Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
Chen Jiqun; Zhao Xiaojun; Zou Ying
(Petroleum Processing Institute, East China University of Science and Technology, Shanghai 200237)
1 Introduction
Naphtha is an important raw material for manufacturing ethylene and propylene, and it also can be used to produce high-octane gasoline or toluene and xylene in the catalytic reforming process. In recent years, the content of organic chlorine species in naphtha has been increasing.Some organic chlorine species can react chemically to form HCl, which would greatly accelerate the corrosion of the equipment. Some organic chlorine species can easily cause poisoning of catalyst, pipeline blockage and other issues[1-3]. Therefore, the study on dechlorination of naphtha has great significance for the production process.There are two sources of chlorine in naphtha[4]. One comes from various crude oil extraction additives, which can reduce the difficulty of oil extraction and increase the crude output. The other comes from some additives,which are used in the crude oil re fining process. They are almost chlorinated alkanes. Most of the inorganic chlorine species can be removed by electric desalting, but the organic chlorine species cannot be completely removed.The residual organic chlorine species still have a significant impact on the downstream processing unit. The removal of organic chlorine species from naphtha is mainly achieved by hydrotreating naphtha to convert organic chlorine species into inorganic chlorine species and then removing
it by alkaline washing or solid dechlorination. There is no mature industrial technology to be applied for the direct removal of organic chlorine compounds. Therefore,effective dechlorination method should be studied in order to remove the organic chlorine compounds.
Some scholars try to use chemical method to remove organic chloride compounds[5]. The principle of this chemical method is to use the strong electro-negativity of chlorine atom to make the carbon atom positively charged. So the nucleophilic material with stronger nucleophilic nature than chlorine atom can be chosen to react with organic chloride species so as to achieve dechlorination. However, the steric hindrance influence of subrogation is relatively large, so there is less research on it. Some scholars have studied the dechlorination process by microbial degradation under anaerobic condition which shows a good effect[6-7], but this method is quite time-consuming and needs harsh conditions.At present, using the adsorbent to remove organic chlorine species is the most popular method. The functional groups of activated carbon adsorbent are mainly divided into basic groups and acidic groups[8-9]. The acidic functionalgroups by means of oxidative modification can improve the dechlorination efficiency[10]. The γ-Al2O3which is loaded with active components CuO by using impregnation method can achieve a dechlorination rate equating to 59.93%[11]. The Y molecular sieve modified by the metal Ce can achieve a dechlorination rate of about 80% at 150 °C,and its adsorption mechanism is a chemical adsorption[12].The X type molecular sieves have the same structure as Y type molecular sieves albeit with different Si/Al ratio. The patents[13-14]have reported that 10X and 13X molecular sieves can be used to remove organic chlorine species. It is reported that the 13X molecular sieve has good dechlorination effect which can reach 74.1% in water containing 55 ppm of organic chlorine species, but the effect of removing organic chlorine species in naphtha and the adsorption mechanism have not been further studied by the author. Therefore, the 13X molecular sieve was chosen as the adsorbent in our study on adsorptive dechlorination process, which used the 13X molecular sieve modified with different metal ions.According to the literature reports[15-17], the most common organic chlorine species in naphtha include chloroform,tetrachloroethane, and o-dichlorobenzene, the total content of which is over 90%. Other kinds of organic chlorine content are too small to affect the experimental results. So a naphtha model oil composed of n-hexane serving as the solvent is used, which also contains chloroform, tetrachloroethane, and o-dichlorobenzene. Then the adsorption effect of molecular sieve before and after modification was studied, with its main dechlorination mechanism explored.
2 Experimental
2.1 Experimental materials
The reagents used in the experiments are listed in Table 1.
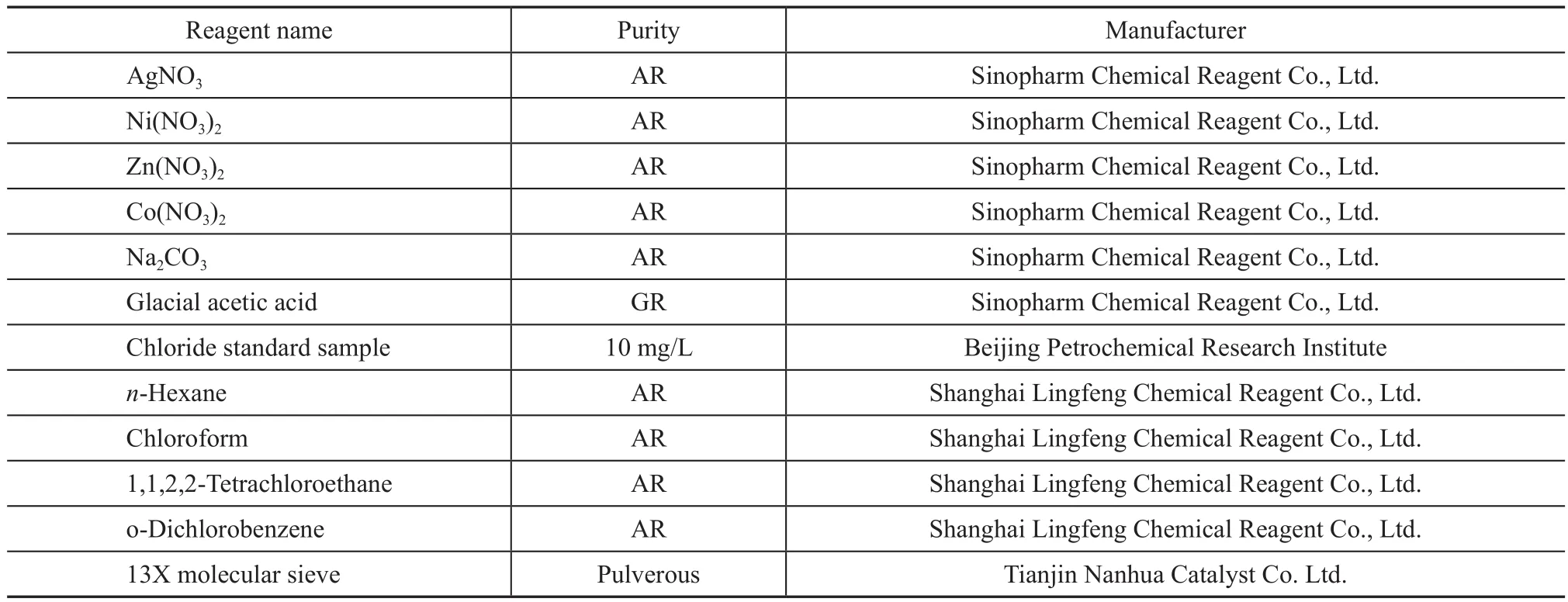
Table 1 Experimental reagents
2.2 Molecular sieve modification
The principle of ion exchange modification of the molecular sieve was adopted. The molecular sieve modification was carried out by using the immersion precipitation method. The 13X molecular sieve was activated at 450 °C for 4 h in a muf fl e furnace to obtain a fresh 13X molecular sieve, which was denoted as 13X.According to the proportion of 1 g∶10 mL, about 4 g of 13X were added to about 40 mL of 0.1 mol/L nitrate solution under continuous stirring at 80 °C for 4 h.Then the mixture was cooled to room temperature, into which a 0.2 mol/L Na2CO3solution was added dropwise.Then through filtering, drying and activating, the metal modified molecular sieve could be obtained, which was denoted as M-13X (in which M was the loaded metal element, include Ag, Ni, Co, and Zn).
2.3 Characterization methods
The phase composition, crystallinity and crystal cell parameters of the adsorbent were analyzed by XRD with a D8 Advance DaVinci polycrystal X-ray diffractometer,using Cu Kα (λ=0.15406 nm) as the radiation source. The working conditions of X-ray tube covered a voltage of 40 KV, a current of 100 mA, a scanning speed of 0.02(°)/s,and a scanning diffraction angle in the range of 5°—50°.The surface morphology of adsorbent was observed by a NOVA Nano SEM450 ultra-high resolution field emission scanning electron microscope (SEM).
The structure of molecular sieve was measured by a Micromeritics ASAP2010 specific surface and pore structure analyzer, which used the BET equation and DFT density function to calculate the surface area and pore size distribution of the adsorbent, respectively, while the BJH method was applied to measure the pore volume of the adsorbent.
The number and distribution of acid sites of the adsorbents were measured by an Auto-Chem II 2920 fully automatic temperature-programmed chemical adsorption instrument.The adsorbent samples were first purged with He at 250 °C for 1 h, followed by cooling to 40 °C. Then the NH3/He mixture with a NH3volume fraction of 10% was adsorbed on the sample at a fl ow velocity of 40 mL/min for 30 min, followed by switching to He purging for 30 min to remove the physically adsorbed NH3. At the same time, the temperature was increased from 40 °C to about 800 °C at a temperature increase rate of 10°C/min,with the signal being collected by the TCD detector.
2.4 Adsorption experiment
The experiment was based on different adsorbent to oil ratio. The concentration of chlorine before adsorption was measured asC0, and then it was adsorbed at room temperature and atmospheric pressure for about 3 h. The concentration of chlorine was measured after reaching the adsorption equilibrium which was recorded asCe.According to the Formula (1) and Formula (2), the rate of dechlorinationΦand equilibrium chlorine capacityQewas calculated, respectively:where,m0is the mass of the solution (g),mais the mass of adsorbent (g),ρis the adsorbed liquid density (g/mL).
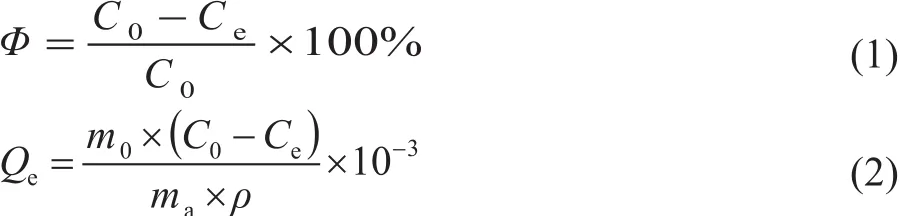
The concentration of organic chlorine in solution was determined by a KY-200 micro-coulometric titration apparatus. The conditions used for measuring the organic chlorine content are as follows: A 70% volumetric acetic acid solution is used as the electrolyte, the bias of the electrolytic cell is about 250 mV, the temperature at
the inlet and in the pyrolysis tube is 650°C and 850°C,respectively, the fl ow rate of the carrier gas N2and O2is about 200 mL/min and 150 mL/min, respectively; and the concentration of the standard sample is 10 mg/L. The average chlorine content should be analyzed three times for each determination.
3 Results and Discussion
3.1 Adsorption experiment
The experimental conditions in this paper covered an adsorbent to oil ratio of 1 g versus 40 mL, a temperature of 20 °C, and a reaction time of 3 h. Figure 1 and Table 2 show the adsorption dechlorination effect of 13X modified by different metal ions. It can be seen that the effect of Ag-13X on adsorption of organic chlorine species was much better than other M-13X. Its rate of chlorine removal was as high as 97%, and its equilibrium adsorption capacity of chlorine was also the largest. The comparison of adsorption dechlorination results and conditions referred to in different literature reports are presented in Table 3. Upon comparing these conditions,the adsorption dechlorination results achieved by Ag-13X are better and the experimental conditions are easier to be implemented. In view of this phenomenon, XRD, SEM, BET and NH3-TPD analyses were used to study the influencing factors of the adsorption of organic chlorine species by Ag-13X, and the adsorption mechanism was explored.
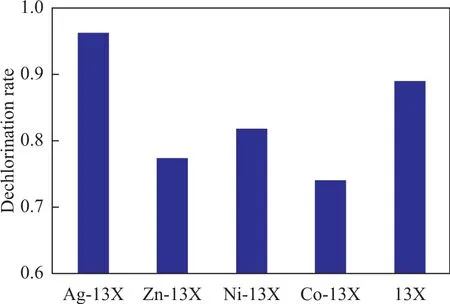
Figure 1 Dechlorination rate of 13X and M-13X
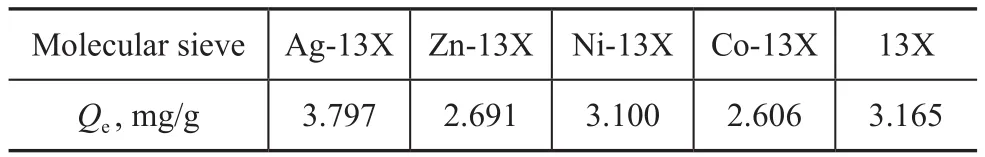
Table 2 Equilibrium capacity for adsorption of organic chlorine species
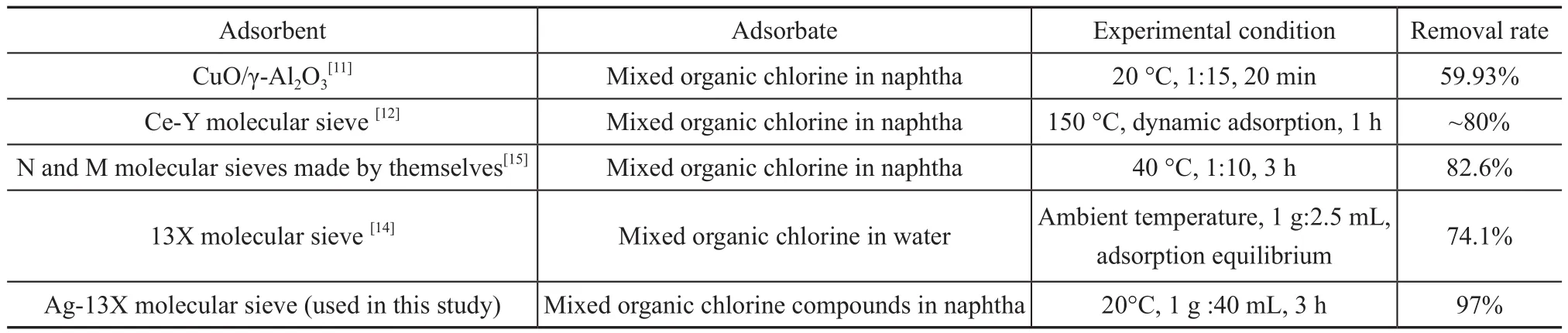
Table 3 Adsorption dechlorination results referred to in different literature reports
3.2 Adsorbent characterization
3.2.1 X ray diffraction analysis (XRD)
Figure 2 shows the XRD patterns of different adsorbents.The results showed that the diffraction patterns of Co-13X, Ni-13X and Zn-13X were almost the same as 13X.There was no additional peak, but the peak intensity varied in different degree. This fact indicated that the metal ions entered the molecular sieve pores and skeleton to replace the original metal ions, which were used for equilibrium of negative charges in the molecular sieves framework. However, Ag-13X displayed a little difference. The intensity of the diffraction peak of Ag-13X was enhanced at 2θ=12.2°, 21.0° and 32.0°, and its diffraction peaks disappeared at 2θ=18.4° and 20.1°. It showed that the diffraction of crystal plane disappeared at the two positions of the modified angle. The fact,which indicated that new diffraction peaks appeared at 2θ=12.3° and 14.2°, could lead to the conclusion that a new crystal diffraction plane appeared. These phenomena were consistent with the diffraction changes of cubic crystal structure[18]. There are two explanations that can account for these phenomena. On the one hand, different metal ions have different influence on the intensity of diffraction peak. The disappearance of diffraction peaks is traceable to the interaction between different diffraction surfaces.On the other hand, it can be possibly ascribed to the collapse of some molecular sieves during the activation process, which would influence the intensity of diffraction peaks.
3.2.2 Scanning electron microscope (SEM)
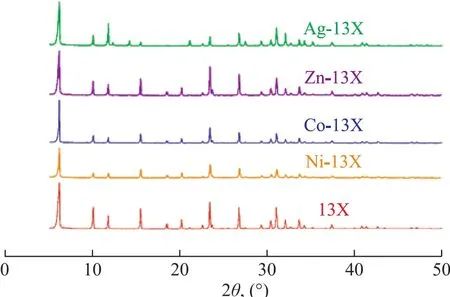
Figure 2 X ray diffraction patterns of 13X and M-13X
In order to confirm whether there was metal oxide adhering to the surface of M-13X, the surface morphology of these molecular sieves was observed by SEM. The results are shown in Figure 3. It can be seen that the surface morphology of Ag-13X was almost the same as 13X, while the surface morphology of other M-13X had shown obvious changes. As shown in Figure 3, there was no accumulation of metal oxide on the surface of Zn-13X,and however, a dense oxide film was seen on the surface which could prevent further oxidation. The surface of Co-13X had accumulation with honeycomb structure which might be ascribed to the oxidation reaction occurring on the surface of the molecular sieve under the experimental conditions, which blocked the pores of molecular sieve so that the subsequent ion exchange reaction could not be carried out and a large number of metal oxide were attached to the surface of the molecular sieve. Figure 3 also shows that the surface structure of Ni-13X was destroyed. This might be caused by the collapse of the internal skeleton of molecular sieves during the activation process at high temperature, leading to collapse of some crystal structures of the molecular sieve.
3.2.3 Specific surface area and pore size analysis(BET)

Figure 3 SEM diagram of 13X and M-13X
Table 4 shows the specific surface area, average pore size and average pore volume of each adsorbent. It can be seen from Table 4 that the specific surface area of M-13X had a little decrease except for the Ni-13X which showed a significant decrease in comparison with 13X. Since the radius of metal ions used for modification of molecular sieves was larger than Na+ion, its specific surface area decreased. The specific surface area of Ni-13X decreased sharply, because the high temperature caused the collapse of the internal skeleton of the molecular sieve during the activation process which could destroy the crystal structure.
The different metal ions had different influence on the aperture of relevant molecular sieve. As shown in Table 4, the size of the pore diameter decreased in the following order: Ag-13X>Co-13X>13X>Zn-13X>Ni-13X. It can be seen from the SEM images that the surface of Co-13X with accumulation of impurities had an impact on the pore size distribution. In this case, only Ag+ions could enlarge the pore volume of molecular sieves. The pore size and pore volume of adsorbent are the important factors affecting the adsorption performance. The larger the pore size, the easier the accessibility of molecular sieve to the adsorbate molecules. The larger the pore volume is, the greater the adsorption capacity of the molecular sieve.Therefore, only Ag-13X which could enlarge the pore hole and pore volume had strong adsorption capacity.
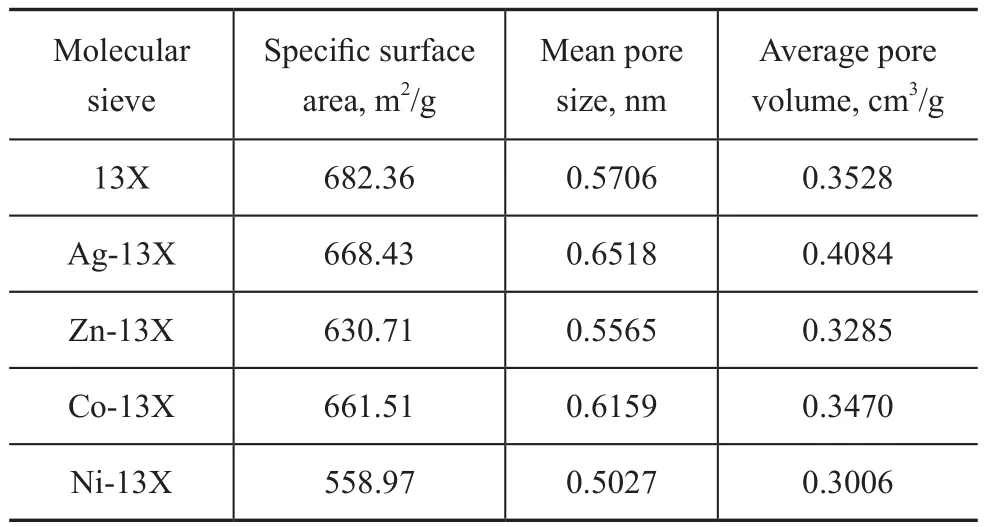
Table 4 BET data of 13X and M-13X
3.2.4 NH3adsorption and temperature programmed desorption (NH3-TPD)
The acid sites of different metal-modified molecular sieves are presented in Figure 4. Figure 4 shows that 13X had only one desorption peak at about 160 °C. The peak area and peak width of different M-13X increased obviously.
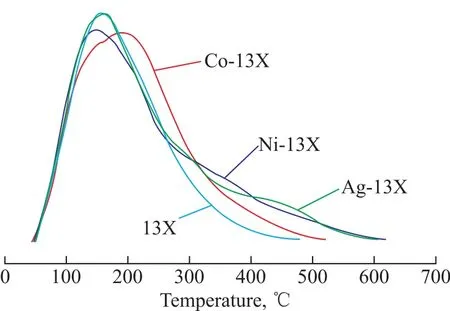
Figure 4 Distribution of acid sites of 13X and M-13X
The peaks of 13X and Ag-13X were almost similar at temperature lower than 300 °C. At temperatures exceeding 300 °C, Ag-13X showed a slowly descending curve which confirmed that the new medium-strong acid sites were generated. However, because the number of medium strong acid sites was too small, it did not form an independent peak. Table 5 lists the total acidity of adsorbent, showing that the total acid amount of M-13X increased. Co-13X and Ni-13X achieved the increase of a large number of weak acid sites, while Ag-13X showed the increase of a small amount of weak acid sites coupled with the formation of new medium-strong acid sites. According to the results of adsorption, the excessive number of weak acid sites may be unfavorable to adsorption process. However, the adsorption of NH3on the molecular sieve was enhanced by the presence of the medium-strong acid sites, denoting that the chemical adsorption capacity was enhanced.
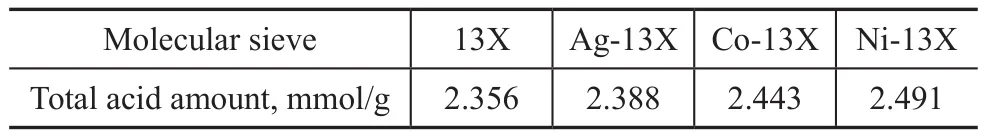
Table 5 Total acidity of 13X and M-13X
Based on the above characterization, the XRD patterns and SEM images showed that some changes had taken place in the surface and interior of the molecular sieve.Among them, Ag-13X displayed a new crystal plane,while some impurities were more or less attached to other M-13X samples. The results of BET data showed that only Ag+ions could enlarge the pore size and the pore volume of molecular sieve at the same time. The NH3-TPD diagram showed that the acid sites of the molecular sieve were increased after the modification with the metal ions. However, too many weak acid sites were not conducive to the adsorption process. And the new medium strong acid sites were found in Ag-13X which could strengthen the chemical adsorption capacity of molecular sieve.
3.3 Adsorption mechanism analysis
Characterization of different molecular sieves showed that the structure and properties of these molecular sieves were improved by modification with metal ions, so the adsorption mechanism could be changed slightly.
The equilibrium capacity for adsorption of the model compounds at different concentrations was determined by experiments. Then the data were used to work out the adsorption equilibrium constant K. The thermodynamic parameters of ΔH, ΔSand the adsorption equilibrium constantKcan meet the van’t Hoffs equation:
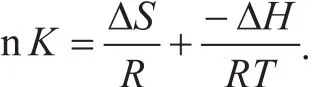
And based on the thermodynamic principle, the following equation is obtained:
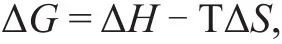
in which ΔHcan be calculated. The experiment results were worked out as shown in Table 6.
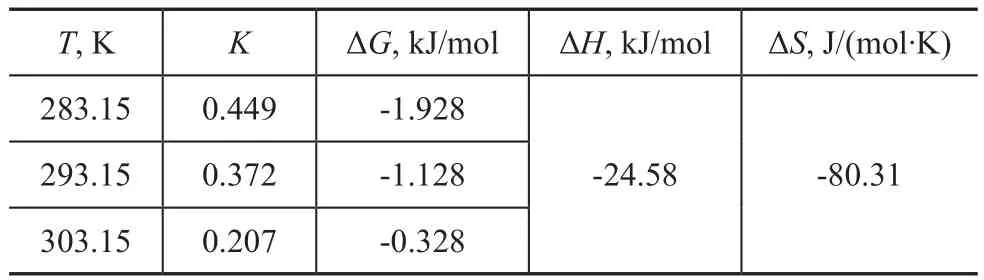
Table 6 Thermodynamic parameters of adsorption on Ag-13X
As indicated in Table 6, ΔH< 0 shows that the adsorption process on Ag-13X was an exothermic process. And ΔG<0 shows that this process was a spontaneous adsorption process.
Then 13X and Ag-13X molecular sieves were used to adsorb organic chlorine species at the same concentration and different temperature conditions. The results are shown in Figure 5. It can be seen from Figure 5 that with the increase of temperature, the adsorption effect of 13X was getting worse and worse. The rate for removal of organic chlorine species decreased gradually. However, Ag-13X showed some difference, and the equilibrium adsorption capacity decreased rapidly at temperature higher than 30°C. The comprehensive characterization and adsorption results show that the surface properties of 13X had changed after being modified by Ag+ions. It is inferred that the mechanism for adsorption of organic chlorine species by Ag-13X was a combination of physical adsorption and chemical adsorption which could have different degree of influence at different temperatures.
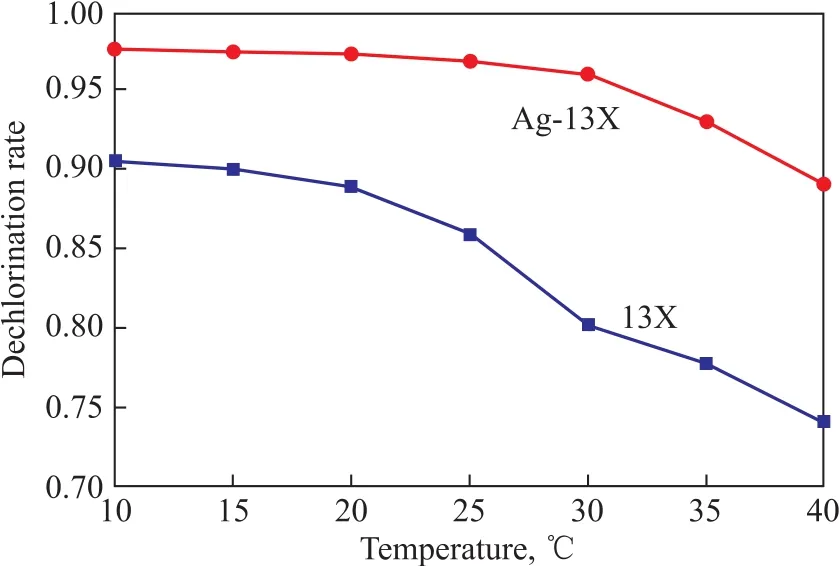
Figure 5 Change of dechlorination rate with temperature
4 Conclusions
In this paper, the adsorptive dechlorination effect of different M-13X was studied. The adsorption mechanism of Ag-13X was especially explored. The following conclusions are summarized:
(1) The effect of Ag-13X on adsorption of organic chlorine species was much better than other M-13X in this study. Under the conditions covering an adsorbent to oil ratio of 1 g/40 ml, a temperature of 20 °C, and an adsorption time of 3 h, the rate of removal of organic chlorine species could reach as high as 97%.
(2) Different metal ions had different impact on the structure and properties of molecular sieves, which were the reason responsible for the different adsorption results. Only the 13X modified by Ag+ions could expand its pore size and pore volume, leading to an increased equilibrium capacity for adsorption of organic chlorine. And Ag-13X had generated new medium-strong acid sites, which could enhance the chemical adsorption capacity of the molecular sieve.
(3) The adsorption experiments conducted at different concentration in combination with the thermodynamic principle had shown that the adsorption process of organic chlorine species over Ag-13X was a spontaneous exothermic process. The adsorption equilibrium capacity curve of Ag-13X at different temperature was significantly different from that of 13X. The equilibrium adsorption capacity was almost constant below 30°C, and then decreased rapidly at temperature exceeding 30°C. The comprehensive characterization and adsorption results show that the surface properties of 13X had changed after being modified by Ag+. It is inferred that the adsorption mechanism of Ag-13X was a combination of physical adsorption and chemical adsorption which showed different degree of influence at different temperatures.
(4) In this paper, the adsorption experiment was a static adsorption study, so although Ag-13X shows a very good dechlorination effect at room temperature and atmospheric pressure, there are many areas that need further study, including the dynamic adsorption and regeneration study.
[1]Liu Kefei, Han Jianmin, Ye Xiaodong, et al. Analysis and countermeasures of the salt in the fractionator of heavy oil catalytic cracking [J]. Petroleum Processing and Petrochemicals, 1994, 25 (1): 46-49 (in Chinese)
[2]Wu Depeng. Cause analysis of fouling in heat exchanger of hydro fining unit [J]. Liaoning Chemical Industry, 2008,37 (11): 773-775 (in Chinese)
[3]Li Ning. The harm of chlorine in crude oil to catalytic fractionator and its solution [J]. Natural Gas and Oil, 2005,23 (3): 52-54 (in Chinese)
[4]Zhang Xiaojing. The source and distribution of chloride in crude oil and its control measures [J]. Petroleum Refinery Engineering, 2004, 34 (2): 14-16 (in Chinese)
[5]Liu Zhe, Cong Xiangqin. Study on removal of organic chlorides in crude oil [J]. Corrosion & Protection in Petrochemical Industry, 2012, 29 (1): 6-8 (in Chinese)
[6]Munakata M J, Matheson V G, Forney L J, et al. Longterm biodegradation of trichloroethylene influenced by bioaugmentation and dissolved oxygen in aquifer microcosms [J]. Environment Science & Technology,1997, 31(3): 786-791
[7]Lu Xiaoxia, Li Guanghe, Zhang Xu, et al. Microbial dechlorination of vinyl chloride under different redox conditions [J]. Environmental Sciences, 2002, 23(2): 29-33[8]Lahaye J Q. The chemistry of carbon surfaces [J]. Fuel,1998, 77(6): 543-547
[9]Boehm H P. Some aspects of the surface chemistry of carbon black and other carbons[J]. Carbon, 1994, 32: 759-769
[10]Li Jingyan, Nan Guozhi, Fan Weiyu, et al. Modification of activated carbon and its adsorption performance of organic chloride in naphtha [J]. Petroleum Processing and Petrochemicals, 2009, 40 (6): 61-64 (in Chinese)
[11]Li Ruili, Zhang Ping, Lü Benzhen. Removal of chloride in straight-run naphtha by adsorption [J]. Petrochemical Technology, 2015, 44 (4): 477-482 (in Chinese)
[12]Ge X, Shi L, Wang X. Dechlorination of reformate via chemical adsorption reactions by Ce–Y zeolite [J]. Ind Eng Chem Res, 2014, 53(15): 6351-6357
[13]Robert E R, Bruce C B, Homer J S, et a1. Removal of chemically combined chlorine and other impurities: The United States, US3864243[P]. 1975-02-02
[14]Reusser R E.Removal of chemically combined chlorine and other impurities from hydrocarbon: US, 3862900[P].1975-01-28
[15]Fan Xiuju, Zhu Jianhua, Song Haifeng, et al.Morphological identification and removal of organic chlorides in naphtha from Liaohe Oil field [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2011, 27 (1): 107-111 (in Chinese)
[16]Shi Junsong, Yang Defeng, Han Jianghua. Study on the speciation and content analysis of organic chlorides in naphtha [J]. Petroleum Processing and Petrochemicals,2013, 44 (8): 85-89 (in Chinese)
[17]Nan Guozhi, Fan Weiyu. Analysis of chlorides in naphtha[J]. Research and Exploration in Laboratory, 2009, 28 (12):40-42 (in Chinese)
[18]Cai Xiane, He Qiaojuan. Preparation and properties of AgNaX zeolite [J]. Journal of Fudan University (Natural Science), 1981 (3): 105-111 (in Chinese)
- 中國煉油與石油化工的其它文章
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Emission-Reductive and Multi-Objective Coordinative Optimization of Binary Feed for Atmospheric and Vacuum Distillation Unit
- A pH Model for Calculating the pH Value of Mixed–Acid–Base Equilibria of Overhead Condensing Systems in Crude Distillation
- Simultaneous Biological Removal of p-Cresol, Sulfide and Nitrate by Denitrification
- The Effects of Ultrasonic Treatment on the Molecular Structure of Residual Oil
- Degradation of Nitrobenzene Wastewater via Iron/Carbon Micro-electrolysis Enhanced by Ultrasound Coupled with Hydrogen Peroxide

