Experimental Study of the Absorption and Regeneration Performance of Several Candidate Solvents for Post-Combustion CO2 Capture
Gao Jie; Chen Xin; Tong Ming; Kang Wanzhong; Zhou Yanbo; Lu Jun
(1. Key Laboratory of Coal Gasi fi cation and Energy Chemical Engineering of Ministry of Education,East China University of Science & Technology, Shanghai 200237;2. SINOPEC Ningbo Engineering Co., Ltd., Ningbo 315103)
1 Introduction
In recent years, because of the gradual increment of greenhouse gases, such as carbon dioxide (CO2), methane,nitrous oxide and chlorofluorocarbons, the climate change and global warming will be the most serious environmental issues human beings must face[1]. In China,about 50% of the CO2emission comes from coal-fired power plants and it will not change for a long time due to the important role of coal in China’s energy structure.Hence, it becomes a crucial issue to develop improved CO2capture technologies. A literature review of CO2capture technologies indicates that a variety of methods are under investigation, including the chemical absorption(such as by means of amine compounds), the physical adsorption and the membrane separation[2-3]. Among these technologies, the absorption method using chemical solvents is considered to be the most favorable technology for application in coal fired power plants since it is suitable for capturing the CO2concentration which is as low as 15% in the flue gases, and it has high capture efficiency,high selectivity and scale-up feasibility[4]. But a major obstacle hindering the application of chemical absorption is the high capital cost and a considerable amount of energy losses of up to approximately 10% in commercial coal fired power plants[5]. Many improvements using the standard 30% MEA-H2O adopted in this process have been made such as intercooling and lean vapor compression, and the energy required for it is still high[6]. So it is essential to find more efficient solvents, which are considered to have fast reaction kinetics, high absorption capacity,low regeneration energy consumption, high stability andlow corrosiveness to secure the economic feasibility of chemical absorption processes[7-8].
Numerous studies on chemical absorption phenomena with alkanolamines such as MEA, DEA, AMP, TEA,MDEA and their related solvents have been developed for CO2capture processes[9-10]. Recently the hybrid solvents which are formed by blending chemical solvents and physical solvents have been proposed for CO2absorption such as aniline in ethanol, 3-amino-1-propanol in methanol and ethanol. These studies showed that the hybrid solvents have significant advantages over the corresponding chemical solvents[11-12]. Though a variety of hybrid solvents for CO2capture have been studied, such as aniline/cyclohexamine/hexamine-ethanol,ethylenediamine, 3-amino-1-propanol in methanol and ethanol, piperazine (PZ)-diethylene glycol (DEG),diethanolamine (DEA)-ionic liquid([hmim][Tf2N]), there is still not a systematic comparison of different solvents on their absorption and regeneration performance and physical properties.
In this work, a hybrid solvent MEA-methanol was studied and evaluated in a semi-batch bubbling reactor in comparison with aqueous solvents composed of MEA, DEA, and TEA.Also the physical properties, absorption performance and regeneration performance of aqueous MEA and MEA-methanol with different concentrations were studied. A 30%MEA-methanol solvent was studied in a pilot plant CO2capture test bed and compared with 30% MEA. The results of this study could provide a basic method to compare different solvents for CO2capture and select appropriate solvents candidates for application in pilot plants.
2 Theories
2.1 Mechanism
The reactions of CO2with MEA, DEA, TEA have been well-established by other researchers[13-14]. And the reaction of CO2with MEA-methanol can be explained by zwiterion mechanism. The reaction involves two steps. First step is the formation of the zwitterions and the second step is the deprotonation of the zwitterions (in which AmH denotes amines).

2.2 Data analysis
2.2.1 Mass transfer coefficient (KGav)
Mass transfer occurs when a component A in a gas phase transfers across a gas-liquid interface into a liquid phase.The mass fl ux of component A (NA) at a steady state can be represented in terms of the gas-side mass transfer coefficient (kG), the total pressure (P), and the gas phase driving forces (yA-yA,i):

The mass flux can also be expressed in terms of the overall mass transfer coefficient (KG) and the equilibrium mole fraction of component A in gas phase (yA*) as follows:

Then, KGcan be expressed as follows:

In a gas absorption apparatus such as a packed column,it is more useful to represent the rate of absorption in terms of the volumetric overall mass transfer coefficients,represented by the term KGav, instead of the one based on the interfacial area unit because the gas-liquid interfacial area cannot be measured accurately[15]. Therefore, the mass-transfer coefficient based on the unit volume of the absorption column is expressed as follows:

Upon considering that the absorption takes place in any height element (dz), the overall differential mass balance can be given as follows:
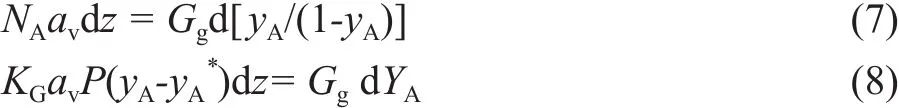
In which YArepresents the mole ratio of component A in the bulk gas and Ggrepresents the molar fl ow rate of inert gas. The final equation that determines the overall mass transfer coefficient, KGav, can then be defined as follows:

2.2.2 Regeneration energy
The regeneration energy for the CO2capture facility is mainly composed of three components, namely the solvent heating energy, the water vaporization energy, and the desorption energy. More precisely, it is composed of the heat required for evaporating the water, the sensible heat to heat up the solvent to reboiler temperature (Qsh)and the heat of CO2desorption (Qdes). The heat required for evaporating the water is actually equivalent to the latent heat of water condensation which can be measured directly around the condenser (Qcond) as shown below:
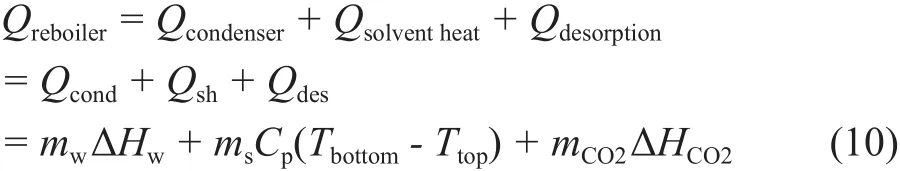
where Qrebotleris the reboiler heat duty; mwis the amount of water fl owing into the condenser; ΔHwis the latent heat of water condensation; msis the solvent fl ow rate; cpis the heat capacity of the solvent; Tbottomis the temperature of hot lean solvent going out from the bottom of stripper column; Ttopis the temperature of hot rich solvent entering the top of stripper column; mCO2is the amount of CO2produced from the stripper column, and ΔHCO2is the enthalpy of CO2desorption.
3 Results and Discussion
3.1 Comparison of MEA, DEA TEA and MEA-methanol solvents
In order to compare the properties of different solvents,10% MEA-H2O, 10% DEA-H2O, 10% TEA-H2O, and 10% MEA-methanol were studied in the absorption and regeneration apparatus, respectively.
3.1.1 Absorption properties
Figure 1 shows the absorption rate (a), absorption capacity (b), and CO2capture efficiency (c) curves of different solvents. It can be observed that at the beginning of the reaction, the CO2absorption rate of MEA-methanol solvent is the highest, and that of MEA is the second,while that of DEA is the third, with that of TEA being the lowest. And the reaction rate of MEA-methanol solvent is higher than that of MEA solvent because of the following reasons: (1) The density of methanol is smaller than water,which leads to a faster mass transfer. (2) The solubility of CO2in methanol is higher than in water, which leads to a higher absorption capacity of CO2[16-17]. As the reaction time prolongs, the absorption rates of all solvents decrease until saturation. After 45 min, the slope of the curve of the MEA-methanol solvent decreases rapidly than other solvents and at 60 min, the reaction rate of the MEA-methanol solvent is less than aqueous solvent of MEA, since methanol cannot restore carbamate to MEA to absorb more CO2as the water does, which can also be validated by the curves of CO2absorption capacity[18]. The curves of CO2capture efficiency are similar to the curves of absorption rate. Also it can be seen that within 45 min,the CO2capture efficiency of MEA-methanol solution is above 90 %, which is perfect in terms of CO2capture.And the CO2capture efficiency of DEA and TEA solvents is too low to be applicable in the industry.
Therefore, the CO2absorption property of the MEA-methanol solvent is better than MEA, DEA, and TEA solvents within a definite reaction time.
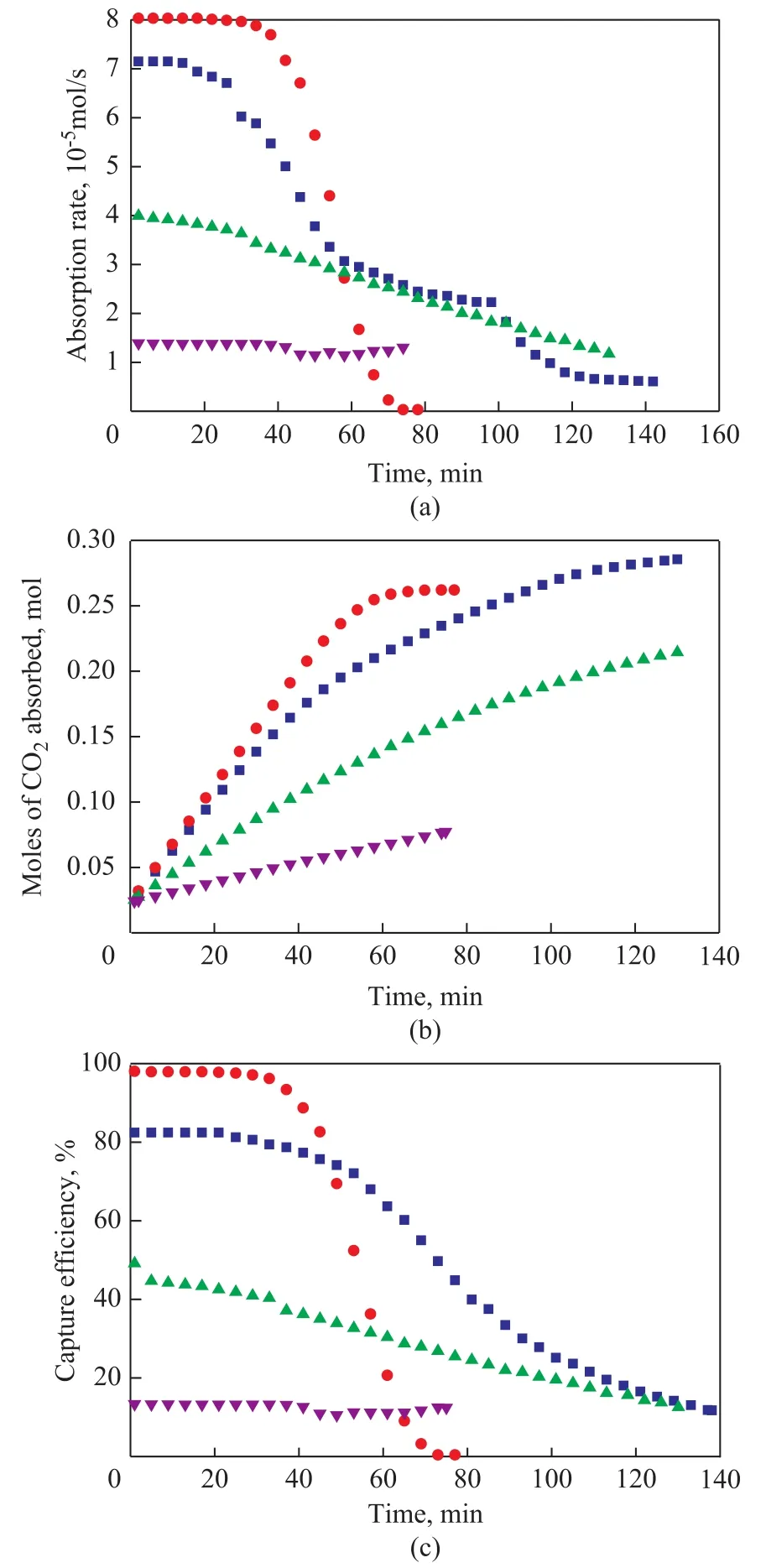
Figure 1 CO2 absorption rate (a), CO2 absorption capacity(b), and CO2 capture efficiency (c)■—10 % MEA; ●—10 % MEA(Methanol);▲—10 % DEA; ▼—10 % TEA
3.1.2 Regeneration properties
The regeneration temperature is directly associated with the energy required for regenerating the solvents and is an important factor for selecting solvents[19]. Figure 2(a)shows the regeneration rates of different solvents with the change in regeneration temperature. It is observed that at a low temperature of 47oC, the regeneration process of the MEA-methanol solvent starts firstly, and its maximum regeneration rate is achieved at 60oC.However, the maximum regeneration rate of MEA, DEA and TEA solvents is achieved at 90oC, 90oC and 96oC,respectively. Therefore, the MEA-methanol solvent requires a relatively low regeneration energy as compared to other solvents[20].
Due to the recycled use of solvents in the industry, the cyclic absorption capacity becomes a very important factor for evaluating a solvent suitable for the industrial use. The CO2absorption capacity of the fresh solvents( first time), the solvents after the first time of regeneration(second time), and the solvents after the second time of regeneration (third time), which are tested in the same reaction time, are studied in this work. The results are shown in Figure 2(b). Based on these data, we can know that the cycle performance of the TEA solvent is the best and that of the MEA solvent is the worst, and the cycle performance of the MEA-methanol solvent is better than that of the MEA solvent.
In conclusion, the MEA-methanol solvent gives an advantage both on the performance of absorption and regeneration as compared to other solvents.
3.2 Comparison of MEA and MEA-methanol solvents in a bubbling reactor
3.2.1 Densities and viscosities
It can be learned from the above-mentioned data that the MEA-methanol solvent has better absorption and regeneration properties as compared to the MEA solvent.But to adopt it into an industrial-scale CO2capture process, a proper concentration of the solvent with good CO2capture performance coupled with low density and viscosity should be determined.

Figure 2 CO2 regeneration rates of different solvents with the change in regeneration temperatures (a), and CO2 cyclic absorption capacity of different amines (b)(a)—10 % MEA; ●—10 % MEA(Methanol);▲—10 % DEA; ▼—10 % TEA(b)— first time; —second time; —third time
The overall assessment of a solvent for CO2capture requires the knowledge of its physical properties. Hence the density and viscosity data are very important physical properties which are related to the pumping energy cost for circulating the solvent in the absorption-regeneration system.
The density and viscosity of lean and rich MEA solvents at 40oC and MEA-methanol solvents at 25oC with different MEA concentration ranging from 10% to 30% are measured. The results are shown in Table 1.Generally, the density and viscosity of the solvent are found to increase with the increase of concentration of amine and load of CO2. And it can be seen that even at a low temperature, the density of the MEA-methanol solvent is much lower than that of MEA at the same amine concentration. On the other hand, the viscosity of the MEA-methanol solvent is higher than the MEA solvent at the same amine concentration, showing that the temperature has a significant effect on the viscosity.Since a high viscosity will lead to a high operating cost,an appropriate solvent concentration should be used.Compared to 30% MEA-H2O solvent, which is a standard solvent used in industry, the 30% MEA-methanol solvent has a higher absorption rate and a lower density achieved at a lower reaction temperature, which will save a lot of energy.

Table 1 Density and viscosity of MEA and MEA-methanol solvents
3.2.2 Kinetics of CO2capture by MEA-methanol and aqueous MEA
(1) Physicochemical properties
The solubility of CO2in aqueous MEA solvent is well established. And it shows that the solubility of CO2does not depend on the MEA concentration. According to Versteeg, et al.[21], the solubility of CO2HCO2in water can be calculated as follows:


(2) Order of reaction with respect to CO2
To evaluate the order of reaction with respect to CO2, the effect of CO2concentration on the absorption rate was investigated. And the results were presented as a plot of absorption rate against the partial pressure of CO2in the logarithm scale as shown in Figure 4. It can be seen that both the aqueous MEA solvent and the MEA-methanol solvent showed a linear relationship with a slope of almost unity which indicated that the order of reaction with respect to CO2can be determined to be unity.
(3) Order of reaction with respect to MEA
When the reaction is regarded as the m-order with respect to CO2and the n-order with respect to MEA, and the absorption reaction is under the fast reaction regime, then the absorption rate can be expressed as:

Figure 4 Rate of absorption of CO2 into aqueous MEA (a)and MEA-methanol solvent (b)■—CMEA=1 mol/L; ●—CMEA=2 mol/L; ▲—CMEA=3 mol/L;▼—CMEA=4 mol/L; ◆—CMEA=5 mol/L
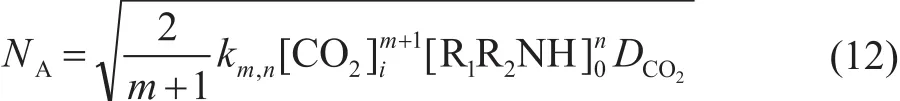
When the order of reaction with respect to CO2is unity as shown above, then Equation (12) can be reduced as follows:

The reaction rate constant k1,nshould be dependent on the concentration of MEA. But if the concentration of MEA changes not so significantly, then the plot of logNA/versus log[R1R2NH]0can be regarded as linear. Therefore, the slope of this relationship is equal to n/2. The results are presented in Figure 5. It can be seen that the reaction order with respect to the aqueous MEA solvent was 1.07, but that with respect to the MEA-methanol solvent was 1.72. The reason can be explained as follows: (1) MEA is a strong base that can be easily ionized in water and the order of reaction with respect to MEA is close to unity; and (2) in the MEA-methanol solvent, the deprotonation is not as easy as in water,therefore the deprotonation step increases the order of reaction with respect to MEA.

(4) Effect of methanol on the absorption rate
According to the mechanism presented in Equations 11 and 12, the reaction rate constant k1,nin Equation 13 should be a function of MEA concentration and is composed of the second-order and the third-order rate constants. As for the regime of the fast-pseudo- first-order chemical reaction, the absorption rate is expressed as:
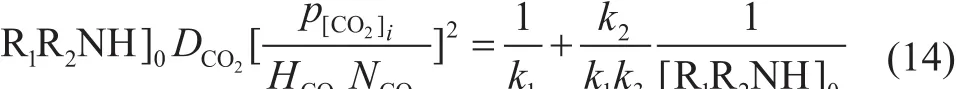
So by using Equation 14, the second-order and the third-order reaction rate constants can be evaluated,respectively, through the intercept on a slope of[R1R2NH]0DCO2(p[CO2]i/HCO2NCO2)2versus 1/[R1R2NH]0.The results are shown in Figure 6. It can be seen that k1of the aqueous MEA solvent and the MEA-methanol solvent remained the same, but k2/k3of the MEA-methanol solvent was higher than that of the aqueous MEA solvent.It can be explained that the deprotonation of zwitterion molecules was difficult, and they had enough time to change to CO2and MEA. So it can be concluded that methanol cannot ameliorate the absorption reaction, and on the contrary, methanol detains the reaction by reducing the rate of deprotonation reaction.
3.2.3 Absorption and regeneration properties
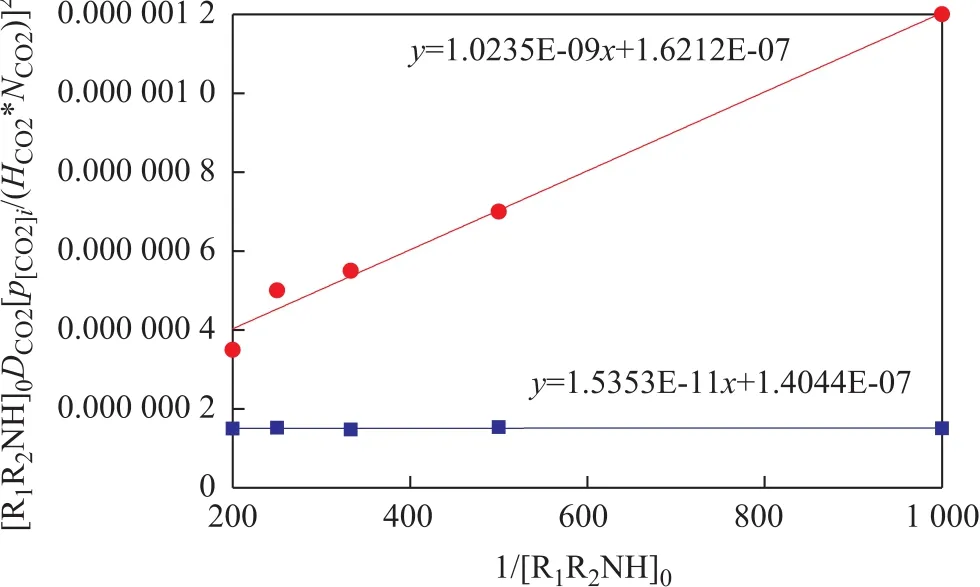
Figure 6 Second-order and third-order reaction rate constants of MEA-CO2 reaction in aqueous MEA solvent and MEA■—MEA aqueous; ●—MEA-Methanol
Figure 7 shows the absorption rates (a), mole of CO2absorbed (b) and CO2capture efficiency (c) of MEA and MEA-methanol solvents under the concentrations of 10%, 20% and 30%. The results show that an increase in the concentration of solvents leads to an increase in the absorption rates, absorption capacity and capture efficiency and the effective reaction time which were in accord with Lu, et al.[22]Also, it is observed that when the concentration of both MEA solvent and MEA-methanol solvent increases from 10% to 20%, only a little increase is obtained on the absorption performance, but when it increases from 20% to 30%, a more increase can be obtained. So to achieve a better absorption performance, a higher concentration is demanded.
3.3 Comparison of MEA and MEA-methanol solvents under basic operating conditions in a test bed
3.3.1 Absorption properties
The absorption performance of a 30% MEA-methanol solvent was compared with that of a 30% aqueous MEA solvent to evaluate the industrial potential of MEA-methanol for CO2capture. Table 2 gives the detailed operating parameters of MEA and MEA-methanol.
Table 2 Operating parameters of MEA and MEA-methanol solvents under basic operating condition
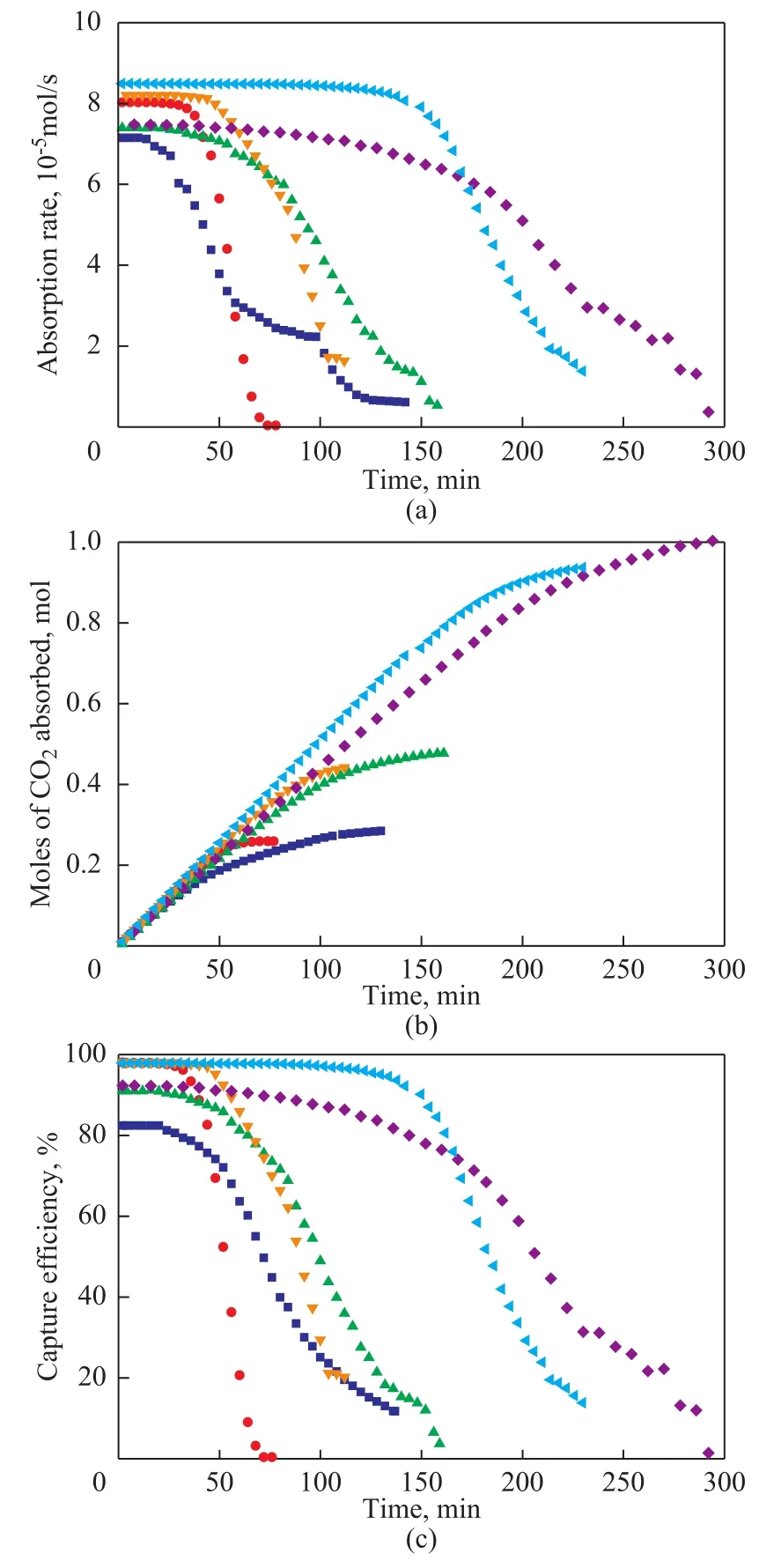
Figure 7 CO2 absorption rates (a) and CO2 absorption capacity(b) and CO2 capture efficiency (c) under different concentrations■—10%MEA; ●—10%MEA(methanol); ▲—20%MEA;▼—20%MEA(methanol); ◆—30%MEA;—30%MEA(methanol)
As shown in Figure 8(a), the outlet CO2concentration decreases quickly along the height of the absorber operating with both the aqueous MEA solvent and the MEA-methanol solvent. At a height of 1.36 m, the CO2recovery achieved by the aqueous MEA solvent is maintained at a constant level of about 98.5% and it almost does not change with the increase of the height.But for the MEA-methanol solvent, at a height of 0.95 m,the CO2recovery is about 98.5% and does not change with the increase of the height. So it can be concluded that at the same flue gas flow rate and solvent fl ow rate,the absorption of CO2into the MEA-methanol solvent is faster than the absorption of CO2into the aqueous MEA solvent. It can also be observed in Figure 8(b), the kGavvalue of the MEA-methanol solvent is higher than that of the aqueous MEA solvent, indicating that the MEA-methanol solvent has a better absorption performance than the aqueous MEA solvent which is in agreement with the study of Fu, et al.

Figure 8 Comparison of CO2 outlet concentration and CO2 recovery (a) and kGav value (b) between CO2 absorption into aqueous MEA and MEA-methanol solvents■—30%MEA; ●—30%MEA-methanol
3.3.2 Regeneration properties
In the present work, the regeneration heat duty Qregas well as the heat duty of its three components Qvap, Qsenand Qdesof the MEA-methanol solvent were compared with those of the aqueous MEA solvent in terms of their ability to regenerate the same amount of CO2in the stripper column. The results are shown in Figure 9. It can be seen that Qregof the MEA-methanol solvent is lower than the aqueous MEA solvent by about 80 %, which indicated that the regeneration performance of the MEA-methanol solvent was better than the aqueous MEA solvent,especially in the area of Qshand Qvap.

Figure 9 Comparison of regeneration heat duty of MEA and MEA-methanol
3.4 Comparison of reaction heat of CO2 with MEA-methanol and MEA
To well understand the absorption and desorption behavior of CO2in the MEA-methanol solvent, it is necessary to measure its absorption heat. In regard to the MEA-methanol solvent, the reaction is carried out in accordance with Equations (11) and (12). But in regard of the aqueous MEA solvent, the absorption reaction becomes more complicated due to the ionization of water,and the reactions proceed as follow:
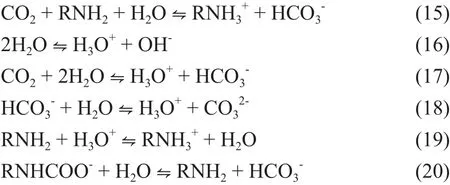
It can be seen that bicarbonate, the product of CO2absorption into the aqueous MEA solvent, is more stable than carbamate, which would dramatically increase the regeneration energy. And in the MEA-methanol system,the regeneration energy is only used for the decomposition of the carbamate, which would significantly improve the energy utilization.
The absorption heat can be estimated by the Gibbs-Helmholtz equation:

According to the relationship between the absorption heat and the partial pressure of CO2, Equation (21) can be transformed into the following equation:

According to Equation (22), the reaction heat can be obtained, and in order to get a more accurate value, the model fitting of Bruder, et al.[23]was used.

wherepco2is the partial pressure of CO2, α is moles of CO2absorbed,AandBare constants, andk1,k2andk3are functions of temperature. Through the above equation, the absorption heat can be obtained, with the results shown in Table 3. It can be seen that the absorption heat in the MEA-methanol solvent was always lower than that in the aqueous MEA solvent. For example, when the CO2loading α was equal to 0.4 mol/mol, the absorption heat of CO2into the MEA-methanol solvent was -60.6 kJ/mol,and that of CO2into the aqueous MEA solvent was -83.6 kJ/mol, which indicated that the desorption heat of rich MEA-methanol is only about 72 % of rich aqueous MEA solvent in the regeneration process. In addition, the absorption heat was gradually reduced with the increase of CO2loading.
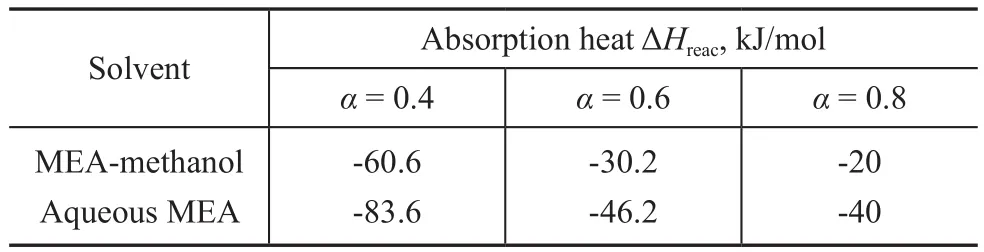
Table 3 Reaction heat of CO2 with MEA in aqueous and non-aqueous solvent
5 Conclusions
The CO2absorption and desorption performance of MEAH2O, DEA-H2O, TEA-H2O solvents and MEA-methanol solvent were experimentally studied at the concentration of 10%. It was found that MEA-methanol solvent had the highest absorption rate and the lowest regeneration temperature. And then to study more properties of MEA-methanol solvent and determine the best concentration,absorption experiment, regeneration experiment,densities and viscosities experiment were studied under the concentrations of 10%, 20% and 30%, compared to aqueous MEA solvents. It was found that 30% MEA-methanol had almost a same absorption capacity, a higher absorption rate and a lower density compared to MEA solvent. And in the test bed, MEA-methanol has a higherKGavvalue, a higher CO2absorption ef fi ciency and a lower regeneration energy consumption than aqueous MEA.
Through the study of kinetics of CO2capture by MEA-methanol solvent and aqueous MEA solvent, it can be concluded that methanol cannot ameliorate the absorption reaction, and on the contrary, methanol retards the reaction by reducing the rate of deprotonation step. And through the study of the reaction heat of CO2into MEA-methanol and aqueous MEA, it can be concluded that the desorption heat of rich MEA-methanol is only about 30 %of rich aqueous MEA solvent in the regeneration process.From the overall experiments, it can be concluded 30%MEA-methanol is a more favorable solvent for CO2capture in comparison with aqueous MEA.
Acknowledgement: This work was supported by the Sinopec Ningbo Engineering Co., Ltd. (No.l4850000-14-ZC0609-0003,H8XY-0032)
[1]Chen P C, Luo Y X, Cai P W. CO2capture using monoethanolamine in a bubble-column scrubber [J].Chemical Engineering Technology, 2015, 38(2): 274-282
[2]Lee J H, Kwak N S, Lee I Y. Performance and economic analysis of domestic supercritical coal-fired power plant with post-combustion CO2capture process [J]. Korean Chemical Engineering Research, 2012, 50(2): 365-370
[3]Li H, Yan J. Performance comparison on the evaporative gas turbine cycles combined with different CO2-capture options [J]. International Journal of Greenhouse Gas Control, 2009, 6(5): 512-526
[4]Liang Y J, Liu H L, Wong W R. Solubility, absorption heat and mass transfer studies of CO2absorption into aqueous solution of 1-dimethylamino-2-propanol [J]. Fuel, 2015,144: 121-129
[5]Knudsen J N, Jensen J N, Andersen J. Evaluation of process upgrades and novel solvents for CO2post combustion capture in pilot-scale [J]. Energy Procedia,2001, 4: 1558-1565
[6]Plaza J M, Chen E, Rochelle G T. Absorber intercooling in CO2absorption by piperazine-promoted potassium carbonate [J]. AIChE Journal, 2010, 56(4): 905-914
[7]Goto K, Okabe H, Chowhury F A. Development of novel solvents for CO2capture from blast furnace gas [J].International Journal of Greenhouse Gas Control, 2011,5(5): 1214-1219
[8]Wu S Y, Liu Y F, Chu C Y. Optimal absorbent evaluation for the CO2separating process by absorption loading,desorption ef fi ciency, cost, and environmental tolerance [J].International Journal of Greenhouse Gas Control, 2015,12(10): 1025-1030
[9]Thee H, Nicholas N J, Smith K H. A kinetic study of CO2capture with potassium carbonate solutions promoted with various amino acids: glycine, sarcosine and proline [J].International Journal of Greenhouse Gas Control, 2014,20: 212-222
[10]Dubois L, Thomas D. CO2absorption into aqueous solutions of monoethanolamine, methyldiethanolamine,piperazine and their blends [J]. Chemical Engineering Technology, 2009, 32(5): 710-718
[11]Fu K Y, Wong W R, Liang Z W. Experimental analyses of mass transfer and heat transfer of post-combustion CO2absorption using hybrid solvent MEA-MeOH in an absorber[J]. Chemical Engineering Journal, 2015, 260: 11-19
[12]Barzagli F, Lai S, Mani F. Novel non-aqueous amine solvents for reversible CO2capture [J]. Energy Procedia,2014, 63: 1795-1804
[13]Sema T, Naami A, Fu K. Comprehensive mass transfer and reaction kinetics studies of CO2absorption into aqueous solutions of blended MDEA-MEA [J]. Chemical Engineering Journal, 2012, 209: 501-512
[14]Brown A S, Freeman B C. Analysis and status of postcombustion carbon dioxide capture technologies [J].Environmental Science Technology, 2011, 45(20): 8624-8632.
[15]Naami A, Edali M, Sema T. Mass transfer performance of CO2absorption into aqueous solutions of 4-diethylamino-2-butanol, monoethanolamine, and N-methyldiethanolamine[J]. Industrial Engineering and Chemical Research, 2012,51(18): 6470-6479.
[16]Gao J, Yin J, Zhu F F. Study on Absorption and Regeneration Performance of Novel Hybrid Solutions for CO2Capture [J]. China Petroleum Processing and Petrochemical Technology, 2016, 18(1): 1-7.
[17]Xu H J, Zhang C F, Zheng Z S. Selective H2S removal by non-aqueous N-methyldiethanolamine solutions in an experimental apparatus [J]. Industrial Engineering and Chemical Research, 2002, 90(12): 2953-2956.
[18]Puxty G, Rowland R, Attalla M. Comparison of the rate of CO2absorption into aqueous ammonia and monoethanolamine [J]. Chemical Engineering Science,2010, 65(2): 915-922.
[19]Kim Y E, Moon S J, Yoon I Y. Heat of absorption and absorption capacity of CO2in aqueous solutions of amine containing multiple amino groups [J]. Separation and Purification Technology, 2014, 122: 112-118
[20]Zhang J F, Misch R, Tan Y. Novel thermomorphic biphasic amine solvents for CO2absorption and low-temperature extractive regeneration [J]. Chemical and Engineering Technology, 2011, 34(9): 1481-1489
[21]Versteeg G F, Van Dijck L A J, Van Swaaij W P M. On the kinetics between CO2and alkanolamines both in aqueous and non-aqueous solutions: An overview[J]. Chemical Engineering Communications, 1996, 144(1): 113-158.
[22]Lu J G, Chun H A, Lan B L. Performance evaluation on complex absorbents for CO2capture [J]. Separation and Purification Technology, 2011, 82: 87-92.
[23]Bruder P, Grimstvedt A, Mejdell T, et al. CO2capture into aqueous solutions of piperazine activated 2-amino-2-methyl-1-propanol [J]. Chem Eng Sci, 2011, 66(23): 6193-6198
- 中國(guó)煉油與石油化工的其它文章
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Emission-Reductive and Multi-Objective Coordinative Optimization of Binary Feed for Atmospheric and Vacuum Distillation Unit
- A pH Model for Calculating the pH Value of Mixed–Acid–Base Equilibria of Overhead Condensing Systems in Crude Distillation
- Simultaneous Biological Removal of p-Cresol, Sulfide and Nitrate by Denitrification
- The Effects of Ultrasonic Treatment on the Molecular Structure of Residual Oil
- Degradation of Nitrobenzene Wastewater via Iron/Carbon Micro-electrolysis Enhanced by Ultrasound Coupled with Hydrogen Peroxide

