Behavioral and physiological photoresponses to light intensity by intertidal microphytobenthos*
DU Guoying (杜國英), YAN Hongmei (閆紅梅), LIU Chunrong (劉春蓉),MAO Yunxiang (茅云翔)
Key Laboratory of Marine Genetics and Breeding, Ocean University of China, Ministry of Education, Qingdao 266003, China
1 INTRODUCTION
Microphytobenthos (MPB) assemblages inhabiting intertidal sediments are important primary producers,and may contribute up to 50% of total primary production in estuarine ecosystems (Underwood and Kromkamp, 1999). MPB assemblages are largely dominated by motile diatom species, although other groups of microalgae can be present, such as cyanobacteria and euglenophytes, also including motile forms. They often exhibit vertical migratory rhythms within the upper sediment layers, which are associated with diurnal and tidal cycles (Guarini et al., 2000; Consalvey et al., 2004a; Du et al., 2010a).As a key factor controlling short-term variability in photosynthetic productivity, MPB vertical migration and its relationship with photosynthetic capability have been increasingly well studied in recent years(Pinckney and Zingmark, 1991; Ser?dio et al., 2001,2012; Ser?dio and Lavaud, 2011).
Inhabiting intertidal flats, MPB faces oscillatory environmental factors because of the tidal cycle and diverse weather conditions. In temperate areas, the light intensity on the sediment surface varies from less than 100 to more than 1 400 μmol/(m2·s) PAR seasonally when the sediments emerge and the exposure time can be long as 6–7 h depending on the tidal cycle (Ser?dio and Catarino, 1999; Du et al.,2009). Because of the large variation of light intensity and possible prolonged irradiance time, and the direct effects of light on the function of the photosynthetic apparatus, migratory and physiological responses of MPB to light are particularly interesting (Ser?dio and Catarino, 2000; Ser?dio et al., 2006; Du et al., 2012).Vertical migration is considered to provide behavioral photoprotection by positioning motile microalgae at a sediment depth for optimum light conditions to avoid photoinhibitory light levels (Kromkamp et al., 1998;Perkins et al., 2001; Ser?dio et al., 2006; Du et al.,2012). Additionally, diatoms, the dominant group in MPB assemblages, physiologically divert excessive light energy from photosystem II reaction centers via a non-photochemical quenching (NPQ) mechanism in the form of heat through processes including the xanthophyll cycle (Ser?dio et al., 2005, 2008; Jesus et al., 2008; Goss and Jakob, 2010; Perkins et al., 2010).
In undisturbed MPB assemblages, the characterization of photophysiological responses to high light is complicated by behavioral photoprotection-vertical migration (Ser?dio et al.,1997; Perkins et al., 2002; Ser?dio, 2004; Jesus et al.,2006). The use of a diatom motility inhibitor,Latrunculin A (Lat A) (Cartaxana and Ser?dio, 2008),has allowed the effects of vertical migration on MPB photophysiology to be demonstrated (Perkins et al.,2010; Cartaxana et al., 2011; Ser?dio et al., 2012).However, these studies have focused on the response to short periods of light (Mouget et al., 2008; Ser?dio et al., 2012; Laviale et al., 2016) or lower light intensities (Perkins et al., 2010; Cartaxana et al.,2011), and revealed only certain aspects of the actual behavioral or physiological photoprotective mechanisms.
This study used fluorescence techniques (Imaging-PAM, pulse amplitude modulated fluorometer) to monitor the vertical migration and photosynthesis of MPB assemblages over a prolonged illumination period. Additionally, the motility inhibitor Lat A was used to evaluate the effect of light on non-migratory MPB assemblages, by comparing with migratory assemblages. The aim was to explore the behavior and physiology of natural MPB assemblages in response to different light intensities.
2 MATERIAL AND METHOD
Sediment samples were collected from the intertidal mudflat of Jiaozhou Bay (36°12.07′N, 120°17.28′E),Qingdao, China, on 28thMarch, 2014. The sampling site is composed of fine muddy sediments(92%<63 μm) where microphytobenthic biofilms are largely dominated by communities of epipelic diatoms, especially species ofGyrosigma,Navicula,andNitzschia(observed by the author). During the low tide period, samples of the surface layers of the sediment (the top ~0.5 cm) were collected in situ using a spatula. In the laboratory, the sediment was sieved through a 63-μm mesh to remove the meioand macrofauna, such as nematodes and sandhoppers.The sediment was thoroughly mixed and spread in shallow trays, covered with site water, and left undisturbed overnight. The next morning, the sediment was homogenized again, and 1.5 mL amounts were transferred into the wells of a 48-well plate using a pipette. In each plate, at least 12 wells were filled with sediment. To six of the wells with sediment, 50 μL 20 μmol/L Lat A solution (dissolved in dimethylsulfoxide and diluted using 0.22-μm filtered site water) was added by careful pipetting directly onto the sediment surface to form a continuous thin layer completely covering the sample. To the other six wells with sediment, 50 μL 0.22-μm filtered site water was added as a control/normal treatment.The minimum effective concentration and dosage of Lat A to inhibit vertical migration was determined by referring to Cartaxana and Ser?dio (2008).Application of the motility inhibitor Lat A enabled the nonmigratory photoresponse to be investigated by comparing migratory and non-migratory MPB assemblages. A minimum of 60 min was allowed for the inhibitors to diffuse and for the biofilms to stabilize before measurements started.

Fig.1 Migratory light response of microphytobenthos to different irradiance levels (represent by numbers)
Chlorophyll fluorescence was measured using an Imaging-PAM fluorometer (Max/L, Walz, Germany).Before measuring fluorescence, areas of interest(AOIs) were defined under Live Video Mode, with circular samples of the same size. The same AOIs were used consistently over the course of the experiment. Fluorescence was induced by royal blue(450 nm) 3 W Luxeon LEDs, which have a standard intensity of 0.5 μmol/(m2·s) and a modulation frequency between 1 and 8 Hz. Induction curves were determined after 2 min dark adaptation by applying a saturation pulse at intervals of 30 s for a total of 315 s.The fluorescence levelF0(minimum fluorescence of a dark-adapted sample) was taken as a proxy for the surface microalgal biomass. NPQ images derived from the induction curves were used to evaluate the photosynthetic status. The NPQ was calculated by(Fm?Fm’)/Fm’, whereFmis the maximum fluorescence excited by the first saturating light pulse after dark adaptation, andFm’ is the maximum fluorescence of the illuminated samples, including those assessed at the plateau level reached during the induction curve measurement. The maximal quantum yield of PSII was calculated by the ratio ofFv/Fm=(Fm?F0)/Fm(Genty et al., 1989). Rapid light curves (RLCs) were used to assess the photosynthetic activities, for which samples were exposed to twelve incremental steps of irradiance (10 s per step) ranging from 0 to 701 μmol/(m2·s). For each irradiance level,E, the relative ETR was calculated from the product ofEand the PSII effective quantum yield, ETR=E×(Fm’?F)/Fm’ (Genty et al., 1989). Three photosynthetic parameters, the maximum relative electron transport rate (rETRmax),minimum saturating irradiance (Ek) and light utilization coefficient (α), were derived from the RLCs fitted to the model of Platt et al. (1980). The variable migratory response to changes in irradiance can be adequately described by biomass vs. light curves (BLCs). After measuring the initial (t=0 h)curves, six wells in the sample plates were simultaneously exposed to six different irradiance levels: 0, 50, 100, 250, 500 and 1 000 μmol/(m2·s).Homogeneous light was provided by LED panels,while 0 μmol/(m2·s) was achieved by covering the well with a black case. Both Lat A and no-Lat A treatments had six replicates for each of the six light intensities. After 2.5, 4, 5.2, 6.2 and 8.0 h irradiance,induction curves and RLCs were measured by Imaging-PAM. All of the sample wells were kept saturated with filtered site water throughout the experimental period.
Statistical analysis was carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). To compare the effects of different light intensities, MANOVA(General Linear Model of Multivariate, SPSS) was run for each irradiance time on the variables includingF0, NPQ,Fv/Fm, rETRmax,Ekandα, followed by the Tukey’s HSD- post-hoc tests to compare pairs of different intensity levels. To test the effect of Lat A treatment on migration, separate one-way ANOVAs were run onF0for each irradiance level. To compare the different effects of no-Lat A and Lat A treatments,at-test was first performed for each irradiance time and irradiance level, and then comparisons between pairs of different light intensities were performed using Tukey’s HSD-test, separated for no-Lat A and Lat A treatments.
3 RESULT
3.1 Migratory photoresponse
3.1.1 Surface biomass variation

Fig.2 Non-photochemical quenching of fluorescence (NPQ, a) and PSII maximal quantum yield ( F v/ F m, b) under different irradiance levels (represent by numbers, μmol/(m 2 ·s))
Upon exposure to different light intensities, MPB assemblages presented a large range of migratory responses. The distinctive variation in the migratory response with incident irradiance, which was monitored byF0using Imaging-PAM, is illustrated in Fig.1a. During the first 4 h of irradiation,F0increased under 50–250 μmol/(m2·s), indicating upward migration and accumulation of microalgae at the surface of the sediment. Conversely, after 4 h,F0decreased to varying degrees under 50–250 μmol/(m2·s), indicating downward migration of microalgae from the surface of the sediment. The maximum upward migration was induced by 100 μmol/(m2·s)(Fig.1a, b), implying that this was the best light intensity for the MPB community. In contrast, light intensities of 500 and 1 000 μmol/(m2·s) consistently induced a downward migratory response, especially 1 000 μmol/(m2·s), with a ca. 50% decrease in the initialF0values in the first 2.5 h, which was more rapid than under the other light irradiance levels.Under darkness,F0oscillated slightly after a decrease of 14±3.3% and was not different significantly to that under 500 μmol/(m2·s) throughout the incubation time (Tukey’s HSD test,P=0.90).
By constructingF0vs. light curves (Fig.1b), i.e.BLCs, the variable migratory response to changes in irradiance can be adequately characterized. The main features of the migratory photoresponse of intertidal MPB were rapid biomass accumulation under low irradiances (50 and 100 μmol/(m2·s)) and a continuous decrease under higher irradiances. This pattern showed similar variation with treatment time:unimodal curves showed the highest accumulation at 8.0 h under 50 μmol/(m2·s) and at other treatment times under 100 μmol/(m2·s) irradiance (Fig.1b).
3.1.2 Non-photochemical quenching (NPQ) and PSII maximal quantum yield (Fv/Fm)
Similar variation patterns in NPQ were observed under light intensities of 50–250 μmol/(m2·s) over the incubation time, although there was a slight rise under 100 μmol/(m2·s) after 4.0 h of illumination with a significant difference between 2.5 and 4.0 h (Tukey’s HSD test,P=0.02) (Fig.2a). Additionally, the NPQ in dark conditions showed no difference to that under light of 50–250 μmol/(m2·s), except that it was significantly lower than that under 100 μmol/(m2·s)after 6.5 h of illumination (P=0.01). NPQ steeply decreased within 2.5 h under high light at 500 μmol/(m2·s) and remained low at 0.76±0.09 until the end of the illumination period. Under high light at 1 000 μmol/(m2·s), NPQ dropped to zero at 2.5 h and showed no difference to that under 500 μmol/(m2·s) at all irradiance times (Tukey HSD test,P≥0.94).
As illustrated as Fig.2b,Fv/Fmvalues were highest under 50 and 100 μmol/(m2·s) irradiance and were not significantly different between the two light intensities at any illumination time (Tukey’s HSD test,P≥0.72). TheFv/Fmvalues were a little lower under 0 and 250 μmol/(m2·s), and there was also no significant difference between these light intensities at any illumination time (Tukey’s HSD test,P>0.05).Conversely,Fv/Fmkept decreasing under 500 and 1 000 μmol/(m2·s) significantly over time until 6.5 h(Tukey’s HSD test,P<0.01, exceptP=0.999 for 6.5 vs. 8.0), and the decrease ofFv/Fmunder 1 000 μmol/(m2·s) was twice that under 500 μmol/(m2·s).
The NPQ images derived from the induction curves presented four different patterns after the first 2.5 h of illumination (Fig.3). Type I under dark incubation:NPQ increased strongly during first minute of illumination, and then showed a slow decrease until it reached a stable phase. Type II under 50–250 μmol/(m2·s) light: NPQ was high during the first minute of illumination and then maintained a slight increase until the end of the experiment. Type III under high light at 500 μmol/(m2·s): NPQ increased in the first minute and remained slightly elevated until the end of the experiment, with values less than half of those under type II. Type IV under 1 000 μmol/(m2·s) (the bottom curve): NPQ remained at 0 throughout the measurement.

Fig.3 NPQ images of induction curves measured by Imaging-PAM after 2.5 h illumination under different irradiance levels (represent by numbers, μmol/(m 2 ·s))
3.1.3 Photosynthetic parameters
Long periods of light exposure (6.5 and 8.0 h) led to significant differences for all three parameters(Tukey’s HSD test,P<0.05). In particular, after 6.5 h the rETRmaxandαdecreased to values even lower than at the beginning of experiment under all light treatments. At 2.5 h, the rETRmaxincreased rapidly with light intensities up to 500 μmol/(m2·s) (Fig.4a).Ek, calculated by rETRmax/α, followed a similar variation pattern to rETRmax (Fig.4c). Except thatαunder 500 μmol/(m2·s) was significantly different to those under 50, 100 and 250 μmol/(m2·s) (P=0.02,0.00 and 0.00 respectively), all three parameters showed no significant pairwise difference among 0,50, 100, 250 and 500 μmol/(m2·s) light treatments(P>0.05) at any illumination time (Fig.4b). However,excluding 1 000 μmol/(m2·s) (which failed to produce RLCs), with regard to particular illumination times,there were significant differences between some light intensities for one or two parameters; for example,rETRmaxandEkunder 0 and 50 μmol/(m2·s) differed significantly from those under 100, 250 and 500 μmol/(m2·s) at 2.5 h illumination time (Tukey’s HSD test,P<0.05), and rETRmaxunder 50 μmol/(m2·s),αunder 0 and 50 μmol/(m2·s), andEkunder 0 μmol/(m2·s) were significantly different to those under other light treatments at 5.2 h irradiance time(Tukey HSD test,P<0.05).
3.2 Non-migratory photoresponse

Fig.4 Variations of ETR parameters with illumination time under different irradiance levels (represent by numbers, μmol/(m 2·s))
Surface biomass variation indicated byF0was compared between non-Lat A and Lat A-treated samples. The motility of MPB assemblages was obviously inhibited by Lat A under all light intensity treatments, as indicated by relatively consistentF0values (an average of 0.96±0.09 when normalized to initial values, ANOVA,F=1.907, df=179,P=0.11)for all Lat A treatments over the experimental period,in contrast to MPB in non-Lat A treatments, which migrated up or down at different light intensities and illumination times.
Migratory responses were affected, particularly theFv/Fmvs.Ecurves, by the higher light illumination levels. In non-Lat A treated samples, inherentFv/Fmvalues were significantly higher than in samples inhibited by Lat A under 500 and 1 000 μmol/(m2·s) at all irradiance times (t- and Tukey’s HSD tests,P<0.01), and under 250 μmol/(m2·s) when illuminated for longer than 5.2 h (t- and Tukey’s HSD tests,P<0.02, Fig.5 left). However, under low irradiance(≤100 μmol/(m2·s)) and shorter illumination times,there was no difference between non-Lat A and Lat A treatments (P>0.05).

Fig.5 Comparisons of non-Lat A and Lat A treatments on fluorescence values vs. light curves (left: F v/ F m; right: NPQ)
NPQ values in Lat A treatments were lower than or equal to those under normal treatments until 6.5 h illumination, but were not significantly different(Fig.5 right,t- and Tukey’s HSD tests,P>0.05 for all irradiance times). However, as the incubation time was prolonged and light intensity increased, the NPQ in the Lat A treatment gradually increased to values close to (6.5 h) and even higher than those in the non-Lat A treatment at 8.0 h under 50 to 250 μmol/(m2·s)irradiance.
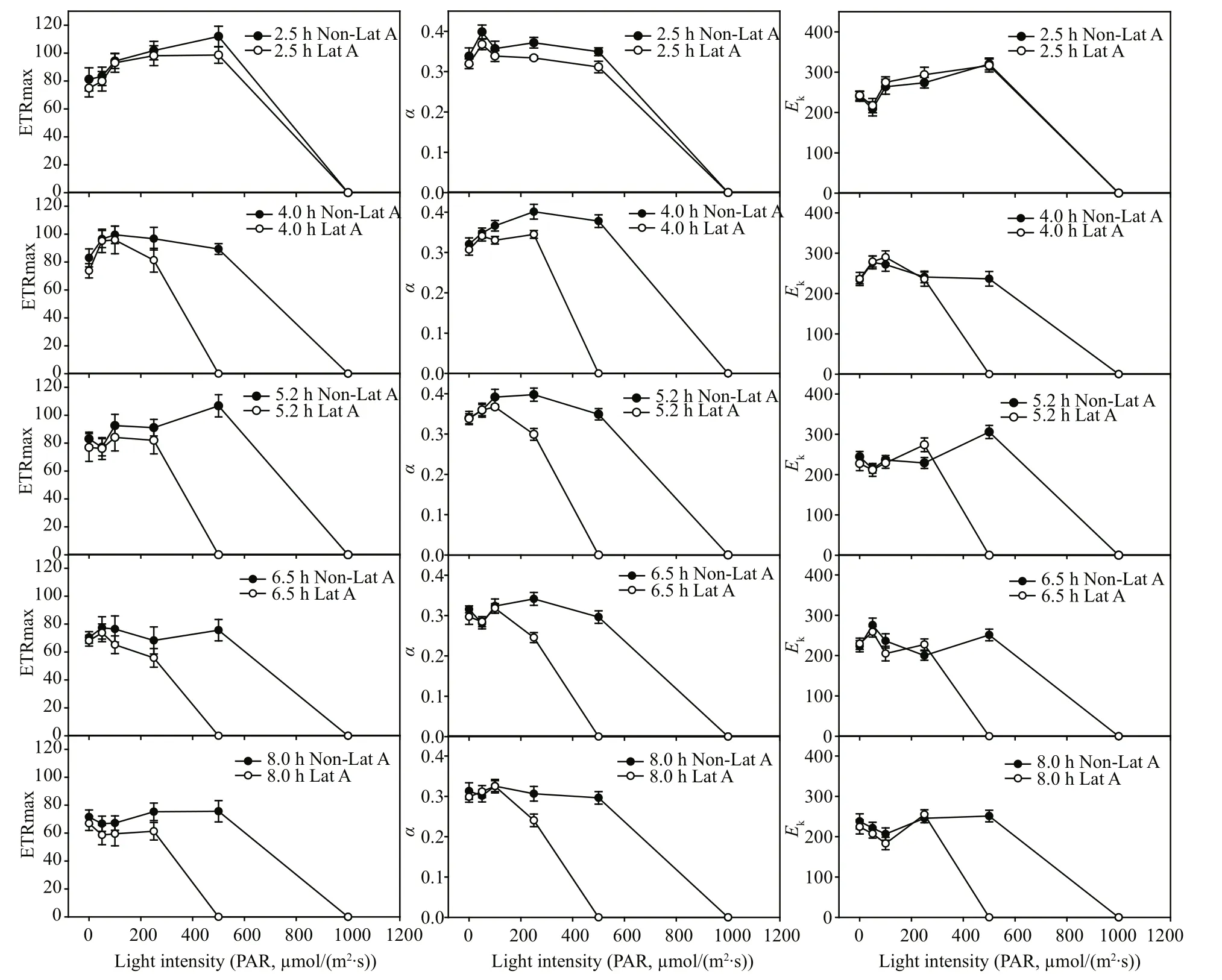
Fig.6 Comparisons of non-Lat A and Lat-A treatments for photosynthetic parameters vs. light curves (left: rETR max;middle: α; right: E k)
The ETR parameters rETRmaxandαpresented lower values in the Lat A than in the non-Lat A treatment, with similar variation patterns in their light curves, but more quickly dropped to their lowest values under high light intensity (Fig.6). In general,theEkvs. light curves showed an opposite pattern toαvs. light curves. There was not much difference inEkvs. light curves between the Lat A and non-Lat A treatments throughout the experimental period (t- and Tukey’s HSD test,P>0.05 for all cases); slightly higherEkvalues were only observed under 250 μmol/(m2·s) incubation at 5.2 and 6.5 h in the Lat A treatment. After 4.0 h, the rETRmaxandαwere significantly different between the Lat A and non-Lat A treatments under light intensities higher than 250 μmol/(m2·s) (t- and Tukey HSD test,P<0.05 for all cases). Compared with non-Lat A treated samples,which failed to produce RLC fluorescence signals only at 1 000 μmol/(m2·s) irradiance, all Lat A-treated samples failed to produce signals earlier under 500 μmol/(m2·s) after 2.5 h illumination.
4 DISCUSSION
4.1 Behavior photoresponse
Vertical migration is considered an endogenous phototaxis and geotaxis mainly induced by light and nutrient availability for MPB physiological requirements or cell division (Consalvey et al.,2004a). The typical migration frequently presents a periodic rhythm according to light and tidal cycles,but there is no universal pattern for migration(Consalvey et al., 2004a). For instance, in subtidal zones with nearly no tidal cycle influence, MPB migration only followed the diel cycle of light (Ní Longphuirt et al., 2006). This behavior has also been found to persist in the lab as endogenous rhythms were shown to continue in the absence of various stimuli in lab systems for 3 to 21 days (Paterson,1986; Ser?dio et al., 1997). In this study, MPB was collected from the upper part of the intertidal zone of a mud flat where the sediments are seldom influenced by the tidal cycle. On the experiment day (29thMarch),the tide cycle during the daytime was low tide at 10:01 (25 cm over the tidal datum line) and high tide at 15:39 (421 cm). During the experimental period(from 9:10 to 18:12), the surface biomass indicated byF0under dark incubation (absence of light and tidal stimulation) presented variation without significant differences. This implied that the prolonged illumination period in the experiment had no effect on the endogenous rhythms of the samples collected from the field.
In this study, 2 min dark-adaptation was used for Imaging-PAM measurements, which may not have been enough for full NPQ reversal and complete QAoxidation for all treatments (from 50 to 1 000 μmol/(m2·s) and up to 8.0 h illumination). It was reported that prolonged periods of dark adaptation up to 23 h were not sufficient to fully reverse NPQ; on the contrary, prolonged exposure to dark/far-red light led to downwards migration of cells in natural assemblages (Consalvey et al., 2004b). Furthermore,the latest research has shown that vertical migration can occur on a scale of a few minutes; surface biomass decreased by more than 30% of the total induced change during the first 2.5 min of exposure to light above 250 μmol/(m2·s) (Laviale et al., 2016). In this study,F0also decreased in the first 2.5 h under dark incubation. Therefore, confounding effects of vertical migration or physiological processes on fluorescence measurements are unavoidable for migratory MPB assemblages. However, as a comparative index,F0can still be used to show relative differences among light treatments of 50 to 1 000 μmol/(m2·s) with a dark treatment as a control. The results of this study indicated that variation inF0could sufficiently characterize the migratory photoresponse of MPB assemblages over time, and the BLCs could adequately describe the variable migratory response to changes in irradiance.
Clear phototaxis of MPB assemblages under low to moderate irradiance of 50–250 μmol/(m2·s) and a marked downward migratory response under higher light above 500 μmol/(m2·s) were observed in this study. Dark conditions induced a little downward movement at the beginning of the experiment,consistent with results of Laviale et al. (2016). These results corroborated that MPBs can adjust their position through migration or micromigration within their biofilm to avoid irradiance that is too strong and to achieve the optimum light intensity for photosynthesis (Kromkamp et al., 1998; Du et al.,2012). With regard to the decrease of surface biomass after 4 h of illumination at 50–500 μmol/(m2·s) and 2.5 h at 1 000 μmol/(m2·s), we suppose that diatoms would leave the surface sediment after sufficient photosynthesis or as a result of increased photoinhibition during prolonged high-light treatment; that is, a prolonged irradiation period induces a negative-migratory response in MPB assemblages.
Vertical migratory responses to different irradiance levels have also been observed in intact biofilms of estuarine sediments (Ser?dio et al., 2006); specifically,the surface biomass increases under irradiances below 100 μmol/(m2·s), reaches a maximum under 100–250 μmol/(m2·s), and gradually decreases under higher irradiances. Benthic diatoms move within the sedimentary environment in response to varying light levels. For example, diatoms concentrated at a depth of 1 mm can migrate up to the surface in 1.5 h(Hopkins, 1963). For the individual diatom speciesCylindrothecaclosteriumandAmphoracoff eaeformis,the greatest surface migration occurred at different light intensities (250 and 100 μmol/(m2·s),respectively) and sediment grain sizes (63–125 μm and 125–350 μm, respectively) with a difference of more than two times in speed (Du et al., 2010b).Therefore, this pattern is species-specific and varies according to different light histories and is related to light dosage, which is determined by the light intensity and duration of exposure (Paterson, 1986; Hay et al.,1993; Underwood et al., 2005; Du et al., 2012).
4.2 Physiological response
Three parameters extracted from the RLCs characterized the physiological response to changes in absorbed light energy. These parameters generally declined, corroborating that light exposure resulted in a decrease in photosynthetic activity. After exposure to higher light over certain time periods (250 and 500 μmol/(m2·s) for 2.5 h), non-migratory MPBs exhibited lower light use efficiency (lowerα) and lower maximum photosynthetic capacity (lower rETRmaxandFv/Fm) than migratory MPBs. This result is consistent with a previous study by Cartaxana et al.(2011) on mud benthic diatom communities.Furthermore, except under high light stress of 500 and 1 000 μmol/(m2·s), the inhibition of migration had no significant effect on the minimum saturating irradiance (Ek) under the same illumination treatment.In Cartaxana et al. (2011),Ekwas also not significantly affected by different treatments, especially for sand assemblages.
Clear physiological downregulation of MPB assemblages was observed in response to high light(super-saturating light) in this study. This regulation is often referred to as NPQ in diatoms, and involves de-epoxidation of diadinoxanthin to diatoxanthin(Jesus et al., 2008; Ser?dio et al., 2008). The results of the present study showed that NPQ steeply decreased under light stress over 500 μmol/(m2·s), and hardly recovered with increased exposure time. Compared with the migratory treatment, the NPQ induction in non-migratory Lat A treated samples presented an increasing trend over time except under high light at 500 and 1 000 μmol/(m2·s). This might imply that MPB assemblages with inhibited migratory ability increased their physiological photoprotection capacity to attenuate the damage from the cumulative light dose, but not under high light stress because photoinhibition was predominant.
The different NPQ image patterns revealed the effects of different light irradiation levels on the MPB photosynthetic system. For dark incubation, without the irradiance effect, the variation of NPQ in the induction curve represented the normal state of the photosynthetic system, viz., O2-dependent electron flow in the first minute was replaced by CO2-dependent electron flow when the Calvin-Benson cycle was activated, ATP was consumed and the transthylakoidal ΔpH decreased (Schreiber and Berry,1977; Type I, Fig.3). In this study, because of the limitation of the measuring time spent on induction and light curves for the six plate wells, as well as artificial cooperation, the first measurement was carried out after 2.5 h incubation. Therefore, after such a long period of illumination even under low and medium light at 50–250 μmol/(m2·s), NPQ still slowly increased following the strong increase in the first minutes of the induction curve (Type II, Fig.3),because O2-dependent electron flow did not relax, as the Calvin-Benson cycle might have been suppressed.High light at 500 μmol/(m2·s) resulted in NPQ values less than half of those under 50–250 μmol/(m2·s)beginning from the third measurement of induction curve (Type III, Fig.3), which might have partly been because O2-dependent electron flow was suppressed.Under extreme high light of 1 000 μmol/(m2·s), the very low NPQ values, on the one hand, were due to the suppression of not only CO2- but also O2-dependent electron flow, resulting in no ΔpH, such as in Lat-A treated samples, and, on the other hand,might have been because of migration away from the measurement depth, such as for migratory MPB assemblages. Researchers have seldom used NPQ images to compare the physiological effects of different light intensities. More research needs to be carried out using shorter periods and more detailed photo-physiological processes.
Generally, there was no significant difference in NPQ andFv/Fmbetween low light treatments (50–250 μmol/(m2·s)), indicating that possibly only high light can influence the photosynthetic capability of MPB.
4.3 Photoprotection and photoinhibition
Generally speaking MPB assemblages show hardly any photoinhibition (Blanchard et al., 2004; Waring et al., 2007; Mouget et al., 2008), which is related to the ability of MPB assemblages to adjust their photosynthetic activity not only through physiological regulation of the use of absorbed light energy by the photosynthetic apparatus, but also through behavioral control of light absorption through migration(Cartaxana et al., 2011; Ser?dio et al., 2012). Besides these two photoprotective mechanisms (vertical migration and the xanthophyll cycle) in MPB (Ser?dio et al., 2005, 2008; Jesus et al., 2006; Mouget et al.,2008; Perkins et al., 2010), other processes have been suggested to be responsible for the observed low rates of photoinhibition; including the cyclic electron flow around PSII (Lavaud et al., 2002a, b, 2004; Lavaud,2007), the efficient scavenging of reactive oxygen species (Roncarati et al., 2008; Waring et al., 2010),and high turnover rates of the PSII protein D1 (Wu et al., 2011). However, Ser?dio et al. carried out a series of studies demonstrating that the rates of photoinhibition could reach up to ca. 18% in situ(Ser?dio et al., 2008) and ca. 20% in laboratory experiments (Ser?dio et al., 2012). Besides light history and species-specific differences, the extent of photoinhibition is directly related to the light dosage,determined by the light intensity and duration of exposure. Furthermore, potential (or some degree of)photoinhibitory damage cannot be completely prevented by the photoprotective mechanisms available to MPB (Lavaud et al., 2002b, 2004; Ser?dio et al., 2012).
In the present study, behavioral photoprotection by vertical migration was clearly shown by the evasion of high light at 500–1 000 μmol/(m2·s) and downward migration after receiving a sufficient light dosage.Conversely, the MPB assemblages inhibited by Lat A that lost the capacity for behavioral photoprotection showed lower photosynthetic capability under light stress. However, the physiological photoprotection was not clear because of strong photoinhibition after 2.5 h illumination, especially at 500 and 1 000 μmol/(m2·s). Additionally, the decrease ofFv/Fmunder 500 and 1 000 μmol/(m2·s) implied that high light stress had photoinhibitory effects on the photosynthetic capability or induced reversible downregulation.However, the increase of NPQ in Lat A treatments implied that the MPB assemblages could recover their physiological photoprotection ability to adapt to light stress. The photoprotective role of the xanthophyll cycle could be further analyzed by the recovery kinetics of photosynthetic activity following exposure to high light stress (Horton and Hague,1988; Walters and Horton, 1991; Müller et al., 2001;Ser?dio et al., 2012). Besides using specific inhibitors for vertical migration, inhibitors of xanthophyll cycle activity (Lavaud et al., 2002a; Ser?dio et al., 2012)could be used to estimate the relative contribution of each process to the overall photoprotection of the biofilm. Because of the limitations of operating time in this experiment, the recovery kinetics need to be examined separately in other sets of experiments.Despite its limitations, the laboratory experimental approach used in the present study still has the advantage over studies carried out under in situ conditions (e.g. Ser?dio et al., 2008; Perkins et al.,2010) of controlled and reproducible conditions,making it possible to simultaneously measure and directly compare the migratory and physiological responses of samples under different treatments.
5 CONCLUSION
In this study, MPB presented distinct variation in behavioral and physiological responses to different light intensities during a prolonged illumination period of 8.0 h. High light at 500 and 1 000 μmol/(m2·s) induced downward migration and decreased photosynthetic ability in terms of NPQ and the effective quantum yieldFv/Fm. Low and medium light levels (50–250 μmol/(m2·s)) promoted MPB upward migration followed by downward migration and decreased photosynthetic capability after the accumulation of sufficient light dose to meet the photosynthesis requirement. Non-migratory MPB assemblages exhibited lower light use efficiency(lowerα) and lower maximum photosynthetic capacity (lower rETRmaxandFv/Fm) than migratory MPB assemblages under high light after certain illumination times. The increase of NPQ in nonmigratory MPB along over time implied that the MPB can recover its physiological photoprotection capacity to adapt to light stress.
Blanchard G F, Guarini J M, Dang C, Richard P. 2004.Characterizing and quantifying photoinhibition in intertidal microphytobenthos.J.Phycol.,40(4): 692-696.
Cartaxana P, Ruivo M, Hubas C, Davidson I, Ser?dio J, Jesus B. 2011. Physiologicalversusbehavioral photoprotection in intertidal epipelic and epipsammic benthic diatom communities.J.Exp.Mar.Biol.Ecol.,405(1-2): 120-127.
Cartaxana P, Ser?dio J. 2008. Inhibiting diatom motility: a new tool for the study of the photophysiology of intertidal microphytobenthic biofilms.Limnol.Oceanogr.Meth.,6(9): 466-476.
Consalvey M, Jesus B, Perkins R G P, Brotas V, Underwood G J C, Paterson D M. 2004b. Monitoring migration and measuring biomass in benthic biofilms: the effects of dark/far-red adaptation and vertical migration on fluorescence measurements.Photosyth.Res.,81(1): 91-101.
Consalvey M, Paterson D M, Underwood G J C. 2004a. The ups and downs of life in a benthic biofilm: migration of benthic diatoms.DiatomRes.,19(2): 181-202.
Du G Y, Li W T, Li H B, Chung I K. 2012. Migratory responses of benthic diatoms to light and temperature monitored by chlorophyll fluorescence.J.PlantBiol.,55(2): 159-164.
Du G Y, Oak J H, Chung I K. 2010b. effect of light and sediment grain size on the vertical migration of benthic diatoms.Algae,25(3): 133-140.
Du G Y, Son M, An S, Chung I K. 2010a. Temporal variation in the vertical distribution of microphytobenthos in intertidal flats of the Nakdong River estuary, Korea.Estuar.Coast.ShelfSci.,86(1): 62-70.
Du G Y, Son M, Yun M S, An S, Chung I K. 2009.Microphytobenthic biomass and species composition in intertidal flats of the Nakdong River estuary, Korea.Estuar.Coast.ShelfSci.,82(4): 663-672.
Genty B, Briantais J M, Baker N R. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence.Biochim.Biophys.Acta,990(1): 87-92.
Goss R, Jakob T. 2010. Regulation and function of xanthophyll cycle-dependent photoprotection in algae.Photosynth.Res.,106(1-2): 103-122.
Guarini J M, Blanchard G F, Gros P, Gouleau D, Bacher C.2000. Dynamic model of the short-term variability of microphytobenthic biomass on temperate intertidal mudflats.Mar.Ecol.Prog.Ser.,195: 291-303.
Hay S I, Maitland T C, Paterson D M. 1993. The speed of diatom migration through natural and artificial substrata.DiatomRes.,8(2): 371-384.
Hopkins J T. 1963. A study of the diatoms of the Ouse Estuary,Sussex I. The movement of the mud-flat diatoms in response to some chemical and physical changes.J.Mar.Bio.Assoc.U.K.,43(3): 653-663.
Horton P, Hague A. 1988. Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts.IV. Resolution of non-photochemical quenching.Biochim.Biophys.Acta,932: 107-115.
Jesus B, Mouget J L, Perkins R G. 2008. Detection of diatom xanthophyll cycle using spectral reflectance.J.Phycol.,44(5): 1 349-1 359.
Jesus B, Perkins R G, Consalvey M, Brotas V, Paterson D M.2006. effects of vertical migrations by benthic microalgae on fluorescence measurements of photophysiology.Mar.Ecol.Prog.Ser.,315: 55-66.
Kromkamp J, Barranguet C, Peene J. 1998. Determination of microphytobenthos PSII quantum efficiency and photosynthetic activity by means of variable chlorophyll fluorescence.Mar.Ecol.Prog.Ser.,162: 45-55.
Lavaud J, Rousseau B, Etienne A L. 2002b. In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching.FEBSLett.,523(1-3): 163-166.
Lavaud J, Rousseau B, Etienne A L. 2004. General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae).JPhycol.,40(1): 130-137.Lavaud J, Rousseau B, van Gorkom H J, Etienne A L. 2002a.Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum.PlantPhysiol.,129(3):1 398-1 406.
Lavaud J. 2007. Fast regulation of photosynthesis in diatoms:mechanisms, evolution and ecophysiology.Funct.Plant Sci.Biotech.,1(2): 267-287.
Laviale M, Frankenbach S, Ser?dio J. 2016. The importance of being fast: comparative kinetics of vertical migration and non-photochemical quenching of benthic diatoms under light stress.Mar.Biol.,163: 10, https://doi.org/10.1007/s00227-015-2793-7.
Mouget J L, Perkins R, Consalvey M, Lefebvre S. 2008.Migration or photoacclimation to prevent high irradiance and UV-B damage in marine microphytobenthic communities.Aquat.Microb.Ecol.,52: 223-232.
Müller P, Li X P, Niyogi K K. 2001. Non-photochemical quenching. A response to excess light energy.Plant Physiol.,125(4): 1 558-1 566.
Ní Longphuirt S, Leynaert A, Guarini J M, Chauvaud L,Claquin P, Herlory O, Amice E, Huonnic P, Ragueneau O.2006. Discovery of microphytobenthos migration in the subtidal zone.Mar.Ecol.Prog.Ser.,328: 143-154.
Paterson D M. 1986. The migratory behaviour of diatom assemblages in a laboratory tidal micro-ecosystem examined by low temperature scanning electron microscopy.DiatomRes.,1(2): 227-239.
Perkins R G, Lavaud J, Ser?dio J, Mouget J L, Cartaxana P,Rosa P, Barille L, Brotas V, Jesus B M. 2010. Vertical cell movement is a primary response of intertidal benthic biofilms to increasing light dose.Mar.Ecol.Prog.Ser.,416: 93-103.
Perkins R G, Oxborough K, Hanlon A R M, Underwood G J C,Baker N R. 2002. Can chlorophyll fluorescence be used to estimate the rate of photosynthetic electron transport within microphytobenthic biofilms?.Mar.Ecol.Prog.Ser.,228: 47-56.
Perkins R G, Underwood G J C, Brotas V, Snow G C, Jesus B,Ribeiro L. 2001. Responses of microphytobenthos to light: primary production and carbohydrate allocation over an emersion period.Mar.Ecol.Prog.Ser.,223: 101-112.
Pinckney J, Zingmark R G. 1991. effects of tidal stage and sun angles on intertidal benthic microalgal productivity.Mar.Ecol.Prog.Ser.,76(1): 81-89.
Platt T, Gallegos C L, Harrison W G. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton.J.Mar.Res.,38(4): 687-701.
Roncarati F, Rijstenbil J W, Pistocchi R. 2008. Photosynthetic performance, oxidative damage and antioxidants inCylindrothecaclosteriumin response to high irradiance,UVB radiation and salinity.Mar.Biol.,153(5): 965-973.
Schreiber U, Berry J A. 1977. Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus.Planta,136(3):233-238.
Ser?dio J, Catarino F. 1999. Fortnightly light and temperature variability in estuarine intertidal sediments and implications for microphytobenthos primary productivity.Aquat.Ecol.,33(3): 235-241.
Ser?dio J, Catarino F. 2000. Modelling the primary productivity of intertidal microphytobenthos: time scales of variability and effects of migratory rhythms.Mar.Ecol.Prog.Ser.,192: 13-30.
Ser?dio J, Coelho H, Vieira S, Cruz S. 2006. Microphytobenthos vertical migratory photoresponse as characterised by light-response curves of surface biomass.Estuar.Coast.ShelfSci.,68(3-4): 547-556.
Ser?dio J, Cruz S, Vieira S, Brotas V. 2005. Non-photochemical quenching of chlorophyll fluorescence and operation of the xanthophyll cycle in estuarine microphytobenthos.J.Exp.Mar.Biol.Ecol.,326(2): 157-169.
Ser?dio J, da Silva J M, Catarino F. 1997. Nondestructive tracing of migratory rhythms of intertidal benthic microalgae usinginvivochlorophyllafluorescence.J.Phycol.,33(3): 542-553.
Ser?dio J, da Silva J M, Catarino F. 2001. Use ofinvivochlorophyllafluorescence to quantify short-term variations in the productive biomass of intertidal microphytobenthos.Mar.Ecol.Prog.Ser.,218: 45-61.
Ser?dio J, Ezequiel J, Barnett A, Mouget J L, Méléder V,Laviale M, Lavaud J. 2012. Efficiency of photoprotection in microphytobenthos: Role of vertical migration and the xanthophyll cycle against photoinhibition.Aquat.Microb.Ecol.,67(2): 161-175.
Ser?dio J, Lavaud J. 2011. A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence.Photosynth.Res.,108(1): 61-76.
Ser?dio J, Vieira S, Cruz S. 2008. Photosynthetic activity,photoprotection and photoinhibition in intertidal microphytobenthos as studiedinsituusing variable chlorophyll fluorescence.Cont.ShelfRes.,28(10-11):1 363-1 375.
Ser?dio J. 2004. Analysis of variable chlorophyll fluorescence in microphytobenthos assemblages: implications of the use of depth-integrated measurements.Aquat.Microb.Ecol.,36: 137-152.
Underwood G J C, Kromkamp J. 1999. Primary production by phytoplankton and microphytobenthos in estuaries.Adv.Ecol.Res.,29: 93-153.
Underwood G J C, Perkins R G, Consalvey M C, Hanlon A R M, Oxborough K, Baker N R, Paterson D M. 2005.Patterns in microphytobenthic primary productivity:species-specific variation in migratory rhythms and photosynthetic efficiency in mixed-species biofilms.Limnol.Oceanogr.,50(3): 755-767.
Walters R G, Horton P. 1991. Resolution of components of non-photochemical chlorophyll fluorescence quenching in barley leaves.Photosynth.Res.,27(2): 121-133.
Waring J, Baker N R, Underwood G J C. 2007. Responses of estuarine intertidal microphytobenthic algal assemblages to enhanced ultraviolet B radiation.Glob.ChangeBiol.,13(7): 1 398-1 413.
Waring J, Klenell M, Bechtold U, Underwood G J C, Baker N R. 2010. Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species.J.Phycol.,46(6):1 206-1 217.
Wu H Y, Cockshutt A M, McCarthy A, Campbell D A. 2011.Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms.PlantPhysiol.,156(4):2 184-2 195.
 Journal of Oceanology and Limnology2018年2期
Journal of Oceanology and Limnology2018年2期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Hydroacoustic estimates of fish biomass and spatial distributions in shallow lakes*
- A comparison between benthic gillnet and bottom trawl for assessing fish assemblages in a shallow eutrophic lake near the Changjiang River estuary*
- Morphological beak differences of loliginid squid, Uroteuthis chinensis and Uroteuthis edulis, in the northern South China Sea*
- Muelleria pseudogibbula, a new species from a newly recorded genus (Bacillariophyceae) in China*
- Planaxidae (Mollusca, Gastropoda) from the South China Sea*
