Patients with early recurrence of hepatocellular carcinoma have poor prognosis
Hiroshima, Japan
Patients with early recurrence of hepatocellular carcinoma have poor prognosis
Tomoki Kobayashi, Hiroshi Aikata, Tsuyoshi Kobayashi, Hideki Ohdan, Koji Arihiro and Kazuaki Chayama
Hiroshima, Japan
BACKGROUND: Early recurrence (ER) after hepatic resection (HR) is a poor prognostic factor for patients with hepatocellular carcinoma (HCC). This study aimed to identify the clinicopathological features, outcomes, and risk factors for ER after HR for small HCC in order to clarify the reasons why ER is a worse recurrence pattern.
METHODS: We retrospectively examined 130 patients who underwent HR for small HCC (≤30 mm). Recurrence was classified into ER (<2 years) and late recurrence (LR) (≥2 years). The clinicopathological features, outcomes, and risk factors for ER were analyzed by multivariate analysis.
RESULTS: ER was observed in 39 patients (30.0%). The survival rate of the ER group was significantly lower than that of the LR group (P<0.005), and ER was an independent prognostic factor for poor survival (P=0.0001). The ER group had a significantly higher frequency (P=0.0039) and shorter interval (P=0.027) of development to carcinoma beyond the Milan criteria (DBMC) compared with the LR group, and ER was an independent risk factor for DBMC (P<0.0001). Multi-nodularity, non-simple nodular type, and microvascular invasion were independent predictors for ER (P=0.012, 0.010, and 0.019, respectively).
CONCLUSIONS: ER was a highly malignant recurrence pattern associated with DBMC and subsequent poor survival after HR for small HCC. Multi-nodularity, non-simple nodular type, and microvascular invasion predict ER, and taking these factors into consideration may be useful for the decision of the treatment strategy for small HCC after HR.
(Hepatobiliary Pancreat Dis Int 2017;16:279-288)
early recurrence; small hepatocellular carcinoma; risk factors; beyond the Milan criteria
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and it ranks sixth in cancer incidence and third in cancer mortality worldwide.[1]The establishment of surveillance for patients at high risk of HCC, such as those with hepatitis B or C virus (HBV/HCV) infection and liver cirrhosis, and recent advances in diagnostic modalities, have led to an increased detection rate of tumors at an early stage. Specifically, these tumors are solitary and small, and they represent an opportunity for radical treatment.[2-4]However, the prognosis of HCC patients remains poor because of the high incidence of recurrence, even in patients with small HCC.[5]Thus, it is critical in the management of HCC to predict recurrence patterns (interval until postoperative recurrence) before treatment decisions are made and to identify their mechanisms because the prognosis of recurrent HCC after hepatic resection (HR) strongly depends on recurrence patterns.[5-10]Therefore, strategies based on recurrence patterns should be considered in order to improve the prognosis of HCC patients.
Recurrence of HCC is thought to have two main mechanisms: one is intrahepatic metastasis (IM) from the primary tumor caused by dissemination of tumor cells in the portal vein, and the other isde novomulti-centric carcinogenicity as a result of persistent hepatitis and fibrosis in the remnant liver.[7]Early recurrence (ER) within 2 years after HR is mainly related to IM and is associated with aggressive tumor biology.[7-9]Further, ER is the leading cause of early death after HR[5,8]and the interval from HR to recurrence is an independent prognostic factor for survival after recurrence.[6]On the other hand, late recurrence (LR) more than 2 years after HR is mainly related to multicentric carcinogenicity and is associated with a background of chronic inflammatory liver disease and cirrhosis.[7]LR has a relatively good prognosis because of the establishment of effective treatments for underlying liver disease and cirrhosis, namely, anti-viral therapy for HBV and HCV, such as interferon (IFN) therapy,[10,11]nucleoside analogue (NA) therapy,[12]and direct-acting antiviral agents (DAAs). Furthermore, most patients with LR have carcinoma within the Milan criteria and many treatment options are available such as re-resection, percutaneous ablation therapy, and salvage liver transplantation (LT).[13]Accordingly, the strategies for ER may be the key to improving the prognosis of HCC patients, and the identification of clinicopathological features and risk factors for ER may be useful for guiding the management of HCC. On the other hand, it is unclear why ER is a worse recurrence pattern, and so far, no study has investigated the reasons for this, although ER is well known as a poor prognostic factor.
The aim of this study was to identify the clinicopathological features, outcomes, and risk factors for ER after HR for small HCC. In particular, we focused on the differences of clinical course after recurrence (recurrence features) between the ER and LR groups in order to clarify the reasons why ER is a worse recurrence pattern.
Methods
Patients
Five hundred thirty-seven HCC patients who received curative HR as initial treatment at Hiroshima University Hospital between October 2003 and November 2012 were considered as candidates for this study. Curative HR was defined as resection of all macroscopic residual tumors. Inclusion criteria were as follows: (1) diagnosis of HCC confirmed by typical radiological imaging (the tumor demonstrates hepatic arterial enhancement and fades during the portal venous and equilibrium phases) along with postoperative pathological examination; (2) no radiological evidence of vascular invasion or extrahepatic metastasis; (3) tumors ≤30 mm in maximum diameter; (4) tumor number ≤3; and (5) no preoperative treatment for HCC such as transarterial chemoembolization (TACE) or radiofrequency ablation (RFA). In patients with multiple HCCs, the largest tumor was analyzed. Finally, 130 patients met these criteria and were enrolled (Fig. 1). This study did not require approval from the institutional ethics committee or informed consent, and complied with the principles ofthe Declaration of Helsinki.
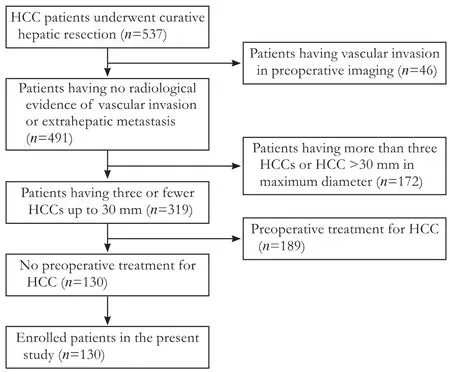
Fig. 1. Flow chart of the patient selection process in this study.
Clinicopathological variables
Data on clinicopathological variables such as age, gender, etiology of liver disease, presence or absence of cirrhosis, Child-Pugh classification, platelet count, alanine aminotransferase (ALT), total bilirubin (T-Bil), prothrombin time (PT), albumin (Alb), indocyanine green retention rate at 15 minutes (ICG-R15), α-fetoprotein (AFP), lens culinaris agglutinin A-reactive AFP (AFP-L3), des-γ-carboxy-prothrombin (DCP), surgical procedure, tumor size, tumor number, macroscopic classification, tumor differentiation, intrahepatic micrometastasis, microvascular invasion (MVI), surgical margin, and tumor stage were obtained and analyzed. All tumor-related factors were determined by pathological assessment of resected tissue. We defined tumor size as the maximum diameter of the resected tumor specimen. When different tumor grades were found within the same tumor, the predominant grade was used in the analysis. Macroscopic classification was divided into five types according to the classification of the Liver Cancer Study Group of Japan (LCSGJ):[14](i) small nodular type with indistinct margins (SN-IM), (ii) simple nodular type with distinct margins (SN-DM), (iii) simple nodular type with extranodular growth (SN-EG), (iv) confluent multinodular type (CMN), and (v) infiltrative type (IF). Further, SN-IM and SN-DM were grouped as simple nodular (SN)-type, and SN-EG, CMN and IF as non-simple nodular (non-SN)type because they have higher malignant potential than SN-IM and SN-DM.[15,16]Tumor stage were assessed using the TNM stage definition proposed by the LCSGJ (Table 1).[14]
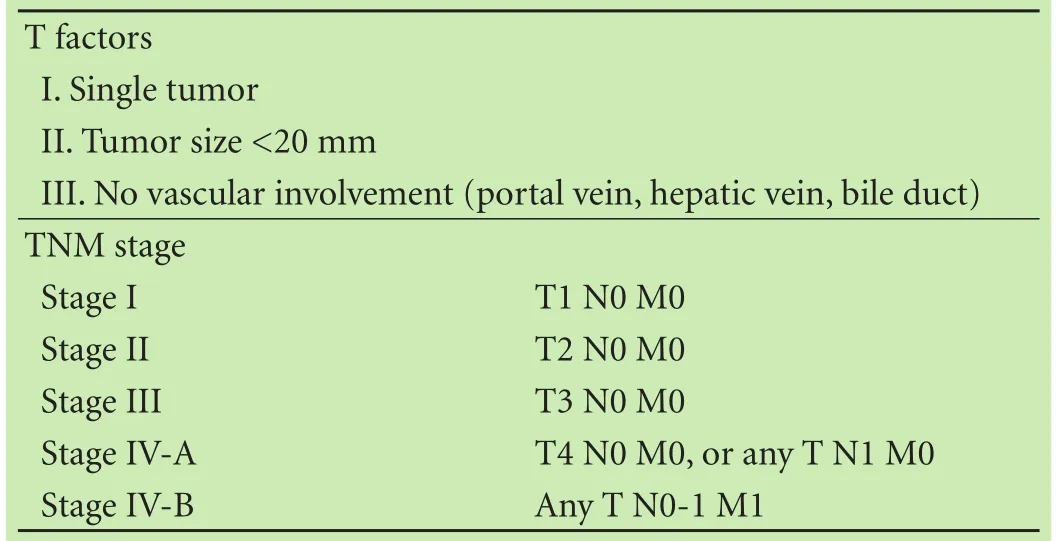
Table 1. Definition and criteria of the TNM stage for HCC according to the LCSGJ[14]
Surgical procedures
Hepatectomy was indicated when it was judged on preoperative imaging that all tumors could be resected with preservation of suf ficient hepatic function. The type of hepatectomy selected was based on liver function and tumor extent.[17,18]Liver function was assessed using the Child-Pugh classi fication and ICG-R15.[19]If liver function was suf ficient, anatomic resection (segmentectomy, sectionectomy or hemihepatectomy) was perfomed.[20,21]In patients with insuf ficient hepatic reserve, limited (non-anatomical) resection was performed. The procedures of hepatectomy were the same as those described previously.[17]
Follow-up and treatment of recurrence
All patients were regularly followed to identify postoperative recurrence by assessing tumor markers such as AFP, AFP-L3, and DCP or ultrasonography (US) or contrast-enhanced computed tomography (CT) every 3 months in the first year and every 3-6 months in the subsequent years after surgery. If recurrence was suspected, contrast-enhanced CT, contrast-enhanced US (CEUS), or gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) was performed to con firm the diagnosis. If necessary, CT angiography or18F- fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET-CT) were also performed. Recurrence was de fined as the detection of a new lesion with typical radiological imaging findings of HCC (the tumor demonstrates hepatic arterial enhancement and fades during the portal venous and equilibrium phases), and histopathological con firmation of tumor recurrence if the patient underwent hepatic reresection.
Patients were divided into the ER group, LR group, and no-recurrence group. With regard to the pattern of recurrence, patients were divided into those within the Milan criteria (WMC; absence of extrahepatic metastasis and a single tumor nodule with a diameter of up to 5 cm, or three or fewer tumors with the largest tumor of diameter less than or equal to 3 cm) and those beyond the Milan criteria (BMC).
When recurrence was diagnosed, the therapeutic strategy was evaluated regardless of the time and pattern of recurrence. If the recurrence was in patients with good hepatic functional reserve, there was no problem with underlying disease, and the patient consented, reresection or LT was performed. Further, RFA, or stereotactic body radiation therapy was considered if re-resection and LT was impossible. On the other hand, if the recurrence was diffuse or patients did not have enough hepatic functional reserve for curative treatment, TACE, hepatic arterial infusion chemotherapy, or systemic chemotherapy as a palliative procedure was selected as appropriate. Follow-up of all patients was concluded by the end of March 2015 or if the patient died.
Statistical analysis
All values were expressed as mean±SD, median (range), or number (%). For paired groups, continuous variables were compared by Mann-WhitneyUtest and categorical variables by Chi-square test or Fisher’s exact test. Cumulative survival rate, development to carcinoma beyond the Milan criteria (DBMC) rate, and ER rate were analyzed by the Kaplan-Meier method and the differences were analyzed by the log-rank test. The Cox proportional hazards model was used to identify the risk factors for survival and DBMC. Independent risk factors for ER and LR were identi fied by univariate logistic regression analysis. Factors that were signi ficant in univariate analysis withP〈0.05 were included in multivariate analysis. APvalue of 〈0.05 was considered statistically signi ficant. All statistical analyses were performed using SPSS version 11 for Windows (SPSS Chicago, IL, USA).
Results
Prognosis and treatment after recurrence
All patients’ characteristics are shown in Table 2. Recurrence was observed in 74 patients (56.9%) with amean time to recurrence of 36.9±29.5 months (range 1.1-126.0). Of these 74 patients, 39 (52.7%) were ER and 35 (47.3%) were LR. There were 40 deaths (30.8%). The causes of death were tumor recurrence or progression (n=20, 15.4%), liver failure (n=9, 6.9%), and non-liver related-causes (n=11, 8.5%). There was only 1 death (2.9%) among 35 patients in the LR group during the if rst 5-year follow-up after HR, whereas 13 (33.3%) of 39 patients died in the ER group (P=0.008). The cumulative survival rates for the ER group, LR group, and norecurrence group were 76.4%, 100.0%, and 93.5% at 3 years, 58.5%, 94.0%, and 93.5% at 5 years, and 20.5%, 66.9%, and 93.5% at 10 years, respectively (Fig. 2). The survival rate of the no-recurrence group was signi ficantly higher than that of other groups (bothP〈0.0001), while the survival rate of the LR group was signi ficantly higher than that of the ER group (P〈0.005). Forty-nine (66.2%) of the 74 patients with recurrence underwent additional curative treatment after the diagnosis of recurrence, including re-resection (n=33, 67.3%), RFA (n=5, 10.2%), TACE+RFA (n=5, 10.2%), TACE+stereotactic body radiation therapy (n=5, 10.2%), and LT (n=1, 2.0%), while the remaining 25 patients underwent a palliative procedure, namely, TACE (n=22, 88.0%), hepatic arterial infusion chemotherapy (n=2, 8.0%), or systemic chemotherapy (n=1, 4.0%).

Table 2. Clinicopathological features of 130 patients with small HCC who underwent curative resection
Comparison of clinicopathological and recurrence features between the ER and LR groups
Table 3 shows the clinicopathological features of the ER and LR groups. No significant differences were detected between the two groups with respect to gender, age, etiology, background liver disease, Child-Pugh grade, platelet count, ALT, T-Bil, PT, Alb, ICG-R15, AFP, DCP, anti-viral therapies and response, surgical procedure, tumor differentiation, IM, and surgical margin. However, patients in the ER group had a higher AFP-L3 level and more frequent multiple tumors, non-SN type, MVI, and pTNM (III or IV) compared with the LR group. Table 4 shows the features of recurrence of the LR and ER groups. No significant differences in the features of initial recurrence and re-recurrence were detected betweenthe two groups. However, the cumulative DBMC rate of the ER group was significantly higher than that of the LR group (12.8% and 0% at 1 year, 42.7% and 2.9% at 3 years, and 55.5% and 11.7% at 5 years, respectively,P=0.039, Fig. 3). Furthermore, the interval from initial recurrence to DBMC of the ER group was significantly shorter than that of the LR group (9.8±9.5 vs 21.8±17.7 months,P=0.027).

Fig. 2. Cumulative survival rates after hepatectomy of patients with small hepatocellular carcinoma in the no-recurrence, early recurrence (ER), and late recurrence (LR) groups. The survival rate of the no-recurrence group was significantly higher than that of the other groups (both P〈0.0001), while the survival rate of the LR group was significantly higher than that of the ER group (P〈0.005).
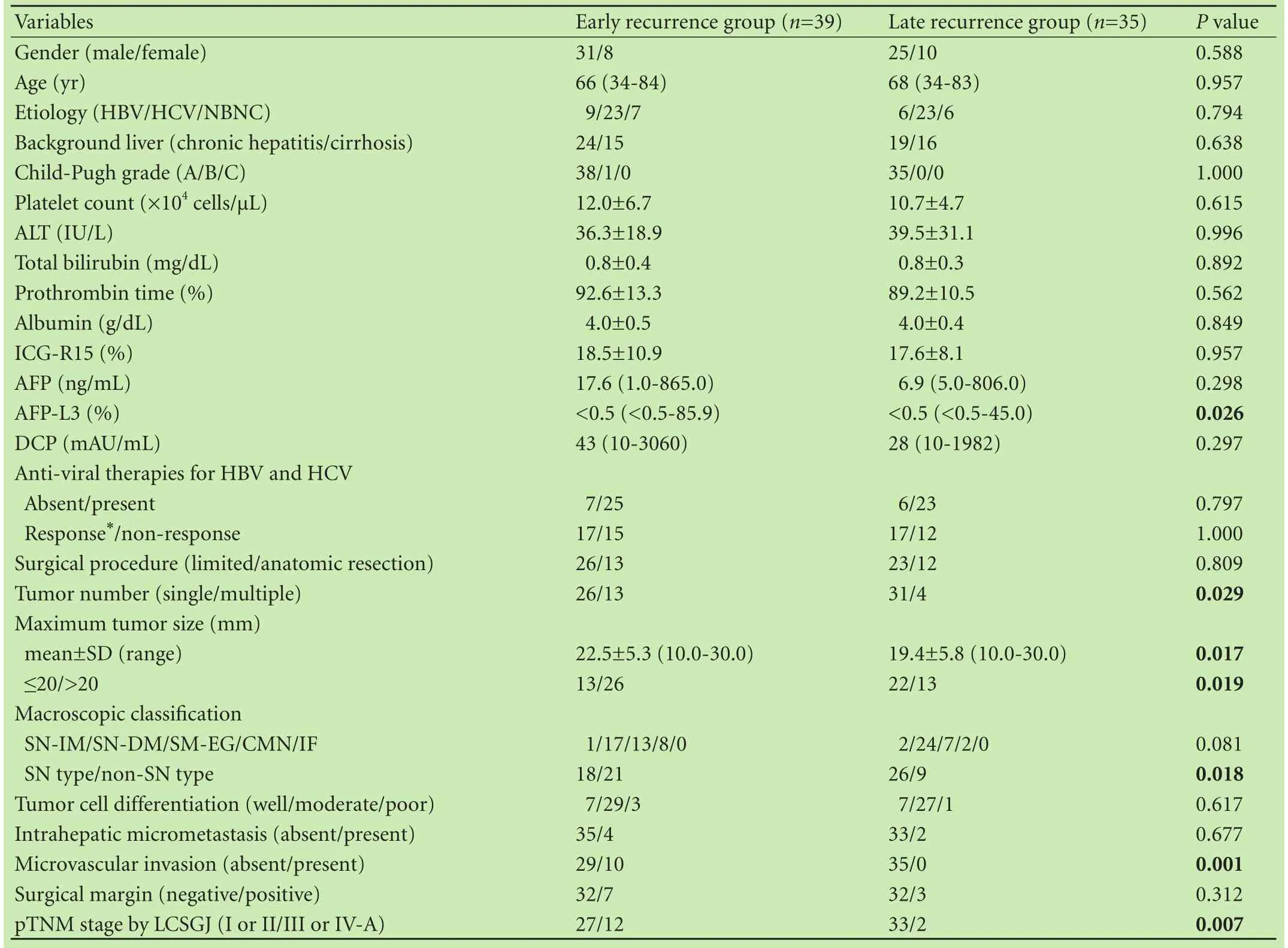
Table 3. Comparison of clinicopathological features between the early and late recurrence groups
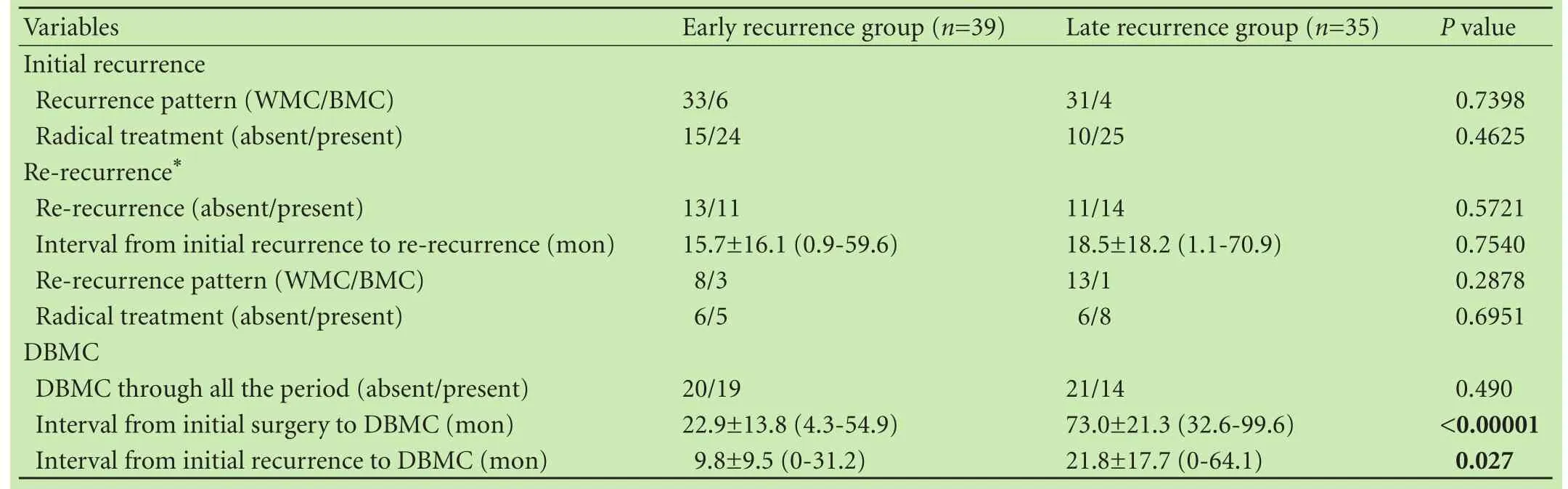
Table 4. Comparison of recurrence features between the early and late recurrence groups

Fig. 3. Cumulative rates of development to carcinoma beyond the Milan criteria (DBMC) after hepatectomy for small hepatocellular carcinoma in the ER and LR groups. The cumulative DBMC rate of the ER group was significantly higher than that of the LR group (P=0.039).
Univariate and multivariate analysis of risk factors for DBMC
Univariate analysis showed that AFP ≥500 ng/mL (P=0.0007), AFP-L3 ≥10% (P=0.0003), DCP ≥1000 mAU/mL(P=0.0132), non-response to anti-viral therapy (P=0.0067), positive surgical margin (P=0.0026), poorly differentiated tumor (P=0.021), MVI (P=0.0115), pTNM stage (P〈0.00001), ER (P〈0.00001), and radical treatment at initial recurrence (P〈0.00001) were associated with DBMC after HR for small HCC. Multivariate analysis revealed that AFP ≥500 ng/mL (P〈0.0001), DCP ≥1000 mAU/mL (P=0.002), non-response to anti-viral therapy (P=0.002), pTNM stage (P=0.019), ER (P〈0.0001), and radical treatment at initial recurrence (P=0.013) were independent risk factors for DBMC after HR for small HCC (Table 5).

Table 5. Results of univariate and multivariate analysis for identifying prospective factors associated with DBMC, survival, early recurrence, and late recurrence after resection for small HCC
Univariate and multivariate analysis of prognostic factors for survival
Univariate analysis showed that ICG-R15 ≥30% (P=0.0303), Alb 〈3.5 g/dL (P=0.0403), platelet count〈10×104cells/μL (P=0.0241), AFP-L3 ≥10% (P=0.0177), non-response to anti-viral therapy (P=0.0003), nonanatomic resection (P=0.0441), positive surgical margin (P=0.0008), pTNM stage (P=0.0266), ER (P=0.0001), and radical treatment at initial recurrence (P=0.0003) were associated with survival after HR for small HCC. Multivariate analysis revealed that non-response to antiviral therapy (P=0.0002) and ER (P=0.0001) were independent factors that predict survival after HR for small HCC (Table 5).
Univariate and multivariate analysis of risk factors for ER and LR
Univariate analysis showed that tumor size ≥20 mm (P=0.007), multiple tumors (P=0.011), non-SN type (P=0.005), and MVI (P=0.008) were associated with ER after HR for small HCC. Multivariate analysis revealed that multiple tumors (P=0.012), non-SN type (P=0.010), and MVI (P=0.019) were independent risk factors for ER after HR for small HCC. On the other hand, univariate analysis showed that ICG-R15 ≥20% (P=0.048) and platelet count 〈10×104cells/μL (P=0.033) were associated with LR of small HCC. Multivariate analysis revealed that platelet count 〈10×104cells/μL (P=0.027) was independent risk factor for LR of small HCC (Table 5).
Relative risks according to number of independent risk factors for ER
The patients were categorized into four levels of risk for ER according to the number of independent risk factors and patients with no risk factors were set as the reference group. The relative risks (RRs) of other groups were compared with that of the reference group. In the group with no risk factors (n=63), 9 patients (14.3%) had ER after HR; in the group with 1 risk factor (n=49), 17 (34.7%; RR=2.960; 95% CI: 1.318-6.646;P=0.009) had ER; in the group with 2 risk factors (n=16), 10 (62.5%; RR=6.482; 95% CI: 2.627-15.995;P〈0.0001) had ER; in the group with 3 risk factors (n=2), 2 (100.0%; RR=12.692; 95% CI: 2.726-59.104;P=0.001) had ER after HR (Table 6). Further, the ER rates were stratified according to the number of risk factors for ER (P〈0.00001, Fig. 4).
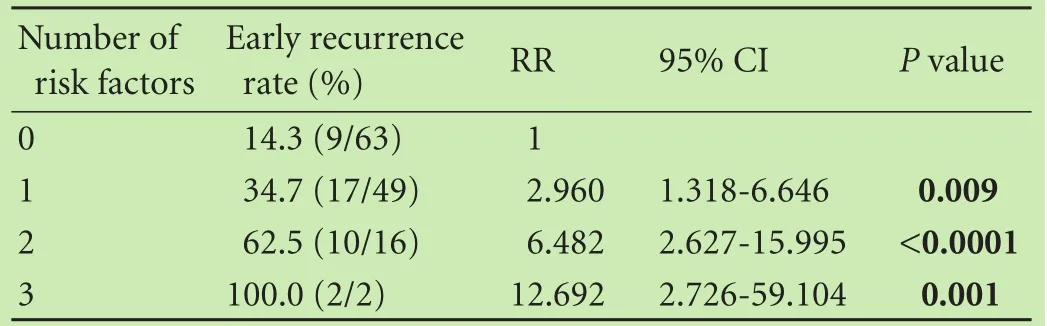
Table 6. Relative risk associated with the number of risk factors for early recurrence

Fig. 4. Early recurrence (ER) rates after hepatectomy for small hepatocellular carcinoma according to the number of risk factors for ER. The ER rates were significantly higher with a greater number of risk factors for ER (P〈0.00001).
Discussion
This study demonstrated that ER is a highly malignant recurrence pattern that impacts prognosis even in patients with small HCC after curative HR. First, the ER group had a significantly higher cumulative DBMC rate and a shorter interval from initial recurrence to DBMC compared with the LR group. Furthermore, ER was an independent risk factor for DBMC after HR for small HCC. On the other hand, multi-nodularity, MVI, and non-SN type, associated with aggressive tumor biology, were independent risk factors for ER after HR for small HCC, consistent with the findings of previous reports.[5,7,8,22,23]These results suggest that ER is associated with worse recurrence kinetics derived from IM, and strategies for IM are necessary for the prevention of ER. Second, with these results in mind, the cumulative survival rate in the ER groupwas significantly lower than that in the LR group and the early-death rate during the first 5-year period after HR in the ER group was significantly higher than that in the LR group. Furthermore, ER was an independent poor prognostic factor for survival after HR for small HCC. Accordingly, strategies for ER, and for IM in particular, should be considered in order to improve the prognosis of patients with small HCC.
Identifying patients who are at high risk of ER and subsequent determination of appropriate treatment are important in the preoperative period. This study demonstrated that even patients with one risk factor had a high ER rate of 34.7% and patients with multiple risk factors had a very high ER rate of greater than 62.5%. Thus, the identification of multi-nodularity, MVI, and non-SN type may be useful for the selection of high-risk patients and determination of treatment. However, MVI and non-SN type cannot be used to guide preoperative selection because they are pathological risk factors. Thus, a precise prediction of MVI and non-SN type using preoperative factors is necessary. Previous studies have identified various preoperative predictors of MVI, including tumor size and number, macroscopic classif ication, tumor markers, and MRI findings.[24]However, a useful surrogate marker of MVI has not been established. Several recent studies have suggested that preoperative18F-FDG PET-CT could allow for a more precise prediction of MVI in HCC.[25-27]We previously demonstrated that18F-FDG PET-CT, AFP-L3, and the combination of these two factors may be useful for predicting MVI in small HCC.[27]In particular, the combination is useful for identifying MVI-positive cases with a sensitivity of 88.9% and a specificity of 82.4%. On the other hand, Gd-EOB-DTPA-enhanced MRI, CEUS, and both modalities combined have been reported to be useful for predicting macroscopic findings because the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI and the Kupffer phase of CEUS can clearly demonstrate the border between the tumor and the surrounding parenchyma.[28,29]In particular, the combined use of these two modalities enables high-quality assessment for identifying the non-SN type of small HCC, with a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 84.6%, 95.7%, 94.3%, 88%, and 90.6%, respectively.[29]Accordingly, preoperative imaging evaluation by multiple imaging modalities along with tumor markers is important for the selection of patients who are at high risk of ER and subsequent determination of treatment.
Patients with risk factors for ER should undergo a more aggressive curative therapy, considering its mechanism of recurrence. Although percutaneous ablation therapy could be a treatment option for small HCC, patients with risk factors may not be good candidates for percutaneous ablation therapy, considering its lower curability and higher incidence of recurrence after treatment compared with HR.[30]Further, it does not enable pathological evaluation which is necessary for accurate assessment of the risk of ER and planning of the follow-up interval or treatment option for recurrence. On the other hand, theoretically, LT is the most curative and effective treatment for small HCC, which not only achieves radical tumor resection, but also deals with the frequent concurrent end-stage diseases. However, the problems of severe shortage of donor organs, risk of disease progression during the long waiting period, higher cost, perioperative risks, and long-term immunosuppression associated with LT exist. Thus, HR is the mainstay treatment for patients with resectable early HCC and well-preserved liver function, and it can achieve a favorable 5-year overall survival rate compared with LT.[31-34]Accordingly, HR is thought to be a more reasonable treatment choice even in patients at a high risk of ER. Especially, systematized HR may be preferable as a more curative treatment for prevention of recurrence, considering the mechanism of recurrence if patients have a good hepatic functional reserve.[35]
The early detection of recurrence before DBMC and subsequent additional radical treatment are important in the postoperative period because once BMC developed, the disease is fatal because of the resistance to treatment and lower rates of additional radical treatment.[13]Accordingly, patients at high risk of ER should be closely monitored in the first 2 years after HR. On the other hand, the maintenance of adequate liver function by antiviral therapy to allow performance of additional radical treatment is also important because this study demonstrated that non-response to viral therapy was an independent risk factor for DBMC and subsequent survival.
Although the above mentioned pre- and postoperative strategies are important for patients at high risk of ER, other novel strategies to prevent ER are required because their prognosis is still unsatisfactory. Thus, the risk factors for ER could be considered as potential predictors of the necessity of further adjuvant treatment. Currently, a number of previous studies, including randomized controlled trials, have reported the efficacy of adjuvant chemotherapy after curative treatment. However, suff icient evidence has not been established for a single treatment protocol of adjuvant chemotherapy at present.[11]Furthermore, some studies reported that adjuvant chemotherapy had worsened the prognosis.[36]The problems of previous studies are examination of a small number of patients with various backgrounds at a single center, and incorporation of various treatment regimens.[11]On the other hand, some randomized controlled trials which targeted advanced HCC with portal vein invasion or multiple intrahepatic metastases demonstrated the efficacy of adjuvant chemotherapy.[37,38]Therefore, multicenter studies appropriately designed to target patients at high risk of ER and examination of an adequate number of patients for statistical evaluation are needed.[11]Recently, the usefulness of sorafenib, a multikinase inhibitor, for advanced HCC has been demonstrated in two RCTs (the SHARP trial and the Asia-Paci fic trial[39,40]). On the basis of these results, the phase III STORM trial of sorafenib as adjuvant therapy for HCC after HR or ablation therapy was designed. Unfortunately, sorafenib showed no bene fit as an adjuvant therapy for HCC after HR or local ablation therapy.[41]The establishment of adjuvant chemotherapy is an urgent issue for the management of HCC, and patients with small HCC with multinodularity, MVI, and non-SN type tumors may become appropriate candidates for adjuvant chemotherapy.
In conclusion, ER was a highly malignant recurrence pattern with a higher frequency and shorter interval of DBMC and led to poor survival after HR, even in the setting of a small HCC. Multi-nodularity, MVI, and non-SN type predicted ER after HR for small HCC, and consideration of these factors may guide the management of small HCC.
Contributors:KTo wrote the main body of the article and collected data under the supervision of AH. AH provided advice on medical aspects. All authors contributed to the design and interpretation of the study and to further drafts. CK is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-2917.
2 Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-422.
3 Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001;48:251-259.
4 Tanaka H, Nouso K, Kobashi H, Kobayashi Y, Nakamura S, Miyake Y, et al. Surveillance of hepatocellular carcinoma in patients with hepatitis C virus infection may improve patient survival. Liver Int 2006;26:543-551.
5 Kaibori M, Ishizaki M, Saito T, Matsui K, Kwon AH, Kamiyama Y. Risk factors and outcome of early recurrence after resection of small hepatocellular carcinomas. Am J Surg 2009;198:39-45.
6 Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-222.
7 Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500-507.
8 Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol 2004;85:36-41.
9 Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol 2009;16:792-794.
10 Tanimoto Y, Tashiro H, Aikata H, Amano H, Oshita A, Kobayashi T, et al. Impact of pegylated interferon therapy on outcomes of patients with hepatitis C virus-related hepatocellular carcinoma after curative hepatic resection. Ann Surg Oncol 2012;19:418-425.
11 Kobayashi T, Ishiyama K, Ohdan H. Prevention of recurrence after curative treatment for hepatocellular carcinoma. Surg Today 2013;43:1347-1354.
12 Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015;261:56-66.
13 Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol 2012;19:156-162.
14 Liver Cancer Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 5th ed. Tokyo: Kanehara, 2008 (in Japanese).
15 Hui AM, Takayama T, Sano K, Kubota K, Akahane M, Ohtomo K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 2000;33:975-979.
16 Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142-147.
17 Itamoto T, Katayama K, Nakahara H, Tashiro H, Asahara T. Autologous blood storage before hepatectomy for hepatocellular carcinoma with underlying liver disease. Br J Surg 2003;90:23-28.
18 Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304.
19 Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-649.
20 Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-350.
21 Yamamoto M, Takasaki K, Ohtsubo T, Katsuragawa H, Fukuda C, Katagiri S. Effectiveness of systematized hepatectomy with Glisson’s pedicle transection at the hepatic hilus for small nodular hepatocellular carcinoma: retrospective analysis. Surgery 2001;130:443-448.
22 Zhu WJ, Huang CY, Li C, Peng W, Wen TF, Yan LN, et al. Riskfactors for early recurrence of HBV-related hepatocellular carcinoma meeting Milan criteria after curative resection. Asian Pac J Cancer Prev 2013;14:7101-7106.
23 Kwon SK, Yun SS, Kim HJ, Lee DS. The risk factors of early recurrence after hepatectomy in hepatocellular carcinoma. Ann Surg Treat Res 2014;86:283-288.
24 Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325-339.
25 Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging 2015;40:843-851.
26 Shirabe K, Toshima T, Kimura K, Yamashita Y, Ikeda T, Ikegami T, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int 2014;34:937-941.
27 Kobayashi T, Aikata H, Honda F, Nakano N, Nakamura Y, Hatooka M, et al. Preoperative Fluorine 18 fluorodeoxyglucose Positron emission tomography/computed tomography for prediction of microvascular invasion in small hepatocellular carcinoma. J Comput Assist Tomogr 2016;40:524-530.
28 Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Kaneoka Y, et al. Utility of contrast-enhanced ultrasound with perflubutane for diagnosing the macroscopic type of small nodular hepatocellular carcinomas. Eur Radiol 2014;24:2157-2166.
29 Kobayashi T, Aikata H, Hatooka M, Morio K, Morio R, Kan H, et al. Usefulness of combining gadolinium-ethoxybenzyldiethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for diagnosing the macroscopic classification of small hepatocellular carcinoma. Eur Radiol 2015;25:3272-3281.
30 Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y, Miao Y. Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol 2012;10:163.
31 Cucchetti A, Cescon M, Ercolani G, Morelli MC, Del Gaudio M, Zanello M, et al. Comparison between observed survival after resection of transplantable hepatocellular carcinoma and predicted survival after listing through a Markov model simulation. Transpl Int 2011;24:787-796.
32 Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. Longterm outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol 2012;19:826-833.
33 Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg 2011;254:527-538.
34 Yi NJ, Suh KS, Kim T, Kim J, Shin WY, Lee KU. Current role of surgery in treatment of early stage hepatocellular carcinoma: resection versus liver transplantation. Oncology 2008;75:124-128.
35 Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg 2011;396:1109-1117.
36 Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, et al. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology 2006;44:891-895.
37 Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, et al. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994;20:295-301.
38 Tanaka S, Shimada M, Shirabe K, Maehara S, Harimoto N, Tsujita E, et al. A novel intrahepatic arterial chemotherapy after radical resection for advanced hepatocellular carcinoma. Hepatogastroenterology 2005;52:862-865.
39 Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.
40 Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Paci fic region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34.
41 Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16: 1344-1354.
April 9, 2016
Accepted after revision December 26, 2016
Author Affiliations: Department of Gastroenterology and Metabolism (Kobayashi To, Aikata H and Chayama K), and Department of Anatomical Pathology (Arihiro K), Hiroshima University Hospital, Hiroshima, Japan; Department of Gastroenterological and Transplant Surgery, Applied Life Sciences, Institute of Biomedical and Health Sciences (Kobayashi Ts and Ohdan H), and Liver Research Project Center (Chayama K), Hiroshima University, Hiroshima, Japan
Hiroshi Aikata, MD, PhD, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan (Tel: +81-82-257-5192; Fax: +81-82-257-5194; Email: aikata@hiroshima-u.ac.jp)
? 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60181-9
Published online February 24, 2017.
 Hepatobiliary & Pancreatic Diseases International2017年3期
Hepatobiliary & Pancreatic Diseases International2017年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Circulating autoantibodies to endogenous erythropoietin are associated with chronic hepatitis C virus infection-related anemia
- Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance
- A clinical analysis of acute pancreatitis in pregnancy
- Hepatobiliary & Pancreatic Diseases International
- Traditional surgical planning of liver surgery is modified by 3D interactive quantitative surgical planning approach: a single-center experience with 305 patients
- Combined cavo-atrial thrombectomy and hepatectomy in hepatocellular carcinoma
