Study of interaction between metal ions and quercetin
Taiane Souza de Castilho,Tatiane Brescovites Matias,Keller Paulo Nicolini,Jaqueline Nicolini
Instituto Federal do Paraná–IFPR,Lacoppi–Laboratório de Corantes e Processos Pirolíticos,Departamento de Química,Palmas,PR,Brazil
ABSTRACT A displacement test based on the interaction between the flavonoid quercetin and an excess of metal chloride allows the determination of the binding constant for the reaction between quercetin and Ca2+,Mg2+ and Ni2+.The values obtained were 2.20±1.77×103 for Ca2+,1.37±0.59×103 for Mg2+ and 7.03±1.04×104 for Ni2+,and all interactions showed type 1:1 stoichiometry,as determined by titration and by the method of continuous variations (Job’s method).The complexion effect was observed qualitatively through a colorimetric change in the medium (yellow →neon yellow) and spectroscopically through a bathochromic shift in the absorption band of quercetin in the presence of metals.This investigation serves as a tool for the development and testing of materials capable of capturing toxic metal ions or favoring the absorption of beneficial ions(in relation to the human metabolism)through the construction of efficient bioorganic systems.The results reported herein allow understanding of this detection system,indicating the following ascending order of the binding constants(Mg2+<Ca2+<Al3+<Ni2+).
Keywords:Binding constants Quercetin Antioxidant properties
1.Introduction
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is one of the most abundant flavonoids and it is found mainly in medicinal plants,fruits,leaves and vegetables [1].It shows band I absorption(~300–380 nm),which is considered to be associated with the B-ring cinnamoyl system,and band II absorption (~240–280 nm)related to the A-ring benzoyl system[2,3](Fig.1).With the presence of chelating sites in its structure,quercetin is capable of forming complexes with a series of cations[2,4–6],which show antioxidant activity [7],DNA protection [8,9],antitumor and anticarcinogenic activity[10,11]and anti-inflammatory action[12].
Some cations are important to human metabolism,such as calcium and magnesium,which are involved in the constitution of bones and muscles [13].Calcium strengthens the matrix material [14]while magnesium provides sites for coordination with adenosine triphosphate (ATP) and myosin through electrostatic interactions in muscles [15].Studies highlight that in the metabolism the presence of quercetin promotes calcium absorption by the intestine,which does not occur in its absence when menadione is present[16].However,other cations,such as nickel,show carcinogenic activity,causing damage to DNA [17],due to their ability to oxidize peptides and enzymes,generating free radicals which cause cell damage,mainly in the lungs [18].Thus,quercetin can capture these free radicals,which cause damage to DNA[19].
In this study,quercetin was used to test a cationic chemosensor based on the formation of a complex between the chelating sites of quercetin and chlorides of K+,Ca2+,Mg2+and Ni2+.These cations were selected due to their biological importance.K+participates in sodium transport in the metabolism,in a process known as the sodium-potassium pump,via Na+/K+ATPase[20–22].Ca2+and Mg2+are indispensable in the maintenance of various biological processes,for instance,Ca2+is important for maximizing bone mineral mass,especially during adolescence[23],and Mg2+plays a role in regulating muscle contractions and transmitting nerve impulses[24].However,Ni2+ions show carcinogenic activity and can damage DNA [17].The data obtained show that the chelating system of quercetin in methanol presents a higher association constant for Ni2+compared with Mg2+and Ca2+.This is because the formation of a higher number of rings leads to greater stability of the complex formed,suggesting that in the event of intoxication this system can be used to remove the Ni2+cations from the human metabolism.
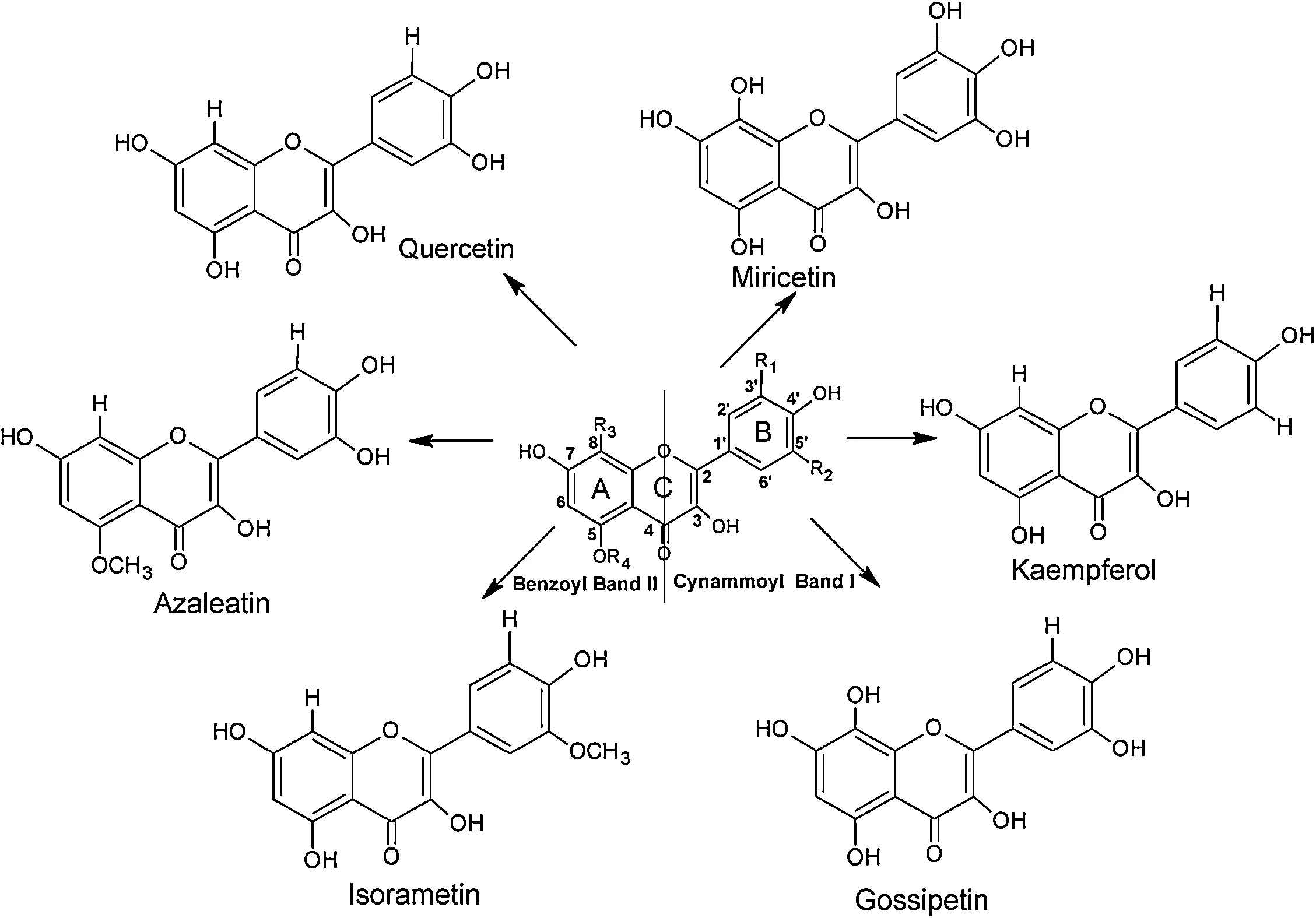
Fig.1.Structures of quercetin and main flavonoids.
2.Material and methods
2.1.Reagents and equipment
The following reagents were used:quercetin (98.0%,Quercegen),aluminum chloride hexahydrate (AlCl3.6H2O,97.0%,Neon),sodium chloride (NaCl,99.6%,Neon),potassium chloride (KCl,99.6%,Neon),calcium chloride dihydrate (CaCl2·2H2O,95.0%,Neon),nickel chloride hexahydrate (NiCl2·6H2O,97.2%,Neon),magnesium chloride hexahydrate (MgCl2·6 H2O,≥98.0%,Neon)and methanol (99.8%,Vetec).The spectra were obtained on a PerkinElmer Lambda 365 Spectrophotometer at between 200 and 550 nm.
2.2.Determination of the binding constant and stoichiometry of the complex formed
Stock solution:To determine the binding constant,5 ml of a stock solution of quercetin (1.0×10-2mol L-1) in methanol was prepared.The tests were performed in triplicate.The binding constants were calculated using the OriginPro 6.1 program.
2.3.Obtainment of binding constants(titration experiments)
Solution 1:for the preparation of the 4.0×10-5mol L-1quercetin solution,a 40 μL aliquot of the stock solution was transferred to a 10 ml volumetric flask,containing NaCl to adjust the ionic strength of the medium [25](I=0.1 mol L-1),and the volume was made up with methanol.The maximum wavelength of quercetin is obtained at 372 nm.
Solution 2:the solution of each cation(K+,Ca2+,Mg2+and Ni2+)was prepared at a concentration of 7.0×10-3mol L-1in a 5 ml volumetric flask from Solution 1.
In the next step,2 ml of Solution 1 was added to a quartz cuvette,hermetically closed with a rubber lid to avoid solvent evaporation,at 25°C.With the aid of a micropipette,Solution 2 was gradually added to a cuvette containing Solution 1 to obtain the spectra.The spectral data were collected and analyzed applying Eq.(1),as described by Valeur et al.[26],which is used when the concentration of free metal cations[M]is much higher than the concentration of complexed cations,whereAis the absorbance obtained after each addition of the metal complex(Solution 2)to the solution containing only quercetin(Solution 1),A0is the absorbance of the quercetin solution(Solution 1)before the start of the titration,Alimis the maximum absorbance reached at the end of the titration,CMis the metal concentration added,Kis the constant obtained andnindicates the stoichiometry of the complex obtained [26].The tests were performed in triplicate.The binding constants were calculated using the OriginPro 6.1 program.

2.4.Method of continuous variations
The method of continuous variations (Job’s method) was also used to determine the stoichiometry of the complex formed.Equimolar solutions of the cations and the quercetin in methanol were prepared in a concentration of 4.0×10-5mol L-1.In the tests carried out with the methanolic solutions,NaCl(I=0.1 mol L-1)was added to maintain the ionic strength of the medium constant.In the next step,based on the maximum wavelength(λmax)of each cation,the maximum absorption intensities of each mixture versus the molar fractions were plotted.The tests were performed in triplicate.
3.Results and discussion
This new approach to investigating the interaction of metals and quercetin shows a strong bathochromic shift of the maximum absorption band with the formation of the complex between quercetin and the metal (M),that is,the maximum wavelength of the quercetin complex formed is greater than that of the free quercetin.This occurs due to the π →π*transitions of the enone system of quercetin.As the solvent polarity increases with the addition of metals,a bathochromic shift of the absorption band occurs due to a reduction in the stability of the energy level of the excited state,which accompanies the dipole-dipole interactions,in relation to the fundamental state [27,28].The addition of increasing amounts of different cations(Ca2+,Mg2+and Ni2+)turned the color of the solution to neon yellow when there was interaction between the quercetin and the tested cation.This behavior was not observed in tests using K+,which is related to certain factors [29]:i) electronegativity,K (0.82)<Ca (1.00)<Mg (1.31)<Ni (1.91); ii) ionic radius,which follows the inverse order of the electronegativity,K+(138)>Ca2+(106)>Mg2+(78)>Ni2+(69);and iii)periodic group(alkaline earth and transition metals).Quercetin acts as a chelating agent for the metals tested and the K+ion has a larger ionic radius and lower electronegativity,being energetically favorable,and thus it remains free in solution and does not form a complex with quercetin under the conditions tested.
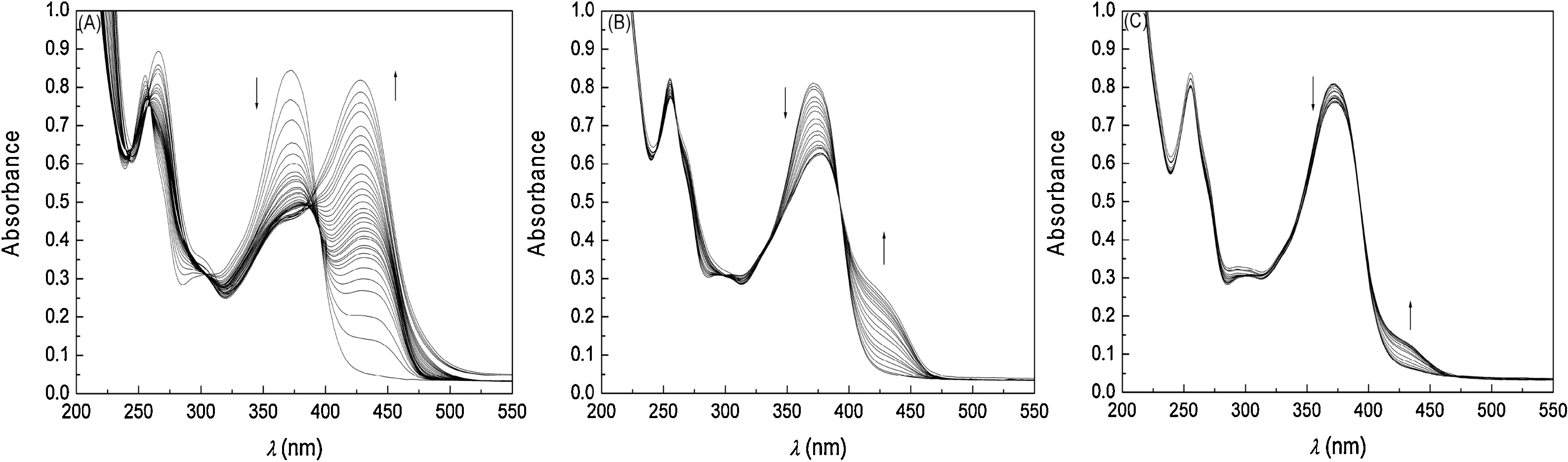
Fig.2.UV–vis spectra taken at 25°C of methanolic solutions of quercetin(I=0.01 mol L-1)containing metal chlorides at maximum concentrations of:(A)3.51×10-3 mol L-1 of nickel;(B)3.24×10-3 mol L-1 of magnesium;and(C)3.20×10-3 mol L-1 of calcium.The stock solution of metals was 7.0×10-3 mol L-1.
The most pronounced behavior was noted with the addition of Ni2+,for which there was a bathochromic shift in the maximum wavelength of quercetin from 371.9 to 428.3 nm,with a 1:1 stoichiometry and binding constant of 7.03±1.04×104L mol-1.The tests to obtain the binding constant for the reaction between quercetin and nickel were carried out with a nickel excess of the order of 88 times in relation to the quercetin concentration.In the case of Ni2+,the binding constant was 17 times higher than that determined for the reaction between quercetin and Al3+ions under the same experimental conditions [30]with a 2:1 stoichiometry[25,30].The titration experiments allowed the binding constants for Ca2+,Mg2+and Ni2+to be obtained and also the influence of the addition of M2+metals on the UV–vis spectrum for quercetin to be investigated.Nickel showed the best result in the formation of the complex,with the highest binding constant values.It is a transition metal with coordination at the sub-level d,while for the other metals the interaction occurs at the sub-levels s and p,favoring the acid-base interaction that occurs between Ni2+(Lewis acid)and quercetin(Lewis base).
Fig.2 shows the sequence of UV–vis spectra obtained for quercetin as a function of the increase in the metal concentration.An analysis of the data reveals the presence of well-defined isosbestic points with the addition of Ni2+at 389.1 nm (Fig.2A) and Mg2+at 393.5 nm(Fig.2B).With the addition of Ca2+to the system there is an isosbestic point at approximately 385.8 nm(Fig.2C),but it is not well defined.There was a bathochromic shift in the absorption band in all tests carried out.This is due to electronic transitions that occur in the B-ring of quercetin,through the formation of stable metal complexes with flavonoids in methanol,causing changes in the absorption band region of the B-ring of quercetin[3,31].
The experiments were carried out in methanol,maintaining the ionic strength of the medium constant (I=0.1 mol L-1) with the addition of NaCl[25].The same tests were carried out in unbuffered medium and the data indicate that the complex is more stable when the ionic force of the medium is kept constant.This behavior has been described for other systems reported in the literature [32].Methanol was used as a solvent in the experiments because it is a hydrogen bond donor(HBD),which favors intermolecular interactions between the solute and the solvent,resulting in the high solvation of quercetin in methanol[33].
The variations in the quercetin concentration with the addition of metal chlorides are shown in Figs.3A–C for Ni2+,Mg2+and Ca2+,respectively.For nickel,magnesium and calcium,respectively,the chloride concentrations were 88,81 and 46 times higher than the quercetin concentration at the end of the titration tests.All of the experimental data were plotted with a nonlinear fit according to Eq.(1).These results are given in Table1 and they showed a good fit with Eq.(1)(with standard deviations below 6.5×10-4).Applying Eq.(1),the system containing quercetin and nickel chloride showed a stoichiometry of 1.5:1 (quercetin:nickel).The continuous variations method (Job’s method) was then applied,which indicated a 1:1 stoichiometry for the complex(Fig.3A,inset),which is in agreement with data reported in the literature[6].The Job’s method was also applied to magnesium chloride and calcium chloride.However,since these tests are equimolar,the concentration range(4.0×10-5mol L-1)does not allow good results to be obtained using the Job’s method.The Job’s method presents better fits when the association constants are higher,which is reflected in greater spectral changes (Fig.2).The orders of magnitude of the association constants observed in Table1 for the tested species indicate that for Ni2+the association constants are at least 10 times higher than those for the other species investigated in this study.This explains the good fit obtained through the continuous variations method for Ni2+and quercetin.
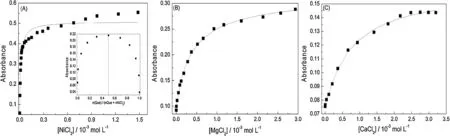
Fig.3.Variations in the absorbance of the quercetin in methanol (I=0.01 mol L-1) with the addition of increasing amounts of:(A) NiCl2,(B) MgCl2 and (C) CaCl2.The concentration of quercetin was 4.0×10-5 mol L-1,and absorbances were collected at 371.9 nm for nickel chloride,415.0 nm for magnesium chloride and 423.2 nm for calcium chloride.(—)Curve fitted with Eq.(1).

Table1 Binding constants and stoichiometry of complexes formed between quercetin and metals in methanolic solution at 25°C.
The formation of complexes between quercetin and metals has been extensively reported in the literature and complexion sites are present at the positions O3/O4,O4/O5 and O3’/O4’(Fig.1),with the complexion commonly occurring between quercetin and nickel at the positions O4/O5 and O3’/O4’ [6]and at O3’/O4’ and O3/O4 for Mg2+[6,34].Evidence of the formation of a complex between quercetin and calcium has also been reported[35].The complexion effect was observed qualitatively through a colorimetric change in the medium(yellow →neon yellow)and spectroscopically through a bathochromic shift in the absorption band of quercetin in the presence of M2+metals,due to an increase in the polarity of the medium.The values obtained for the binding constants follow the ascending order Mg2+<Ca2+<Ni2+,with a 1:1 stoichiometry in all cases(Table1).On comparing these results with those previously obtained by our research group,under the same conditions,it can be noted that Al3+has a binding constant of 3.94±0.34×103with a standard deviation of 2.72×10-2[30].Thus,a new ascending order of stability can be established as Mg2+<Ca2+<Al3+<Ni2+.However,the nonlinear fit for the quercetin-aluminum complex followed the description by Castro and Blanco [25],since it shows a lower standard deviation and higher binding constant than that obtained based on the equation reported by Valeur et al.[26].This equation is best applied in situations when there is a higher concentration of metal ions in solution in relation to complexed ions.
4.Conclusions
In this study it was possible to obtain the binding constants for quercetin and M2+metals.The binding constants obtained indicate stability in the ascending order of Mg2+<Ca2+<Ni2+.This study highlights that the greatest variation in the maximum wavelength(Δλ) on comparing quercetin with the metal-quercetin complex formed occurs for Ni2+with Δλ=56.4 nm (371.9 nm–428.3 nm),followed by Ca2+with Δλ=51.3 nm (371.9 nm–423.2 nm) and Mg2+with Δλ=43.1 nm (371.9 nm–415 nm).The formation of an absorption band is evident only in the case of the quercetinnickel complex.In the case of calcium and magnesium,there is an absorption shoulder.The determination of the binding constant associated with the binding stoichiometry shows importance from the biological-environmental point of view.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank the Instituto Federal do Paraná (IFPR),the IFPR by Programa de Apoio ao Desenvolvimento Tecnológico e Inovac??o (PRADI),and Conselho Nacional de Desenvolvimento Científico e Tecnológico(CNPq-Brazil).
- 食品科學(xué)與人類健康(英文)的其它文章
- Behavioral assessment of hippocampal function following dietary intervention
- Prophylactic effect of Kudingcha polyphenols on oxazolone induced colitis through its antioxidant capacities
- Nutraceutical support for respiratory diseases
- Role of calpain system in meat tenderness:A review
- Corn phytochemicals and their health benefits
- GUIDE FOR AUTHORS

