Silencing of syndecan-binding protein enhances the inhibitory effect of tamoxifen and increases cellular sensitivity to estrogen
Jun Zhang, Xiaolong Qian, Fangfang Liu, Xiaojing Guo, Feng Gu, Li Fu
Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s Clinical Research Center for Cancer; Key Laboratory of Breast Cancer Prevention and Therapy, Ministry of Education, Tianjin 300060, China
Introduction
Breast cancer (BCa) is a heterogeneous disease, and approximately 75% of all BCa cases show overexpression of estrogen receptors (ER) and/or progesterone receptors (PR)1.The estrogen pathway affects the expression of hundreds of genes involved in proliferation, differentiation, survival,invasion, metastasis, and angiogenesis, all of which are particularly relevant to cancer2.
Apart from surgery, endocrine therapy is considered a complementary treatment in most patients and has shown consistent clinical benefits, particularly for ER-positive patients with respect to inducing tumor remission3. Among all endocrine therapies, tamoxifen is the most extensively used drug and functions as a selective ER modulator4that competitively blocks estrogen binding5. However, many breast tumors show either primary resistance to endocrine therapies or develop secondary resistance after initial responsiveness6-10. Approximately 20%–30% of patients who received adjuvant tamoxifen experienced relapse, and most patients with advanced disease who showed an initially positive response to tamoxifen eventually experienced disease progression11. The mechanism of this resistance involves cross-talk between ER and alternative signaling pathways involved in cell survival and proliferation, such as those for epidermal growth factor receptor and human epidermal growth factor receptor 212-15.
Melanoma differentiation-associated gene 9 was discovered through screening of differentially expressed genes upon treatment of melanoma16. This protein, also known as syntenin, interacts with syndecan family members and is therefore also known as syndecan-binding protein(SDCBP). The syndecan family belongs to a group of cell surface molecules and is involved in cell–cell and cell–matrix adhesion. SDCBP has a total of 298 amino acids and contains two PDZ domains, PDZ-1 (amino acids 110–193) and PDZ-2(amino acids 194–274)17. The PDZ domain is found in a family of proteins that controls diverse and central physiologic processes such as migration and lipid binding18-20.Through cross-talk with protein kinase C alpha via adhesionmediated activation downstream of the fibronectin signal,SDCBP activates focal adhesion kinase to take part in cellular migration and invasive BCa development21. Moreover,activation of integrin β1 and extracellular signal-related kinase 1/2 was shown to be required for syntenin-mediated migration and invasion of BCa cells22.
Our previous study showed that SDCBP expression was positively correlated with histologic grade and tumor staging,but negatively correlated with ERα expression. In ER-negative BCa cells, SDCBP silencing increased cell populations in G1 phase of the cell cycle and resulted in upregulation of p21 and p27 while down-regulating cyclin E,thereby arresting the cell cycle and prohibiting cell proliferation23. In the present study, we examined the effects of SDCBP on ER- positive BCa cells. To determine the role of SDCBP expression in ER-positive BCa development and whether SDCBP down-regulation can be used as a targeted treatment, we evaluated the expression profile of SDCBP in ER-positive cases. Using the RNAi technique, we analyzed the mechanisms underlying the involvement of SDCBP in ER-positive BCa development and its correlation with the estrogen-signaling pathway as well as its impact on endocrine therapy.
Material and methods
Sample collection
ER-positive breast tissue samples (n = 99) were obtained from patients who underwent surgical excision at the Department of Breast Cancer Pathology and Research Laboratory at Tianjin Medical University Cancer Institute and Hospital (China) from January to March of 2010. These samples were used in our previous study23.
Immunohistochemistry
Staining of ERα, PR, and SDCBP was performed as described in our previous publication23. Table S1 lists information regarding the antibodies used.
The expression levels of ERα, PR, and SDCBP were semiquantified using a modified scoring system, where the intensity score (0 = negative; 1 = low; 2 = medium; 3 = high)was multiplied by the percentage of cells that were stained.This scoring system gives a final score ranging from 0 to 300.In the presence of cytoplasmic staining, SDCBP status was classified according to this modified scoring system: negative(0–50), weak (51–100), moderate (101–200), or strong(201–300). ERα and PR status were categorized in the same manner as SDCBP signals in the presence of nuclear staining.All cases were evaluated by two pathologists independently and any discrepancy was resolved by group discussion. The PR/ERα ratio was calculated as the PR staining score/ERα staining score. The correlation between SDCBP status and pathologic features were analyzed using a non-parametric Spearman correlation test.
Cell culture
The human BCa cell lines MCF-7 and T47D were purchased from American Type Culture Collection (ATCC? HTB-22TMand ATCC? HTB-133 respectively, Manassas, VA, USA).
To deplete estrogen, cells were cultured in phenol red-free RPMI 1640 containing 2.5% HyClone Charcoal/Dextran-Treated Fetal Bovine Serum (SH30068.03, Thermo Scientific,Waltham, MA, USA) for 24 h. Next, 17-β estradiol (E2,Sigma-Aldrich, St. Louis, MO, USA) in ethanol was added to the culture medium at a final concentration of 0, 0.1, 1, or 10 nM, and the cells were cultured for another 24 h. Tamoxifen was purchased from Sigma and added to the culture medium at a final concentration of 2 μM.
Real-time quantitative reverse transcriptase PCR (qRT-PCR)
Total RNA extraction was performed as previously reported23. Primers for pS2, PR, and SDCBP are listed in Table S2 and β-actin was used as an internal control. The real-time qRT-PCR assay was performed using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). pS2 and PR mRNA transcription levels were normalized against β-actin mRNA expression.
Establishment of SDCBP- silenced MCF-7 cells
The shRNA used to silence SDCBP and negative control shRNA are listed in Table S3 [both were designed by Genepharma Co., Ltd (Shanghai, China)]. The procedures for screening the SDCBP-silenced stable MCF-7 cell line were performed as previously reported23. Subcultures showing maximal SDCBP silencing were designated as “MCF-7 shRNA”, while control shRNA-transfected subcultures were designated as “MCF-7 NC”.
SDCBP-overexpression BCa cell line construction
SDCBP-overexpressing and control cell lines were constructed as described previously24. Corresponding exogenous protein overexpression was evaluated by Western blot after the cells were cultured for 8 and 6 weeks for MCF-7 and T47D cells, respectively, in the appropriate medium containing 0.5 mg/mL of G418 (Sigma-Aldrich).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The MTT assay was performed as previously reported23,except that MCF-7 and T47D cells were seeded at 2,000 and 1,500 cells per well in a 96-well plate, respectively.
Flow cytometric cell-cycle analysis
Cell-cycle analysis was performed on a BD FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) as described previously25.
Western blot assay
Holoproteins in cell lysates were extracted, quantified, and immunoblotted as previously described23. The information and usage of antibodies are listed in Table S1. Protein expression levels were quantified using intensities normalized to β-actin. The expression levels calculated from three repeated immunoblots of all groups followed a normal distribution and were presented as the mean ± standard deviation. Student’s t-test was used to examine differences between groups.
Luciferase assay
Cells were co-transfected with the ERα luciferase reporter plasmid PGMER-Lu (Genomeditech Co., Ltd., Shanghai,China) and wide-type Renilla luciferase reporter gene control plasmid pGMR-TK in 24-well plates. Luciferase activities in cell lysates were measured using the Dual-Luciferase Reporter Assay in triplicate and normalized to Renilla luciferase activity. pGM-CMV-Lu-transfected cells were used as positive controls and the average relative luciferase activity of transfected MCF-7 NC/MCF-7 Neo/T47D Neo cells was defined as “1”. Student’s t test was used to examine the differences between these normally distributed groups.
Results
Silenced/overexpressed SDCBP influences the effects of tamoxifen on BCa proliferation
As shown in Figure 1A, SDCBP shRNA silenced most target proteins compared to MCF-7 NC in either the presence or absence of tamoxifen. However, SDCBP silencing alone did not affect cellular proliferation in the absence of tamoxifen,but rather enhanced the suppressive effect of tamoxifen(Figure 1B). Although SDCBP silencing did not affect MCF-7 cell-cycle kinetics in the absence of tamoxifen, it consistently contributed to the arrest of more cells in G1 in the presence of tamoxifen (P < 0.001, Figure 1C).
As shown in Figure 1D and 1G, SDCBP was significantly overexpressed in both MCF-7 and T47D cells; SDCBP overexpression accelerated cellular proliferation in both the absence and presence of tamoxifen in both cell lines. Under conditions of SDCBP overexpression, the effect of tamoxifen on cell proliferation was significantly attenuated (Figure 1E and 1H). Accordingly, in both cell lines, SDCBP overexpression reduced cells in G1 phase in both the absence and presence of tamoxifen and weakened the effects of tamoxifen on the cell cycle (Figure 1F and 1I).
Effects of SDCBP silencing/overexpressing on cell-cycle regulators in MCF-7 cells in the presence of tamoxifen
In ER-positive MCF-7 cells, tamoxifen treatment alone significantly increased p21 levels but attenuated the levels of phosphorylated Rb and cyclin D1. However, SDCBP silencing alone did not influence levels of p21, p27, cyclin D1,cyclin E, or phosphorylated Rb. In contrast, SDCBP silencing significantly up-regulated the levels of p21 and p27, but down-regulated the levels of phosphorylated Rb and cyclin E beyond that of tamoxifen alone. However, SDCBP silencing failed to further decrease cyclin D1 compared to tamoxifen alone (Figure 2A–2G). SDCBP overexpression alone did not influence p21 levels, but significantly down-regulated p27.Tamoxifen treatment did not recover the levels of p27, but up-regulated the levels of p21 under conditions of SDCBP overexpression (Figure 2H–2K).
Effects of SDCBP silencing/overexpression on estrogen responsiveness in ER-positive BCa cell line

Figure 1 Effect of tamoxifen treatment and SDCBP silencing/overexpressing on proliferation of ER-positive breast cancer cells. (A, D, G)Expression of SDCBP in the absence or presence of 2 μM tamoxifen as shown by Western blot assay. β-actin was used as an internal reference. (B, E, H) Proliferation was examined by the MTT assay. (C, F, I) Cell-cycle progression was determined by flow-cytometric cell-cycle analysis in the absence or presence of 2 μM tamoxifen. Student’s t-test was then used to compare differences (#P > 0.05, *P <0.05, **P < 0.01 and ***P < 0.001).

Figure 2 Effect of tamoxifen treatment and SDCBP silencing/overexpression on G1/S cell-cycle regulators. Western blot was conducted to examine the differential expression of p21, p27, phosphorylated Rb (phospho-Rb), cyclin D1, and cyclin E levels in MCF-7 cells with 2 μM tamoxifen treatment and/or SDCBP silencing (A)/overexpression (H). The ratios of Western blot intensities for the examined proteins to βactin were calculated from triplicate experiments (B-G, I-K); Student’s t-test was then used to compare differences (*P < 0.05, **P < 0.01,***P < 0.001, #P > 0.05).

Figure 3 Effects of SDCBP silencing/overexpression on estrogenic responses. (A, D, G) Effects of SDCBP silencing/overexpression on expression of estrogen-response reporter genes after administration of 0, 0.1, 1, or 10 nM E2 were examined using the dual luciferase assay.pGM-CMV-Lu-transfected cells were used as positive controls and the average relative luciferase activity of transfected MCF-7 NC/MCF-7 Neo/T47D Neo cells was defined as “1”; Student’s t-test was used to compare differences. Quantitative analysis of pS2 (B, E, H) and PR (C, F,I) transcription levels in MCF-7 shRNA/MCF-7 SDCBP/T47D SDCBP or MCF-7 NC/MCF-7 Neo/T47D Neo cells under steroid hormone deprivation or 10 nM E2 stimulation was performed by real-time quantitative reverse transcription-PCR. Transcription levels were normalized against β-actin. Each experiment was repeated three times and Student’s t-test was used to compare differences (*P < 0.05,**P < 0.01, ***P < 0.001, #P > 0.05).
The luciferase assay suggested that SDCBP silencing enhanced the estrogenic response when E2 was administrated at concentrations between 0.1 and 10 nM compared to MCF-7 NC counterparts (P = 0.017, P = 0.020 and P = 0.002,respectively) (Figure 3A). qRT-PCR evaluation showed that SDCBP silencing up-regulated pS2 and PR by 40.0% and 62.3% at the mRNA level, respectively, compared to those in MCF-7 NC cells incubated with 10 nM E2 (P = 0.026 and P =0.0011, respectively) (Figure 3B and 3C). The enhanced effect of SDCBP silencing on pS2 and PR transcription depended upon the presence of estrogen, as transcriptional levels were unaffected in the absence of estrogen (P = 0.847 and P = 0.413, respectively).
In contrast, the luciferase assay suggested that SDCBP overexpression in MCF-7 or T47D cells attenuated the estrogenic response compared to their MCF-7 and T47D Neo counterparts, respectively (Figure 3D and 3G). qRT-PCR showed that SDCBP overexpression resulted in downregulation of pS2 and PR in MCF-7 cells (51.6% and 28.1%,respectively) (Figure 3E and 3F) and T47D cells (33.7% and 19.8%, respectively) (Figure 3H and 3I) when incubated with 10 nM E2.
Clinical pathologic characters of ER-positive BCa cases and their correlations with SDCBP expression
Correlations between pathologic characters and SDCBP expression were examined in ER-positive BCa tissues (n =99). Among PR-negative tumors, 26.3% (5/19) demonstrated strong SDCBP staining (Table 1), while no tumors staining strongly positive for PR (0/26) showed strong positive staining for SDCBP (Table 1). Negative correlations between SDCBP expression and PR status or the PR/ERα ratio were also established (RS= –0.37, P < 0.001; and RS= –0.24, P =0.017, respectively) (Figure 4 and Table 1). This experiment also showed that SDCBP expression was negatively correlated with ERα (RS= –0.29, P = 0.004) (Table 1). There were no significant differences among the different levels of SDCBP staining in lymph node involvement and pTNM stage(Table 1). Detailed information for each case is shown in Table S4.
Discussion
Tamoxifen is the most commonly used chemotherapeutic agent for patients with ER-positive BCa26, and tamoxifen resistance poses great challenges to BCa treatment. Some patients have presented with intrinsic resistance regardless of showing high levels of ER, while other patients initially respond to tamoxifen but later develop acquired resistance27.
Our previous study showed that expression of SDCBP can be attenuated by estrogen23; in the present study, we found that silencing of SDCBP enhances the inhibitory effect of tamoxifen with regard to cellular proliferation and cell-cycle progression in ER/PR-positive MCF-7 cells. This indicates that SDCBP drives cell proliferation and cell-cycle progression by up-regulating self-expression and activating alternative signaling pathways when estrogen signaling is inhibited. Under conditions of SDCBP overexpression, the function of tamoxifen on cell proliferation was significantly attenuated, suggesting that SDCBP overexpression leads to tamoxifen resistance in ER-positive BCa. Notably, SDCBP silencing alone did not affect cell proliferation or the expression of molecules that control the cell cycle in ER-positive MCF-7 cells; however, SDCBP overexpressionaccelerated cellular proliferation in both the absence and presence of tamoxifen. This indicates that ER signaling counteracts some of the SDCBP-signaling lost in malignancy development.
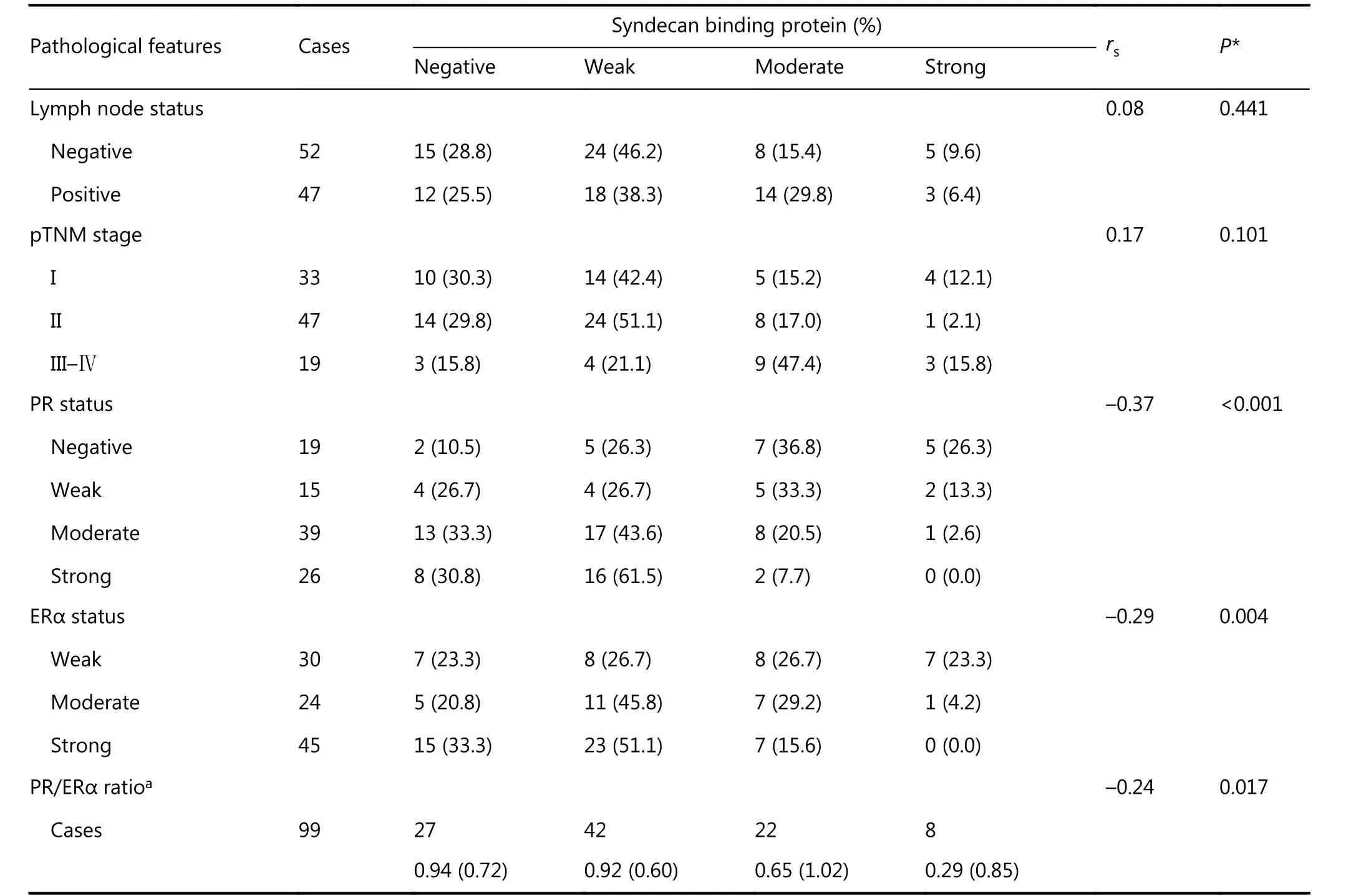
Table 1 Correlation of SDCBP expression with pathologic features in ER-positive breast cancers

Figure 4 ERα, PR, and SDCBP expression in ER-positive breast cancer tissue. Case 1: the sample was stained with high ERα and SDCBP but low PR (H&E staining, 200 ×, respectively). Case 2: the sample was stained with moderate ERα and low SDCBP but high PR (H&E staining, 200 ×, respectively). Scale bar = 50 μm.
In MCF-7 cells, under estrogen-signaling pathway activation, cellular proliferation and cell-cycle progression(including G1/S checkpoint-related regulators) showed no obvious changes when SDCBP expression was silenced by RNA interference. It has been reported that activated estrogen signaling can accelerate cell-cycle progression by limiting p21 expression, increasing phosphorylation levels of Rb protein, and promoting cyclin D1 expression28, all of which are consistent with our results. In contrast, SDCBP silencing further up-regulated the expression of p21 and p27 while down-regulating phosphorylation levels of Rb and expression of cyclin E in MCF-7 cells in the presence of tamoxifen, similar to the effects of SDCBP silencing alone in triple-negative BCa cell lines. Interestingly, SDCBP silencing can either exert its effect in the presence of tamoxifen (such as by promoting p27 expression and inhibiting cyclin E expression) or augment the effect of tamoxifen (such as by further promoting p21 expression and inhibiting phosphorylation levels of Rb), but SDCBP silencing does not change cyclin D1 expression markedly beyond that of tamoxifen alone. SDCBP overexpressing alone did not influence the levels of p21 but significantly down-regulated p27. Tamoxifen treatment did not recover the levels of p27,but up-regulated the levels of p21 under conditions of SDCBP overexpression. This indicates that when the estrogen signal is restrained, SDCBP not only partially substitutes for the estrogen signal, but also is involved in some other regulating mechanism(s) of cell-cycle progression. In the absence of tamoxifen, because SDCBP silencing up-regulates the estrogenic response as shown in Figure 3, the upregulation tendency of p21 and p27 may be counteracted by a larger estrogenic response; however, in the absence of tamoxifen, this counteraction was eliminated. This may partly explain why SDCBP silencing alone did not alter the levels of p21 and p27, while these levels were changed with tamoxifen. We also predicted that p21 levels depend more on the estrogen pathway than on SDCBP in ER-positive BCa cells; however, p27 levels may be closely related to the interaction between SDCBP and c-src as previously reported in triple-negative breast cancer cells24. In addition, SDCBP silencing enhances the effects of tamoxifen and may be useful as a targeted treatment in ER-positive BCa.
PR is an ER-regulated gene that mediates the effects of progesterone on the development of both the normal mammary gland and BCa29. Compared to ER/PR doublepositive BCa, patients with BCa who are ER-positive but PR-negative suffered a poorer prognosis and were more prone to developing resistance against endocrine treatment30-32. The 21-gene recurrence score assay (Oncotype DX?) is a multigene assay used to predict the recurrence of tamoxifentreated, node-negative BCa. In this scoring system, the ER group score is negatively correlated with cancer recurrence,and PR carries even more weight than ER in the ER group score33. The pS2 gene was originally identified as an estrogeninducible transcript in the human BCa cell line MCF-7 and was shown to be a direct target of ERs34,35. Our study showed that SDCBP silencing enhanced the estrogen response of MCF-7 cells and further elevated the expression levels of PR and pS2 in response to estrogen, while its overexpression had the opposite effect in both MCF-7 and T47D cells, indicating a role for SDCBP in suppressing estrogenic responses. As indicated by immunohistochemistry analysis, the expression level of SDCBP was negatively correlated with PR status and the PR/ERα ratio. This supports that SDCBP negatively regulates the estrogenic response and may play an important role in developing resistance to endocrine treatment in ER-positive BCa. These results also suggest that SDCBP silencing can be applied as a targeted treatment in ER-positive BCa.
In conclusion, SDCBP promotes cell cycle progression in ER-positive BCa, particularly when the estrogen-signaling pathway is blocked. It also negatively regulates the estrogen response in ER-positive BCa, but its underlying molecular mechanism of action and related signaling pathway(s)remain unclear. Silencing of SDCBP or its downstream signal(s) may improve the therapeutic effect of endocrine treatment in ER-positive BCa, particularly in cases of primary or secondary resistance.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81302292 to Xiaolong Qian,Grant No. 81702629 to Jun Zhang, Grant No. 81672636 and 81272358 to Feng Gu, Grant No. 81672637 to Li Fu). We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.S?rlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al.Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98: 10869-74.
2.Tokunaga E, Hisamatsu Y, Tanaka K, Yamashita N, Saeki H, Oki E,et al. Molecular mechanisms regulating the hormone sensitivity of breast cancer. Cancer Sci. 2014; 105: 1377-83.
3.Love RR, Philips J. Oophorectomy for breast cancer: history revisited. J Natl Cancer Inst. 2002; 94: 1433-4.
4.Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20: 596-604.
5.Higgins MJ, Stearns V. CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr Oncol Rep. 2010; 12: 7-15.
6.Pritchard KI. Endocrine therapy: is the first generation of targeted drugs the last? J Intern Med. 2013; 274: 144-52.
7.García-Becerra R, Santos N, Díaz L, Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: Focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci.2012; 14: 108-45.
8.Moy B, Goss PE. Estrogen receptor pathway: resistance to endocrine therapy and new therapeutic approaches. Clin Cancer Res. 2006; 12: 4790-3.
9.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009; 9: 631-43.
10.Dodwell D, Wardley A, Johnston S. Postmenopausal advanced breast cancer: options for therapy after tamoxifen and aromatase inhibitors. Breast. 2016; 15: 584-94.
11.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004; 11: 643-58.
12.Jordan VC, O'Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25: 5815-24.
13.Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions.J Med Chem. 2003; 46: 883-908.
14.Hutcheson IR, Knowlden JM, Madden TA, Barrow D, Gee JMW,Wakeling AE, Nicholson RI. Oestrogen receptor-mediated modulation of the EGFR/MAPK pathway in tamoxifen-resistant MCF-7 cells. Breast Cancer Res Treat. 2003; 81: 81-93.
15.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000; 60: 5887-94.
16.Lin JJ, Jiang H, Fisher PB. Characterization of a novel melanoma differentiation associated gene, mda-9, that is down-regulated during terminal cell differentiation. Mol Cell Differ. 1996; 4: 317-33.
17.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G,Dürr J, et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997; 94: 13683-88.
18.Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002; 277: 5699-702.
19.Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD, Lee JH. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002; 21: 4080-8.
20.Meerschaert K, Bruyneel E, De Wever O, Vanloo B, Boucherie C,Bracke M, et al. The tandem PDZ domains of syntenin promote cell invasion. Exp Cell Res. 2007; 313: 1790-804.
21.Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase Cα and the PDZ adapter protein mda-9/Syntenin. Cancer Res. 2010; 70: 1645-55.
22.Yang Y, Hong Q, Shi PC, Liu ZB, Luo JM, Shao ZM. Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res. 2013;15: R50
23.Qian XL, Li YQ, Yu B, Gu F, Liu FF, Li WD, et al. Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation.PLoS One. 2013; 8: e60046
24.Qian XL, Zhang J, Li PZ, Lang RG, Li WD, Sun H, et al. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP). PLoS One. 2017; 12: e0171169
25.Wu X, Zhu ZM, Li WD, Fu XY, Su D, Fu LY, et al. Chromodomain helicase DNA binding protein 5 plays a tumor suppressor role in human breast cancer. Breast Cancer Res. 2012; 14: R73
26.Ziauddin MF, Hua D, Tang SC. Emerging strategies to overcome resistance to endocrine therapy for breast cancer. Cancer Metastasis Rev. 2014; 33: 791-807.
27.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group).Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365: 1687-717.
28.Chen XM, Danes C, Lowe M, Herliczek TW, Keyomarsi K.Activation of the estrogen-signaling pathway by p21WAF1/CIP1in estrogen receptor-negative breast cancer cells. J Natl Cancer Inst.2000; 92: 1403-13.
29.Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003; 8: 205-14.
30.Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C,et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007; 25: 1239-46.
31.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM.Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003; 21: 1973-9.
32.Stendahl M, Rydén L, Nordenskj?ld B, J?nsson PE, Landberg G,Jirstr?m K. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res. 2006; 12: 4614-8.
33.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, nodenegative breast cancer. N Engl J Med. 2004; 351: 2817-26.
34.Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A,Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982; 10: 7895-903.
35.Berry M, Nunez AM, Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci USA. 1989; 86: 1218-22.
 Cancer Biology & Medicine2018年1期
Cancer Biology & Medicine2018年1期
- Cancer Biology & Medicine的其它文章
- Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences
- Post-irradiation pericardial malignant mesothelioma with deletion of p16: a case report
- Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer
- Modified Blumgart anastomosis without pancreatic ductto-jejunum mucosa anastomosis for pancreatoduodenectomy:a feasible and safe novel technique
- A new combined criterion to better predict malignant lesions in patients with pancreatic cystic neoplasms
- Parkin protein expression and its impact on survival of patients with advanced colorectal cancer
