Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
Md.Sakinul Islam*,Kotaiah Naik Dhanavath Nhol Kao Pradipto K.Bhattacharjee Brahim Si Ali*,Rozita Yusoff
1 Department of Chemical Engineering,School of Engineering,RMIT University,Melbourne,VIC 3001,Australia
2 Department of Chemical Engineering,Faculty of Engineering,University of Malaya,50603 Kuala Lumpur,Malaysia
1.Introduction
Carbon dioxide emissions are commonly found in flue gases( flue gas streams,coal- fired power plants,re fineries,cement manufacturing plants,etc.)and are a major contributor to the greenhouse effect.Amine-based absorption and stripping processes are widely used for sour gas sweetening and flue gas treatment which is shown in Fig.1.Carbon dioxide capture in post-combustion processes using alkanolamines is currently the most advanced technology.In the case of diluted and low-pressure streams,absorption based on chemical reaction with aqueous alkanolamine solutions is the most attractive technology[1].One of the advantages of alkanolamines is that,structurally,they contain at least one hydroxyl group,which helps to reduce their vapor pressures but increases their solubilities in aqueous solution.On the other hand,the amino group provides the necessary alkalinity for absorbing CO2.The most common alkanolamines used in the sour gas absorption technology are monoethanolamine(MEA),diethanolamine(DEA),N-methyldiethanolamine (MDEA),di-isopropanolamine(DIPA),N,N-diethylethanolamine (DEEA),1-dimethylamino-2-propanol(1DMA2P)and aminomethyl propanol(AMP).Among all known solvents,MEA is the benchmark molecule because of its suitable properties toward CO2capture(high absorption capacity,fast kinetics,high watersolubility,low price,etc.).However,MEA is rapidly displaced by more efficient systems,although,for low concentrations of H2S and CO2,it is still preferable.This is especially applicable for low pressures and ensures maximumremoval of H2S and CO2.The advantages include the high alkalinity and ease of recovery from contaminated solutions.On the other hand,an irreversible product is formed with COS and CS2;it is more corrosive having a high heat of reaction with CO2and also has a high vapor pressure[2].
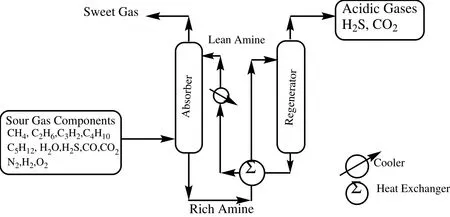
Fig.1.Schematic diagram of amine-based absorption and desorption process.
DEA is used for the treatment of re finery gases with COS and CS2.DEA is less reactive with COS and CS2than MEA and has a lower vapor pressure.The disadvantage of DEA treatment is the need for vacuum distillery that makes the process difficult.And when a high content of CO2is present,DEA is not a good choice because of the formation of corrosive DGPs[2].However DEA has some good advantages over other alkanolamines such as DEEA,DIPA,and AMP.DEA is extensively used as a corrosion inhibitor and surfactantin various industrial applications.In the amine based absorption and stripping processes it is used to remove hydrogen sulfide and carbon dioxide from natural gas.In petroleum re fineries aqueous solution of DEA is commonly used to remove hydrogen sul fide(H2S)from sour gas.It has an advantage over a similar amine ethanolamine in that a higher concentration may be used for the same corrosion potential.This allows re finers to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.MDEA is another alkanolamine which can be used for the removal of CO2.The advantages reported by Appl et al.[3]include low energy requirement,high capacity,and high stability whereas it's obvious disadvantage is associated with its low rate of reaction with CO2.The rate of the reaction can be increased by using promoters,without diminishing the advantages of MDEA.MDEA is the most widely used alkanolamine as reported by Chakma and Meisen in 1988[2].
However,a major disadvantage associated with chemical absorption is solvent degradation,which is defined as the undesirable breakdown of amine to form unwanted by-products by means of reversible or irreversible side reactions due to the presence of CO2and O2.These by-products lead to a significant decrease in efficiency of the process(solvent losses,corrosion,foaming,fouling,and an increase in viscosity).This undesirable breakdown reduces their CO2absorption capacity and introduces unwanted DGPs,thereby forcing the solutions to be eventually discarded.An operational burden such as corrosion is also induced,as it has periodically been reported that the DGPs can lead to severe corrosion[4,5].
Alkanolamine degradation with CO2has been studied for some time:N-methyldiethanolamine(MDEA)and diethanolamine(DEA)are commonly used in the naturalgas treatment,and MEAis the benchmark solventforpost-combustion applications.Contrary to the degradation with CO2,actual plant operating conditions have not been studied in much detail;only Chakma and Meissen established the relative stability of MDEA as a function of temperature[2,4].Many authors were interested in MEA degradation with CO2and identified the main by-products as 2-oxazolidinone,N-(2-hydroxyethyl)-imidazolidinone,and N-(2-hydroxyethyl)-ethylenediamine(HEEDA)[6,7].Luo et al.studied the prediction CO2loading of 1DMA2P solutions with establishing vaporliquid equilibrium by calculating ion concentrations using the pH method[8].DEAdegradation was firststudied by Kennard and Meisen[9-12]followed by Hsu and Kim in 1985[13]and Chakma et al.in 1990[14].MDEA degradation was also investigated,and some reaction mechanisms were suggested by Chakma and Meisen[15,16].Thermal degradation of aqueous DEEA solution at stripper conditions for postcombustion CO2capture was studied by Gao et al.in 2015[17].These studies highlighted similarities between MEA,DEA,and MDEAdegradation:in fact,the main products formed from the degradation reactions include amines,oxazolidinones,and imidazolidinones.On the contrary,carbon dioxide induced degradation of DEA has not yet been described suf ficiently in the literature,and many unknown parameters of the process still need to be identified.Limited information was found on DEA degradation induced by CS2[18],COS[19-21]and CO2[22-25].The mechanism of DEA degradation has not been investigated significantly and is still not under stood properly.In this present work,carbon dioxide induced degradation of DEA is studied under actual plantoperating conditions.The liquid reaction samples were characterized by anion ion chromatography(IC),cation IC and organic acid IC methods in order to identify the unknown ionic DGPs.
2.Materials and Methods
2.1.Materials
DEAwas used in this study to identify the ionic degradation products during the absorption and desorption CO2at 55 °C and 100 °C respectively.DEA and all required standards(acetate,nitrate,nitrite,oxalate,carbonate,ammonium,phosphate)were purchased from Merck and Sigma-Aldrich(Malaysia).The IC evaluation assessment was done by the standard calibration curve verification method.About five different concentrations of each standard were prepared at the ppmlevel.During the preparation of standard solution,it was diluted by deionized distilled water(DDW).
2.2.Experimental set-up of degradation
The degradation experiments were conducted in a 1.25 L jacketed glass reactor equipped with stainless steel top plate,impeller,shaft bearing,1.25 L borosilicate 3.3@Pyrex jacketed glass vessel and a metallic glass vessel holder.The jacketed glass vessel is equipped with sanitation at its bottom and had a polytetra fluoroethylene(PTFE)valve for sample outlet.Other accessories used during the experiments included an analog pressure gauge(0-1.4 MPa),digital pH meter and a Pt-100 temperature probe with thermometer having a digital display that is built on the top plate of the reactor for measuring the reactor's interior pressure,pH,and temperature.The pressure gauge is also used as an indicator for emergency switch off by observing the pressure limit inside the reactor.An overhead stirrer motor is capable of providing agitation speeds of up to 1500 r·min-1with overheat protection,while a digital PID(proportional integral derivative)controller of RPM(rotation per minute)is built in the central position of the top plate.A stainless steel condenser(with built in the coil)having an inlet,outlet and exhaust point is used to prevent solution loss by means of evaporation under high temperature.A stainless steel ring purger that comes with a top headlock,a top plate Viton circular ring,and a top head stainless steel plug circular ring are equipped with the reactor for proper distribution of carbon dioxide during the degradation reaction with amine solution.The heat supply to the reactor was performed by a temperature-controlled water bath.A digital pressure transducer was used to measure the total pressure in the reactor.To monitor the pH of the amine mixture against time,a pH probe is linked to a Metrohm?719S auto-titrator which is controlled by the Tinet?software.Reaction gases(CO2and N2)were introduced into the reactor by needle valve flow meters to check the flow accuracy.Both flow meters are mounted on the stainless steel controlcabinet.Before introducing the gas into the reactor,it was first fed into the gas saturator for separating dust or particulates.The gas saturator is designed with the jacketing system to avoid any thermal deviation from the reactor.The overall experimental setup for the degradation study is shown in Fig.2.
2.3.Degradation experiment
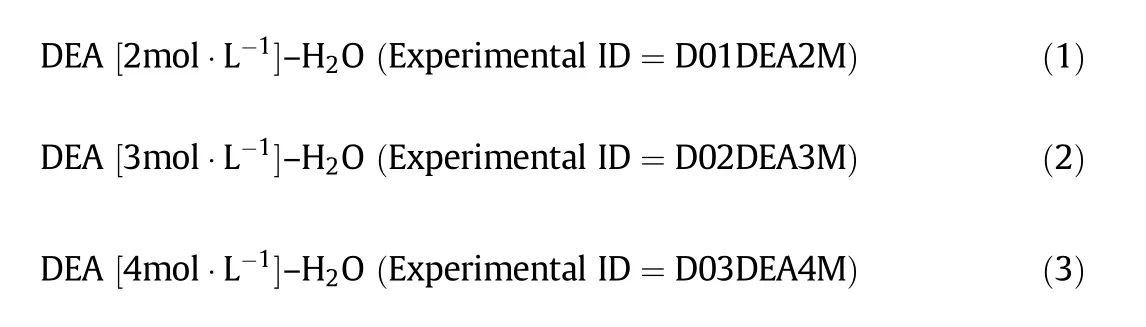
There are three typical experiments(D01DEA2M,D02DEA3M,and D03DEA4M)performed in this degradation study with aqueous solution of DEA.The aqueous amine samples were prepared by diluting DEA in deionized distilled water(DDW)according to the following Eqs.((1)to(3)).Necessary precautions were taken to avoid any exposure of the sample to the air during the preparation and experimental work.
For each degradation experiment,about 1000 ml of an aqueous amine solution was placed in the reactor.Prior to purging gas through the reactor,all control modules were turned on according to the preselected control parameters.The whole system was held for 10-15 min while being heated to the desired temperature.The stirrer was set to a speed of 1200 r·min-1,and oxygen was purged out from the reactor by passing nitrogen through the amine solution for about 25 to 30 min in order to remove any possible contamination that might have been present from previous experiments.Then a known volume of 100%pure CO2was fed into the reactor with a flow rate 200 ml·min-1from a stainless steel cylinder which was connected to the reactor.The gas inlet valve was left open for a certain duration in order to complete six cycles for each experiment.Each cycle configured with absorption and desorption of CO2at 55 °C and 100 °C respectively.For complete degradation,each experimenthad taken about two weeks of duration.The reaction of CO2with amine solvents is exothermic which decrease the alkalinity of amine solution.Thus,the variation of pH of the solution was measured continuously with time until equilibrium was reached.When the equilibrium was achieved in the case of absorption and desorption processes,the experiment was stopped in each cycle,and the reaction sample was collected by the outlet valve.About 50 ml of reaction sample was taken at the end of the experiment.Collected reaction samples were labeled with corresponding IDs and stored for further analysis.To identify the DGPs,three different IC methods(anion,cation,and organic acid)were used in this present work.
2.4.Characterization
To identify the DGPs,ion chromatography was performed using a Metrohm 861 Compact IC instrument with suppressed and nonsuppressed detection(Model 861 Advanced Compact IC,Metrohm,Switzerland).The IC was equipped with a dual-piston pump,863 compact autosamplers,a degas assembly,and a digital conductivity detector.Data processing was performed with IC Net 2.3 software.Separation was carried out under isocratic conditions and at a room temperature of approximately 20°C.Three different modes of chromatography(anion exchange,cation exchange,and ion exclusion)were used in this investigation which simply relate to the different types of columns used to achieve the separation of the ions.The eluent used depends on the column type and also the modes of detailed detections are given below.
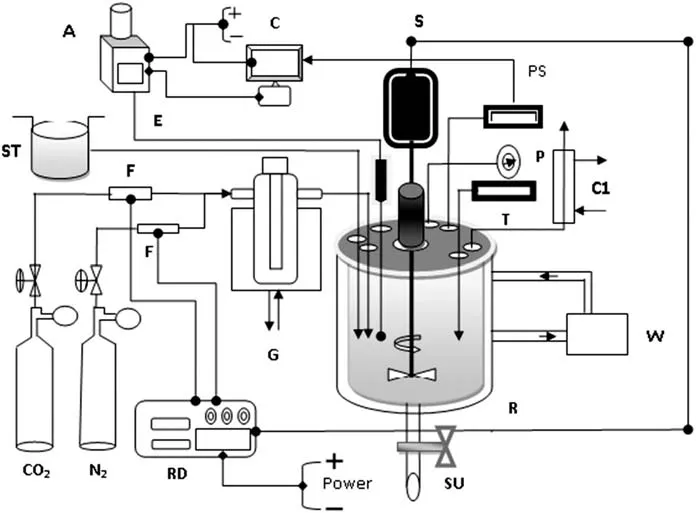
Fig.2.Schematic diagram of experimentalset-up for the degradation aqueous DEA.Here,W—water bath;C1—gas outlet and condenser;A—auto-titrator for continuous observation of pH with time;E—electrode connected with auto-titrator;F— flow meter;PS—pHsensor;P—pressure gauge,T—digital thermometer;S—stirrer(~1500 r·m-1);ST—solvent storage tank;RD—power,gas flow and RPM regulator;C—computer for data collection;G—gas saturator;SU—sample outlet valve;R—jacketed glass reactor(1.25 L).
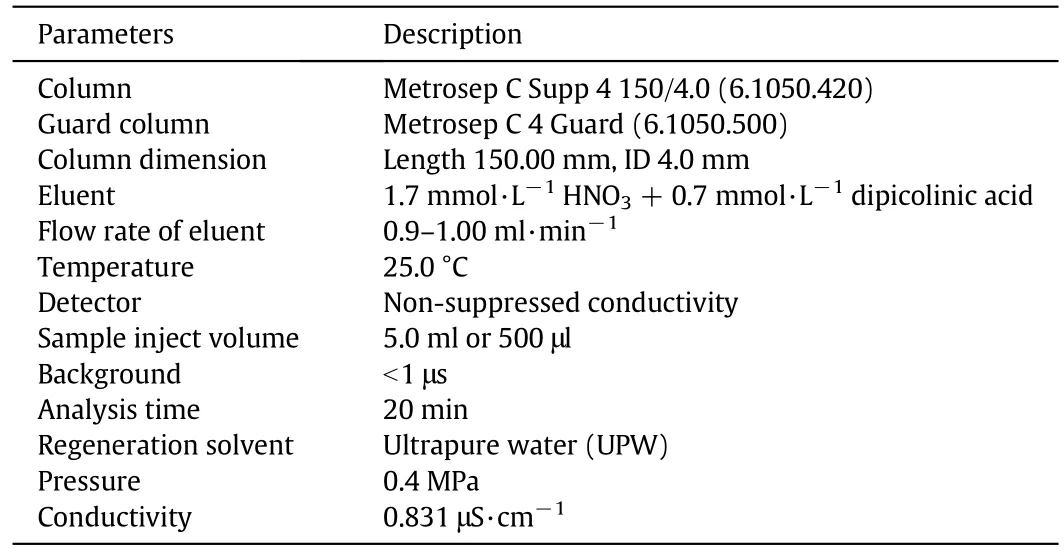
Table 1 Operating condition and details of cation chromatography
Table 1 shows the operating conditions of cationic chromatography for analyzing cationic spaces in the degraded samples of DEA system.This is a non-suppression process of the detector.Eluentand regenerate were stored at atmospheric pressure in 4 L glass reservoirs.
In the anion exchange chromatographic system,acetate,chloride,nitrite,nitrate,phosphate,sulfate and oxalate ions were separated,respectively.The column was operated atambienttemperature.The description of eluent and all operating conditions are as follows in Table 2.The system used a suppression conductivity detector.Eluentand regenerate were stored under atmospheric conditions in 4 L glass reservoirs.
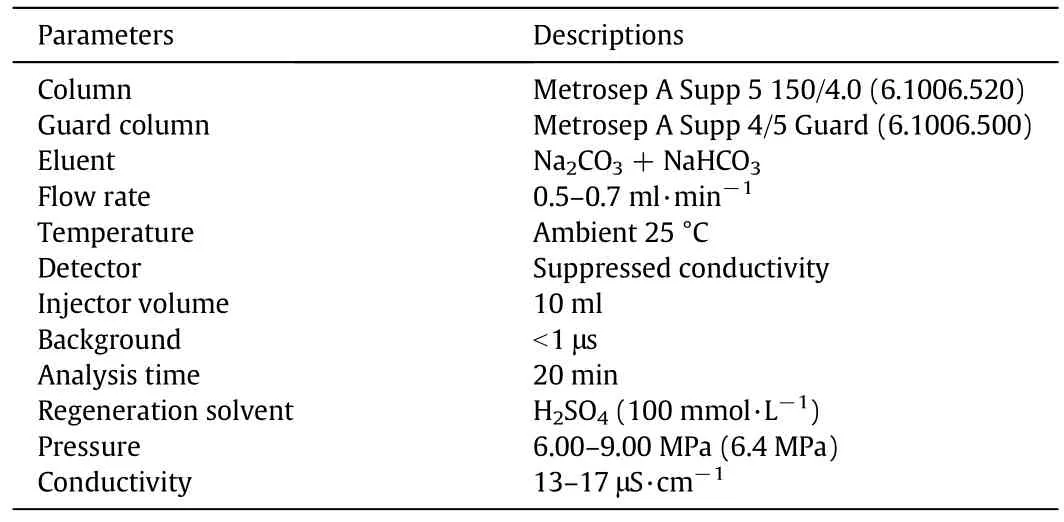
Table 2 Operating condition and details of anion chromatography
Ion exclusion chromatography(IEC)is mainly used for the separation of weak acids or bases.The greatest importance of IEC is for the analysis of weak acids such as carboxylic acids,carbohydrates,phenols or amino acids.In this experiment,carboxylic acids were mainly investigated by this chromatographic method using the same IC equipment.The operating condition of IEC is given in Table 3.
3.Results and Discussion
To identify the ionic DGPs ofthe DEA-H2O-CO2system,three experimental samples from D01DEA2M,D02DEA3M,and D03DEA4M were investigated by ion chromatographic methods;these are illustrated below with possible reaction pathways.
3.1.Degradation products of D01DEA2M
Table 4 provides detailed information on the DGPs for the sample D01DEA2M.By using the anionic ion chromatographic method(AICM),two DGPs were identified as nitrite and phosphate.The characterization was carried out by means of the standard verification process following the retention time of the sample in order to determine whether it matched with the standard or not.The two identifiedDGPs,nitrite and phosphate,are obtained at concentrations of 643.19 and 882.00 μg·g-1respectively.The chromatogram of the detected DGP is shown in Fig.3.
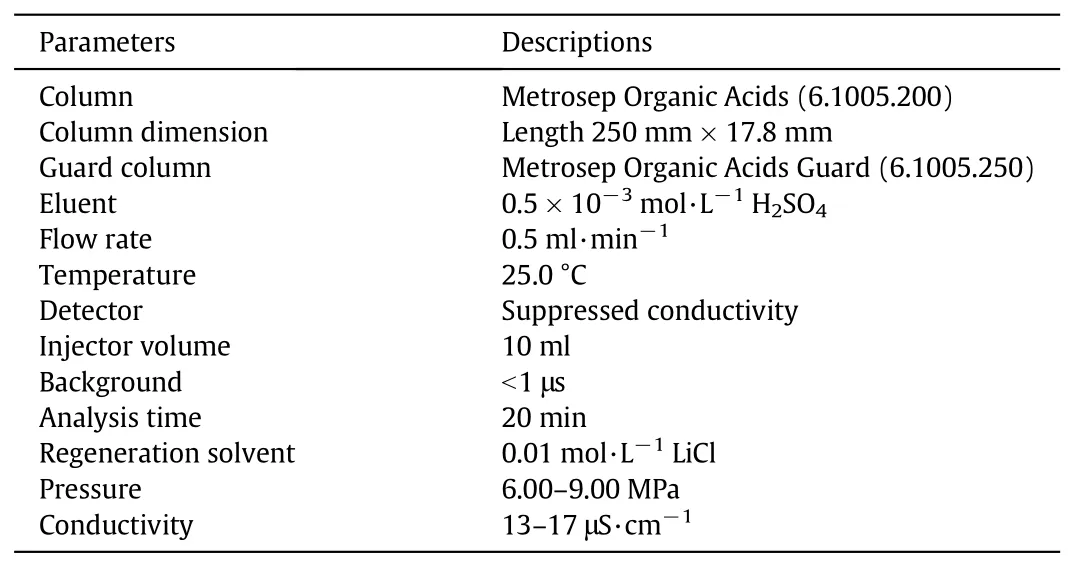
Table 3 Operating conditions and details of ion exclusion chromatography
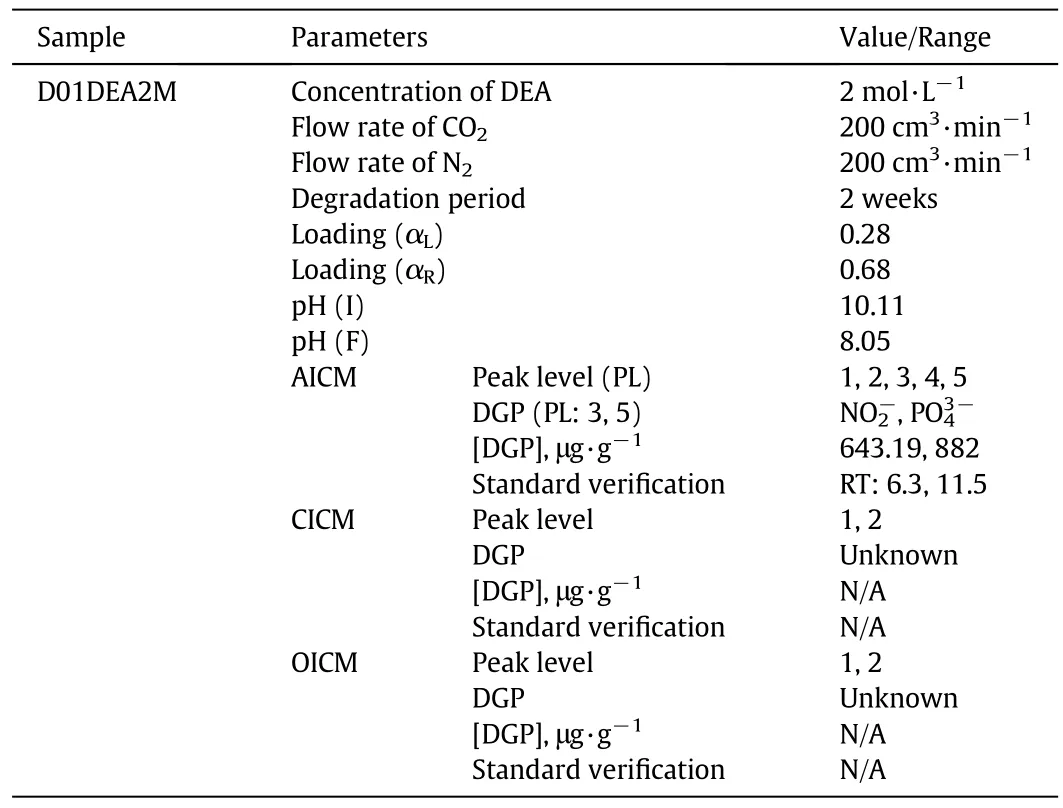
Table 4 Carbon dioxide-induced degradation products of 2 mol·L-1 diethanolamine
These two DGPs are formed by the degradation of amine by CO2during the absorption and stripping processes.It is assumed that the alkanolamines that contain the N atom behave as an essential part of the functional radical.This amine radical and nitrogen react with gaseous impurities or contaminants of mixed water and finally form miscellaneous DGPs.Phosphate and nitrite are the typical ionic DGPs of the a mine system.The same result is found in literature which mentions that amines are degraded into many ionic DGPs during sour gas absorption plants[26].The study of degradation also measures the carbon dioxide loading and pH at every stage of the generic cycles.The rich and lean loading was obtained as 0.68 and 0.28 respectively.On the other hand,pH was observed as 10.11 and 8.05 for initial and final solutions respectively.This sample was also analyzed by cationic ion chromatographic method(CICM)and organic acid ion chromatographic method(OICM),but in both analyses,no significant degradation was found as shown in Figs.4 and 5,respectively.In CICM three peaks were observed,but none of them was detected.In OICM chromatograms two peaks were observed,but the method could not detect either of these.
The overall result of this sample D01DEA2M is given in Table 4.From the obtained results of this sample it was assumed that some DGPs may occur in trace quantities,so it was not possible to identify them by IC or they may possess greater degradation stability.To overcome these problems further analyses such as HPLC,GC,and GCMS are recommended to determine what is produced under this condition.
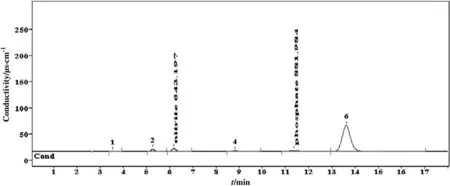
Fig.3.Chromatogram of carbon dioxide induced DGPs of 2 mol·L-1 diethanolamine identified by anionic ion chromatographic method(AICM).
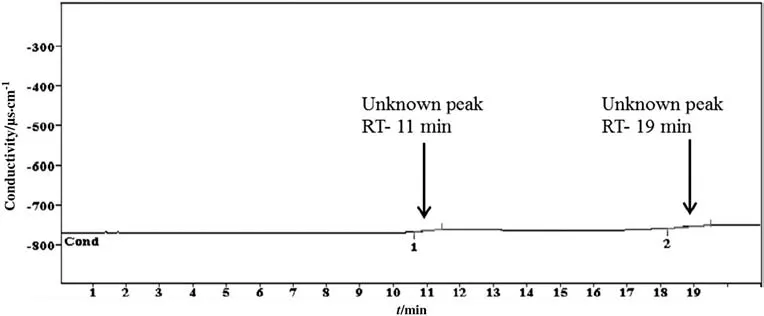
Fig.4.Chromatogram of carbon dioxide is induced DGPs of 2 mol·L-1 diethanolamine identified by cationic ion chromatographic method.
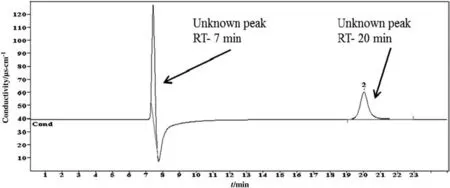
Fig.5.Chromatogram of carbon dioxide induced DGPs of 2 mol·L-1 diethanolamine identified by organic acid ion chromatographic method.
3.2.Degradation products of D02DEA3M
The identified cationic DGPs of the sample D02DEA3M are ammonium based.The concentration of ammonium radical is 0.102 μg·g-1as shown in Fig.6.Alkanolamines are degraded into ammonium radical during natural gas and crude oil purification processes as described by Stewart and Lanning[27].This result is also consistent with several published works in the literature[26,28-30].
In the AICM,six peaks have been determined out of which only three were identified in this work.The identified DGPs are acetate,nitrate,and phosphate(Fig.7).The concentrations of the detected compounds are plotted in Table 5.The obtained concentrations were calculated in ppm level for each.In OICM methods chromatograms showed two peaks,but none were detected.Fig.8 showed two peaks,but to identify all the unknown compounds,further analyses such as HPLC,GC,GC-MS,and LC-MS are recommended.In these degradation studies,CO2loading and pHare also simultaneously determined.From the observed result,it can be concluded that the degraded sample has satisfactory alkalinity to capture impurities from raw natural gas(NG).The observed loading was comparatively significant at 55 and 100°C.The observed regeneration was also better than other conventional amines.The summarized DGPs of this sample D04DEA4M are given in Table 5.
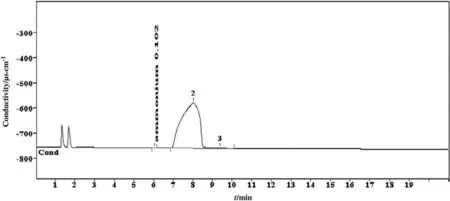
Fig.6.Chromatogram of carbon dioxide induced degradation products of 3 mol·L-1 diethanolamine identified by cationic ion chromatographic method.

Fig.7.Chromatogram of carbon dioxide induced degradation products of 3 mol·L-1 diethanolamine identified by anionic ion chromatographic method.
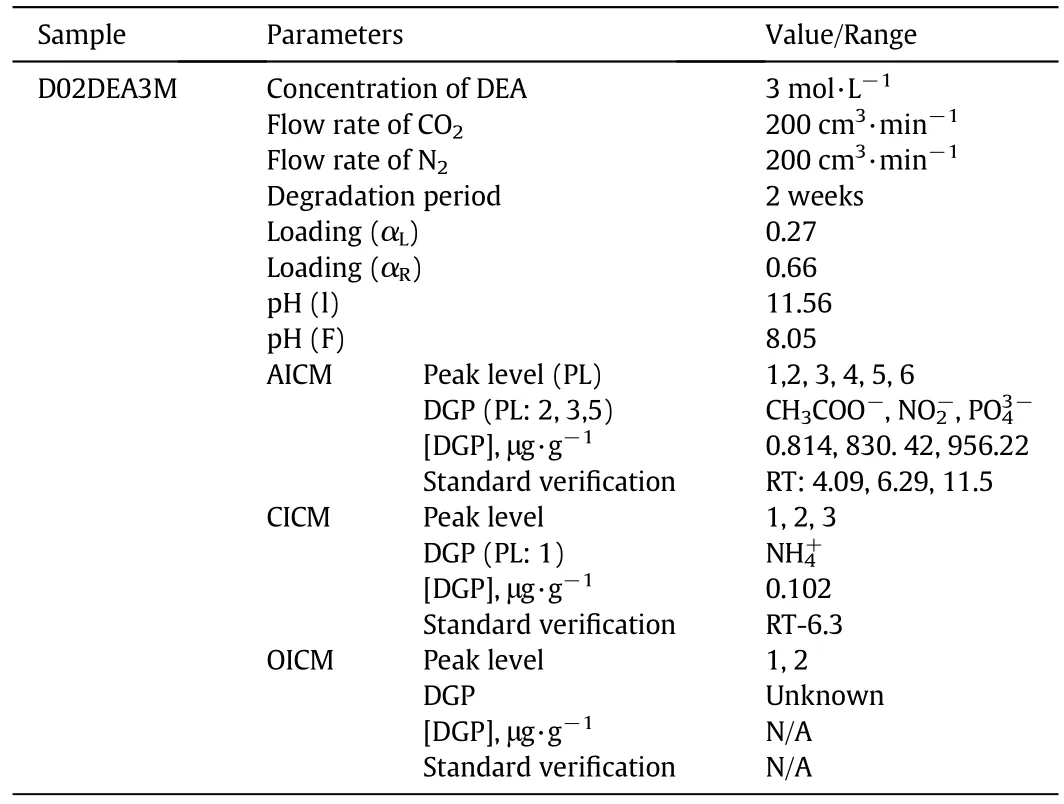
Table 5 Carbon dioxide-induced degradation products of 3 mol·L-1 diethanolamine
3.3.Degradation products of D03DEA4M
To determine the DGPs of D03DEA4M,the sample was analyzed by the same three chromatographic methods i.e.AICM,CICM,and OICM.The concentration of the DGPs obtained was identified by the standard calibration curve assessment.In AICM the chromatograms of the DO5DEA4M samples showed eight peaks,but among these peaks,only two were identified by this method.The detected DGPs were acetate and phosphate.The concentrations of these two detected DGPs were 0.66 and 2.52 respectively.The chromatograms of identified DGP are shown in Fig.9.Table 6 provides the representation of the summarized DGPs determined by three chromatographic methods.
In the cationic IC methods,the same sample was analyzed where the chromatograms showed two peaks.Among these two peaks,only one was identified.The detected reaction product was ammonium.The concentration and retention time were found to be 0.5 μg·g-1and 6.303 min,respectively.Ammonium is the most common degradation product of alkanolamine systems during sour gas absorption and stripping processes.The obtained result is consistent with the literature as many researchers also found NH4+ions in alkanolamine system[27-31].The chromatogram of CICM is shown in Fig.10.For the case of the organic acid chromatograms to peaks were also observed,but none was detected either.There were four standard solutions of organic acids that were prepared such as acetate,butyrate,propionate,and valerate.Among these,none were identified except for the acetate.However,the acetate was detected in anionic ion chromatography only.OICM chromatogram of 4 mol·L-1diethanolamine solution is given in Fig.11.
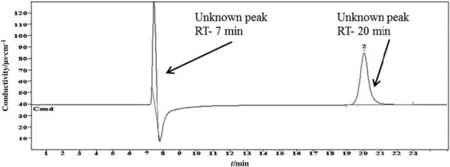
Fig.8.Chromatogram of carbon dioxide induced DGPs of 3 mol·L-1 diethanolamine identified by organic acid ion chromatographic method.
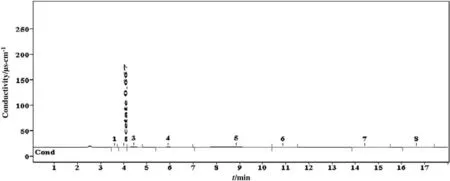
Fig.9.Chromatogram of carbon dioxide induced degradation products of 4 mol·L-1 diethanolamine identified by anionic ion chromatographic method.
Therefore the overall DGPs of these compounds are acetate,ammonium,and phosphate.All of these are charged molecules;they can create enormous problems for plant operation.Many peaks were unidentified in this sample,so to recover these unknown products,further chromatographic analysis is required.
3.4.Possible reaction pathways
Besides MEAand MDEA,DEA is widely used in the CO2capture technology as an absorbent[32,33].DEA is less corrosive and suitable forseparating H2S and CO2from raw natural gas or petroleum.The proposed reaction pathways between CO2and DEA are shown by the following reaction steps.The formation of proton(reactions(4)-(6)),bicarbonate(reaction(5))and carbonate(reaction(6))is mainly shown here.According to the following reaction pathways,carbon dioxide capture will result in the formation of bicarbonate in the liquid amine system.However in the absorber,captured CO2generally reacts with DEAat55°C(reaction(8))to form DEAcarbamate.DEAcarbamate is considered as lean amine solution which is sent to the stripper to eliminate CO2by applying heat at 100-120°C.During this absorption and desorption of CO2,DEA is degraded to DGPs.
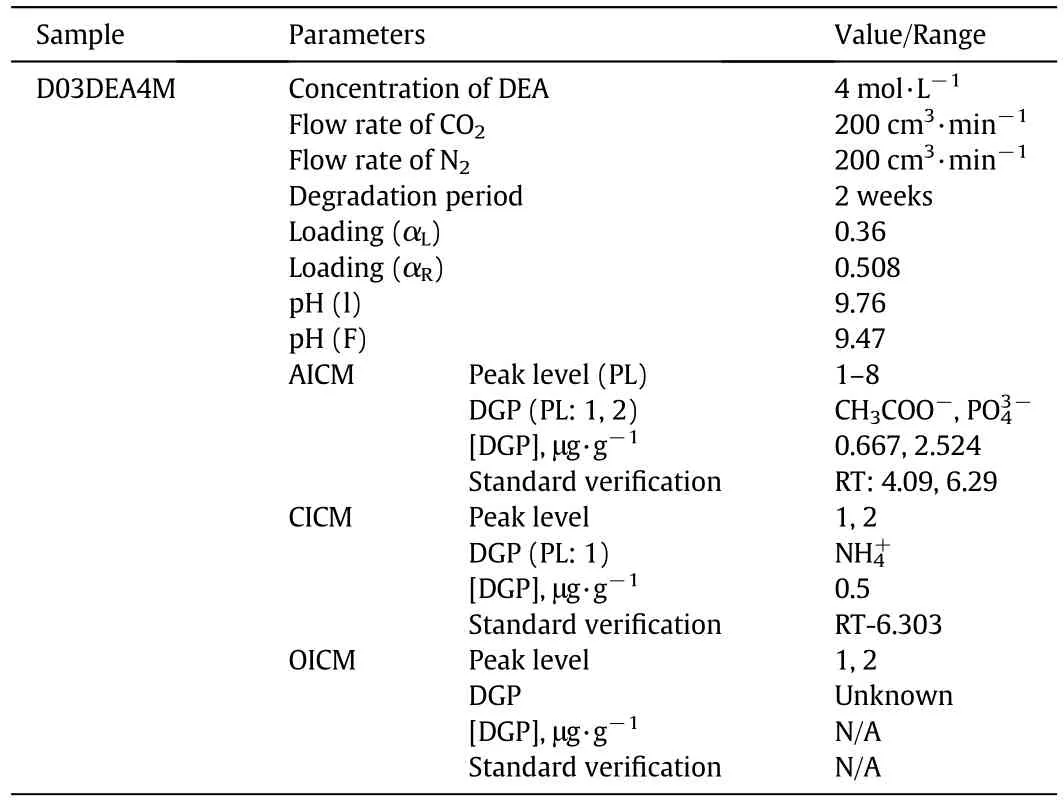
Table 6 Carbon dioxide-induced degradation products of 4 mol·L-1 diethanolamine
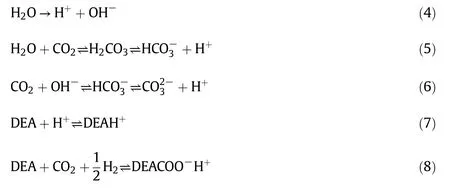
The following reaction pathways are given for the formation of acetate product with associated DGPs which occurred in the amine degradation process((9)-(15)).Amines take part in the oxidation reaction and convert it to organic acid.An example of carboxylic acid formation from amine oxidation is shown by reaction(9).In the amine degradation protonated DEA is also formed as shown in reaction(7).Protonated DEA(DEAH+)reacts with CO2and produces MEA with acetaldehyde.Later on MEA and acetaldehyde react with O2to produce various degradation products.Acetic acid is produced from the oxidation of acetaldehyde[Eq.(11)]which finally generates acetate product by dissociation reaction.In the amine degradation reaction,oxalic acid could be produced from MEA oxidation as shown in reaction(14).Apart from the following reactions,it could be presumed that,various degradation products like acetate,oxalate,ammonia and protonated amines(MEAH+and DEAH+)could further react with other available species.A study on the oxidative degradation of MEA and MDEA reported various degradation products with reaction pathways which support this work[34].
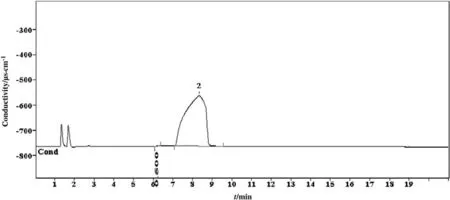
Fig.10.Chromatogram of carbon dioxide induced degradation products of 3 mol·L-1 diethanolamine identified by cationic ion chromatographic method.
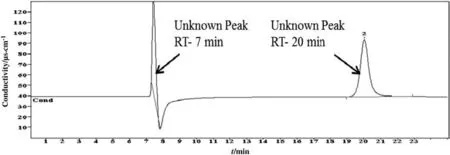
Fig.11.Chromatogram of carbon dioxide induced degradation products of 4 mol·L-1 diethanolamine identified by organic acid ion chromatographic method(OICM).
Nitrate and nitrite are common degradation products of amine systems as reported by many researchers in the case of MEA,DEA,and MDEA[35-37].In the following reactions((16)-(24)),the formation of nitrite and nitrate,both are produced from ammonia due to successive oxidative reaction.Ammonia further reacts with CO2and produces ammonium carbamate leading to urea.However,DEA molecule has--NH2group which could produce NH3.By considering the following reactions,nitrous acid could be formed due to the oxidation of hydroxyl amine(NH2-OH).Finally the dissociation of nitrous acid could produce nitrite.
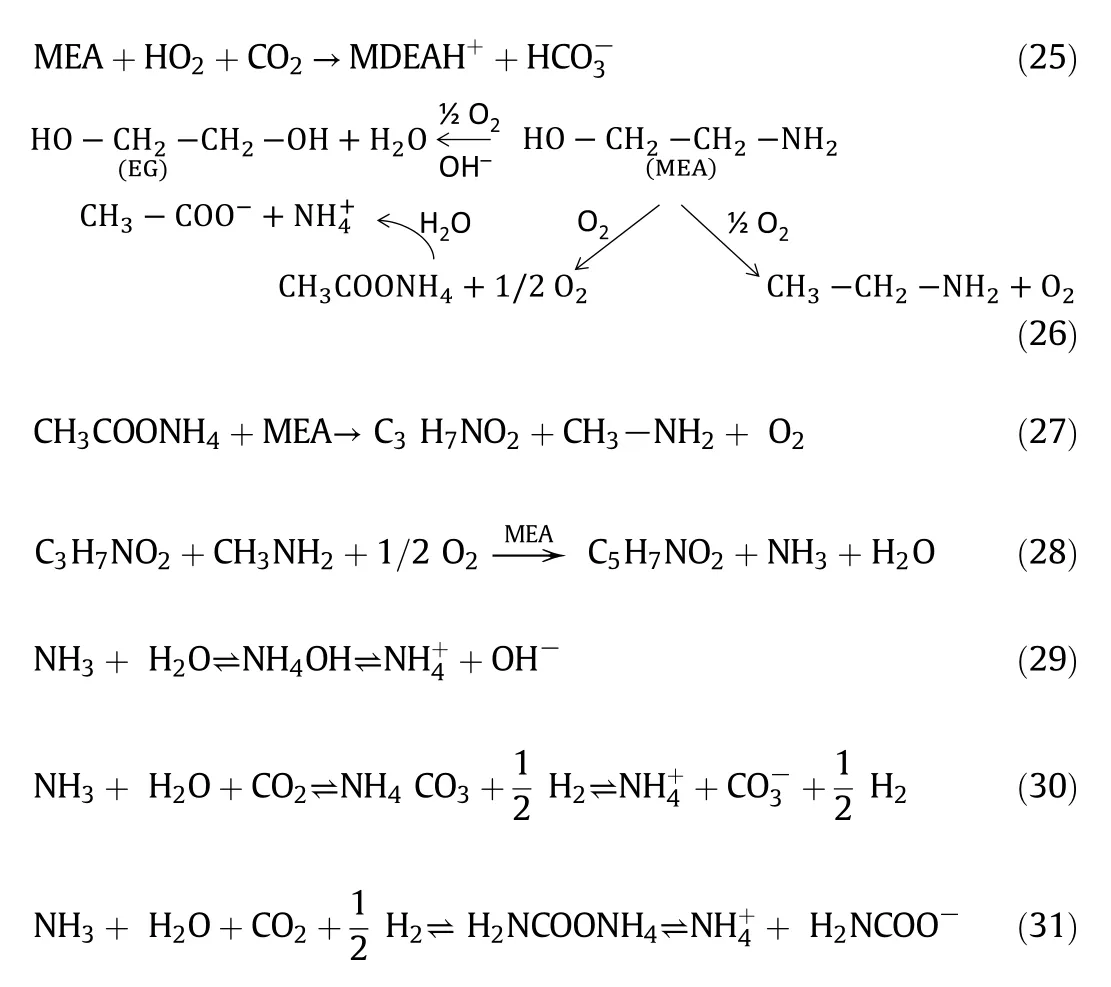
The following reaction pathways((25)-(31))are shown for the formation of ammonium product with associated products in amine degradation.Ammonium products could be produced in many ways in amine degradation,either from the formation of ammonium salts or carbamate.In the amine degradation reactions,MEA is formed due to the reaction between DEAH+and CO2(10).Ethylene glycol(EG)could be formed due to the reaction between oxygen and carbon dioxide(26).MEA could also produce ammonium acetate due to oxidation reaction.The following reaction mechanism also proposed by many researchers in the literature which strongly support this work[34,35].
4.Conclusions
Carbon dioxide-induced DGPs of aqueous diethanolamine were identified by ion chromatographic methods,and the most common ionic DGPs are acetate(CH3COO-),nitrite(NO2-)and ammonium().In this research,phosphate(PO43-)was found as a degradation product of the DEA+H2O+CO2amine system.However,this phosphate might be due to water impurities,not from the degradation of DEA.During the analysis,several peaks were found in AICM and OICM.To identify those unknown peaks of the DEA amine system,the authors recommend further chromatographic analysis on this in the future.The identified DGPs of the DEA+H2O+CO2amine system for three degradation experiments(D01DEA2M,D02DEA3M,and D03DEA4M)are listed below with concentration.
?D01DEA2M:In AICM the identified DPs wereandf concentration 643.19 and 882 μg·g-1respectively.
?D02DEA3M:In AICM the identified DPs were CH3COO-,andof concentration 0.814 μg·g-1,830.42 μg·g-1and 956.22 μg·g-1respectively.On the other hand in CICM,was detected with a concentration of 0.102 μg·g-1as well.
?D03DEA4M:In AICM the identified DPs were CH3COO-,andof concentration 0.667 μg·g-1and 2.524 μg·g-1respectively.On the other hand in CICM,was detected with concentration 0.5 μg·g-1as well.
Recommendations
Three IC methods were used to detect the unknown ionic DGPs of CO2induced degradation of the DEA system in this study.Some limitations were observed in this study,i.e.the ionic DGPs were notidentified,and this may be due to the high concentration or due to limited standards.In the OICM method,DGPs were not detected.To identify these unknown peaks,other chromatographic methods such as HPLC,GC-MS,and LC-MS are recommended.Phosphate product was detected in this study for all samples;however this product may be due to contamination of water.The possible reaction pathways for nitrite,acetate and ammonium were proposed except phosphate product.
Nomenclature
AICM anionic IC method
CICM cationic IC method
DGPs degradation products
[DGP] concentration of degradation products
F final
I initial
IC ion chromatography
L lean
OICM organic acid IC method
PL peak level
R rich
RT retention time
α loading
Acknowledgments
The authors would like to acknowledge the Ministry of Science,Technology and Innovation,Malaysia(MOSTI),for funding the project:RG003/09AET as wellas the University ofMalaya for allowing fullaccess to several key laboratories to perform experimental work.
[1]Z.Liang,K.Fu,R.Idem,P.Tontiwachwuthikul,Review on current advances,future challenges and consideration issues for post-combustion CO2capture using amine-based absorbents,Chin.J.Chem.Eng.24(2016)278-288.
[2]A.M.Chakma,A.Meisen,Identification of methyl diethanolamine degradation products by gas chromatography and gas chromatography-mass spectrometry,J.Chromatogr.457(1988)287-297.
[3]M.Appl,H.J.Henrici,U.Wagner,K.Keussner,K.Volkamer,E.Fuerst,Removal of CO2and/or H2S and/or COS from gases containing these constituents,Canadian Patent 1(1980)90-98.
[4]Q.Zhi,G.Kai,Modeling and kinetic study on absorption of CO2by aqueous solutions of N-methyldiethanolamine in a modified wetted wall column,Chin.J.Chem.Eng.17(2009)571-579.
[5]N.Verma,A.Verma,Amine system problems arising from heat stable salts and solutions to improve system performance,Fuel Process.Technol.90(2009)483-489.
[6]L.D.Polderman,C.P.Dillon,A.B.Steele,Why monoethanolamine solution breaks down in gas-treating service,Oil Gas J.54(1955)180-183.
[7]B.R.Strazisar,R.R.Anderson,C.M.White,Degradation pathways for monoethanolamine in a CO2capture facility,Energy Fuel 17(2003)1034-1039.
[8]H.Liu,X.Luo,Z.Liang,P.Tontiwachwuthikul,Determination of vapor-liquid equilibrium(VLE)plots of 1-dimethylamino-2-propanol solutions using the pH method,Ind.Eng.Chem.Res.54(17)(2015)4709-4716.
[9]C.J.Kim,G.Sartori,Kinetics and mechanism of diethanolamine degradation in aqueous solutions containing carbon dioxide,In.J.Chem.Kinet.16(1984)1257-1266.
[10]A.Chakma,A.Meisen,Degradation of aqueous DEA solutions in a heat transfer tube,Can.J.Chem.Eng.65(1987)264-273.
[11]M.L.Kennard,A.Meisen,Gas chromatographic technique for analyzing partially degraded diethanolamine solutions,J.Chromatogr.267(1983)373-380.
[12]M.L.Kennard,A.Meisen,Mechanisms and kinetics of diethanolamine degradation,Ind.Eng.Chem.Res.Fundam.24(1985)129-140.
[13]C.S.Hsu,C.J.Kim,Diethanolamine(DEA)degradation under gas treating conditions,Ind.Eng.Chem.Prod.Res.Dev.24(1985)630-635.
[14]A.Chakma,E.Chornet,R.P.Overend,W.H.Dawson,Absorption of CO2by aqueous diethanolamine(DEA)solutions in a high shear jet absorber,Can.J.Chem.Eng.68(1990)592-598.
[15]P.E.C.Holub,W.-Y.Su,Amine degradation chemistry in CO2service,48th Annual Laurance Reid Gas Conditioning Conference,Norman,Oklahoma 1998,pp.146-160.
[16]A.Chakma,A.Meisen,Methyl-diethanolamine degradation—Mechanism and kinetics,Can.J.Chem.Eng.75(5)(1997)861-871.
[17]H.Gao,Z.Liang,H.Liao,R.O.Idem,Thermal degradation of aqueous DEEAsolution at stripper conditions for post-combustion CO2capture,Chem.Eng.Sci.135(2015)330-342.
[18]P.D.D.Clark,P.M.Davis,Introduction to sulphur chemistry and sulphur handling,Sulphur 2004 Conference,Barcelona(Spain),October 24,2004.
[19]O.F.Dawodu,A.Meisen,Degradation of aqueous diethanolamine solutions by carbon disulphide,Gas Sep.Purif.10(1996)1-11.
[20]O.F.Dawodu,A.Axel Meisen,Mechanism and kinetics of COS-induced diethanolamine degradation,Ind.Eng.Chem.Res.33(1994)480-487.
[21]O.Dawodu,A.Meisen,The effects of operating conditions on COS-induced degradation of aqueous diethanolamine solutions,Gas Sep.Purif.6(1992)115-124.
[22]O.Dawodu,A.Meisen,Identification of products resulting from carbonyl sulphideinduced degradation of diethanolamine,J.Chromatogr.587(1991)237-246.
[23]G.Versteeg,H.Oyevaar,The reaction between CO2and diethanolamine at 298 K,Chem.Eng.Sci.44(1989)1264-1268.
[24]H.Lepaumier,S.Martin,D.Picq,B.Delfort,P.-L.Carrette,New amines for CO2capture.III.Effect of alkyl chain length between amine functions on polyamines degradation,Ind.Eng.Chem.Res.49(2010)4553-4560.
[25]H.Lepaumier,D.Picq,P.-L.Carrette,New amines for CO2capture.II.Oxidative degradation mechanisms,Ind.Eng.Chem.Res.48(2009)9068-9075.
[26]A.J.Sexton,G.T.Rochelle,Catalysts and inhibitors for oxidative degradation of monoethanolamine,Int.J.Greenhouse Gas Control 3(2009)704-711.
[27]E.J.Stewart,R.A.Lanning,Reduce amine plant solvent losses,Hydrocarb.Process.73(1994).
[28]R.Kadnar,Determination of amines used in the oil and gas industry(upstream section)by ion chromatography,J.Chromatogr.A 850(1999)289-295.
[29]M.Kaminski,D.Jastrz?bski,A.Przyjazny,R.Kartanowicz,Determination of the amount of wash amines and ammonium ion in desulfurization products of process gases and results of related studies,J.Chromatogr.A 947(2002)217-225.
[30]O.Mrklas,A.Chu,S.Lunn,Determination of ethanolamine,ethylene glycol and triethylene glycol by ion chromatography for laboratory and field biodegradation studies,J.Environ.Monit.5(2003)336-340.
[31]F.Closmann,T.Thu Nguyen,G.T.Rochelle,MDEA/Piperazine as a solvent for CO2capture,Energy Procedia 1(2009)1351-1357.
[32]F.Barzagli,F.Mani,M.Peruzzini,Continuous cycles of CO2absorption and amine regeneration with aqueous alkanolamines:A comparison of the efficiency between pure and blended DEA,MDEA and AMP solutions by 13C NMR spectroscopy,Energy Environ.Sci.3(6)(2010)772-779.
[33]M.Hasib-ur-Rahman,M.Siaj,F.Larachi,CO2capture in alkanolamine/roomtemperature ionic liquid emulsions:A viable approach with carbamate crystallization and curbed corrosion behavior,Int.J.Greenhouse Gas Control 6(2012)246-252.
[34]O.Lawal,A.Bello,R.Idem,The role of methyldiethanolamine(MDEA)in preventing the oxidative degradation of CO2loaded and concentrated aqueous monoethanolamine(MEA)-MDEA blends during CO2absorption from flue gases,Ind.Eng.Chem.Res.44(6)(2005)1874-1896.
[35]J.G.Thompson,R.Frimpong,J.E.Remias,J.K.Neathery,K.Liu,Heat stable salt accumulation and solvent degradation in a pilot-scale CO2capture process using coal combustion flue gas,Aerosol Air Qual.Res.14(2)(2014)550-558.
[36]D.K.Lee,Mechanism and kinetics of the catalytic oxidation of aqueous ammonia to molecular nitrogen,Environ.Sci.Technol.37(24)(2003)5745-5749.
[37]S.Bazhenov,V.Vasilevsky,A.Rieder,S.Unterberger,E.Grushevenko,V.Volkov,A.Volkov,Heat stable salts(HSS)removal by electrodialysis:Reclaiming of MEA used in post-combustion CO2-capture,Energy Procedia 63(2014)6349-6356.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
