Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts
Marina Offengenden,Subhadeep Chakrabarti,Jianping Wu
Department of Agricultural,Food&Nutritional Science and the Cardiovascular Research Centre,University of Alberta,Edmonton,AB,Canada
ABSTRACT Collagen is a major extracellular matrix protein.Given the potential anti-inflammatory and antioxidant profiles of these bioactive compounds,there has been increasing interest in using collagen derived peptides and peptide-rich collagen hydrolysates for skin health,due to their immunomodulatory,antioxidant and proliferative effects on dermal fibroblasts.However,all hydrolysates are not equally effective in exerting the beneficial effects; hence,further research is needed to determine the factors that improve the therapeutic applicability of such preparations.We used different enzymatic conditions to generate a number of different collagen hydrolysates with distinct peptide profiles.We found that the use of two rather than one enzyme for hydrolysis generates a greater abundance oflow molecular weight peptides with consequent improvement in bioactive properties.Testing these hydrolysates on human dermal fibroblasts showed distinct actions on inflammatory changes,oxidative stress,type I collagen synthesis and cellular proliferation.Our findings suggest that different enzymatic conditions affect the peptide profile of hydrolysates and differentially regulate their biological activities and potential protective responses on dermal fibroblasts.
Keywords:Chicken collagen Collagen peptides Antioxidant activity Anti-inflammatory activity Human dermal fibroblasts
1.Introduction
Collagen is a major component of extracellular matrices in skin,bones,tendons and cartilage and other connective tissues of the body.There are up to 27 different types of collagen;however,type I collagen is the predominant type in connective tissues.Collagen is composed of three α-chains to form a triple helix that is stabilized by hydrogen bonds.The N-and C-terminal parts do not form a triple helix,but are responsible for intra and intermolecular covalent crosslinking between lysine and hydroxylysine; the rest of structure mostly consists of repeating sequences of Gly-X-Y,where X is(usually)proline(Pro)and Y is hydroxyproline(Hyp)(reviewed in[1]).Degradation and/or hydrolysis of collagen generates a range of bioactive peptides rich in Pro and Hyp,many of which have shown anti-inflammatory,antioxidant,angiotensin converting enzyme(ACE)-inhibitor,antimicrobial activities in addition to being protective on skin,bone and joint health[2-8].Native collagen is relatively resistant to most proteases;however,prior degradation to gelatin can make it more amenable to enzymatic hydrolysis.Gelatin is degraded collagen,produced by thermal degradation under acidic(type A gelatin) or alkaline (type B gelatin) conditions.Following degradation into gelatin,enzymatic treatment can yield a mix of collagen peptides of varying molecular weights,a preparation known as collagen hydrolysate[9,10].Traditionally,the source for collagen,gelatin and their hydrolysates has been the connective tissue from farm animals including pigs and cows[5,11-14];however,there is increasing interest in utilizing the collagen obtained from fishes as well as poultry as alternative sources due to fear of bovine spongiform encephalopathy and religious reasons [15-19].However there is limited research in preparing collagen from chicken and there is no published report of using collagen from spent hens.
One of the potential applications of collagen hydrolysates is in the field of skin health.Skin is the largest organ in the human body.An intact healthy skin not only protects the internal organs and tissues but also serves important functions in the body’s defences against invading micro-organisms and maintaining fluid balance[20,21].Skin aging is caused by a combination of factors,both biological as well as environmental such as exposure to extremes of temperature and ultraviolet radiation.Local and systemic inflammatory and infectious disease can also affect the skin health as does the effects of aging [22-24].Treatment and management of skin aging and its complications is an area where the use of natural compounds has recently attracted a lot of attention[19,25,26].Wound healing is another condition where optimal skin health can make for faster and better recovery compared to an aged and/or damaged skin.Given the potential anti-inflammatory,antioxidant and other beneficial properties of many collagen derived peptides,there is increasing interest in utilizing collagen hydrolysates for the maintenance and improvement of skin health[19,27,28].
However,all collagen hydrolysates are not created equal;different preparations are enriched in peptides with particular properties that may affect the final biological outcomes.For example,peptides with lower molecular weight and better aqueous solubility are suited for topical use,while those rich in proline/hydroxyproline show significant bioavailability even upon oral ingestion [2,8,29-31].Indeed,a recent publication by Schadow et al.has demonstrated the stark differences in biological effects of several commercially available collagen hydrolysates on articular cartilage,suggesting only some but not all the hydrolysates would have any beneficial effects on joint health [32].The factors and production methods that may ensure a more therapeutically beneficial hydrolysate are still poorly understood.
Given this background,the purpose of this study was to determine the factors(both enzymatic preparation methods and profiles of constituent peptides)underlying the ability of different collagen hydrolysates to exert beneficial effects on inflammatory molecule expression,oxidative stress,type I collagen synthesis and cell proliferation,using cultured human dermal fibroblasts (HDF) as a model system.
2.Materials and methods
2.1.Chicken collagen isolation
Chicken meat was excised manually from spent hens and trimmed of external fat.The meat was homogenized with distilled water (1:4; weight:volume) using a Waring heavy duty blender (Waring commercial,CT,USA) at maximum speed for 1 min.Homogenized meat slurry was filtered through a metal fine sieve(1 mm mesh).The filtrate was collected for other experiments and the retentate was collected as connective tissue sample.
Freeze dried connective tissue was mixed with 5%hydrochloric acid (HCl) (at 5% weight/volume) and incubated for 24 h at room temperature.After incubation,excess acid was drained (using a sieve to prevent material loss)and washed 2 times with water(5%weight/volume based on original weight of connective tissue)using a centrifuge.The purpose was to remove acid,soluble proteins and fat.Afterwards the sample was mixed same volume of water and immersed in a water bath at 90°C for 1 h.After cooling to room temperature,the sample was homogenized at 15,000 rpm for several min using a T25 digital Ultra-Turrax homogenizer(IKA works Inc.,Wilmington,NC,USA)and then subjected to enzymatic treatments.
2.2.Enzymatic hydrolysis of chicken collagen
Protein hydrolysates were prepared by digestion with 2% w/w enzyme for 2 h according to conditions in Table1.Where two enzymes were applied,the digestions were carried out consecutively for 2 h each.Before digestion the samples were incubated at the digestion temperature (50°C) for 10 min.This time was used to adjust the pH and to allow the sample to reach the digestion temperature.Following digestion,the enzymes were inactivated by boiling at 95°C for 10 min in a water bath.After cooling to room temperature,it was centrifuged at 10,000gfor 20 min at 4°C,the supernatant was filtered through #1 Whatman’s filter paper and freeze dried.The yield was calculated based on the dry matter of the original sample.Dry matter was determined by drying the samples at 110°C overnight and ash content was determined by keeping samples at 550°C for 24 h.
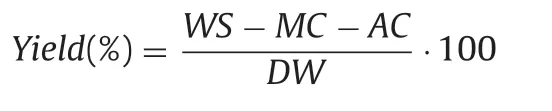
WS-weight of freeze dried supernatant;MC-moisture content;AC-ash content;DW-dry weight ofinitial sample(water and ash content were subtracted).
Protein content of the samples was estimated by a LECO TruSpec machine (LECO Corporation,St Joseph,MI,USA) according to the manufacturer’s instructions using 5.55 as the protein factor for gelatin,the denatured form of collagen.Protein concentration was determined by the modified Lowry Protein Assay Kit(Pierce,Rockford,Ill,U.S.A.)using bovine serum albumin as the standard.
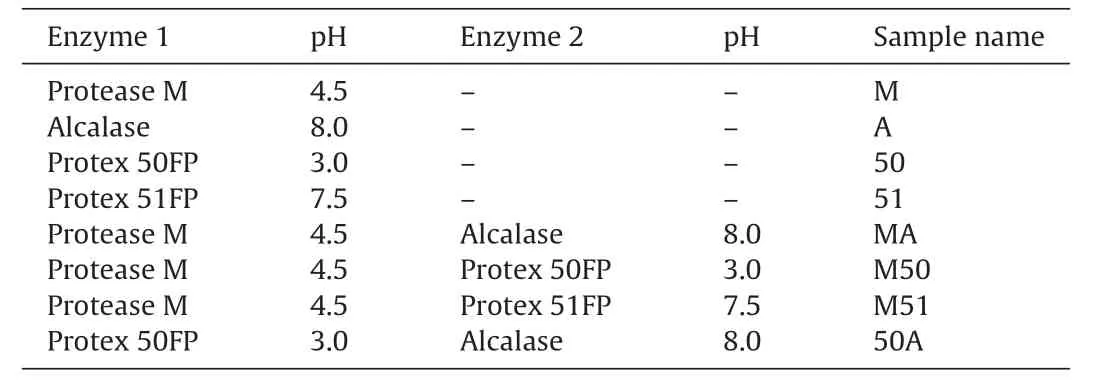
Table1 Enzymatic hydrolysis conditions for generating chicken collagen hydrolysate(CCH)samples.
2.3.Gel filtration chromatography
Each preparation of collagen hydrolysate is composed of a number of different peptides.The molecular weight distribution of the constituent peptides was analyzed by gel filtration chromatography on a Superdex peptide 10/300 GL column coupled to an AKTA explorer 10XT system(GE Healthcare,Uppsala,Sweden).The samples were dissolved in 30% aqueous acetonitrile containing 0.1%trifluoroacetic acid (TFA),at a concentration of 1 mg/mL.100 μL of the sample were injected onto the column and eluted with the acetonitrile solution; chromatograms were monitored at 215 nm.Standard molecular mass markers were injected at the same conditions of the samples.The markers were the peptide Gly-Gly-Gly(189 Da),vitamin B12 (1355 Da),aprotinin (6500 Da),cytochrome C(12,400 Da).
2.4.Reverse phase chromatography
To determine the relative hydrophilic and hydrophobic nature of the constituent peptides,5 μL of each hydrolysate (10 mg/mL)was injected onto BEH C18(1.7 μm 2.1×100 mm)column(Waters,Mississauga,ON,Canada)attached to Waters Acquity UPLC system(Waters).Column temperature was 35°C and the peptides were eluted by a gradient of 100%water containing 0.1%TFA to 30%acetonitrile with 0.1%TFA over 45 min.The peptides were detected by PDA detector at 254 nm.A later time point of elution indicated a greater hydrophobic nature.
2.5.Hydroxyproline assay
Hydroxyproline was determined using a hydroxyproline assay kit (Sigma-Aldrich,St.Louis,MO,USA) according to manufacturer’s technical bulletin.Aqueous solutions of 1 mg/mL of each hydrolysate were mixed 1:1 with concentrated HCl (~12 M) and hydrolyzed for 24 h at 110°C (in oven).Afterwards,10 μL were transferred to a 96-well plate,dried and 100 μL of Chloramine T in oxidation buffer was added.After 5 min 100 μL of dimethylamine borane(DMAB)reagent was added to each well and the plate was incubated in an oven at 60°C for 90 min.The absorbance was read at 560 nm and the concentration of the samples was determined according to a standard curve of hydroxyproline.
2.6.Cell culture
Commercially available adult HDFs were obtained from Cell Applications Inc.(San Diego,CA,USA; catalogue #:107-25a).The cells were grown in Dulbecco’s modified Eagle medium (DMEM)supplemented with 10%heat-inactivated fetal bovine serum(FBS)and penicillin-streptomycin.The cells were transferred into a quiescing medium(DMEM with 1%FBS and antibiotics)prior to performing the actual experiments.All experiments were performed on 70-80%confluent cells grown in tissue culture grade plastic 48-well plates.All collagen hydrolysates were de-salted,freeze-dried and reconstituted in sterile distilled water prior to use in cell culture studies.
2.7.Oxidative fluorescence assay
The confluent monolayers of HDF were incubated for 2 h with quiescing medium containing 2.5 mg/mL of different collagen hydrolysate samples.At the end of the incubation period,the cells were washed once and then incubated for 30 min with the reactive oxygen species(ROS)sensitive fluorescent dye dichlorofluorescein diacetate (DCF-DA,10 μM) in quiescing medium.Afterwards,the nuclei were counterstained with the Hoechst 33342 nuclear dye(Molecular Probes,Eugene,OR,USA).The cells were then washed 3 times and visualized under an Olympus IX81 fluorescent microscope(Carson Scientific Imaging Group,Ontario,Canada)using the Slidebook 2D,3D Timelapse Imaging Software (Intelligent Imaging Innovations Inc.,CO,USA).For each data point,images from three randomly chosen fields were taken.The total green fluorescence intensity and the number of cells in each field were noted and the mean fluorescence intensity per cell(MFI/Cell)was determined similar to our previous studies on oxidative fluorescence[33,34].ROS level was measured as percentage change in MFI/Cell over the untreated control.A stronger green fluorescence indicated a higher level ofintracellular ROS activity.All images presented are in(X100)magnification.
2.8.Western blotting
Confluent HDF monolayers were pre-treated for 20 h in quiescing medium with/without 2.5 mg/mL of collagen hydrolysate prior to 4 h stimulation with the pro-inflammatory cytokine tumor necrosis factor(TNF,also known as TNFα,used at 10 ng/mL).At the end of the incubation period,the culture medium was removed and the cells lysed in boiling hot Laemmle’s buffer containing 50 μM dithiothreitol (DTT,a reducing agent) and 0.2% Triton-X-100 to prepare samples for western blot.These cell lysates were run in SDS-PAGE,blotted to nitrocellulose membranes and immunoblotted with antibodies against vascular cell adhesion molecule-1(VCAM-1,rabbit polyclonal antibody from Santa Cruz Biotechnologies,Santa Cruz,CA,USA),intercellular cell adhesion molecule-1(ICAM-1,mouse monoclonal antibody from Santa Cruz Biotechnologies) and the loading control α-tubulin (rabbit polyclonal antibody from Abcam,Cambridge,MA,USA).Anti-tubulin was used at 0.4 μg/mL,while all others were used at 1 μg/mL Goat anti-rabbit and Donkey anti-mouse fluorochrome-conjugated secondary antibodies were purchased from Licor Biosciences (Lincoln,NB,USA).The protein bands were detected by a Licor Odyssey BioImager and quantified by densitometry using corresponding software (Licor Biosciences).Each band of VCAM-1 or ICAM-1 was normalized to its corresponding band ofloading control.Cell lysates from untreated cells were loaded onto every gel,similar to our previous studies[33,34].The results were expressed as percentage change from the positive control(cells treated with TNF alone).
2.9.Procollagen ELISA
To measure Type I procollagen levels,confluent monolayers of HDF were incubated for 24 h with quiescing medium containing 2.5 mg/mL of different collagen hydrolysate samples.At the end of this incubation period,cell free supernatants were collected and analyzed for their type I procollagen content by a commercially available ELISA kit(Takara Bio Inc,Shiga,Japan).
2.10.BrDU incorporation
HDFs were plated onto 48-well tissue culture plates in DMEM with 10% FBS and incubated for 4 h.Afterwards the medium was changed to DMEM with 1%FBS(quiescing medium)and 2.5 mg/mL of collagen hydrolysate was added.Following 24 h incubation with the hydrolysates,the cells were washed and placed in fresh quiescing medium containing 1%BrDU(Invitrogen,Carlsbad,CA,USA)for 1 h.The cells were then fixed in 70%ethanol(20 min),treated with 1N hydrochloric acid(HCl)for antigen exposure(also 20 min),permeabilized with 0.1% Triton-X-100 in phosphate buffered saline(5 min) and blocked in 1% bovine serum albumin in phosphate buffered saline (60 min) prior to incubation with mouse monoclonal antibody against BrDU(1:1000;Cell Signaling,Beverly,MA,USA) at 4°C.All the steps prior to addition of primary antibody were performed at room temperature.Following overnight incubation with the primary antibody,the cells were treated with anti-mouse secondary antibody(Molecular Probes,Eugene,OR)for 30 min in the dark.Nuclei were stained with the Hoechst33342 nuclear dye (Molecular Probes).Cells were visualized under an Olympus IX81 fluorescent microscope (Carson Scientific Imaging Group; Markham,Ontario,Canada) as described before.For each data point,3 random fields were chosen.The percentage of nuclei positive for BrDU staining was noted in each field and the mean calculated.
2.11.LC-MS/MS analysis of chicken collagen hydrolysate(CCH)
LC-MS/MS was performed by loading 5 μL of the peptide digests onto a nanoAcquity UPLC system with peptide trap (180 μm×20 mm,Symmetry?C18 nanoAcquityTMcolumn,Waters,Milford,MA) and a nano analytical column (75 μm×150 mm,AtlantisTMdC18 nanoAcquityTMcolumn,Waters,Milford,MA).Peptides were separated with a gradient of 1-85% solvent B (acetonitrile,0.1% formic acid) over 45 min at a flow rate of 350 nL/min.The column was connected to a q-Tof Premier (Waters Corporation) for ESI-MS/MS analysis in the positive ion mode.
2.12.Reagents
Dulbecco’s phosphate buffered saline (PBS),and dithiothreitol (DTT) were bought from Sigma Chemical Co (St Louis,MO,USA).Dichlorofluorescein-DA(DCF-DA),DMEM and fetal FBS were obtained from Gibco/Invitrogen (Carlsbad,CA,USA).Type 1 Collagenase used for cell splitting was purchased from Worthington Biochemical Corporation (Lakewood,NJ,USA).Triton-X-100 was from VWR International(West Chester,PA,USA).Protease M,Protex 50FP,Protex 51FP were from Amano Enzyme (Elgin,IL,USA);Alcalase?2.4L(Protease fromBacillus licheniformis)was from Sigma Chemical Co.
2.13.Statistics
All data graphs are presented as mean±SEM (standard error of mean) from 4-5 independent experiments.Each independent experiment was performed using HDF of a different passage number(between 4 and 16).Data are expressed as percentage of either negative control (untreated cells) or the positive control (cells treated with TNF alone).Data were analyzed using one way analysis of variance(ANOVA)with Dunnett’s post-hoc test for comparisons to control.Repeated measures test was used wherever applicable.p<0.05 was taken as significant.
3.Results
3.1.Dry matter,ash content and yield of collagen hydrolysates
The results of dry matter estimation,ash content and determination of protein levels are summarized in Table2.In general,no major difference was noted in moisture level,dry matter or protein content among the different hydrolysate preparations.However,the ash content was much lower in hydrolysates prepared under acidic(low pH)conditions(M,50,M50)compared to those prepared under alkaline pH during either the first or second digestions(A,51,MA,M51,50A).No major differences in hydroxyproline levels were observed,although the samples M50 and M51 showed comparatively lower hydroxyproline abundance(Table2).Whether this represents a selective loss of hydroxyproline residues or conversion of these into proline residues is not clear at this point.
3.2.Peptide profiles
We aimed to characterize the peptide profiles of different hydrolysates based on both peptide size (molecular weight) and hydrophilic versus hydrophobic properties.An analysis of peptide molecular weights by gel filtration chromatography suggested a diversity of peptide size among the different preparations(Fig.1).In general,use of a single enzyme produced hydrolysates with a greater frequency oflarger peptides.Digestion with Protease M in particular yielded more small peptides compared to others.Our results showed that use of a second digestion with another enzyme significantly reduced peptide size and yielded hydrolysates containing shorter peptides(<2000 Da);peptide size is a major contributor to the bioavailability of bioactive peptides.
Peptide profiles were evaluated on a C18column(Fig.2).Among the single enzyme treatments,Alcalase and Protease M appeared to yield more hydrophilic peptides compared to others.Digestion with Protex 50FP generated the most hydrophobic preparation where the majority of peptides eluted at a later time point.However,a second digestion with Alcalase(hydrolysate 50A)showed a predominantly hydrophilic profile,suggesting Alcalase treatment was sufficient to breakdown the hydrophobic peptides in the initial sample into more hydrophilic ones.As with molecular weights,the sequential use of 2 enzymes for hydrolysis yielded samples with increased hydrophilic properties.
Based on these peptide profiles,we predicted that hydrolysates prepared with 2 enzymes would demonstrate more pronounced biological effects compared to the products of a single enzyme digestion.
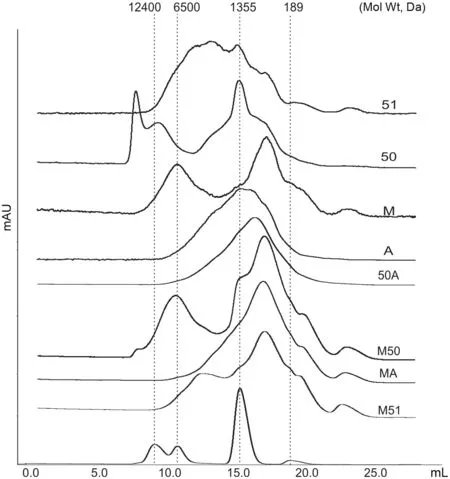
Fig.1.Gel chromatography showing differences in molecular weight profiles of different collagen hydrolysate preparations.The hydrolysates were dissolved in 30%aqueous acetonitrile containing 0.1% trifluoroacetic acid (TFA),at a concentration of 1 mg/mL.Sample was injected onto the Superdex peptide 10/300 GL column and eluted with the acetonitrile solution.The chromatograms were monitored at 215 nm.Molecular weight standards were injected(their molecular weights,in Da,are shown at the top)and analyzed under conditions identical to those used for the samples.A representative set ofimages is shown.
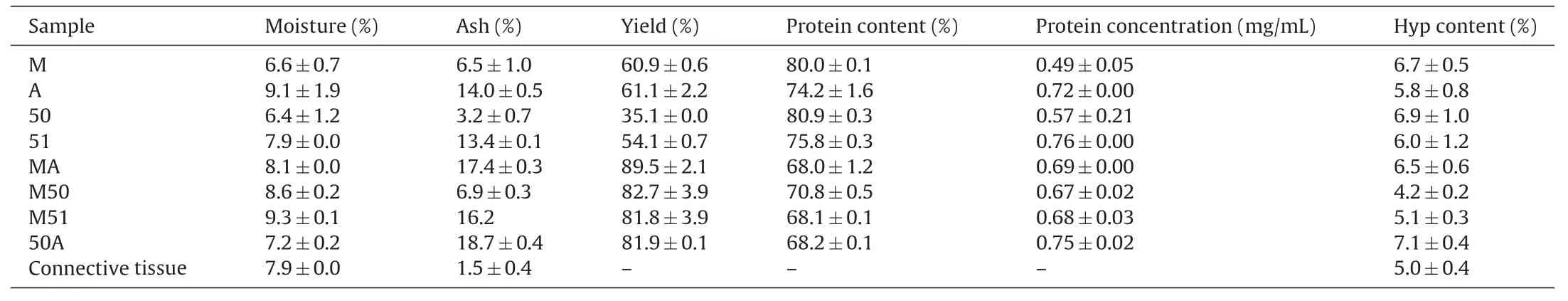
Table2 Dry matter,ash content,hydroxyproline(Hyp)content and yield of different CCH preparations.The average protein content was determined by LECO TruSpec machine and based on gelatin protein factor of 5.55.The protein concentration was estimated by a modified Lowry method.All values are in Mean±SD.
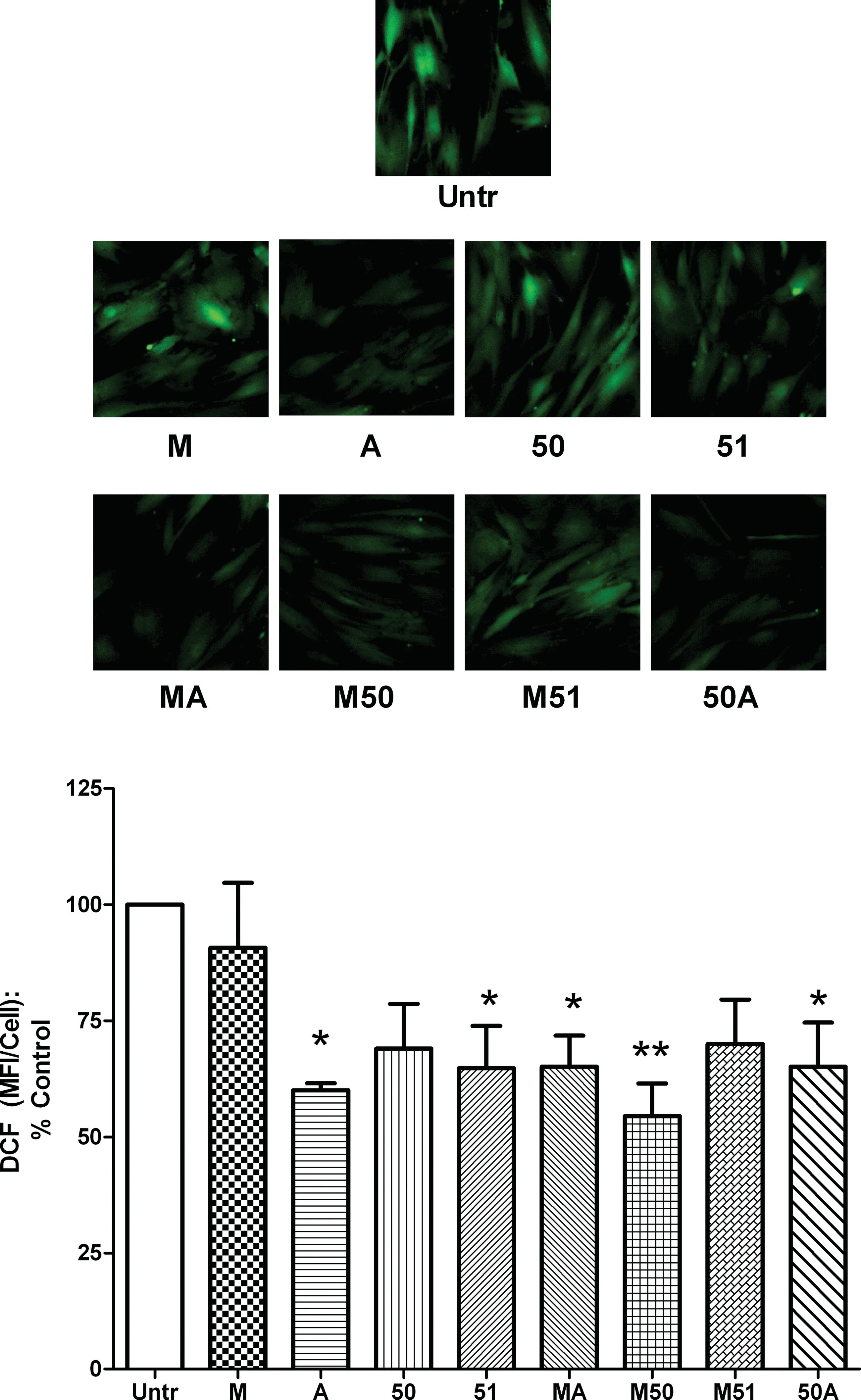
Fig.3.Antioxidant effects of different collagen hydrolysates on cultured dermal fibroblasts.Confluent monolayers of human dermal fibroblasts were treated for 24 h with 2.5 mg/mL of different collagen hydrolysate preparations.Reactive oxygen species levels were determined by staining with DCF-DA for 30 min and expressed as mean fluorescence intensity(MFI)per cell.A representative set ofimages is shown.Data are expressed as mean±SEM of 4 independent experiments.*and**indicate p <0.05 and p <0.01respectively,compared to the untreated control.
3.3.Antioxidant effects
Higher levels of reactive oxygen species (ROS) in the tissues lead to oxidative stress which is associated with the pathogenesis of chronic inflammatory conditions.The dermal fibroblasts are known to show increased oxidative stress under stressful conditions;so,we first tested the ability of our collagen hydrolysates to modulate ROS levels in these cells(Fig.3).Of the 4 single enzyme treated hydrolysates,only A and 51 showed anti-oxidant effects with a reduction in basal oxidative stress while the 2 other preparations(M and 50)had no effect.In contrast,3 of the 4 dual enzyme treated hydrolysates (MA,M50 and 50A) showed significant antioxidant activity.Interestingly,neither Protease M nor Protex 50FP could generate anti-oxidant peptides on their own,but using both these enzymes generated anti-oxidant effects in their hydrolysate(M50).
3.4.Cytokine responses
Next we tested the anti-inflammatory capability of the collagen hydrolysates in inflamed HDFs.The pro-inflammatory cytokine TNF,involved in various inflammatory conditions,was chosen to induce upregulation ofinflammatory molecules such as VCAM-1 and ICAM-1 in the HDFs (Fig.4).In contrast to the anti-oxidant effects,both M and 50 significantly reduced TNF mediated expression of ICAM-1 and VCAM-1,while A and 51 had no such effect.All 4 of the dual enzyme treated preparations (MA,M50,M51 and 50A) showed similar anti-inflammatory effects.These results suggest that different enzymes generate peptides with distinct anti-inflammatory and anti-oxidant profiles while combining more than one enzyme can yield hydrolysates consisting of a range of peptides with different actions.Given the beneficial profile of dual enzyme generated hydrolysates,we decided to use only these 4 preparations for our subsequent studies.
3.5.Type I collagen synthesis
Procollagen is the native form of collagen that is synthesized by the cells and then undergoes enzymatic cleavage to yield the actual collagen molecule [35].Since fibroblasts synthesize,cleave and store collagen molecules under resting conditions,the release of procollagens can be taken as a measure ofde novosynthesized molecules [26,36,37].We examined the effects of collagen hydrolysates on type I collagen biosynthesis in HDFs(Fig.5).Incubation with MA caused a modest but significant increase in type I procollagen from resting HDFs.However,treatment with the other 3 preparations had no significant effect on basal collagen synthesis,suggesting this effect was not universal among the hydrolysates.
3.6.HDF proliferation
Next,the collagen hydrolysates were investigated for their ability to stimulate HDF proliferation.While there is a constant turnover of HDF and other skin cells in the normal skin,stimulating their proliferation might be beneficial under stressful conditions such as wound healing.We found that only 2 of the 4 dual enzyme generated hydrolysates,namely,M50 and 50A,could induce increased HDF proliferation while the other 2(MA and M51)had no significant effects (Fig.6).These data suggest that only certain enzyme combinations are able to generate peptides with particular functions while other combinations do not show similar capacities.The results from these different experiments are summarized in Table3.
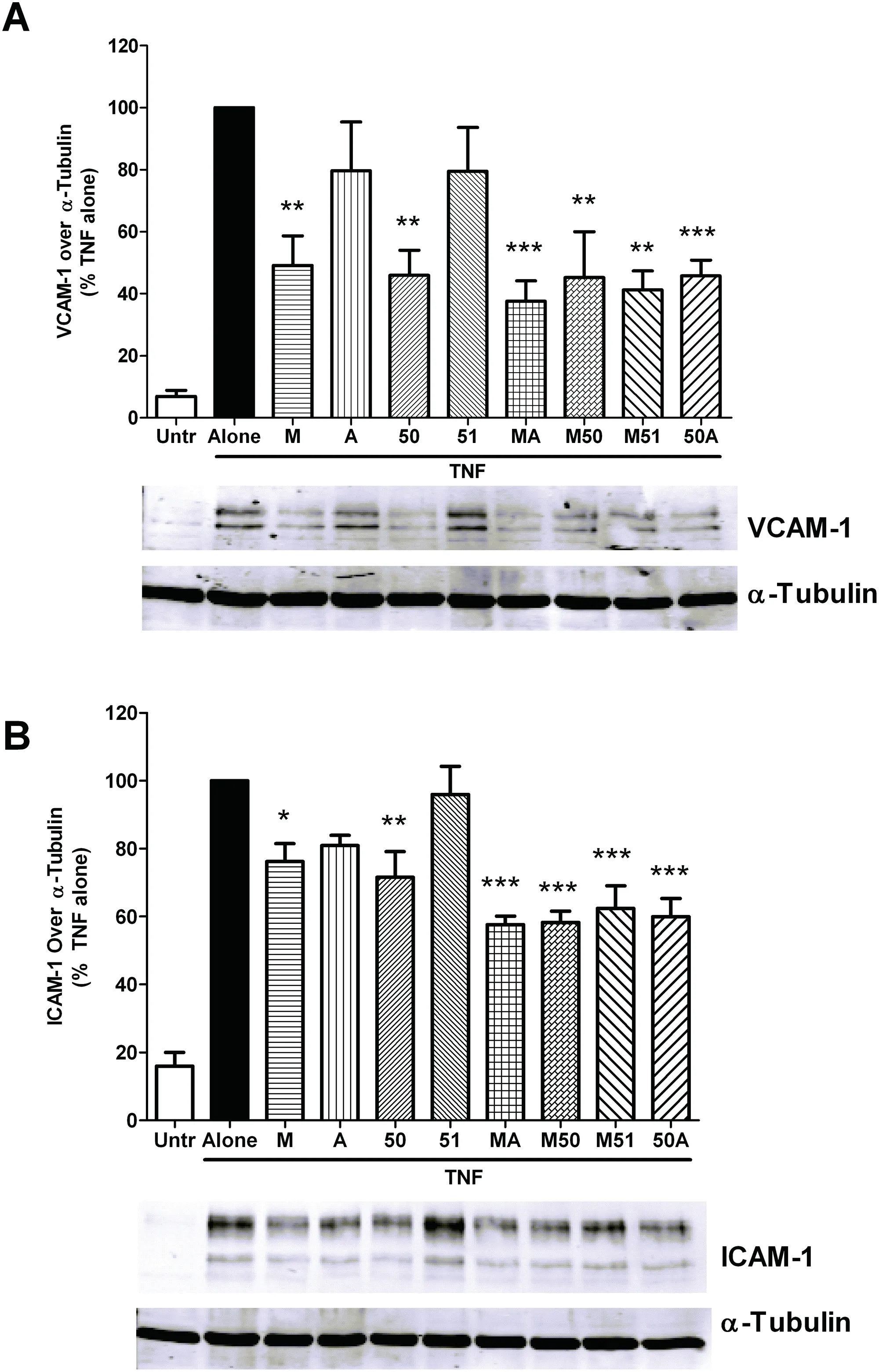
Fig.4.Anti-inflammatory effects of collagen hydrolysates on cytokine stimulated dermal fibroblasts.Confluent monolayers of human dermal fibroblasts were treated for 24 h with 2.5 mg/mL of different collagen hydrolysates prior to 4 h stimulation with TNF (10 ng/mL).The cells were lysed and the lysates used for immunoblotting with anti-VCAM-1 (A) and anti-ICAM-1 (B) antibodies.The protein bands were normalized to their corresponding bands of the loading control,α-tubulin.A representative set ofimages is shown.Data are expressed as mean±SEM of 4-5 independent experiments.*,**and***indicate p <0.05,p <0.01 and p <0.001respectively,compared to the untreated control.
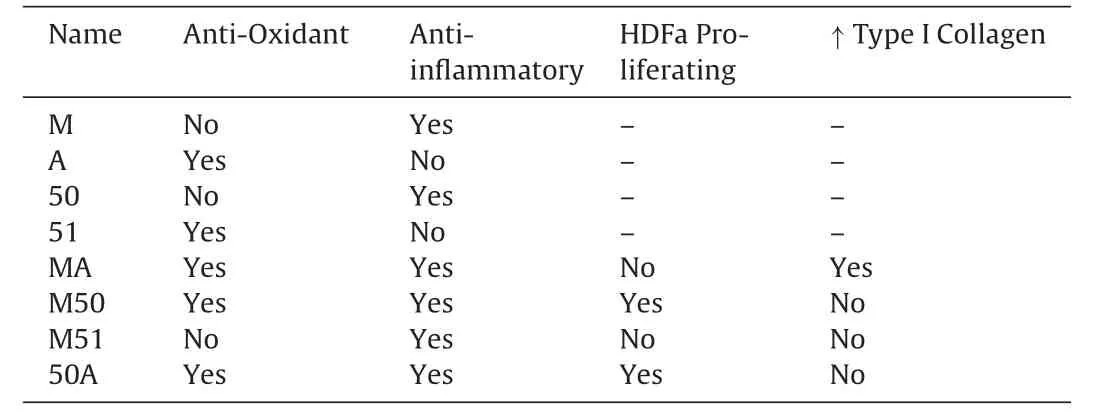
Table3 Summary of the effects of CCH preparations on fibroblast functions.
3.7.LC-MS/MS identification
Finally,two of the CCH preparations (MA and 50A) were further analyzed by LC-MS/MS to identify their source-protein andthe sequences.Database search of the raw data was performed by Mascot (www.matrixscience.com) and the sequences were confirmed by analyzing the raw data [38].In both hydrolysates the identified peptides were matched to α1 and α2 chains of type I collagen (precursor).These precursors are further processed into mature collagen form.α1 chain (accession number P02456) is a product of the gene CO1A1CHICK and consists of 1453 amino acids,while α2 chain(accession number P02467)is a product of the gene CO1A2CHICK and consists of 1362 amino acids(Uniprot database).The identified peptides from hydrolysate MA and 50A are presented in Tables 4 and 5,respectively.
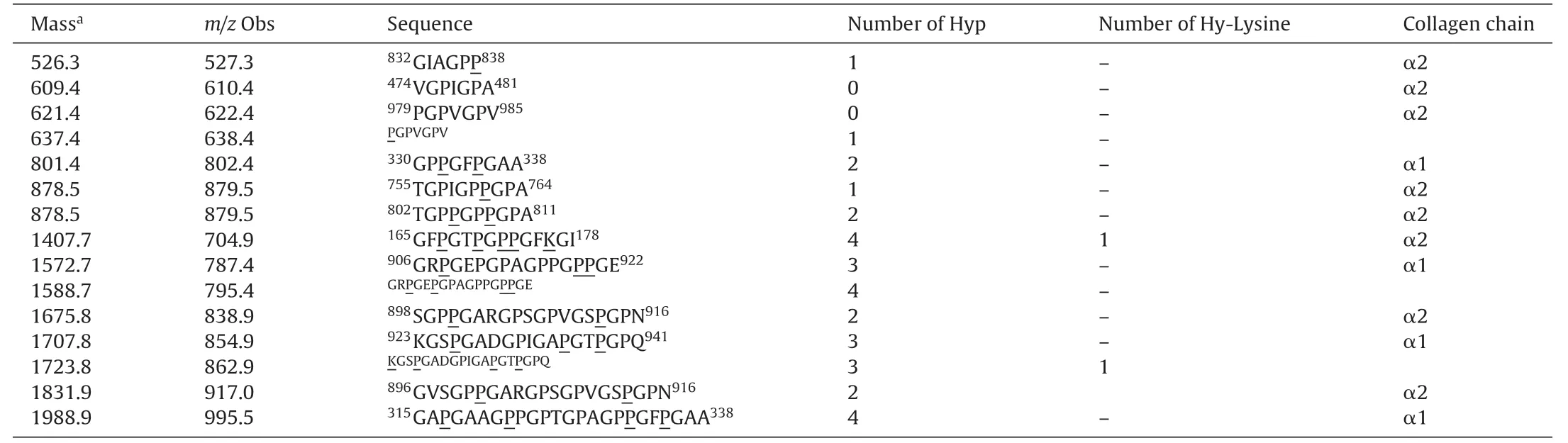
Table4 Collagen peptides identified from MA by Mascot.Hydroxylated prolines and lysines are underlined.The super-scripted numbers indicate the location of the peptide on the α1 or α2 chain.
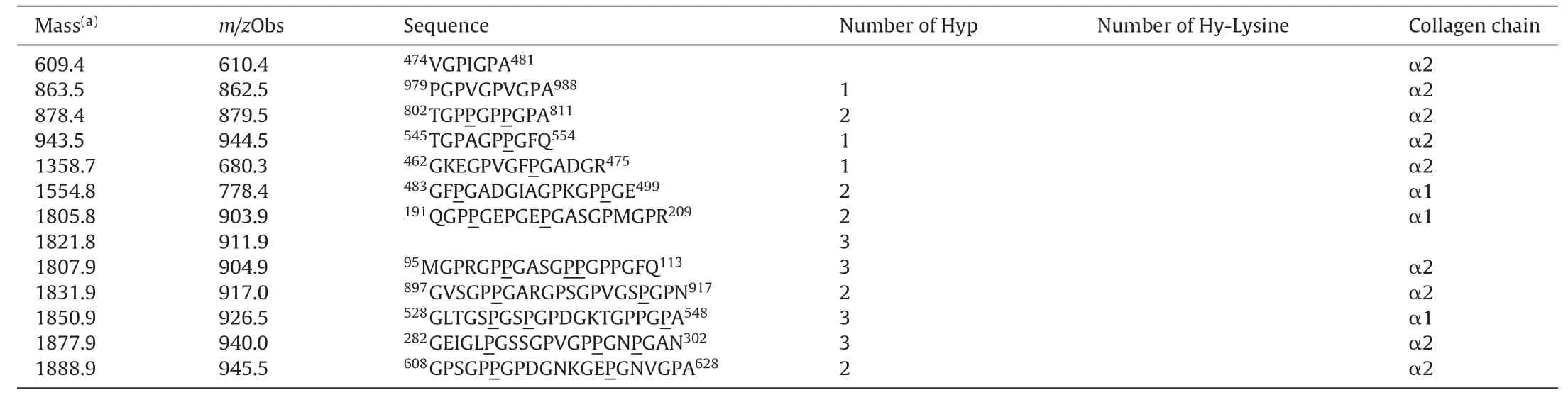
Table5 Collagen peptides identified from 50A hydrolysate by Mascot.Hydroxylated prolines and lysines are underlined.The super-scripted numbers indicate location of the peptide on the α1 or α2 chain.
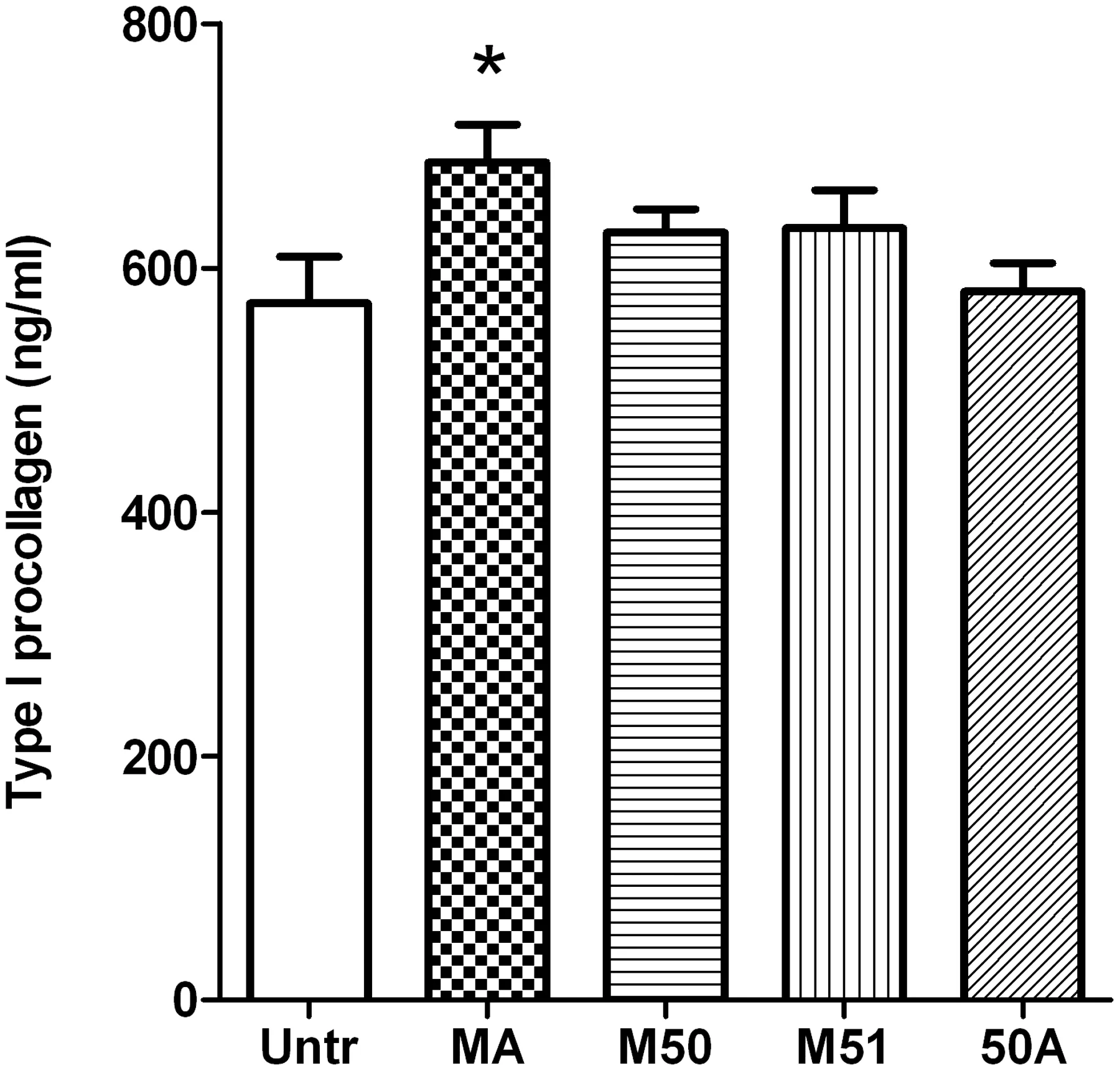
Fig.5.The effects of collagen hydrolysates on type I procollagen release from dermal fibroblasts.Confluent monolayers of human dermal fibroblasts were treated for 24 h with 2.5 mg/mL of different collagen hydrolysate preparations.The cell-free supernatants were collected and analyzed for type I procollagen content with a commercially available ELISA kit.Data are expressed as mean±SEM of 4 independent experiments.*indicates p <0.05 compared to untreated cells.
4.Discussion
In this study,we used different enzymatic conditions to generate a range of collagen hydrolysates,characterize their constituent peptides and finally examine the corresponding biological actions on dermal fibroblasts.Our results suggest that different enzymatic treatments can generate collagen hydrolysates that markedly differ in their biological activities.The key findings were:(a)different enzyme treatments generated peptides that differed in molecular weight and peptide profiles;(b)dual enzyme treatments resulted in abundance oflower molecular weight peptides;(c)dual enzyme generated hydrolysates were more likely to demonstrate antiinflammatory and antioxidant activities and finally,(d)even among dual enzyme treated preparations,only some but not others could induce HDF proliferation,suggesting it was dependent on specific actions of particular peptide/s rather than peptide size/molecular weight alone.
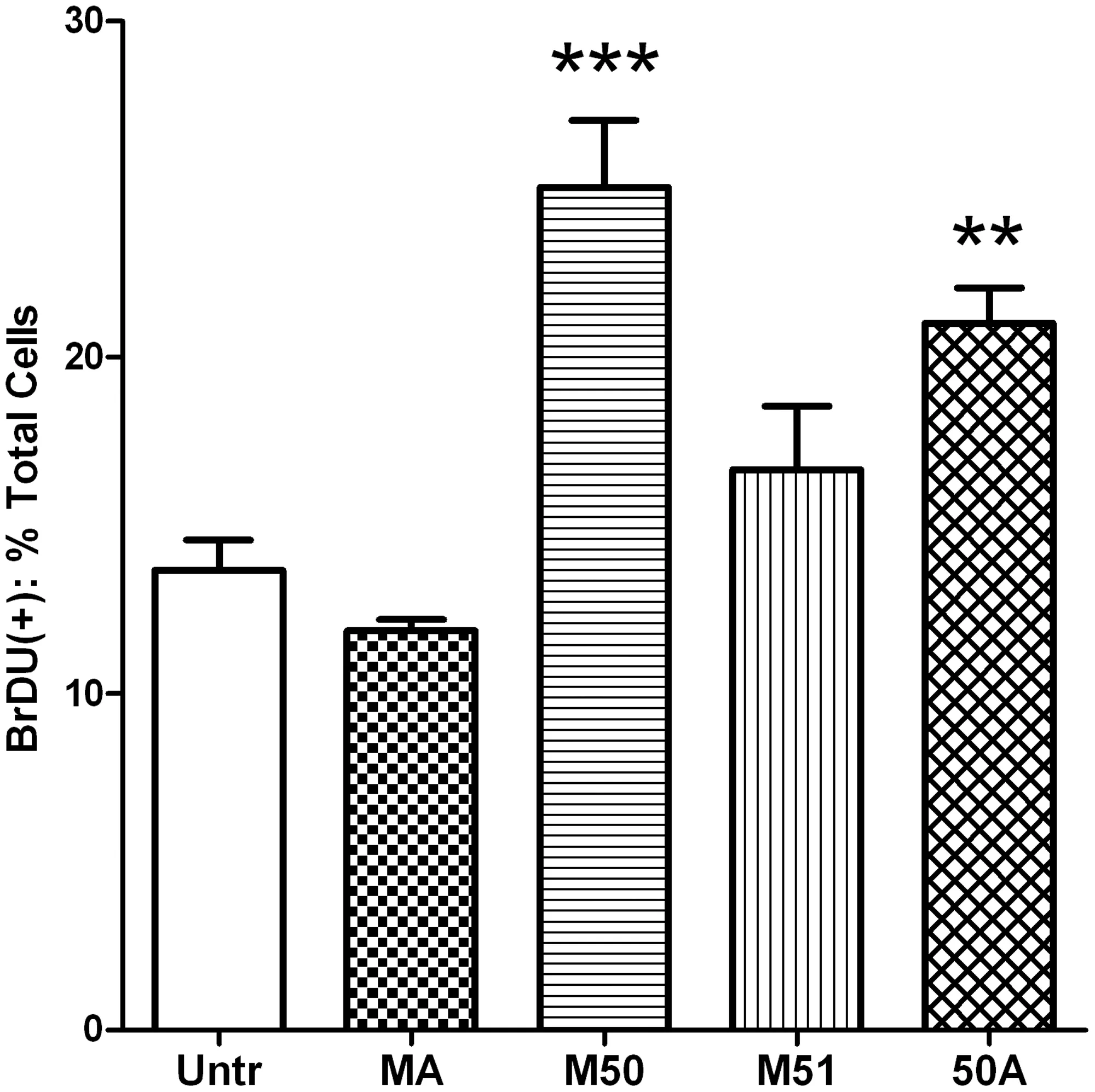
Fig.6.The effects of collagen hydrolysates on dermal fibroblast proliferation.Freshly plated monolayers of human dermal fibroblasts were treated for 24 h with 2.5 mg/mL of different collagen hydrolysate preparations prior to incubation with 1%BrDU in culture medium for 1 h.Afterwards,the cells were fixed,permeabilized and immunostained with anti-BrDU antibody.Nuclei were counter-stained with a nucleus specific dye and the percentage of BrDU-positive nuclei determined.Data are expressed as mean±SEM of 4 independent experiments.** and *** indicate p <0.01 and p <0.001 respectively,compared to the untreated cells.
Collagen hydrolysates are becoming an increasingly popular nutraceutical with potential applications in skin and joint health,wound healing and cosmetics[10,17,39-42].Yet the specific chemical and biological properties ofits constituent collagen derived peptides are still largely unknown.Given the variations in enzymatic and pH conditions during collagen hydrolysis,it is likely that the resulting hydrolysates differ widely in their biochemical properties and biological actions which may hamper their widespread therapeutic usage.Despite this,only a single study on differences among commercially available collagen hydrolysates has been published to date[32].However,specific methods of generating hydrolysates with potentially beneficial properties remain yet to be determined.Similarly,little is known about the specific peptide characteristics that would result in therapeutic advances beyond the role of Pro-Hyp rich moieties[30,43].
Dermal fibroblasts are key cellular components of skin.Located in the dermis layer,these cells regulate functions such as immunomodulation,extracellular matrix protein deposition,proliferation and wound healing that are essential for maintaining skin integrity[36,44,45].A large body of work exists studying the responses of HDFs to various injurious,infectious and inflammatory stimuli that correlate well with medical conditions affecting skin tissue [46-50].Indeed,a number of naturally sourced beneficial products have been validated by their protective actions on dermal fibroblasts,in the absence or presence ofinflammatory cytokines or UV rays[26,46,50-53].Collagen derived peptides have been shown to induce proliferation and protein synthesis in cultured fibroblasts,suggesting their potential as enhancers of skin health[30,54].
Our results suggested that both pH levels and the particular enzymes used during collagen hydrolysis could affect the characteristics of the peptides generated.Single enzyme digestions carried out under low pH (M and 50) appeared to produce fewer low molecular weight peptides compared to those generated under higher pH(A and 51).Protex 50FP treatment also produced a greater abundance of hydrophobic peptides compared to any other preparation.Compared to single enzyme treatment,sequential digestion with 2 different enzymes produced hydrolysates containing a greater frequency of both hydrophilic and low molecular weight peptides(Figs.1 and 2).It appears that the larger molecular weight peptides generated during the first digestion were targeted by the second enzyme and cleaved into smaller peptides.Similarly,the hydrophobic peptides generated by Protex 50FP were also cleaved into more hydrophilic components on subsequent digestion by Alcalase (in 50A).All 4 dual enzyme treated preparations showed similar features with little difference in either molecular weight profiles or hydrophilic properties.Given these apparently beneficial peptide profiles,it was predicted that all such preparations would demonstrate pronounced anti-inflammatory,antioxidant,collagen-generating and proliferative effects on dermal fibroblasts.
Both single and dual enzyme treated samples demonstrated at least some beneficial effects on the studied parameters.Among the single enzyme treated hydrolysates,those generated under acidic conditions (M and 50) exerted antioxidant effects without any discernible anti-inflammatory properties (Figs.3 and 4).In contrast,samples generated under alkaline conditions (A and 51)showed anti-inflammatory effects without affecting oxidative stress.Apart from the different sites of enzyme action,it is quite possible that acidic versus alkaline conditions could also generate specific subsets of antioxidant versus anti-inflammatory peptides,thus affecting the net biological activity of the hydrolysates.Not surprisingly,the sequential use of 2 enzymes generated preparations containing both antioxidant and anti-inflammatory peptides.Indeed,all 4 dual-enzyme treated hydrolysates showed significant anti-inflammatory effects,while only one of these (M51) lacked antioxidant activity.While both Protease M and Protex 50FP were unable to yield antioxidant peptides on their own,sequential use of both enzymes generated a preparation with antioxidant ability,further justifying the use of 2 enzymes to generate a mix of peptides with greater bioactive potential.
While dual enzyme treated preparations with lower molecular weight peptides were successful in antioxidant and antiinflammatory roles,their effects on other biological effects were more complex.For example,only one sample(MA)demonstrated a modest increase in type I procollagen levels while none of the other samples tested had any effect(Fig.5).Skin ageing is known to result in diminished production while increased degradation of extracellular matrix,where collagen is the major constitute.The increased type I procollagen levels in MA sample treated cells indicated its potential application for preventing skin ageing and health.Since normal HDFs were used for our studies,it is probable that the high levels of type I procollagen produced by the resting cells had masked any stimulatory effects of our preparations.Use of diseased or UV treated HDFs(which may have impaired procollagen synthesis)may be necessary to determine any modest stimulatory effect of the hydrolysates similar to previous work by Park et al.[26].Although two of the dual enzyme treated hydrolysates,M50 and 50A,could induce increased fibroblast proliferation,the other 2(MA and M51) had no such effect at all (Fig.6).Specific peptide sequences are known to be involved in this process; for example,the dipeptide Pro-Hyp is able to induce proliferation in fibroblasts[30,43].Given the use of Protex 50FP in both these preparations(M50 and 50A),it is likely that similar specific peptide sequence/s might be present in these hydrolysates that could account for their proliferative actions.Furthermore,the results from studies on cell proliferation and procollagen release also indicate a role for specific peptides rather than general patterns of peptide molecular weight and/or hydrophilic properties.Future studies on sequencing the major responsible peptides are required to identify the mediators of such potentially beneficial functions.
In conclusion,these findings demonstrate that differences in enzymatic treatments generate collagen hydrolysates with distinct chemical and biological profiles.Use of two enzymes in sequential digestion improves the production of bioactive peptides that may be better suited to wound healing and maintenance of skin health.
Conflicts ofinterest
The authors declare that there are no conflicts ofinterest.
Acknowledgements
This study was funded by grants from Alberta Livestock and Meat Agency (ALMA) and the Natural Sciences and Engineering Research Council(NSERC)of Canada to JW.The funders had no role in the study design,data collection and analysis,decision to publish or preparation of this manuscript.
- 食品科學與人類健康(英文)的其它文章
- Protein quality of four indigenous edible insect species in Nigeria
- Identification of sea snake meat adulteration in meat products using PCR-RFLP of mitochondrial DNA
- Development of a reversed dispersive based graphene functionalized with multiwalled carbon nanotubes for detection of β2-agonists in pork by high-performance liquid chromatography
- Glycoalkaloids and phenolic compounds in three commercial potato cultivars grown in Hebei,China
- Volatile components,total phenolic compounds,and antioxidant capacities of worm-infected Gomphidius rutilus
- Anticancer effect of curcumin on breast cancer and stem cells

