Comparison of efficacy of folic acid and silymarin in the management of antiepileptic drug induced liver injury: a randomized clinical trial
Tehran, Iran
Comparison of efficacy of folic acid and silymarin in the management of antiepileptic drug induced liver injury: a randomized clinical trial
Masoumeh Asgarshirazi, Mamak Shariat and Mahdi Sheikh
Tehran, Iran
BACKGROUND: Liver injury associated with antiepileptic drugs accounts for a large proportion of drug-induced liver injuries (DILI) in children. Although withdrawal of the causative agent is the only proved treatment for DILI, in some clinical situations it is not possible. Recent studies have reported promising results of using hepatoprotective drugs with antioxidant actions for the management of DILI. This study aimed to evaluate the efficacy of folic acid versus silymarin treatment in relation to decreasing liver enzymes in patients with DILI due to antiepileptic therapy.
METHODS: This randomized, open-label, clinical trial evaluated 55 children with epilepsy who were on antiepileptic treatment and experienced DILI. The children were randomized to receive either silymarin (5 mg/kg per day) or folic acid (1 mg per day) for one month and were followed up for three months.
RESULTS: Liver enzymes significantly decreased in both groups. The decrease trend in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were stronger in the folic acid group compared to silymarin group (P=0.04 andP=0.007, respectively). At the end of the study patients in the folic acid group had significantly lower ALT (P=0.04), AST (P=0.02), and gamma-glutamyl transferase (GGT) (P<0.001) levels and also higher percentage of normal ALT (30.7% vs 3.4%,P=0.009) and AST (42.3% vs 0%,P<0.001), and GGT (23.1% vs 0%,P=0.008) values compared to the patients in the silymarin group. No rebound elevations in ALT, AST and GGT levels or adverse reactions were noted in neither of the study groups.
CONCLUSION: Although both treatments were safe and effective in decreasing liver enzymes, folic acid seems to be superior to silymarin in the management of DILI.
(Hepatobiliary Pancreat Dis Int 2017;16:296-302)
anticonvulsant; antiepileptic; enzymes; hepatitis; hepatotoxicity
Introduction
Drug-induced liver injury (DILI), is defined as biochemical abnormalities in liver tests or liver dysfunction due to medications or herbs and is usually diagnosed after exclusion of other etiologies.[1]DILI accounts for nearly 10% of cases of acute liver failure (ALF) in adults and 5%-10% of ALF in children,[1-4]and is the most common cause of ALF in the United States.[2,5]
Many drugs cause DILI. However, antimicrobial and antiepileptic drugs are the most recognized.[1,3]Liver injury associated with antiepileptic drugs accounts for as highly as 40% of DILI in children and can present through a wide range of hepatotoxic reactions from mild and transient elevations of liver enzymes to very serious liver failure leading to death or liver transplantation.[3,6,7]The management of DILI is largely supportive and withdrawal of the causative agent is the only proved treatment.[5]However, recent studies involving human and animal subjects reported promising results of using hepatoprotective drugs with antioxidant actions for the management of DILI.[5,8-15]This is especially important in clinical setting when drug withdrawal is not possible or when the patient experiences continuous rising in liver enzymes even after substituting the offensive drug, and also in the milder forms of DILI that manifest only through a moderate increase in liver enzymes before seriously impairing liver function.
Silybum marianum (milk thistle) is one of the oldest used plants in the treatment of hepatobiliary diseases and silymarin is the active constituent of this plant.[16]Silymarin treatment has been studied in patients with different liver injuries;[17]causing a significant improvement and decreased the liver enzymes in alcoholic liver disease,[18]non-alcoholic steatohepatitis (NASH),[19,20]amanita mushroom poisoning,[5]and viral hepatitis.[21,22]Different hepatoprotective activities of silymarin is recognized including antioxidant, cell membrane stabilizing, stimulating liver regeneration, and anti-inflammatory activities.[16]
Folic acid is the synthetic form of vitamin B9, which is an essential nutrient for the body with strong antioxidant activity. Folic acid supplementation has been studied in animal models with liver injury caused by alcohol, paracetanol and arsenic consumption,[14,15,23]and also in human subjects with methotrexate induced liver injury and also in patients with elevated aminotransferases with unknown liver diseases;[24,25]in these studies folic acid supplementation decreased liver enzymes and attenuated the hepatotoxic effects of the drugs.[14,15,23-25]The hepatoprotective effects of folic acid is due to its strong antioxidant and reactive oxygen species (ROS) scavenging activities.[14,15,23-25]
Most of the published studies have evaluated the effects of silymarin and folic acid in animal models of DILI. By our extensive search we could not find enough studies assessing the efficacy of silymarin and folic acid in patients with DILI, especially those caused by antiepileptic drugs. Since liver injury due to antiepileptic drugs accounts for a large number of pediatric cases with DILI,[3]that can potentially progress to life threatening conditions,[6,7]studies are required to evaluate the efficacy of hepatoprotective drug supplementation in children who are on antiepileptic treatment and who experience continuous rising in liver enzymes despite lowering the dose or substituting the offensive drug. Given the fact that antiepileptic treatment is associated with a signif icant decrease in folate levels and folic acid treatment is recommended in epileptic patients,[26]folic acid supplementation in these patients might be beneficial for both diseases control and hepatoprotection.
We conducted this study to evaluate the efficacy of silymarin and folic acid supplementation in relation to decreasing and normalization of liver enzymes in pediatric patients on antiepileptic treatment who experienced a continuous rise in liver enzymes despite lowering the dose or substituting the offensive drug.
Methods
Study population and study designThis prospective, randomized, open-label, clinical trial was conducted on 55 children with epilepsy who were on antiepileptic treatment and experienced DILI. The participants were recruited from the pediatric neurology clinic of our institute from February 2014 to February 2015. Participants were considered eligible when they were less than 18 years of age and were on antiepileptic treatment due to con firmed epilepsy and experienced DILI as a continuous rise in liver enzymes despite decreasing the dose of antiepileptic drugs or changing the antiepileptic drugs when possible. Exclusion criteria were: liver injury caused by viral, autoimmune or metabolic diseases, aminotransferase levels below three times the upper normal limit, spontaneous decrease in aminotransferase levels within two months preceding the beginning of treatment with silymarin or folic acid, having a functional liver failure presenting with icterus, coagulopathy or encephalopathy, anatomic abnormalities, and parents refusal to give an informed consent. After explaining the whole procedure, an informed written consent was obtained from the parents. This study was approved by the Ethics Committee of our institute. This study is registered at the Iranian Registry of Clinical Trials (www.irct.ir) which is a Primary Registry in the WHO Registry Network (Registration Number: IRCT2015010711392N2).
In this study, we used simple randomization with a 1:1 allocation ratio, and sequence generations was carried out using a computerized random number generator which was performed by Sheikh M. Consecutive opaque envelopes was used for the allocation concealment which was carried out by Shariat M. The envelopes were opened sequentially after the participant’s name and other details were written on them. The implementation of assignments was carried out by Asgarshirazi M.
The primary outcome of our study was the decrease and normalization of liver enzymes during the study period. The secondary outcomes were the decreasing trend and the rebound elevation of enzymes after cessation of the treatment. Rebound elevation of liver enzymes was de fined as an elevation of the liver enzymes of more than twice the upper limit of the normal range after cessation of the treatment for DILI.
Data and specimen collection
All the participants were diagnosed with epilepsy and were on antiepileptic treatment at least for three months before recruitment. The participants were referred from the pediatric neurology clinic because of the continuous rise in liver enzymes despite the efforts to reduce the dose of antiepileptic drugs to minimal effective dose or if possible substituting the offensive drug with a saferantiepileptic drug. Upon enrollment complete liver function tests including serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total and conjugated bilirubin, alkaline phosphatase (ALP), albumin, prothrombin time (PT), partial thromboplastin time (PTT) and international normalized ratio (INR) were performed for all participants at one laboratory. DILI was defined based on the FDA Center for Drug Evaluation and Research and the American Association for the Study of Liver Diseases as the following: an ALT and/or AST level of more than three times the upper limit of the normal range, and an ALP level of more than twice the upper limit of normal range after excluding other causes of liver injury.[27]Additionally to rule out other causes of liver injury the following serologic tests were performed on all the participants: hepatitis A virus (HAV) immunoglobulin M (IgM), hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) IgM, hepatitis C virus antibody (HCV Ab), Epstein-Barr virus viral-capsid antigen (EBV-VCA) IgM, cytomegalovirus (CMV) IgM, antinuclear antibody (ANA), anti-mitochondrial antibodies (AMA), anti-smooth muscle antibodies (ASMA), and antineutrophil cytoplasmic antibodies (ANCA). The children then underwent metabolic screening by measuring blood sugar in ortho and glucose oxidase manner, serum amino acids chromatography, and blood ammonia and lactate level. An arterial blood gas (ABG) analysis was performed and urine analysis was carried out for detecting ketones, reducing substances and organic acids. Furthermore, abdominal ultrasound imaging was performed for all the children by the same specialist for the detection of possible structural abnormalities. Two months were spent for the complete investigation before starting the treatment allocation. Any patient who experienced a decrease in aminotransferase levels during this period was excluded from the study. The children then were randomly assigned in two groups: the silymarin treated group and the folic acid treated group. Both groups received the allocated treatment for one month; silymarin (70 mg tablets) was used in a dose of 5 mg/kg once per day, and folic acid (1 mg tablets) was used in a dose of 1 mg once per day. Complete liver function tests were checked before the intervention and then every two weeks during the trial and monthly following the intervention for two months. Possible side effects of silymarin and folic acid including allergic reactions, nausea, epigastric discomfort, arthralgia, pruritus and headache were monitored during the study.
Statistical analysis
Sample size was calculated for a power of 80%, α=0.05, β=20%, and a standard effect size of 0.87, where at least 22 participants would be required in each of the study groups. All the statistical analyses were performed using SPSS statistical software (version 18.0.0: PASW, Chicago, IL, USA). Chi-squared analysis and Fisher’s exact test were used to analyze the dichotomous variables, while the paired-samplesttest, independent-samplesttest, and repeated measures ANOVA were used to analyze the continuous variables. APvalue 〈0.05 was considered statistically significant.
Results
Descriptive statistics
A total of 80 children were considered eligible for this study. Twenty children were excluded according to the exclusion criteria; of these 9 had aminotransferase levels below three times the upper normal limit, 6 had spontaneous decrease in aminotransferase levels within two months before treatment, and 4 had functional liver failure and 1 was excluded due to parental request. Further, at the end of the study 5 were lost to follow-up and were not included in further analysis (Fig. 1).
We evaluated 55 children who were diagnosed with epilepsy and experienced DILI under antiepileptic treatment and who remained to the end of the study. Twentysix children remained in the randomly assigned folic acid treated group and 29 in the silymarin treated group. At enrollment 34 children (61.8%) were male. The mean ± standard deviation (SD) for the participants’ age was 50.8±41.7 months; antiepileptic treatment duration was 5.7±2.3 months; ALT was 106.8±32.1 U/L; AST was83.2 ±29.3 U/L; GGT was 107.0±26.6 U/L; and ALP was 811.7±102.0 U/L.
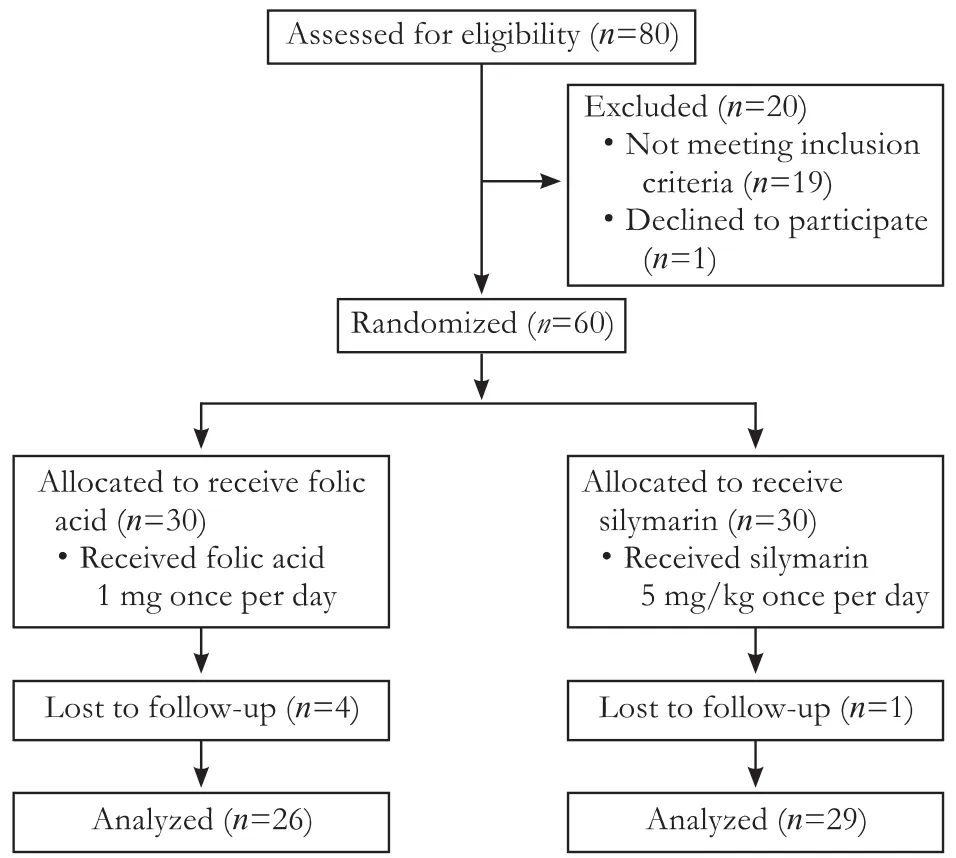
Fig. 1. Flowchart of the study showing patients randomization, intervention and follow-up.
The used antiepileptic drugs were primidone, valproic acid, levetiracetam and phenobarbital alone or any combination of them. There were no significant differences in the demographics or the used antiepileptic drugs between the two study groups (Table 1).
Liver enzymes in the study groups
At the end of the study liver enzymes were signif icantly decreased in both silymarin and folic acid groups. Patients treated with silymarin for three months significant decreased ALT (P〈0.001), AST (P〈0.001), and GGT (P=0.003) values, while the decrease was not statistically significant in the ALP values (P=0.22) (Fig. 2). ALT (P〈0.001), AST (P〈0.001), GGT (P=0.01) and ALP (P=0.002) values (Fig. 2) were significantly decreased in patients treated with folic acid for three months. No rebound elevation was noted in ALT, AST, GGT and ALP levels in both of the treatment groups.

Table 1. Comparison of the demographics, baseline labs and antiepileptic drugs used by the children randomized to folic acid versus silymarin treatment group
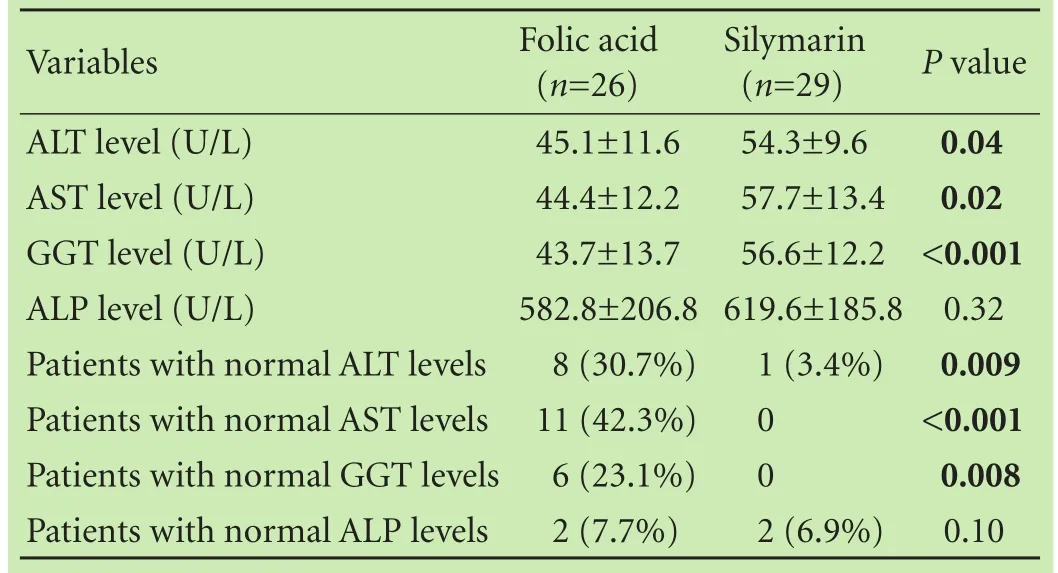
Table 2. Comparison of the liver enzymes and study outcomes at the end of the study between the participants randomized to folic acid versus silymarin treatment groups
Folic acid versus silymarin
At the end of the study higher percentage of children in the folic acid treated group had normal ALT (30.7% vs 3.4%,P=0.009), AST (42.3% vs 0%,P〈0.001) and GGT (23.1% vs 0%,P=0.008) values compared to that in the silymarin treated group (Table 2).
The decrease trend in ALT and AST were stronger in the folic acid group compared to that in the silymarin group (P=0.04 andP=0.007, respectively). The decrease trends were not statistically different in GGT and ALP between the two study groups (P=0.64 andP=0.47, respectively).
At the end of the study patients in the folic acid group had significantly lower ALT (P=0.04), AST (P=0.02), GGT (P〈0.001) levels compared to those in the silymarin group. However ALP levels were not significantly different between the two study groups (P=0.32; Table 2, Fig. 2). No adverse reactions or side effects occurred during the study period in all of the treatment patients.
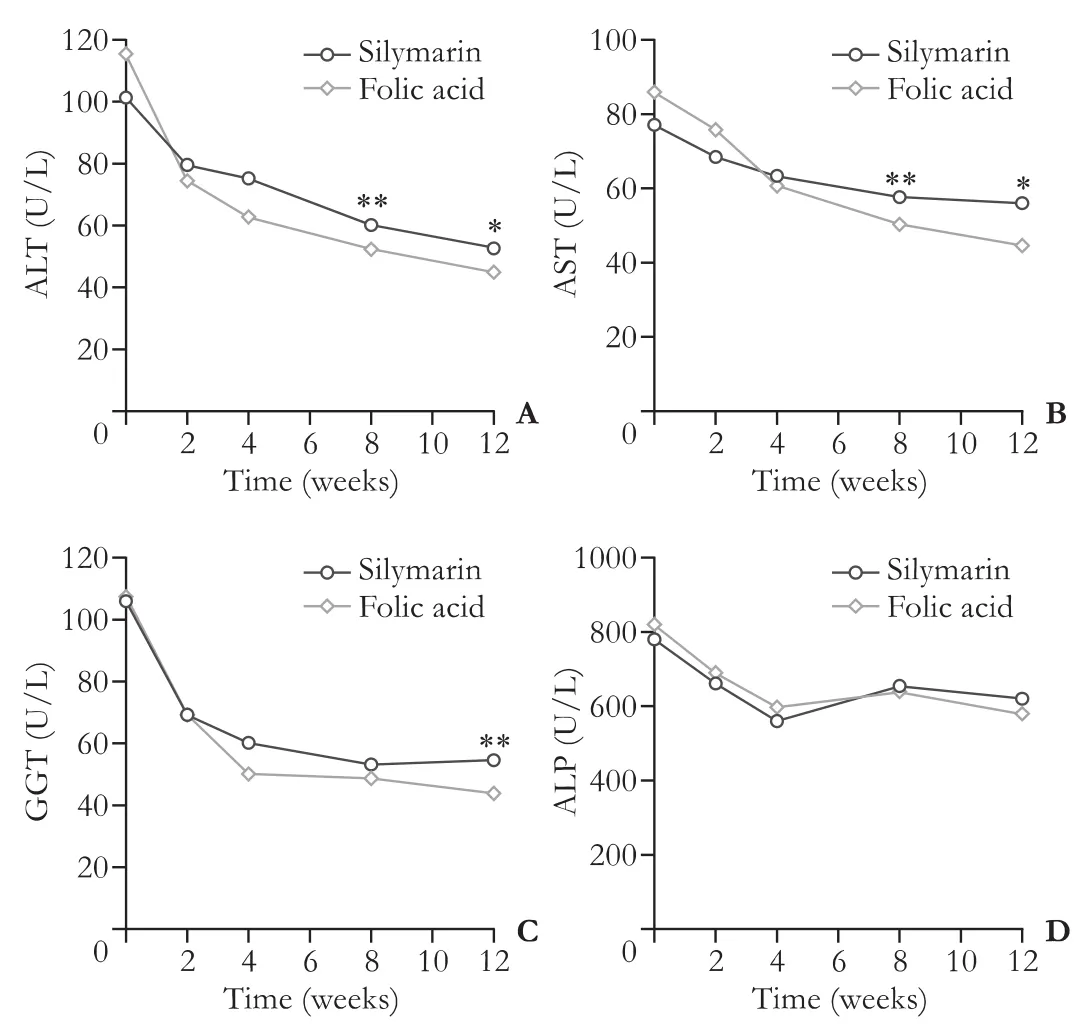
Fig. 2. Comparison of the efficacy of folic acid versus silymarin treatment on decreasing ALT (A), AST (B), GGT (C) and ALP (D) levels. *: P〈0.05, **: P〈0.001.
Discussion
DILI is a common concern in antiepileptic therapy especially when multiple drugs are used. In the hepatic metabolism of the drugs, activation and detoxification are two crucial steps.[28]In the phase 1 reactions (activation) that is mediated by cytochrome P450, oxidation or demethylation occurs.[28]The formed active metabolite is hydrophobic and toxic for cells thus must be changed to hydrophilic, nontoxic molecules during the second phase (detoxification) to be excreted through the urine or bile. Any imbalance between these two successive steps can lead to liver injury.[28]Anticonvulsive drugs are commonly enzyme inducer for cytochrome P450 in liver and can dispose liver parenchyma to injury by produced metabolites. In multiple drugs regimen the mechanism of injury is believed to involve the induction of cytochrome P450 by one agent, which increases the quantity of the toxic metabolite formed from the others. The formation of reactive metabolites plays the key role in the pathogenesis of DILI which can bind to proteins thus triggering the immune response or causing a direct toxicity.[29]These metabolites can also cause cytosolic oxidative stress resulting from an imbalance of ROS formation and their detoxification. ROS directly damages DNA, proteins, enzymes, and lipids and triggers liver damage.[29]
The management of DILI is largely supportive, and withdrawal of the offensive drug is the only proven treatment.[5,8]However, there are some clinical circumstances when drug withdrawal is not possible or when the patient experiences continuous rise in liver enzymes even after substituting the offensive drug, therefore some studies evaluated using hepatoprotective drugs with antioxidant activities on human and animal subjects with DILI that showed promising results.[5,8-15]Except for acetaminophen poisoning which should be treated with N-acetylcysteine as the available antidote, other treatments are still experimental.[5,8]
Our study showed that silymarin treatment signif icantly decreased liver enzymes in patients with DILI due to antiepileptic drugs. Silymarin has been used to treat liver diseases since the 16th century.[16]Many studies have documented the hepatoprotective role of silymarin in different liver diseases,[17]including alcoholic liver disease,[18]NASH,[19,20]and viral hepatitis.[21,22]Silymarin has been also suggested as a suitable candidate for the treatment of iatrogenic and toxic liver diseases.[16]The hepatoprotective role of silymarin is maintained through four different mechanisms: (i) as antioxidant, scavenger and regulator of the intracellular content of glutathione; (ii) as cell membrane stabilizer and permeability regulator that prevent hepatotoxic agent from entering hepatocytes; (iii) as a promoter of ribosomal RNA synthesis, stimulating liver regeneration; and (iv) as an inhibitor of the transformation of stellate hepatocytes into miofibroblasts, the process responsible for the deposition of collagen fibers leading to cirrhosis. Anti-inflammatory and anticarcinogenic properties of silymarin have also been documented.[16]
The current study showed for the first time that folic acid supplementation significantly decreases liver enzymes in patients with DILI due to antiepileptic drugs. Folic acid supplementation had been evaluated recently in some studies involving animal models with toxic liver injuries due to alcohol, arsenic and paracetanol.[14,15,23]These studies showed that folic acid supplementation decreased liver enzymes in animals with toxic liver injury, the results were supported by histopathological examination revealing marked decrease in inflammatory cells numbers at the periportal area and reduction in degenerative changes to reach almost normal hepatic morphology by folic acid supplementation.[14,15,23]Suzuki et al[24]and Qin et al[25]in their studies tried folic acid supplementation in rheumatic and hypertensive patients with sustained elevated liver enzymes without known liver diseases; in both studies folic acid significantly decreased liver enzymes. The hepatoprotective effects of folic acid is believed to be due to its strong antioxidant and ROS scavenging activities eliminating the key step in the pathogenesis of DILI.[14,15,23-25]Another mechanism is believed to be through the role of folate being a methyl donor in methylation reactions that could decrease liver injury caused by the active metabolites of the drugs which were produced in the phase 1 reaction of the hepatic drugs metabolism.[15]
Our study showed that both silymarin and folic acid treatments were safe and well tolerated and no adverse reactions were noted. The safety of silymarin in the treatment of liver diseases has been investigated by several systematic reviews which demonstrated that silymarin is safe.[30,31]Additionally, silymarin reduces liver-related mortality.[30]Majority of the study showed that folic acid supplementation is safe.[26,32-35]However, in special populations such as in patients with epilepsy dosing restric-tion persists and more studies are required to assess the optimal dosage of folic acid supplementation.[26,35]The possible side effects of silymarin and folic acid are listed in Table 3.[26,32-35]

Table 3. The possible adverse effects of silymarin and folic acid treatments
The current study is the first to compare folic acid versus silymarin in the treatment of liver diseases. Although both silymarin and folic acid treatment signif icantly reduced liver enzymes in patients with DILI due to antiepileptic treatment, folic acid was superior to silymairn; participants in the folic acid group experienced faster and more efficient decrease in the enzyme levels compared with those in the silymarin group. In addition to its hepatoprotective roles, folic acid supplementation is recommended in patients on antiepileptic treatment,[26]because more than 90% of patients receiving cytochrome P450 inducer antiepileptic drugs suffer from decreased folate levels.[26]These patients usually have elevated homocysteine levels due to folate deficiency and therefore are at increased risk of vascular and cerebral diseases.[26]Therefore, folic acid supplementation in patients with DILI due to antiepileptic treatment seems to be beneficial for both hepatic protection and folate def iciency and its consequences.
The strength of this study was evaluating for the first time the effect of folic acid treatment in human subjects with DILI due to antiepileptic drugs, this study is also the first to compare the efficacy of folic acid versus silymarin in pediatric population with liver diseases. This study, however, has some limitations that should be considered when interpreting the results; this study was not blinded. However, treatment groups were similar. We don’t have placebo control due to ethical issues, because the children experienced continuous rise in liver enzymes despite lowering the dose of antiepileptic drugs and changing the drug when possible. Another limitation was not measuring folate levels in the study groups before interventions. Not performing liver biopsy for more accurate results and not having information regarding long-term follow-up of the children are other limitations.
In conclusion, both silymarin and folic acid treatments are highly effective in decreasing liver enzymes in pediatric patients with DILI due to antiepileptic drugs. However, folic acid seems to be superior to silymarin because it is associated with a stronger decrease trend and higher improvement rate. These treatments could be applied when withdrawal of the offensive drug is not possible or when the patient experiences continuous rise in liver enzymes even after substituting the offensive drug.
Acknowledgement:The authors thank Dr. Zarrin Keihanidoost for her contribution in data gathering.
Contributors:AM and Sheikh M wrote the first draft. AM, Shariat M and Sheikh M collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. Sheikh M is the guarantor.
Funding:This study was supported by a grant from the research deputy of Tehran University of Medical Sciences.
Ethical approval:This study was approved by the Research Deputy and the Ethics Committee of Tehran University of Medical Sciences (ID: 1391-1075).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol 2012;18:249-257.
2 Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis 2000;4:73-96.
3 Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N, et al. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr 2011;53:182-189.
4 Zhu XX, Zhu Y, Wan CM. Clinical features of drug-induced liver injury in children. Zhongguo Dang Dai Er Ke Za Zhi 2012;14:131-133.
5 Giordano CM, Zervos XB. Clinical manifestations and treatment of drug-induced hepatotoxicity. Clin Liver Dis 2013;17: 565-573.
6 Hussein RR, Soliman RH, Ali AM, Tawfeik MH, Abdelrahim ME. Effect of antiepileptic drugs on liver enzymes. Beni-Suef Univ J Basic Appl Sci 2013;2:14-19.
7 Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity: a review. J Appl Pharm Sci 2012;2:233- 243.
8 Devarbhavi H. An Update on Drug-induced Liver Injury. J Clin Exp Hepatol 2012;2:247-259.
9 Asgarshirazi M, Shariat M, Dalili H, Keihanidoost Z. Ursodeoxycholic acid can improve liver transaminase quantities in children with anticonvulsant drugs hepatotoxicity: a pilot study. Acta Med Iran 2015;53:351-355.
10 Gillessen A, Herrmann WA, Kemper M, Morath H, Mann K. Effect of silymarin on liver health and quality of life. Results of a non-interventional study. MMW Fortschr Med 2014;156:120-126.
11 Upadhyay G, Kumar A, Singh MP. Effect of silymarin on pyrogallol- and rifampicin-induced hepatotoxicity in mouse. Eur J Pharmacol 2007;565:190-201.
12 Jaydeokar AV, Bandawane DD, Bibave KH, Patil TV. Hepatoprotective potential of Cassia auriculata roots on ethanol and antitubercular drug-induced hepatotoxicity in experimental models. Pharm Biol 2014;52:344-355.
13 Suzuki A, Yuen NA, Ilic K, Miller RT, Reese MJ, Brown HR, et al. Comedications alter drug-induced liver injury reporting frequency: Data mining in the WHO VigiBase?. Regul Toxicol Pharmacol 2015;72:481-490.
14 AL-Sowyan NS. Efficacy and safety of folic acid during toxic hepatitis induced by acute overdose of paracetamol. Int J Pharmacol 2009;5:208-214.
15 Chattopadhyay S, Deb B, Maiti S. Hepatoprotective role of vitamin B(12) and folic acid in arsenic intoxicated rats. Drug Chem Toxicol 2012;35:81-88.
16 Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res 2006;124:491-504.
17 Matveev AV, Koniaeva EI, Kurchenko VP, Shchekatikhina AS. Hepatoprotective properties of silymarin. Eksp Klin Gastroenterol 2011:130-135.
18 Nanda V, Gupta V, Sharma SN, Pasricha A, Karmakar AK, Patel A, et al. Effect of Liverubin? on hepatic biochemical profile in patients of alcoholic liver disease: a retrospective study. Minerva Med 2014;105:1-8.
19 Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Caspian J Intern Med 2014;5:9-12.
20 Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol 2013;5:109-113.
21 Wei F, Liu SK, Liu XY, Li ZJ, Li B, Zhou YL, et al. Metaanalysis: silymarin and its combination therapy for the treatment of chronic hepatitis B. Eur J Clin Microbiol Infect Dis 2013;32:657-669.
22 Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat 2005;12:559-567.
23 Lee SJ, Kang MH, Min H. Folic acid supplementation reduces oxidative stress and hepatic toxicity in rats treated chronically with ethanol. Nutr Res Pract 2011;5:520-526.
24 Suzuki Y, Uehara R, Tajima C, Noguchi A, Ide M, Ichikawa Y, et al. Elevation of serum hepatic aminotransferases during treatment of rheumatoid arthritis with low-dose methotrexate. Risk factors and response to folic acid. Scand J Rheumatol 1999;28:273-281.
25 Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. Effect of folic acid intervention on ALT concentration in hypertensives without known hepatic disease: a randomized, double-blind, controlled trial. Eur J Clin Nutr 2012;66:541-548.
26 Morrell MJ. Folic acid and epilepsy. Epilepsy Curr 2002;2:31-34.
27 FDA Working Group. CDER-PhRMA-AASLD Conference 2000: clinical white paper on drug-induced hepatotoxicity, November 2000.
28 Lee WM. Drug-induced hepatotoxicity. N Engl J Med 1995;333: 1118-1127.
29 Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Druginduced liver injury: Interactions between drug properties and host factors. J Hepatol 2015;63:503-514.
30 Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs 2001;61:2035-2063.
31 Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed 2008;15:9-20.
32 Campbell NR. How safe are folic acid supplements? Arch Intern Med 1996;156:1638-1644.
33 Santos Qd, Sichieri R, Marchioni DM, Verly E Jr. Evaluation of the safety of different doses of folic acid supplements in women in Brazil. Rev Saude Publica 2013;47:952-957.
34 Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, et al. Effects of folic acid supplementation on overall and sitespecific cancer incidence during the randomised trials: metaanalyses of data on 50000 individuals. Lancet 2013;381:1029-1036.
35 Asadi-Pooya AA. High dose folic acid supplementation in women with epilepsy: are we sure it is safe? Seizure 2015;27:51-53.
November 21, 2015
Accepted after revision May 19, 2016
Correction
10.1016/S1499-3872(17)60032-8)
Author Affiliations: Department of Pediatrics, Vali-asr Hospital, Tehran University of Medical Sciences, Tehran, Iran (Asgarshirazi M); Maternal, Fetal and Neonatal Research Center, Tehran University of Medical Sciences, Tehran, Iran (Shariat M and Sheikh M)
Mahdi Sheikh, MD, PhD, Maternal, Fetal and Neonatal Research Center, Vali-asr Hospital, Imam Khomeini Hospital complexes, Keshavarz blvd, Tehran 1419733141, Iran (Tel: +98-912-8481663; Fax: +98-21-22834332; Email: mahdisheikh@gmail.com)
? 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
doi: 10.1016/S1499-3872(16)60142-X
Published online September 29, 2016.
In the article entitled “The diversity between pancreatic head and body/tail cancers: clinical parameters and in vitro models” by Ling et al (Hepatobiliary Pancreat Dis Int2013;12:480-487), the No. of Zhejiang Educational Committee in Funding should be Y201120605.
 Hepatobiliary & Pancreatic Diseases International2017年3期
Hepatobiliary & Pancreatic Diseases International2017年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Circulating autoantibodies to endogenous erythropoietin are associated with chronic hepatitis C virus infection-related anemia
- Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance
- A clinical analysis of acute pancreatitis in pregnancy
- Patients with early recurrence of hepatocellular carcinoma have poor prognosis
- Hepatobiliary & Pancreatic Diseases International
- Traditional surgical planning of liver surgery is modified by 3D interactive quantitative surgical planning approach: a single-center experience with 305 patients
