Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
Li Na; Long Jun; Zhao Yi; Tao Zhiping; Dai Zhenyu
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
Li Na; Long Jun; Zhao Yi; Tao Zhiping; Dai Zhenyu
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Using molecular dynamics simulations based on classical mechanic method, the mechanism of competitive adsorption between gasoline detergent and deposit on Fe (110) surface was investigated. The representative simulation relating to the deposit molecule and the gasoline detergent molecule with high market share were selected as the model compound. It was found that when the detergent and deposit molecules exist at the same time, the detergent molecules would compete with the deposit molecules to reduce the adsorption of the deposit on Fe (110) so as to protect the metal surface. In addition, the ESP distribution is further con firmed that the detergent molecule has higher adsorption ability than the deposit molecule with the DFT theory. The essence of competitive adsorption is further revealed in detail, which is very important for the development of new type high-efficiency detergent additives.
gasoline detergent; deposit; competitive adsorption; molecular simulation
1 Introduction
During their use in automobile engines, gasoline molecules tend to react with the oxygen molecules. The formed oxidation products could continue to form sticky gum[1-3]and further generate carbon deposit inevitably[4].The deposit could stick to the metal surface of engine parts, such as the fuel nozzle, the intake valve, and the fuel system, covering the fuel tank to the combustion chamber. The deposit could lead to increased fuel consumption, engine power loss, and poor operation performance, which would have a serious impact on engine efficiency and exhaust emissions[5-7]. Gasoline detergent[8-11]is the effective and low cost method which can inhibit and remove engine deposit, prolong the service life of the engine and improve the exhaust emission performance. However, the mechanism of gasoline detergent was mostly learned from lubricant detergent[10],which is inconsistent with the gasoline system. The action mechanism of detergent is not yet clearly understood.
Essentially, the gasoline detergent is an amphoteric surfactant, containing hydrophobic and hydrophilic moieties[10]. It works mainly by adsorption. Experimental investigations have led to a wide variety of data on the adsorption properties of detergent. Techniques that have been used in these studies include the adsorption isotherm measurements[12], the X-ray photoelectron spectroscopy[13], the calorimetry[14], the ellipsometry[15], the fluorescence spectroscpoy[16], NMR spectrometry[17], and the Fourier transform infrared(FTIR) spectroscopy[18-19].
All the above-mentioned techniques can give useful information, such as the adsorption enthalpy, the adsorption amount or adsorption layer thickness.However, due to the complicated work condition in the internal engine, it is difficult to analyze the intermediate process, and unequivocally to establish the structure of adsorbed detergent layer from the experimental data.Therefore, we use molecular simulation techniques to reveal the details which cannot be obtained through macroscopic tests.
Further revealing the action details of gasoline detergentcould provide a theoretic basis for development of new type high-efficiency detergent additives, so as to improve the engine performance. This work intends to study the mechanism of competitive adsorption between gasoline detergent and carbon deposit on Fe (110) surface using molecular simulation. For this purpose, the adsorption interaction energy, the Mean Square Displacement (MSD),Radial Distribution Function (RDF) and the concentration distribution of detergent and deposit have been calculated by molecular dynamics simulation. In addition, in order to explain the adsorption ability, the ESP charge distribution of the two molecules has also been analysed by the density functional theory (DFT).
2 Computational Details and Models
The adsorption behavior of detergent molecule is very important for understanding the protection behavior on metal surface. The molecular dynamics (MD) simulations are carried out by Forcite Plus in the Material studio 8.0 developed by Daussault Biovia Inc. The interatomic interactions are described by the force field of condensedphase optimized molecular potential for atomistic simulation studies (COMPASS II), which is a general all-atom force field. The MD simulations are performed in NVT (the temperature and the volume are constant)ensemble. The Nose thermostat method was employed to control the system at a temperature. The van der Waals interactions were calculated by group-based, with the cut-off distance equating to 12.5?. The electrostatic interactions were calculated by the Ewald method.
The initial iron lattice is derived from the structural database of MS software. Fe (110) surface is chosen for the simulation study. The MD simulation of the interaction between the molecules and the Fe (110) surface is carried out in a simulation box (37 ? × 37 ? × 48 ?) with periodic boundary conditions to model a representative part of the interface devoid of any arbitrary boundary effects.
The representative simulation deposit molecule[20]and the gasoline detergent molecule[21]with high market share were selected as the model compound. The model compounds in these simulations are both shown in Figure 1(a) and(b). The box consisted of a Fe slab, a molecule slab (10 deposit molecules or 10 deposit molecules+10 detergent molecules) containing the studied compounds and a 40? vacuum layer. During simulations, all the bulk atoms in the Fe (110) systems were kept ‘‘frozen’’, and the detergent molecules and deposit molecules were allowed to interact with the metal surface freely. The MD simulation was performed under 473K, NVT ensemble, with a time step of 1.0 ps and a simulation time of 1 000 ps, and the data were collected every 5 ps. Then the full-precision trajectory was recorded, and the results were analyzed in the next part.
The interaction energy, Einteractionbetween the Fe (110)surface and the adsorbed molecule is calculated according to the following equation (1)[22]:

in which Etotalis the total energy of the simulation system,Esurfaceis the energy of iron surface, and Emoleculeis the energy of the stabilized detergent molecules or deposit molecules. These individual energy components are determined by selecting the respective constituents from the simulation box under the finally equilibrated state (as presented pictorially in Figure 2, Figure 4 and Figure 5).

Figure 1 The molecular structure of model compounds.
The quantum calculations are performed in the present study with Dmol3program in Material Studio 8.0.Geometry optimizations of both detergent molecule and deposit molecule are performed with GGA-PBE functional[23]of DFT theory employing basis sets DNP.The SCF calculations are converged tightly (SCF tolerance: 1×10-6Ha; energy: 1×10-5Ha; max force: 0.0002 Ha/nm; max displacement: 5×10-4nm).
3 Results and Discussion
3.1 MD simulation calculations
3.1.1 Single molecule adsorption calculation
It can be seen from Figure 2 that the adsorption of deposit molecule on Fe (110) surface is mainly in the form of planar ring skeleton structure, with hydroxyl radical being perpendicular to the ring structure. And the detergent is tiled on the surface with the whole molecule. This is related to their structure.
As to the adsorption interaction energy, it is shown in Table1. The interaction energy of single deposit adsorbed on Fe(110) surface is much lower than detergent molecule. But both of the adsorption interaction energy mainly originates from the van der Waals effects, the electrostatic effect of which is very small.

Figure 2 Adsorption on Fe(110) surface

Table 1 Calculated adsorption interaction energies between detergent or deposit molecules and Fe (110) (unit in kJ/mol)
The shadow area of the molecules was calculated by using QSAR program in MS 8.0 software. Then the interaction energy per cross-sectional area was obtained,as shown in Table 2, showing that the deposit is obviously not comparable with the detergent. This further con firmed that the adsorption ability of the detergent molecules on the iron surface is above the deposit molecules.

Table 2 Interaction energy per cross-sectional area
By using the molecular dynamics simulation, MSD of detergent and deposit molecules was calculated. The diffusion behavior of two molecules on Fe (110) surface was investigated.
The MSD[24]at different time was calculated according to Equation (2), and the diffusion coefficient was calculated according to Equation (3).

Generally, the greater the slope of the MSD curve, the stronger the motion of the molecule, and the weaker the adsorption ability of the molecule on the surface would be.It can be seen from Figure 3 that the slope of the curve for the deposit is higher than detergent, which indicates that the diffusion coefficient of deposit is bigger than that of detergent molecules on the iron surface.
Obviously, the deposit molecule moves faster than the detergent. The adsorption of detergent molecules on the iron surface is more stable, so that the adsorbed deposit molecules can be easily removed from the surface. Thus more detergent molecules will occupy the adsorption sites, so as to achieve the protection of the iron surface.

Figure 3 MSD of detergent and deposit on Fe (110) surface
3.1.2 MD simulation calculation
Research[25]showed that the fresh gasoline, which did not contain detergent, could generally produce 7—8 mg of deposit /100 mL. The amount of detergent used was about 300 ppm. Then, the ratio of the deposit and the detergent for molecular dynamics model was about 1:1 after calculation. Therefore, Model 1 was built with 10 deposit molecules, and Model 2 was built with a mix composed of 10 deposit molecules and 10 detergent molecules, as shown in Figure 4 and Figure 5, respectively.
It can be seen from Figure 4 that after 1 000 ps, all the deposit molecules were adsorbed on the surface adequately. The interaction energy was -6 656.02 kJ/mol as shown in Table 3. But for Model 2, it is clear that the detergent molecules competed with the deposit molecules on Fe (110) surface. The interaction energy was-1 588.98 kJ/mol, which was significantly lower than Model 1. However, compared to the deposit, the interaction energy of detergent was -7 501.15 kJ/mol,which was much higher. Therefore, when the detergent and deposit molecules exist at the same time, the detergent molecules would compete with the deposit molecules to reduce the adsorption of the deposit on the iron surface, so as to protect the surface.
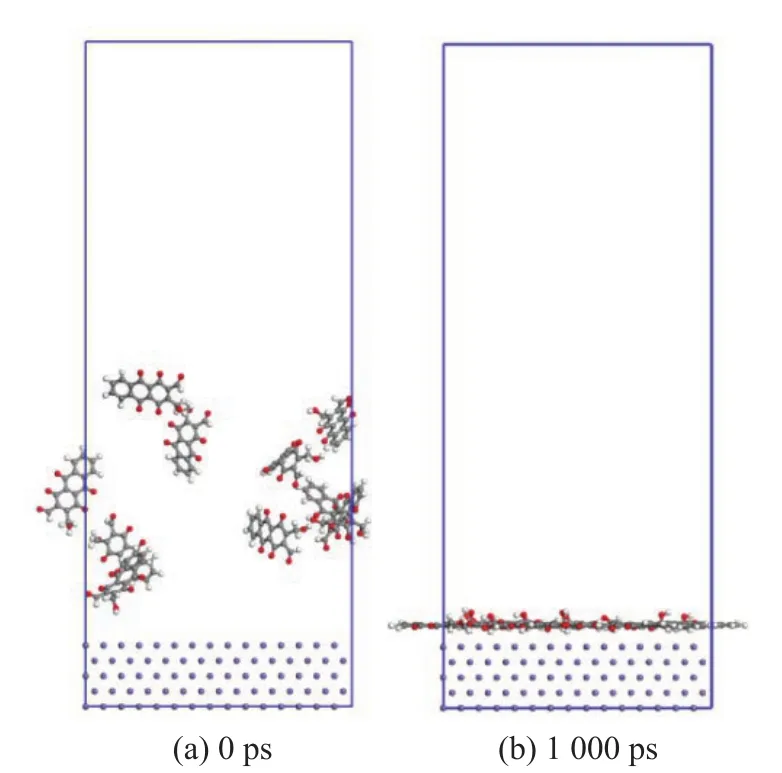
Figure 4 Model 1 (10 deposit molecules only)

Figure 5 Model 2 (a mix composed of 10 deposit molecules and 10 detergent molecules)

Table 3 Calculated adsorption interaction energies of detergent or deposit molecules on Fe (110) (unit in kJ/mol)
The relative concentration distribution of deposit molecules on Fe (110) before and after adding detergent corresponding to Model 1 (deposit only) and Model 2(deposit and detergent mix) is shown in Figure 6. It can be seen from the result that for Model 1, the deposit molecules are mainly located 10 ? away from the iron surface. Since the thickness of the iron layer is about 10 ?,it means that the deposit molecules are close to the iron surface. However, for Model 2, the peak value at about 10 ? is obviously reduced, indicating that the deposit molecules have moved away from the iron surface.
In addition, for Model 2, upon comparing the concentration distribution of the detergent molecules and deposit molecules as shown in Figure 7, the competitive adsorption occurred between the detergent molecules and the deposit molecules. The concentration of detergent molecules adsorbed at about 10 ? is higher than the deposit molecules.On the other hand, the radial distribution was also analyzed. The radial distribution function can be interpreted as the ratio of the area density to the average density of the system. As shown in Figure 8, the black ball in the center could be considered as the reference particle, and the number of particles from r to (r+dr) is dM.

Figure 6 Relative concentration distribution of deposit on Fe (110) for Model 1 and Model 2

Figure 7 Relative concentration distribution of deposit and detergent molecules on Fe (110) for Model 2

Figure 8 Diagrammatic sketch of radial distribution.
The radial distribution function g (r)[26]is de fined as:

The radial distribution of deposit molecules for Model 1 (10 deposit molecules only) and Model 2 (a mix composed of 10 deposit molecules and 10 detergent molecules) is shown in Figure 9. It is indicated that after adding detergent the radial distribution of fe-C atoms in the deposit molecules has changed obviously.The first peak appears in the vicinity of 3.19? which can hardly change. However, the peak height decreased significantly after adding the detergent. And the peak width becomes larger obviously, which shows that the adsorption of deposit on Fe (110) becomes loose and disorderly. Furthermore, the probability of moving the deposit molecules closer to the iron surface is obviously reduced. The competitive adsorption has occurred between the detergent molecules and the deposit molecules, making some deposit molecules move away from the surface.

Figure 9 RDF results of fe-C of deposit for Model 1 and Model 2
3.2 Quantum calculations
The molecular structure determines its property. As for the above results, it is necessary to make further analysis on the two structures to explain the results.The van der Waals effect is mainly related to the charge properties of molecules. ESP (electrostatics potential)distribution was obtained by the property analysis with Dmol3program.
Firstly, the color distribution of ESP is shown in Figure 10. As indicated by the color ruler, blue represents the negative charge region. The electronegativity of deposit molecule is mainly distributed on both sides of the O atoms and the terminal hydroxyl group. As for the detergent molecule, the electronegativity region is mainly concentrated on the N atoms and O atoms in the skeleton.As for the charge value, the ESP charge of O atom in deposit molecule is between -0.3 e— -0.38 e. The ESP charge of N atom in PEA is -0.878 e, and the charge of O atoms is between -0.34 e— -0.42 e.
The general reactivity of a molecule depends on the value of ESP. The lower the ESP, the higher the active molecule is[27-28]. The strength of each site in the detergent is slightly higher than that of the deposit. The adsorption of N atom is stronger than other elements, and more stable.In addition, in terms of the number of possible adsorption sites, there are only 6 sites in the deposit, and there are 14 O and 1 N atoms in the detergent molecule, with a total of 15 sites. Therefore, the interaction energy of detergent on Fe (110) surface would be significantly higher than that of the deposit.From the viewpoint of molecular steric hindrance, the detergent also has more advantages than the deposit. All the active sites of the deposit were distributed on both sides of the plane formed by the cyclic structure of the deposit molecule. When the deposit content is less, the deposit molecules will be more inclined to spread on the surface of iron, so that it has sufficient contact with Fe(110) surface. But if the adsorption quantity changes,when the surface is congested, the deposit molecules will tend to side with the surface. So the number of active sites in deposit molecule would decrease from 6 to 3, thus the adsorption strength will become weaker.
As for the relative flexibility of detergent molecule, the adsorption sites were more evenly distributed, which means a more readily interaction with the surface.When the adsorption quantity increases, there would be also steric hindrance effect. But in any case, the strong adsorption of terminal N atom will work. And due to its relatively large molecular size, the number of adsorption sites will be more than 3.
In conclusion, the adsorption of detergent molecules on Fe (110) is stronger than that of deposit molecules.

Figure 10 ESP distribution
4 Conclusions
By using the molecular dynamics simulations, the mechanism of competitive adsorption between gasoline detergent and deposit on Fe (110) surface was investigated.
(1) As for the single molecule calculation, the adsorption interaction energy of a detergent molecule adsorbed on Fe (110) is significantly higher than that of a deposit molecule. The MSD result showed that the adsorption of deposit molecule on Fe (110) is not as stable as the detergent molecule. As the simulation time increases, the deposit molecule would separate from the adsorption site.(2) As for the MD results, the adsorption interaction energy of the deposit molecules decreased apparently after adding the detergent molecules. Furthermore, the relative concentration distribution and radial distribution results further proved the preferential adsorption for the detergent on Fe (110) over the deposit so as to protect the metal surface.
(3) As for the quantum results, the ESP distribution result showed the number of possible adsorption active sites of detergent molecule was more than that of the deposit. It is further con firmed that the detergent molecule has higher adsorption ability than deposit molecule.
[1] Brooks B T. The chemistry of gasolines - particularly with respect to gum formation and discoloration[J]. Industrial and Engineering Chemistry, 1926, 18(11): 1198-1203
[2] Flood D T, Hladky J W, Edgar G. Chemical nature of gum-forming constituents in gasoline[J]. Industrial and Engineering Chemistry, 1933, 25(11): 1234-1239
[3] Walters E L, Iminor H B, Yabroff D L. Chemistry of gum formation in cracked gasoline[J]. Industrial and Engineering Chemistry, 1949, 41(8): 1723-1729
[4] Nagpal J M, Joshi G C, Singh J. Gum forming olefinic precursors in motor gasoline. A model compound study[J].Fuel Sci Technol Int, 1994, 12(6): 873-94
[5] Kinoshita M, Saito A, Matsushita S, et al. Study of deposit formation mechanism on gasoline injection nozzle[C].SAE Paper 1998, 19:355-357
[6] Lacey P, Gail S, Kientz J M, et al. Internal Fuel Injector Deposits[C]. SAE Paper, 2011-01-1925
[7] Tanaka A, Yamada K, Omori T, et al. Inner Diesel Injector Deposit Formation Mechanism[C]. SAE Paper, 2013-01-2661
[8]. Gibbs L M. Gasoline Additives -When and Why[C]. SAE Paper, 902014
[9] Danilov A M. Progress in research on fuel additives (review)[J]. Petroleum Chemistry, 2015, 55(3): 169-179
[10] Sigworth, H W, Payne J Q. The Cause and Correction of Carburetor Gumming[C]. SAE Paper 540189
[11] Barusch M R, Mingle J G, Payne J Q, et al. The "World's First Detergent-Action Gasoline" (With 36 Years of Perspective)[C]. SAE Paper 900149
[12] González-García C M, González-Martín M L, Gómez-Serrano V, et al. Analysis of the adsorption isotherms of a non-ionic surfactant from aqueous solution onto activated carbons[J]. Carbon 2001, 39 (6): 849-855
[13] Soria-Sánchez M, Maroto-Valiente A, Guerrero-Ruiz A,et al. Adsorption of non-ionic surfactants on hydrophobic and hydrophilic carbon surfaces [J]. Journal of Colloid and Interface Science, 2010, 343 (1): 194-199
[14] Dubois-Clochard M C, Durand J P, Delfort B, et al.Adsorption of polyisobutenylsuccinimide derivatives at a solid-hydrocarbon interface[J]. Langmuir, 2001, 17(19):5901-5910
[15] Kozaka D, Moreton D, Vincent B. The adsorption of nonionic surfactants on carbon black particles in hydrocarbon media[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2009, 347(1/3): 245-250
[16] Pirouz S, Wang Yulin, l Chong J M, et al. Characterization of the chemical composition of polyisobutylene-based oilsoluble dispersants by fluorescence[J]. J Phys Chem B,2014, 118(14): 3899-3911
[17] Pirouz S, Wang Yulin, Chong J M, et al. Chemical modification of polyisobutylene succinimide dispersants and characterization of their associative properties[J]. J Phys Chem B, 2015, 119(37): 12202-12211
[18] Won Y Y, Meeker S P, Trappe V, et al. Effect of temperature on carbon-black agglomeration in hydrocarbon liquid with adsorbed dispersant [J]. Langmuir, 2005, 21(3): 924-932
[19] Alemán-Vázquez L O, Villagómez-Ibarra J R.Polyisobutenylsuccinimides as detergents and dispersants in fuel: Infrared Spectroscopy Application [J]. Fuel, 2001,80(7): 965-968
[20]Li Na, Long Jun, Zhao Yi, et al. Study on the mechanism of oxidation stability of typical gasoline hydrocarbon[J].Acta Petrolei Sinica (Petroleum Processing Section), under review (in Chinese)
[21] Mach H, Oppenlaender K, Rath H P, et al. Gasolineengine fuel containing polyetheramines or polyetheramine additive: US 5112364[P]. 1992-05-12
[22] Saha S K, Dutta A, Ghosh P, et al. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approach[J]. Phys Chem Chem Phys, 2016, 18(27):17898-17911
[23] Yu Yinzhe, Yang Dong, Zhang Daquan, et al. Anti-corrosion film formed on HAl77-2 copper alloy surface by aliphatic polyamine in 3 wt.% NaCl solution[J]. Applied Surface Science, 2017, 392: 768-776
[24] Charati S G, Stern S A. Diffusion of gases in silicone polymers: Molecular dynamics simulations [J].Macromolecules, 1998, 31(16): 5529-5535
[25] Tian Gaoyou, Zhang Chi, Tian Yongzhi. Study on the evaluation of detergent content in gasoline by the glial method [J]. Petroleum Products Application Research,2010 (2): 81-85 (in Chinese)
[26] Sandia National Laboratories, Lammps Users Manual[M].US: Sandia Corporation, 2004: 33-36
[27] Li X, Deng S, Fu H, et al. Adsorption and inhibition effect of 6-benzylaminopurine on cold rolled steel in 1.0 M HCl[J]. Electrochim Acta, 2009, 54(16): 4089-4098
[28] Mishra P M. Electron affinity calculation for selected PAHs using DFT: Effect of cyclopenta ring fusion and aromaticity[J]. Comput Theor Chem, 2015, 1068: 165-171
date: 2017-04-14; Accepted date: 2017-07-06.
Dr. Li Na, E-mail: lina.ripp@sinopec.com.
- 中國(guó)煉油與石油化工的其它文章
- A Study on Tribological Properties of Polypropylene Nanocomposites Reinforced with Pretreated HNTs
- Study on Quantitative Relationship between Surface Wettability and Frictional Coefficient of Liquid Flowing in a Turbulent Horizontal Pipe
- Study on the Hydrodynamic Characteristics of Venturi-Rod Deck Tray
- Behavior and Kinetics of Non-isothermal Pyrolysis of Coal at Different Heating Rates
- Characteristics of Hydrotreating Reaction in VRDS Units Located along the Yangtze River and Overall Solution for Long-cycle Running
- Synthesis of MIL-53(Fe)/MWCNTs Hybrid Material with Enhanced Efficiency for Photocatalytic Degradation of Rhodamine B

