Parkin protein expression and its impact on survival of patients with advanced colorectal cancer
Claudia Caroline Veloso da Silva-Camargo, Rosimeri Kuhl Svoboda Baldin, Nayanne Louise Costacurta Polli,Amanda Pereira Agostinho, Marcia Olandosk, Lúcia de Noronha,, Vanessa Santos SotomaiorGroup for Advanced Molecular Investigation (NIMA), School of Health and Biosciences, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba 805-90, Brazil; Hospital de Clínicas da Universidade Federal do Paraná (HC-UFPR), Curitiba 805-90, Brazil
Introduction
Colorectal cancer comprises neoplasms that affect the colon and rectum1. It is the third most prevalent cancer and the fourth leading cause of cancer-related deaths worldwide1,2.Although the natural history of this cancer is well understood, the 5-year survival rate after diagnosis is < 10%3.Patient prognosis is primarily based on TNM staging, which however, can be highly inaccurate4. Thus, there is a critical need for development of novel and more specific prognostic tools that can be used to improve both prognostic prediction and treatment. Understanding of the pathophysiological mechanisms that underlie this disease constitutes an important step toward this goal.
The Parkin RBR E3 ubiquitin protein ligase (PARK2) gene encodes the parkin protein, an E3 ubiquitin ligase5that induces the mono- and polyubiquitination of unfolded and damaged proteins to regulate their proteasomal degradation6-8,localization, or intracellular trafficking9-14. Parkin is also a key regulator of mitochondrial homeostasis15, with known roles in biogenesis, fusion/fission, mitochondrial DNA repair, and mitophagy16. It can also regulate cell cycle progression via the proteasomal degradation of cyclins D and E to repress G1/M phase transition17,18. Furthermore, parkin is capable of downregulating VEGFR2 (vascular endothelial growth factor receptor 2, encoded by KDR), thereby suppressing angiogenesis18.
Molecular changes affecting this gene have been described mostly in association with autosomal recessive Parkinsonism19; however, recent studies have shown its involve-ment in the tumorigenic process. Low PARK2 expression is often associated with positive lymph node metastasis and poor overall survival20, whereas PARK2 overexpression attenuates cell proliferation and primary tumor growth21,contributing to a prolonged survival7,8,17,18. In colorectal cancer specifically, PARK2 is generally responsible for cyclin E1 degradation22, and PARK2 haploinsufficiency cooperates with adenomatous polyposis coli (APC) gene mutations to accelerate adenoma progression and increased polyp multiplicity in mutant mice7.
In the present study, we investigate the involvement of PARK2 in the pathogenesis of colorectal adenocarcinoma by examining its protein expression status and association with clinicopathological parameters and expression of known proteins involved in colorectal cancer (MLH1, MSH2,MSH6, PMS2, APC, cyclin D1, cyclin E1, TP53, and Ki67),with the goal of identifying a prognostic biomarker that may be used in the development of improved therapeutic strategies.
Materials and methods
Study population and sample collection
This study was reviewed and approved by the Ethics Research Committee of the Hospital de Clínicas da Universidade Federal do Paraná (HC-UFPR) (Registration No. 820.432,informed consent was waived). Seventy-three colon adenocarcinoma samples from patients undergoing elective or emergency surgery from 2007 to 2011 were obtained from the Pathology Department of HC-UFPR. The patient population included both men and women (n = 34 and 39,respectively), aged >18 years. Patients with a history of familial adenomatous polyposis or individuals previously treated with radiotherapy or chemotherapy prior to surgical resection were excluded from our analyses. In addition, 55 matched adjacent normal tissue samples (1.7–56.0 cm away from the tumor site) and 9 non-neoplastic colorectal tissue samples collected from patients undergoing colectomy (for other reasons, including diverticular disease and endometriosis, but excluding inflammatory bowel disease)were also examined in this study.
Histopathological and clinical variables were obtained from patient files stored in the hospital database. The following clinicopathological variables were considered according to World Health Organization criteria23: sex, age(≤45 and >45, per Bethesda classification), tumor location(colon/rectum), tumor side (right colon/left colon or rectum), histological type (mucinous/not mucinous)24,degree of differentiation (less/moderately/well differentiated),angiolymphatic and/or perineural invasion, lymph node status, tumor staging (I–IV), tumor size (T1–T4), type of surgery (elective or emergency), follow-up (from diagnosis to last outcome consultation or death), and overall survival.
Included samples were also examined for protein expression (including parkin, APC, cyclin D1, cyclin E1,TP53, and Ki67) and markers of microsatellite instability(MSI) status (MLH1, MSH2, MSH6, and PMS2) for sample characterization and protein expression.
Tissue microarray (TMA) and immunohistochemistry (IHC)
TMA and IHC analyses were conducted at the Laboratory of Experimental Pathology of the Pontifícia Universidade Católica do Paraná (PUCPR). Hematoxylin and eosin slides were prepared from original paraffin blocks of samples from the patients with colorectal cancer to select areas for TMA construction. The areas selected included superficial (S) areas of the tumor, tumor bulk or intermediate (I) areas, and invasive front or profound (P) tumor areas. The selected tumor areas were isolated using a semi-automated method,and samples were spotted along with normal matched control tissues on the TMAs.
Fifteen TMAs were constructed: 3 samples (S, I, and P)from each of 73 patients were spotted on one of 11 TMAs, 55 samples of matched adjacent normal tissue were spotted on one of 3 TMAs and 9 samples of non-neoplastic colorectal tissue were spotted on one TMA.
For IHC, the TMA slides were subjected to antigen retrieval with Target Retrieval Solution (Dako, Glostrup,Denmark) and then incubated with the following monoclonal antibodies: anti-parkin antibody (mouse, 1:100;Abcam, Cambridge, UK), anti-MLH1 antibody (rabbit,prediluted; Abcam), anti-MSH2 antibody (rabbit, prediluted;Abcam), anti-APC antibody (mouse, 1:200; Abcam), anticyclin D1 antibody (rabbit, 1:100; Abcam), anti-cyclin E1 antibody (rabbit, 1:200; Abcam), anti-protein P53 antihuman (mouse, 1:200; Spring Bioscience Corp., Pleasanton,CA, USA), anti-MSH6 antibody (mouse, prediluted; Spring Bioscience Corp.), anti-PMS2 (mouse, prediluted; Spring Bioscience Corp.), and anti-Ki67 antibody (mouse, 1:150;Dako). Subsequently, disclosure polymer (Spring Bioscience Corp.) was used as a secondary antibody. Slides were incubated with diaminobenzidine complex and substrate and then counterstained with Harris hematoxylin. Positive and negative controls were included in the IHC analysis for each antibody.
Morphological analysis of protein expression
Parkin expression morphology was examined using an Olympus microscope CH30. Expression of parkin in tumor and normal colorectal tissues was classified by cell location(nuclear, basal cytoplasm, or apical cytoplasm) and by the percentage of immunoreactivity; MLH1, MSH2, MSH6,PMS2, and TP53 protein expression were evaluated visually and classified as positive or negative based on nuclear immunostaining, with any detectable staining scored as positive (using the Allred scoring system for breast cancer;Allred score > 1 was considered as positive) or negative based on nuclear immunostaining25.
For the morphometric analysis, representative images of parkin and APC immunostaining for each of the three tumor regions and the matched normal colorectal tissue were captured with a BX40?Olympus microscope equipped with a 40× objective and a Dino Eye?camera using DinoCapture 2.0 software (Figure 1). Images were optimized in Adobe Photoshop CS6 v 13.0 software by removing the stroma,mucin lakes, and white areas. The remaining regions of interest were then analyzed in Image Pro Plus?software with the color morphometric tool (Figure 1) by high-power field(HPF). Cyclin D1, cyclin E1, and Ki67 expression was determined by counting 100 cells in hot spot areas and calculating percentage (following breast cancer guidelines).Positive staining was defined as nuclear immunoreactivity,regardless of the intensity25.
Statistical analysis
Statistical analysis was performed using the SPSS Statistics v.20 software. To compare clinicopathological and quantitative variables, Student’s t-test, analysis of variance(ANOVA), and the nonparametric Kruskal-Wallis test were used to identify significant differences between two or more groups, respectively. The degree of association between two variables was evaluated by Pearson correlation coefficients.Median survival was determined by Kaplan-Meier analysis with log-rank testing. Cox regression and Wald testing were used for the multivariate analysis. Data represent the mean ±standard deviation (SD). P < 0.05 was considered statistically significant.
Results
The descriptive statistics for the patient population and clinicopathological parameters are shown in Tables 1–3.Patients had a median age of 61 years (range, 18–90).
IHC analysis of MSI markers (MLH1, MSH2, MSH6,PMS2, and P53) showed positive expression in the majority of cases (Table S1). Parkin and APC immunoreactivity in the different tumor regions showed higher expression of both in the intermediate areas, and higher expression of parkin but lower expression of APC in non-neoplastic mucosa (Table 2).
No correlation was observed between parkin subcellular localization and any of the clinicopathological variables evaluated; however, higher levels of parkin protein were found in the cytoplasm of tumor samples, whereas higher levels were localized in the nuclei in normal cells. Moreover,increased parkin expression was significantly associated with tumors in men (P = 0.019, 0.049), tumors of the mucinous type (P = 0.028), and tumors at higher stages (III + IV) (P =0.041) (Table 3).
No correlation was found between parkin immunoreactivity and other clinicopathological variables, such as age,tumor location, tumor side, degree of differentiation,angiolymphatic and/or perineural invasion, lymph node status, tumor size, or type of surgery.
Parkin expression was also not associated with MLH1,MSH2, MSH6, PMS2, cyclin D1, cyclin E1, or Ki67 immunoreactivity (data not shown). However, a significant association was observed between parkin and APC expression; a positive correlation between the expression of these proteins was observed in the superficial, intermediate,and profound regions of all colorectal tumors (ρ = 0.37, P =0.001) and in non-neoplastic mucosa (ρ = 0.30, P = 0.032).
To determine whether any of the clinicopathological variables had a significant effect on patient overall survival,patients were classified into two groups based on a cutoff survival time of 5 years. Eleven patients with censored survival who were followed for < 5 years were excluded from the analysis; therefore, our sample population consisted of 20 and 42 patients with survival times of < 5 years and ≥ 5 years,respectively.
Independent variable analysis revealed no correlation between overall patient survival and any of the clinicopathological variables evaluated.
In relation to parkin localization, there was no correlation between parkin subcellular localization and patient survival.However, there was a significant correlation between survival and parkin expression in the isolated tumor regions. Notably,patients with survival of ≥ 5 years exhibited high levels of parkin expression (22.4%, HPF) in the profound tumor region when compared to those with survival of < 5 years(12.2%, HPF; P = 0.019).

Figure 1 Parkin and APC expression (IHC staining, 40 x). (A) Parkin expression in colorectal adenocarcinoma. (B) Parkin expression in nonneoplastic mucosal samples. (C) APC immunoexpression in colorectal adenocarcinoma. (D) APC immunoexpression in non-neoplastic mucosal samples. (E) Adobe Photoshop CS6 software v 13.0?-optimized image. (F) Image submitted to the mask for morphometric analysis.

Table 1 Clinicopathological characterization of colorectal cancer patients (n = 73)
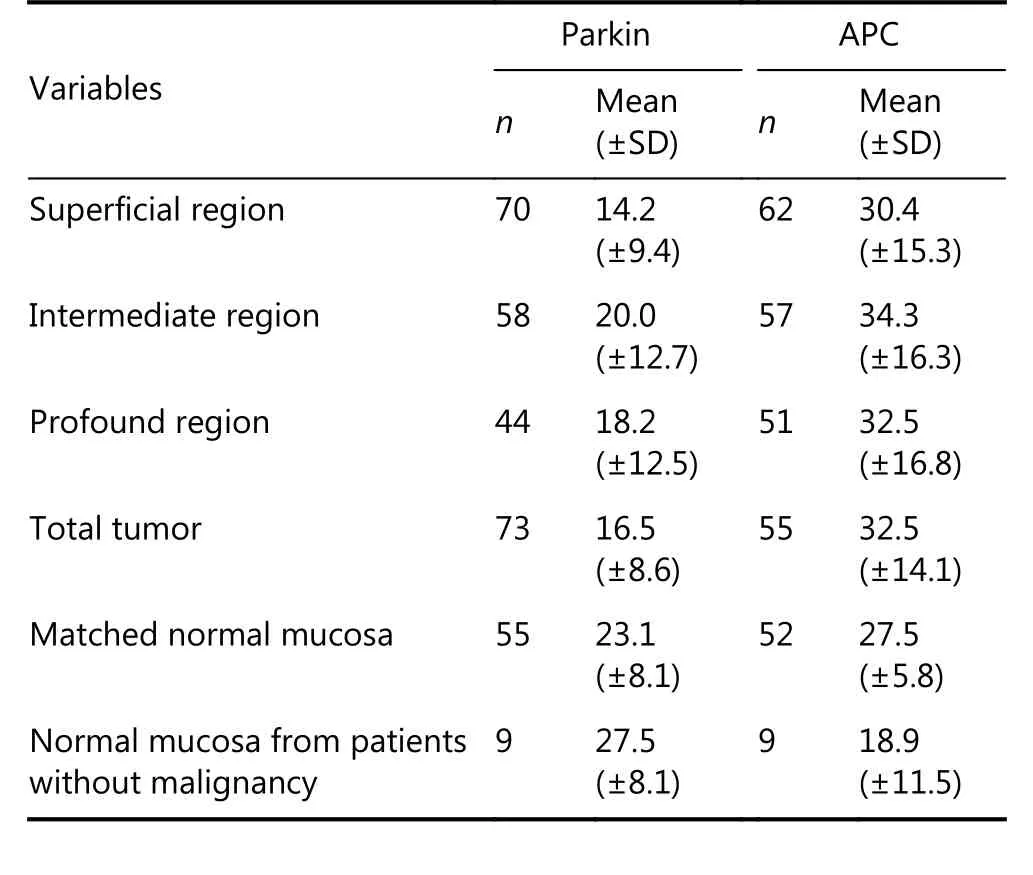
Table 2 Parkin expression in colorectal cancer (n = 73) and normal samples (n = 55)
In addition, hazard ratio (HR) analysis of the association between parkin expression in the profound tumor region and survival was performed using a 10% cutoff to define the area of parkin expression (Figure 2). Results of this analysis indicated a significant difference in survival for patients with> 10% or without (≤ 10%) parkin expression [P = 0.025; HR,4.98; 95% confidence interval (CI), 1.48–16.68]. Multivariate analysis factoring parkin expression in the profound tumor region and the clinicopathological variables showed no association (Table 4), indicating the independent prognostic value of parkin expression in these tumor areas.
Discussion
In the present study, we demonstrated lower parkin protein expression levels in localized colon tumors compared to adjacent normal tissue. These levels appeared to increase in advanced colorectal adenocarcinomas and to be an independent predictor of increased survival. As PARK2 is a putative tumor suppressor gene17, one would expect its expression to be lower in advanced cases; however, our data demonstrated that higher parkin protein levels in the deep tumor region correlated with prolonged survival, suggesting that parkin may play a protective or inhibitory role in tumorigenesis7,8,18,22,26-35and may have prognostic value in patients with advanced disease in cases when the tumor has reached the deep region.
In fact, multivariate Cox regression analysis indicated that parkin expression in the deep region is an independent predictor of patient survival. Consistent with our data, Yeo et al.18demonstrated that parkin pathway activation could be a favorable predictor of prognosis, a finding that is furthersupported by its proposed inhibitory actions on cell cycle progression and angiogenesis through regulation of cyclin D and VEGFR2 expression, respectively22. Although no association was found between parkin and cyclin D1/cyclin E1 expression in the present study, this mechanism of action may facilitate the formation of aberrant polyps, or contribute to other processes that occur in later stages of the disease. We also found a correlation between higher expression of both APC and parkin proteins in tumor and normal tissue.Previous data showed that APC mutation and parkin deletion cooperate to accelerate progression of colorectal adenocarcinoma7. It has been suggested that initial APC suppression can influence a subsequent change in parkin expression; this may be another mechanism by which the protein could be involved in cancer pathophysiology.However, while the results found in the present studyconfirm the correlation between APC and parkin proposed in the previous study, we observed high expression of the proteins, and we cannot confirm the occurrence or lack of mutation or the mechanism of the correlation.

Table 3 Association of parkin expression with gender, histological type, and tumor staging
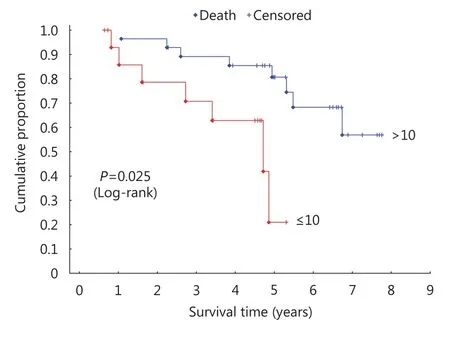
Figure 2 Kaplan-Meier survival curves for parkin expression in the profound tumor region.
Table 4 Cox regression model of parkin mean expression in the profound tumor region and its association with clinicopathological variables
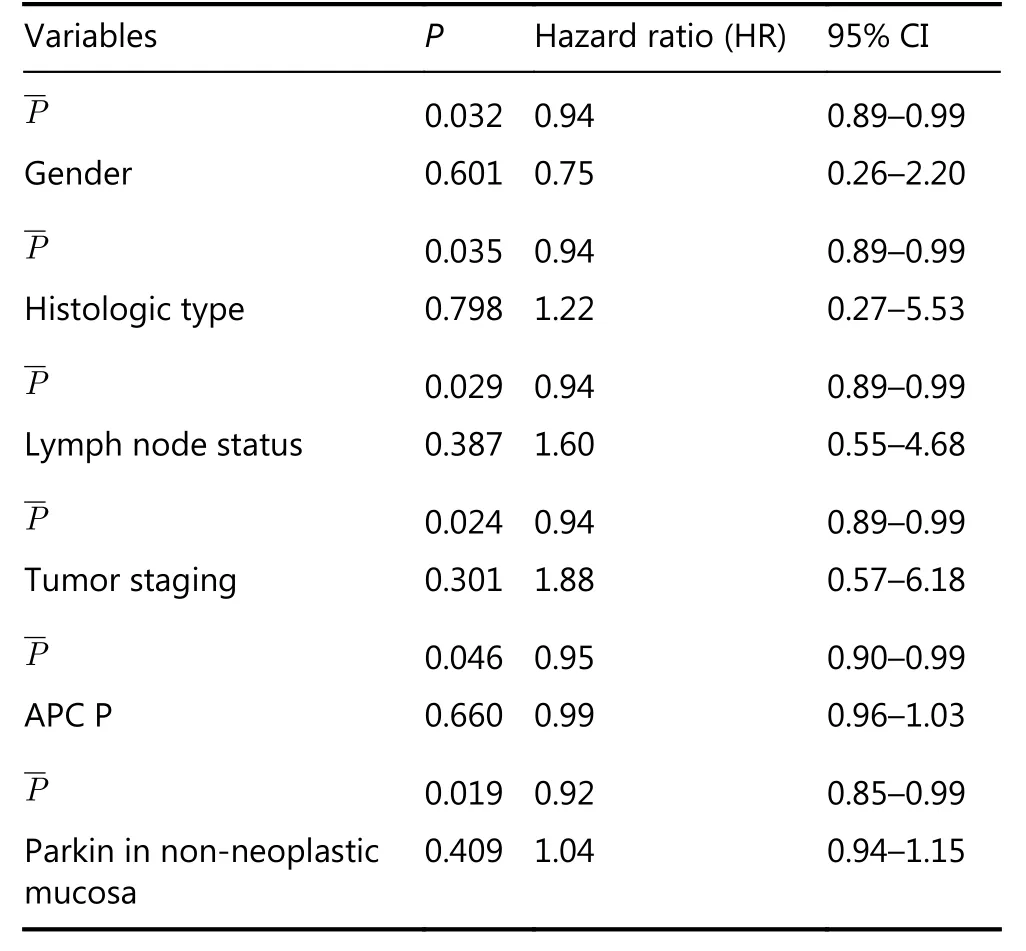
Table 4 Cox regression model of parkin mean expression in the profound tumor region and its association with clinicopathological variables
Our analysis of parkin subcellular localization revealed more pronounced nuclear and cytoplasmic expression in normal tissue and adenocarcinomas, respectively, suggesting that the protein may play a functional role in the cytoplasm specifically in colorectal cancer, such as by acting as a regulator of mitochondrial homeostasis and/or mitophagy.Mitochondria function to produce cellular energy and are thus required for cell proliferation. Tumor cells are often subject to changes in mitochondrial DNA and metabolism to support their accelerated proliferation36. As such, these cells exhibit increased DNA damage, oxidative stress, and chromosomal instability owing to the accumulation of dysfunctional mitochondria and deficiencies in mitophagy,which usually is associated with poor prognosis in patients with colorectal cancer4. Similarly, mitophagy also plays an important role in the induction of cellular senescence37.Moreover, parkin plays a major role in controlling mitophagy, in which mitochondrial alterations can lead to membrane depolarization and PINK1 accumulation to facilitate the mitochondrial recruitment of parkin15,38,39.Gong et al.40demonstrated that parkin has an important role in regulating apoptosis mechanisms through the control of BLC-XL protein stability, ubiquitinating this protein for degradation via the proteasome. Thus, when PINK1 recruits parkin, it regulates mitochondrial outer membrane permeability and apoptosis by controlling the stability of BCL-XL protein. As parkin can elicit mitophagy15,38,39,PINK1, parkin, and BCL-XL may act cooperatively to repair slightly damaged mitochondria or promote the synthesis of new mitochondria when necessary39,41, and consequently,parkin can promote apoptotic activity. The degradation of damaged mitochondria contributes to improved survival in colorectal cancer cells42; thus, based on the literature, parkin could function similarly by inducing mitophagy and thereby controlling cell proliferation and apoptosis. This may be the explanation for which patients with higher parkin levels have a longer survival than others who are also in the more advanced stages of colorectal cancer; it may be that parkin interacting with BCL-XL is increasing the apoptosis of tumor cells and consequently the survival of patients.
A multiple-level validation identifies that PARK2 has antiinflammatory functions, and loss of PARK2 maintains higher expression of cytokines for inflammation. PARK2 suppresses NF-κB activation through ROS/Akt regulation in both E3 ligase-dependent and -independent manners43. Tran et al.44demonstrated that parkin protein and mRNA are detectable in peripheral macrophages. Other E3 ubiquitin ligases are described as having a role in the immune response, such as natural killer lytic-associated molecule (NKLAM), which is expressed in macrophages and natural killer (NK) cells.Macrophage NKLAM expression is driven by proinflammatory cytokines; therefore, considering that parkin is an E3 ubiquitin ligase, it may also have a similar function to NKLAM. Moreover, parkin can be regulated by lipopolysaccharide, a cell membrane component in gram-negative bacteria45, and PARK2 polymorphisms are associated with an increased susceptibility to infection46. For instance, mice and flies with disruptions in parkin function are sensitive to intracellular bacterial infections, suggesting that parkin plays a protective role in cellular immunity47. The recognition of tumor antigens involves multiple immune cell types and molecules, and activated macrophages, NK cells, and CD4+and CD8+ T cells, as well as tumor-specific peptides and immunoglobulins29. It is known that patients with tumors that contain cellular infiltrates showing these molecules present a better prognosis48. Thus, the protective function of parkin could be attributed to its involvement in immune cell recruitment; however, based on the current literature, parkin is more closely linked to other mechanisms, such as cyclin degradation.
In summary, our results suggest that parkin expression analysis could be an independent prognostic marker of survival, used after surgery in combination with classic prognostic factors such as TNM staging to improve the accuracy of survival prediction in patients with advanced colorectal cancer. A prospective study including a larger patient population with a diverse genetic background, with well annotated clinical data and long-term follow-up, would be critical to confirm the prognostic value of parkin protein expression across distinct cohorts of patients with colorectal cancer. In any event, our data provide the groundwork and a promising foundation for increased accuracy of clinical prognosis and for the continued development of cancer therapies and personalized treatment strategies for this disease.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.INCA (Internet). Ministério da Saúde. Colorretal. 2016. (Accessed January 17, 2016) Available from:http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/hom e/colorretal/definicao+
2.International Agency for Research on Cancer (Internet). Estimated incidence, mortality and prevalence worldwide in 2012. (Accessed Juny 10, 2014), Available from:http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63: 11-30.
4.Yang MP, Zhao H, Guo L, Zhang QY, Zhao L, Bai SP, et al.Autophagy-based survival prognosis in human colorectal carcinoma. Oncotarget. 2015; 6: 7084-103.
5.Gorgoulis VG, Vassiliou LVF, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434: 907-13.
6.Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, et al.PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005; 24: 1477-80.
7.Poulogiannis G, McIntyre RE, Dimitriadi M, Apps JR, Wilson CH,Ichimura K, et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci USA. 2010; 107: 15145-50.
8.Veeriah S, Taylor BS, Meng SS, Fang F, Yilmaz E, Vivanco I, et al.Somatic mutations of the Parkinson’s disease–associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet.2010; 42: 77-82.
9.Doss-Pepe EW, Chen L, Madura K. α-synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem. 2005; 280: 16619-24.
10.Fallon L, Bélanger CML, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006; 8: 834-42.
11.Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O.Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin – protein ligase with monoubiquitylation capacity. Hum Mol Genet. 2006; 15: 2059-75.
12.Lim KL, Chew KCM, Tan JMM, Wang C, Chung KKK, Zhang Y,et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005; 25: 2002-9.
13.Sakata E, Yamaguchi Y, Kurimoto E, Kikuchi J, Yokoyama S,Yamada S, et al. Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 2003;4: 301-6.
14.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RLA, Kim J,et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010; 107: 378-83.
15.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008; 183: 795-803.
16.Chen H, Chan DC. Mitochondrial dynamics-fusion, fission,movement, and mitophagy-in neurodegenerative diseases. Hum Mol Genet. 2009; 18: R169-76.
17.Tay SP, Yeo CWS, Chai C, Chua PJ, Tan HM, Ang AXY, et al.Parkin enhances the expression of cyclin-dependent kinase 6 and negatively regulates the proliferation of breast cancer cells. J Biol Chem. 2010; 285: 29231-8.
18.Yeo CW, Ng FS, Chai C, Tan JM, Koh GR, Chong YK, et al. Parkin pathway activation mitigates glioma cell proliferation and predicts patient survival. Cancer Res. 2012; 72: 2543-53.
19.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y,Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998; 392: 605-8.
20.Sun XD, Liu M, Hao JH, Li DW, Luo YG, Wang XC, et al. Parkin deficiency contributes to pancreatic tumorigenesis by inducing spindle multipolarity and misorientation. Cell Cycle. 2013; 12:1133-41.
21.Wang F, Denison S, Lai JP, Philips LA, Montoya D, Kock N, et al.Parkin gene alterations in hepatocellular carcinoma. Genes Chromosomes Cancer. 2004; 40: 85-96.
22.Ikeuchi K, Marusawa H, Fujiwara M, Matsumoto Y, Endo Y,Watanabe T, et al. Attenuation of proteolysis-mediated cyclin E regulation by alternatively spliced Parkin in human colorectal cancers. Int J Cancer. 2009; 125: 2029-35.
23.Bonhin RG, de Carvalho GM, Guimar?es AC, Chone CT, Crespo AN, de Almeida Milani Altemani AM, et al. Histologic correlation of expression of Ki-67 in squamous cell carcinoma of the glottis according to the degree of cell differentiation. Braz J Otorhinolaryngol. 2014; 80: 290-5.
24.Hugen N, Brown G, Glynne-Jones R, de Wilt JHW, Nagtegaal ID.Advances in the care of patients with mucinous colorectal cancer.Nat Rev Clin Oncol. 2016; 13: 361-9.
25.Love RR. On the road to precision, there is more practical medicine to be implemented. Breast. 2016; 29: 188-91.
26.Blenkinsopp WK, Stewart-Brown S, Blesovsky L, Kearney G,Fielding L P. Histopathology reporting in large bowel cancer. J Clin Pathol. 1981; 34: 509-13.
27.Chew MH, Yeo SAE, Ng ZP, Lim KH, Koh PK, Ng KH, et al.Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010; 25: 1221-9.
28.Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM,et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012; 19: 2814-21.
29.Chen JX, Tang XD, Xiang DB, Dong XL, Peng FY, Sun GY. TNM stages and prognostic features of colorectal and mucinous adenocarcinoma patients: a meta analysis. Asian Pac J Cancer Prev.2012; 13: 3427-30.
30.Chen JS, Chen KT, Fan CW, Han CL, Chen YJ, Yu JS, et al.Comparison of membrane fraction proteomic profiles of normal and cancerous human colorectal tissues with gel-assisted digestion and iTRAQ labeling mass spectrometry. FEBS J. 2010; 277: 3028-38.
31.Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012; 65: 381-8.
32.Nitsche U, Zimmermann A, Sp?th C, Müller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013; 258: 775-83.
33.Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E,Abeliovich A. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from Kainate excitotoxicity. Neuron. 2003; 37: 735-49.
34.Gong YX, Zack TI, Morris LGT, Lin K, Hukkelhoven E, Raheja R,et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet. 2014; 46: 588-94.
35.Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H,et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25–q27. Proc Natl Acad Sci USA. 2003; 100:5956-61.
36.Ussakli CH, Ebaee A, Binkley J, Brentnall TA, Emond MJ,Rabinovitch PS, et al. Mitochondria and tumor progression in ulcerative colitis. J Natl Cancer Inst. 2013; 105: 1239-48.
37.Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW.Implications of cellular senescence in tissue damage response,tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008; 73: 513-22.
38.Geisler S, Holmstr?m KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010; 12: 119-31.
39.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al.PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010; 189: 211-21.
40.Gong YX, Schumacher SE, Wu WH, Tang FY, Beroukhim R, Chan TA. Pan-cancer analysis links PARK2 to BCL-XL-dependent control of apoptosis. Neoplasia. 2017; 19: 75-83.
41.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA.Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014; 33:282-95.
42.Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP,MacCoss MJ, et al. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci USA. 2013; 110: 6400-5.
43.Lee SB, She J, Deng B, Kim J, de Andrade M, Na J, et al. Multiplelevel validation identifies PARK2 in the development of lung cancer and chronic obstructive pulmonary disease. Oncotarget. 2016; 28:44211-23.
44.Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee J, Tansey MG.Lipopolysaccharide and Tumor Necrosis Factor Regulate Parkin Expression via Nuclear Factor-Kappa B. PLoS One. 2011; 6: e23660
45.Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. 4th ed. Philadelphia: Saunders, 2000.
46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420: 860-7.
47.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS,et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013; 501: 512-6.
48.Zitvogel L, Casares H, Péquignot MO, Chaput N, Albert ML,Kroemer G. Immune response against dying tumor cells. Adv Immunol. 2004; 84: 131-79.Cite this article as:da Silva-Camargo CCV, Svoboda Baldin RK, Costacurta Polli NL, Agostinho AP, Olandosk M, de Noronha L, et al. Parkin protein expression and its impact on survival of patients with advanced colorectal cancer. Cancer Biol Med. 2018; 15: 61-9. doi: 10.20892/j.issn.2095-3941.2017.0136
 Cancer Biology & Medicine2018年1期
Cancer Biology & Medicine2018年1期
- Cancer Biology & Medicine的其它文章
- Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences
- Post-irradiation pericardial malignant mesothelioma with deletion of p16: a case report
- Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer
- Modified Blumgart anastomosis without pancreatic ductto-jejunum mucosa anastomosis for pancreatoduodenectomy:a feasible and safe novel technique
- A new combined criterion to better predict malignant lesions in patients with pancreatic cystic neoplasms
- Calcium channel α2δ1 subunit as a novel biomarker for diagnosis of hepatocellular carcinoma
