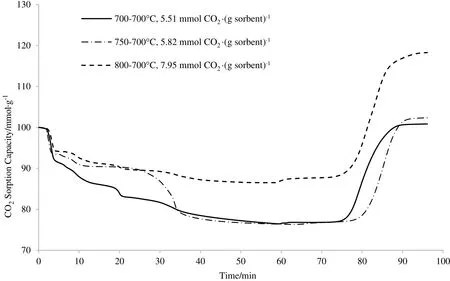
Fig.5.TGA profiles at different calcination temperatures(T calcination=700,750 and 800°C).
3.3.2.Carbonation temperature,Tcarbonation
Since the calcination temperature was unable to treat the sintering problem of the sorbent after CO2sorption,another operating condition was studied,which was the carbonation temperature.Carbonation temperature can affect both the kinetics and thermodynamics of the reaction.The carbonation step was able to produce a dense,non-porous layer of carbonate materials around the reacting particles.The carbonation reaction for Li4SiO4sample was conducted under dry condition with pure CO2flow.The CO2sorption test was run at Tcalcination=800°C(which had produced the highest CO2sorption capacity in the previous step)and Tcarbonation=500,600,and 700°C.
During the carbonation process,the CO2gas was incorporated into the Li4SiO4compound and underwent a reaction that produced a new material as the by-product.As shown in Fig.6,Li4SiO4sorbent had poorly adsorbed CO2at carbonation temperature of lower than 700°C.Due to the low temperature,CO2gas and Li4SiO4sorbent had a hard time undergoing the chemisorption process.Temperature has a pronounced effecton the diffusivity since diffusivity ofCO2isdependent on the temperature.
CO2-Li4SiO4reaction had proceeded under two controlling reaction regimes,namely,the chemical reaction and the diffusion controlled regimes.When the molecules collide,the kinetic energy of the molecules can be used to stretch,bend,and ultimately break the bonds,which can induce the chemical reactions.If the molecules are moving too slow with little kinetic energy,or collide with an improper orientation,they will not react and just simply bounce off each other.However,if the molecules are moving at a fast enough velocity with a proper collision orientation,such that the kinetic energy upon collision is greater than the minimum energy barrier,then a reaction will occur.From the kinetic theory of gas,the gas molecules'velocity will increase as the temperature increases,and more molecules will have high kinetic energies.Thus,the fraction of molecules that have high enough kinetic energy to exceed the minimum energy needed for a reaction will also increase.
3.4.Characterisation of Li4SiO4 sorbent
3.4.1.X-ray diffraction(XRD)
The XRD diffractograms for Li4SiO4,before and after CO2sorption,are illustrated in Fig.7.These diffraction patterns are in agreement with those published in the literature.According to Fig.7(a),the fresh sorbent(before CO2sorption)exhibited a pattern with major peaks of the Li4SiO4phase(●).These major peaks were found at 2θ =22.2°,22.5°,24.0°,28.0°,33.7°,34.5°,38.0°,49.3°,and 50.5°.Another XRD analysis was performed on the same sorbent,which had undergone the CO2sorption process,and its XRD diffraction pattern is shown in Fig.7(b).It can be observed that no Li4SiO4phase was detected in this pattern,which signified that all Li4SiO4particles had reacted with CO2and converted to product phases.The most intense peak was detected for the lithium metasilicate(Li2SiO3)phase(Δ),which was in agreement with Eq.(1),while the lithium carbonate(Li2CO3)phase(□),was detected as the second phase.The existence of the Li2SiO3and Li2CO3compounds confirmed the reaction between Li4SiO4and CO2.However,two side products were also detected in small amounts,which were silicon oxide(SiO2)and lithium oxalate(Li2C2O4).These phases were produced from the incomplete reactions during the cyclic process.
3.4.2.Scanning electron microscopy(SEM)
The surface morphology of Li4SiO4,before and after CO2sorption,is shown in Fig.8(a)and(b),respectively.The difference of the surface morphology can be detected from both images by comparing the SEM micrographs.
The microstructure of fresh Li4SiO4had exhibited single,nonagglomerated particles with some open porosity between the grain boundaries,as depicted in Fig.8.The morphology of the sorbent then changed after CO2sorption.Agglomeration occurred during the carbonation step when the sorbent reacted with CO2at high temperatures.This led to the growth in particle size,while open porosity can lead to bigger surface areas of the particles and reduction on the surface area of the sorbent.
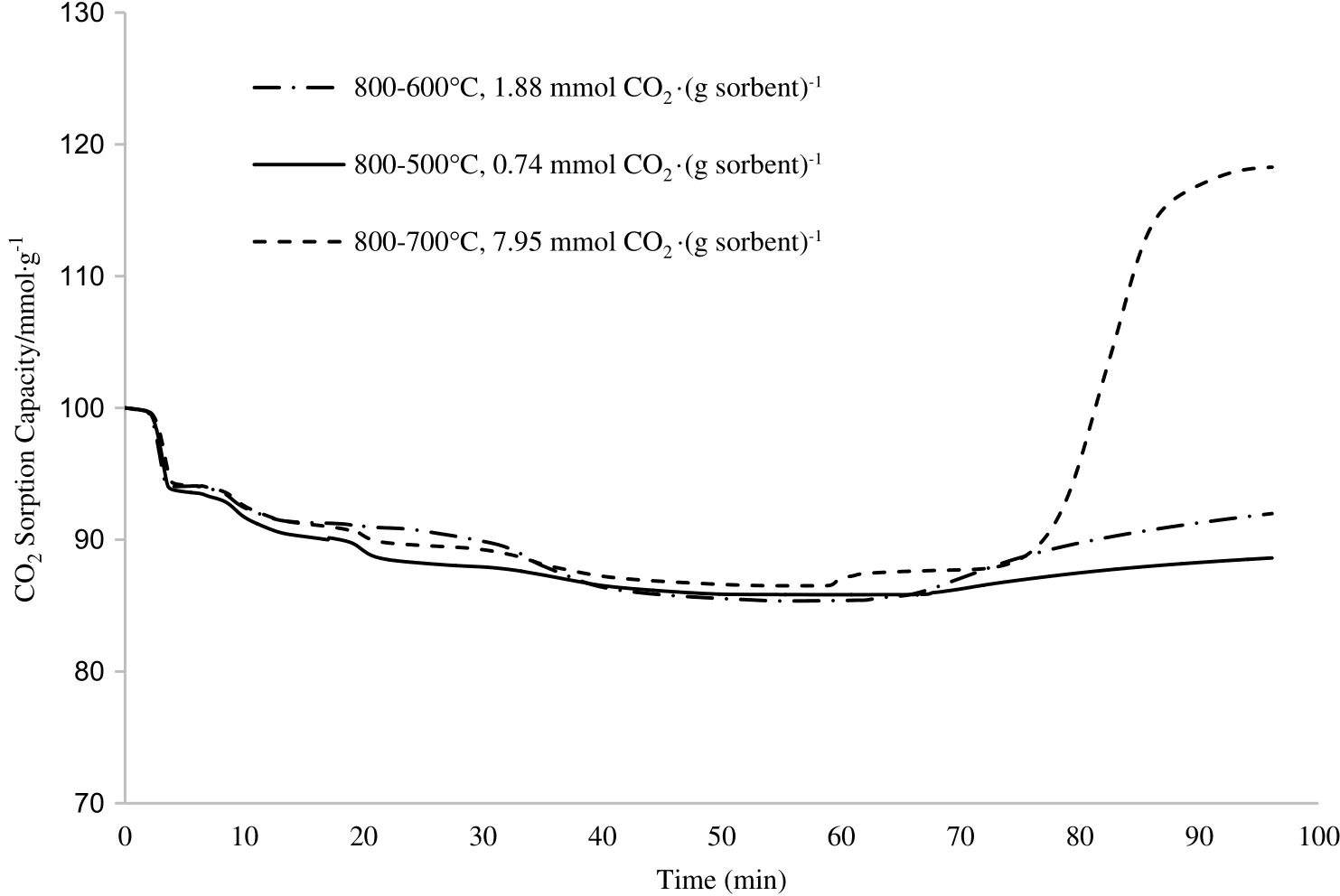
Fig.6.TGA profiles at different carbonation temperatures(T carbonation=500,600 and 700°C).

Fig.7.XRD diffractograms of Li4SiO4(a)before and(b)after CO2 sorption.
Depositions ofthe reaction's producthad accumulated on the surface,as shown in Fig.9,thus giving an image of a dense structure,with a portion of polygonal-shaped particles.Additionally,the empty pores between the grain structures had almost disappeared due to the growth ofthe particles and the formation ofreaction products after CO2sorption.
3.4.3.Energy dispersive X-ray(EDX)
Together with SEM analysis,the Energy Dispersive X-ray(EDX)analysis was conducted to confirm the presence of carbonate species according to the SEM images.The EDX analysis results are shown in Fig.10.
The concept of charge neutrality was employed since Li+ion is not detectable by the EDX.By using this concept,it is found that the stoichiometry of EDX analysis was in good agreement with the designated stoichiometry.The carbon(C)and oxygen(O)species detected by the EDX had confirmed the formation of carbonate,as a result of the reaction between the sorbent and CO2.
3.5.Cyclic CO2 sorption–desorption studies
The cyclic test was conducted to measure the sorbent's durability and stability through several calcination–carbonation cycles.This section presents the results of the cyclic tests that were conducted using a TGA equipment.The cyclic test was conducted up to ten cycles using the standard gas flow and pure CO2concentration(100%CO2)atcertain temperatures.From the previous step,the calcination and carbonation temperatures were optimised to Tcalcination=800°C and Tcarbonation=700°C,respectively.The sample was heated from room temperature to 800°C and this temperature was held for 15 min to let the moisture and other residues vaporises,for the purpose of producing a particle with high surface area and high porosity.Then,the temperature was decreased to 700°C and was held for 30 min to allow CO2gas and Li4SiO4sorbent to react.The calcination and carbonation steps were repeated continuously until ten cycles have been completed.Fig.11 shows the ten-cycle performance of the Li4SiO4sorbent.

Fig.8.SEM image of sorbent Li4SiO4 before CO2 sorption.(a)600× magnification(b)20000× magnification.
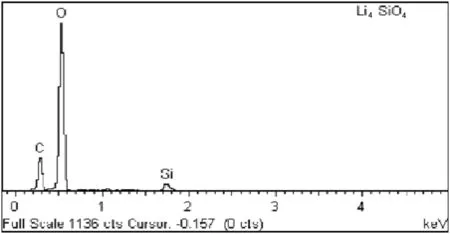
Fig.10.EDX spectrum of Li4SiO4 sorbent.
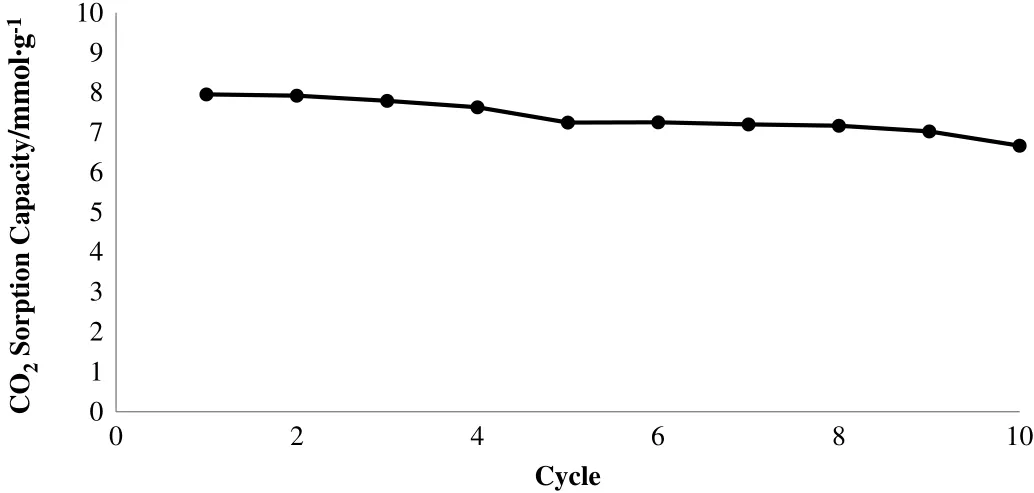
Fig.11.CO2 sorption capacities of sorbent Li4SiO4 throughout 10 cycles of CO2 sorption–desorption.
By conducting the cyclic performance of Li4SiO4,it was found that the CO2sorption capacity of the sorbent had slightly decreased for the first four cycles.However,the sorption capacity became stable during the fifth to ninth cycles.During the tenth cycle,the sorption capacity had dropped from 7.02 mmol CO2·(g sorbent)?1to 6.02 mmol CO2·(g sorbent)?1.Throughout the whole cyclic process,the CO2sorption capacity of the sorbent had decreased by 16.16%from the first to the tenth cycle,which was deemed reasonable.
3.5.1.Scanning electron microscopy(SEM)
SEM analysis was performed to examine the surface morphology of the sorbent,before and after the ten cycles of sorption–desorption.The comparisons between the fresh sorbent and the used sorbent after the ten cycles of sorption–desorption are shown in Fig.12.
According to Fig.12(a),the fresh Li4SiO4sorbent showed the appearance of moderate porosity before undergoing the CO2sorption process.There was an obvious difference between the fresh and the used sorbents,where the particles of the used sorbent were observed to grow and changed into rod structures after 10 cycles of CO2sorption–desorption study.The rod structures were measured to be approximately 15–20 μm in average.The particles of the used Li4SiO4sorbent had also shown decay after the cyclic process when they changed from spherical structures into rodlike structures.There was a noticeable sintering effect caused by the decrement of CO2sorption capacity throughout the cyclic process.
4.Conclusions
A parametric study was conducted using several parameters during the preparation of Li4SiO4as a sorbent.The effects of these parameters towards CO2sorption performance were studied and optimised for maximum CO2capture capacity.The sorbent that was prepared using Li:Si=1:1 molar ratio and calcined at 700°C for 5 h was found to have the highest capacity for CO2capture(7.76 mmol CO2·(g sorbent)?1).The process study for CO2sorption was conducted using a TGA equipment,and calcination and carbonation temperatures were the process variables studied.Tcalcination=800 °C and Tcarbonation=700 °C were chosen as the best operating conditions,which had resulted in the sorption capacity of 7.95 mmol CO2·(g sorbent)?1.Characterizations were performed to analyse the properties of the Li4SiO4sorbent,before and after CO2sorption.The XRD analysis has shown the existence of the desired product(Li4SiO4)in the sorbent sample.In the meantime,the XRD results for this sample have also shown that the excellent reaction between Li4SiO4and CO2,whereby XRD detected Li2SiO3and Li2CO3materials in the used sorbent sample as the products of the carbonation reaction.This was followed by a cyclic CO2sorption–desorption study for the Li4SiO4sorbent.Ten cycles of the sorption–desorption process were conducted using the TGA equipment.The efficiency drop throughout the ten cycles of CO2sorption–desorption process was calculated to be 16.16%.
Acknowledgments
This work is fully sponsored by the Ministry ofEducation ofMalaysia and Universiti Sains Malaysia through LRGS-USM NanoMITe Grant(203/PJKIMIA/6720009).
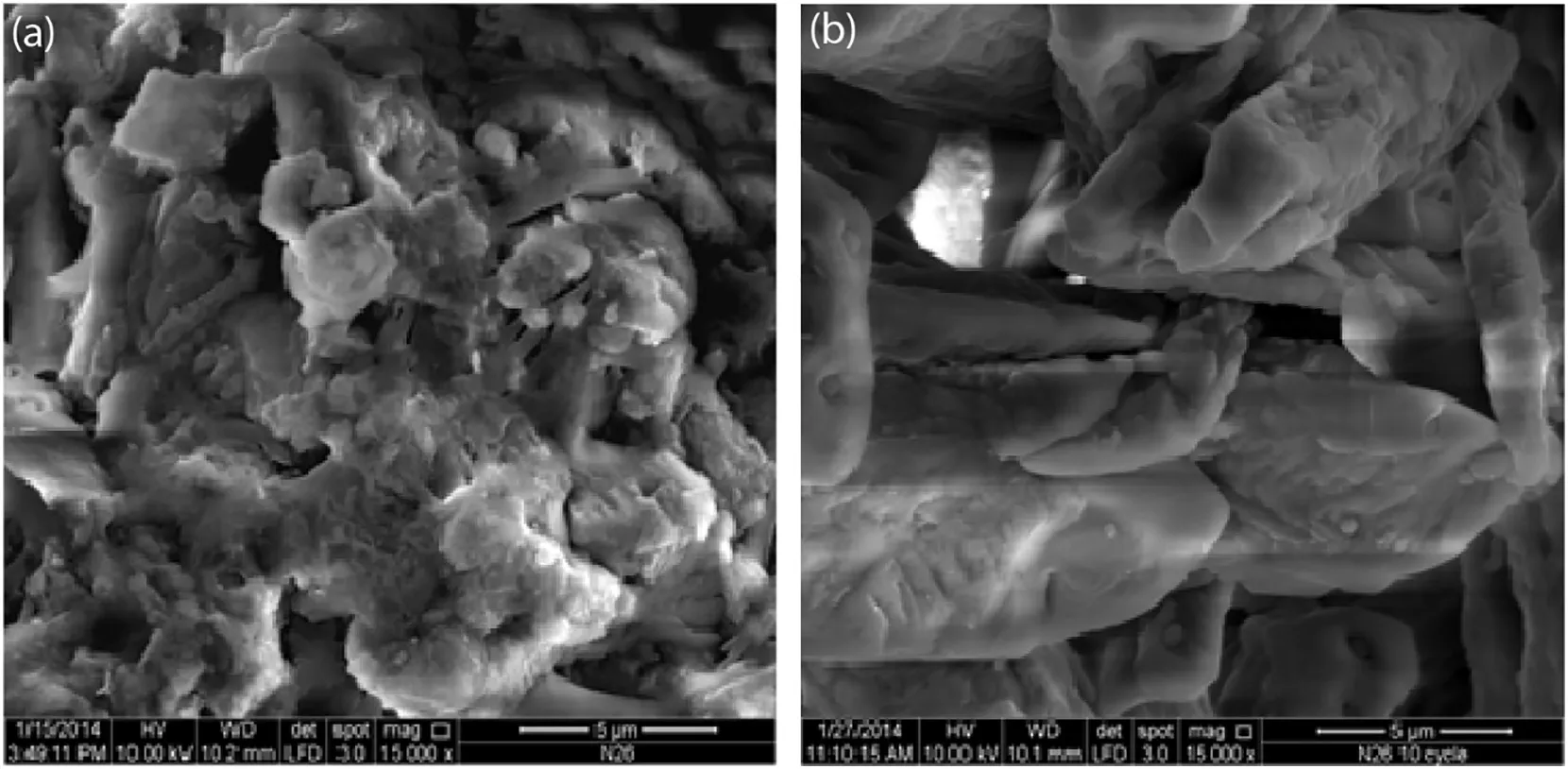
Fig.12.SEM image of(a)fresh Li4SiO4 sorbent and(b)Li4SiO4 sorbent after 10 cycles of CO2 sorption–desorption(15000× magnification).
[1]R.H.R.H.Moss,J.A.J.A.Edmonds,K.A.K.A.Hibbard,M.R.Manning,S.K.S.K.Rose,D.P.D.P.van Vuuren,T.R.T.R.Carter,S.Emori,M.Kainuma,T.Kram,et al.,The next generation of scenarios for climate change research and assessment,Nature 463(7282)(2010)747–756.
[2]IEA-International Energy Agency,Redrawing the Energy–climate Map:World Energy Outlook Special Report,2013.
[3]Y.E.Kim,J.H.Park,S.H.Yun,S.C.Nam,S.K.Jeong,Y.Yoon II,Carbon dioxide absorption using a phase transitional alkanolamine–alcohol mixture,J.Ind.Eng.Chem.20(4)(2014)1486–1492.
[4]A.S.Lee,J.C.Eslick,D.C.Miller,J.R.Kitchin,Comparisons of amine solvents for postcombustion CO2capture:a multi-objective analysis approach,Int.J.Greenhouse Gas Control 18(2013)68–74.
[5]Y.S.Sistla,A.Khanna,Carbon dioxide absorption studies using amine-functionalized ionic liquids,J.Ind.Eng.Chem.20(4)(2013)2497–2509.
[6]E.A.Roth,S.Agarwal,R.K.Gupta,Nanoclay-based solid sorbents for CO2capture,Energy Fuel 27(8)(2013)4129–4136.
[7]C.Zhao,X.Chen,C.Zhao,Y.Liu,Carbonation and hydration characteristics of dry potassium-based sorbents for CO2capture,Energy Fuel 23(3)(2009)1766–1769.
[8]S.Ma'mun,H.F.Svendsen,K.A.Hoff,O.Juliussen,Selection of new absorbents for carbon dioxide capture,Energy Convers.Manag.48(1)(2007)251–258.
[9]M.Auta,N.D.Amat Darbis,A.T.Mohd Din,B.H.Hameed,Fixed-bed column adsorption of carbon dioxide by sodium hydroxide modified activated alumina,Chem.Eng.J.233(2013)80–87.
[10]J.C.Hicks,J.H.Drese,D.J.Fauth,M.L.Gray,G.Qi,C.W.Jones,Designing adsorbents for CO2capture from flue gas-hyperbranched aminosilicas capable of capturing CO2reversibly,J.Am.Chem.Soc.130(10)(2008)2902–2903.
[11]P.Monica,S.Maurizia,V.Sandra,CO2capture at high temperature and low concentration on Li4SiO4based sorbents,Chem.Eng.Trans.2013(32)(2005)1279–1284.
[12]H.Pfeiffer,C.U.V,D.Coyoaca,C.Universitaria,D.Coyoaca,Toward Understanding the Effect of Water Sorption on Lithium Zirconate(Li2ZrO3)during Its Carbonation Process at Low Temperatures,J.Phys.Chem.C. 114(2010)9453–9458.
[13]M.Seggiani,M.Puccini,S.Vitolo,Alkali promoted lithium orthosilicate for CO2capture at high temperature and low concentration,Int.J.Greenhouse Gas Control 17(2013)25–31.
[14]S.Shan,Q.Jia,L.Jiang,Q.Li,Y.Wang,J.Peng,Novel Li4SiO4-based sorbents from diatomite for high temperature CO2capture,Ceram.Int.39(5)(2013)5437–5441.
[15]R.Xiong,J.Ida,Y.S.Lin,Kinetics of carbon dioxide sorption on potassium-doped lithium zirconate,Chem.Eng.Sci.58(19)(2003)4377–4385.
[16]W.Yinjie,L.Jiping,The In fluencing factor for CO2absorption of Li4SiO4at high temperature,2011 Third Int.Conf.Meas.Technol.Mechatronics Autom,No.1,2011,pp.839–841.
[17]C.Gauer,W.Heschel,Doped lithium orthosilicate for absorption of carbon dioxide,J.Mater.Sci.41(8)(2006)2405–2409.
[18]K.Nakagawa,M.Kato,S.Yoshikawa,K.Essaki,H.A.Uemoto,Novel CO2absorbent using lithium-containing oxides,2nd Annu.Conf.Carbon Sequestration,May,6 2003.
[19]H.Kim,H.D.Jang,M.Choi,Facile synthesis of macroporous Li4SiO4with remarkably enhanced CO2adsorption kinetics,Chem.Eng.J.280(2015)132–137.
[20]S.Zhang,Q.Zhang,H.Wang,Y.Ni,Z.Zhu,Absorption behaviors study on doped Li4SiO4under a humidified atmosphere with low CO2concentration,Int.J.Hydrog.Energy 39(31)(2014)17913–17920.
[21]E.Carella,M.T.Hernandez,High lithium content silicates:A comparative study between four routes of synthesis,Ceram.Int.(2014)9499–9508.
[22]K.Wang,Z.Yin,P.Zhao,Z.Zhou,Z.Su,J.Sun,Development of metallic elementstabilized Li4SiO4sorbents for cyclic CO2capture,Int.J.Hydrog.Energy(2016)1–9.
[23]R.B.Khomane,B.K.Sharma,S.Saha,B.D.Kulkarni,Reverse microemulsion mediated sol-gel synthesis of lithium silicate nanoparticles under ambient conditions:scope for CO2sequestration,Chem.Eng.Sci.61(10)(2006)3415–3418.
[24]C.-C.Chang,C.C.Wang,P.N.Kumta,Chemical synthesis and characterization of lithium orthosilicate(Li4SiO4),Mater.Des.22(7)(2001)617–623.
[25]K.Wang,Z.Zhou,P.Zhao,Z.Yin,Z.Su,J.Sun,Synthesis of a highly efficient Li4SiO4ceramic modified with a gluconic acid-based carbon coating for high-temperature CO2capture,Appl.Energy,183(2016)1418–1427.
[26]H.Pfeiffer,P.Bosch,S.Bulbulian,Synthesis of lithium silicates,J.Nucl.Mater.257(3)(1998)309–317.
[27]Z.Qi,H.Daying,L.Yang,Y.Qian,Z.Zibin,Analysis of CO2sorption/desorption kinetic behaviors and reaction mechanisms on Li4SiO4,AICHE J.59(3)(2013)901–911.
[28]M.J.Venegas,E.Fregoso-Israel,R.Escamilla,H.Pfeiffer,L.Sio,S.From,R.Husk,J.Wang,Kinetic and reaction mechanism of CO2sorption on Li4SiO4:study of the particle size effect,Ind.Eng.Chem.Res.46(8)(2007)2407–2412.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of butter fly-like BiVO4/RGO nanocomposites and their photocatalytic activities☆
- Fabrication of chitosan microspheres for efficient adsorption of methyl orange☆
- The combined effects of lysozyme and ascorbic acid on microstructure and properties of zein-based films☆
- Effects of nitrogen doping on surface-enhanced Raman scattering(SERS)performance of bicrystalline TiO2 nano fibres☆
- Effect of heat flux and inlet temperature on the fouling characteristics of nanoparticles☆
- Variation of toxic pollutants emission during a feeding cycle from an updraft fixed bed gasifier for disposing rural solid waste☆
