Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats
Yazd, Iran
Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats
Razieh Kabirifar, Zohreh-al-sadat Ghoreshi, Fatemeh Safari, Alireza Karimollah, Ali Moradi and Ebrahim Eskandari-nasab
Yazd, Iran
BACKGROUND: Bile duct ligation (BDL) and subsequent cholestasis are correlated with oxidative stress, hepatocellular injury and fbrosis. Quercetin is a favonoid with antifbrotic, and hepatoprotective properties. However, the molecular mechanism underlying quercetin-mediated hepatoprotection is not fully understood. The current study was to evaluate mechanisms of hepatoprotective effect of quercetin in BDL rat model.
METHODS: We divided male Wistar rats into 4 groups (n=8 for each): sham, sham+quercetin (30 mg/kg per day), BDL, and BDL+quercetin (30 mg/kg per day). Four weeks later, the rats were sacrifced, the blood was collected for liver enzyme measurements and liver for the measurement of Rac1, Rac1-GTP and NOX1 mRNA and protein levels by quantitative PCR and Western blotting, respectively.
RESULTS: Quercetin signifcantly alleviated liver injury in BDL rats as evidenced by histology and reduced liver enzymes. Furthermore, the mRNA and protein expression of Rac1, Rac1-GTP and NOX1 were signifcantly increased in BDL rats compared with those in the sham group (P<0.05); quercetin treatment reversed these variables back toward normal (P<0.05). Another interesting fnding was that the antioxidant markers e.g. superoxide dismutase and catalase were elevated in quercetin-treated BDL rats compared to BDL rats (P<0.05).
CONCLUSION: Quercetin demonstrated hepatoprotective activity against BDL-induced liver injury through increasingantioxidant capacity of the liver tissue, while preventing the production of Rac1, Rac1-GTP and NOX1 proteins.
(Hepatobiliary Pancreat Dis Int 2017;16:88-95)
quercetin;
Rac1;
NOX1;
liver fbrosis;
oxidative stress
Introduction
Liver fbrosis is characterized by the excessive production of extracellular matrix proteins (collagen) that occurs in most types of liver injury. Advanced liver fbrosis leads to cirrhosis, liver failure, and hepatocellular carcinoma (HCC).[1,2]Cholestasis is a clinically substantial event that contributes to liver fbrosis. Cholestatic liver fbrosis is identifed by excessive collagen production and deposition, which is mediated by reactive oxygen species (ROS). Continuous cholestasis leads to damage of hepatocytes and subsequent liver fbrosis, cirrhosis and death.[3]
Cellular oxidative damage advances when the balance between ROS-generating systems and ROS scavenging ones tilts in favor of the former.[4-6]Among all the antioxidants that are available in the body, thiols constitute the major portion which plays a signifcant role in defense against ROS.[7,8]Carbonylation of proteins and malondialdehyde (MDA) are two important oxidative stress markers.[9]Carbonylation of proteins is an indicator of severe oxidative damage and disease-derived protein dysfunction that can be promoted by ROS.[4]MDA is the most abundant aldehyde produced during lipid peroxidation, and its measurement is indicative of oxidative stress.[10]
Nicotinamide adenine dinucleotide phosphate oxidase (NOX) is a major intracellular producer of ROS. NOXis an enzyme system that induces the reduction of molecular oxygen to superoxide and plays a key role in the pathogenesis of liver fbrosis.[11]The NOX family consists of seven different members (NOX1-5 and the dual oxidases Duox1 and 2). Among the NOX family, NOX1, NOX2 and NOX4 are expressed on hepatic stellate cells (HSCs) and may contribute to liver fbrosis. One integral component in the activation of NOX is Ras-related C3 botulinum toxin substrate 1 (Rac1).[12,13]Rac1 belongs to a subfamily of small GTP-binding proteins, and regulates many cellular functions including proliferation, gene expression, infammation, apoptosis and tumor progression.[14,15]Upon Rac1-GTP (biologically active form of Rac1) translocation to the membrane-bound cytochrome complex, enzymatically active NOX1 and NOX2 are released. Increased HSC-NOX activity through Rac1 induces liver fbrogenesis.[12]
Use of herbal drugs in the treatment of liver diseases has a long tradition. Flavonoids are plant-derived antioxidants with major suppressive effects on liver fbrosis through their antioxidant, anti-fbrotic and anti-carcinogenic properties.[16]The most abundant favonoid in nature, quercetin presents in large amounts in vegetables, fruits, tea and olive oil. Recent evidence has demonstrated the therapeutic effects of quercetin against cholestasis liver injury.[17-19]Its therapeutic characteristics have been attributed to its phenolic hydroxyl groups.[17,20]
Bile duct ligation (BDL) is the most common model used to induce obstructive cholestatic damage in mice and rats. BDL models can give valuable information about cholestasis and subsequent liver fbrosis.[17]Based on evidence that Rac1 and NOX1 expressions are associated with oxidative stress and liver fbrogenesis, we investigated the impact of quercetin on Rac1, Rac1-GTP, and NOX1 expression in BDL rat model. As the indices of oxidative stress, we also quantifed protein carbonylation and total reduced thiols, and also assessed enzymatic activities of superoxide dismutase (SOD) and catalase.
Methods
Animals and experimental procedures
Adult male Wistar rats (200-250 g, Pasteur Institute, Tehran, Iran) were used in this research. Rats were kept in an air-conditioned room at 25 ℃ with a 12-hour darkness/light cycle, and had free access to rat food diet and drinking water. All of the study's protocols agree with the current ethical considerations of local ethical committee of animal use. The 32 rats were randomly divided into 4 groups: sham, sham+quercetin (30 mg/kg per day), BDL and BDL+quercetin (30 mg/kg per day). Quercetin was suspended in 5% CMC (Sigma Chemicals Co., USA). Quercetin or the same volume/weight of the 5% CMC vehicle was gavaged once a day from the day after surgery for 28 days.[21]BDL procedure was performed as described previously.[22]Briefy, under general anesthesia, the common bile duct was exposed by a midline abdominal incision under sterile conditions. It was then ligated in two places with a silk thread and sectioned between the ligatures.[23]At the end of the 4-week period, blood samples were collected by puncturing the heart under deep anesthesia and they were centrifuged at 3000 g for 15 minutes. The serum was separated and kept at -70 ℃until next experiments. Liver tissues were divided into three parts, one part frozen in liquid nitrogen for RNA extraction, the second part was kept at -70 ℃ to make a homogenized tissue for assessment of antioxidant parameters such as Rac1, Rac1-GTP, NOX1 and Western blotting analyses, and the last part was fxed with 10% neutral formalin for histology.
Histopathological evaluation
The liver specimens were fxed in 10% neutral formalin individually, dehydrated in alcohol and embedded in paraffn and then sections were stained with hematoxylin and eosin (HE). Lobular and portal infammation, focal hepatocyte necrosis, ductular proliferation, and portal and septal fbrosis were investigated by a pathologist. The rats in the BDL group with no signifcant histopathological fndings were excluded from further analysis.
Blood chemistry
The collected serum samples were examined for alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) as indicators of the liver injury by using standard animal diagnostic kits (Pars Azmon Diagnostic Co., Iran) and a Roche BT3000 Auto Analyzer.
Determination of hydroxyproline level in liver tissue
An automated procedure for quantitative assay of hydroxyproline in tissue is based on the oxidation of hydroxyproline by chloramine T in aqueous solution. The oxidation product reacts with Ehrlich's reagent, and the obtained chromogen is registered in a recorder connected to the colorimeter. The amount of hydroxyproline is expressed as μg/mg tissue.[24]
Determination of MDA in tissue
The levels of MDA were determined spectrophotometrically by measuring thiobarbituric acid-reactive substances.[25]One hundred μL homogenized liver tissue supernatant were incubated with 15% trichloroacetic acid, 0.375% thiobarbituric acid, 0.25 mol/L HCl and 6.8mmol/L 2, 6-ditert-butyl-4-methylphenol for one hour in a boiling water bath. After cooling the mixtures were centrifuged at 3000 rpm for 15 minutes, the absorbance of the supernatant was recorded at 532 nm.[26]For the standard curve 1, 1, 3, 3-tetraethoxypropane was used. The results were expressed as nmol/μg protein of the tissue.
Determination of thiol groups in liver tissue
Ellman's reagent 5, 5'-dithiobis (2-nitrobenzoic acid) (DTNB) was frst used for the estimation of thiol groups. The process is based on the reaction of the DTNB with thiol to produce the mixed 2-nitro-5-thiobenzoic acid (TNB) and disulfde which is quantifed by the absorbance of the TNB2 at 412 nm.[27]Thiol groups were measured by adding: 25 μL DTNB, 50 μL Tris and 420 μL water (495 μL initial volume take blank)+5 μL sample (500 μL fnal volume). The solution was mixed gently using pipette, and the cuvette was put into UV-Vis spectrophotometer and reading was done at 412 nm. Absorbance was taken for each sample and then the results were expressed in nmol/mg protein.
Determination of protein carbonylation in liver tissue
Homogenized tissue samples were treated with 2, 4-dinitrophenylhydrazine (10 mmol/L) in HCl (2.5 mol/L) for 1 hour at dark and room temperature. After treatment with 20% trichloracetic acid and separation by centrifugation, the precipitate was washed three times with a mixture of absolute ethylic alcohol and ethylic acetate 1:1 (v/v). Subsequently, protein precipitate was dissolved in guanidine hydrochloride (6 mol/L). Protein concentration was determined in these samples by measuring the absorbance at 355 nm and ultimate results were expressed in nmol/mg protein.
Catalase activity in liver tissue
Catalase activity was assessed by the method previously described by Beers and Sizer[28]in which the disappearance of peroxide is followed spectrophotometrically at 240 nm. The incubation mixture contained potassium phosphate (0.05 mol/L, pH 7.0), hydrogen peroxide (0.02 mol/L) and a sample (50 μL) of the supernatant fuid. The decrease in absorbance was measured at 240 nm for 3 minutes. The rate of absorbance reduction per minute was calculated from the initial linear portion of the curve. The value of 0.0394 cm-1·mol-1proposed by Nelson and Kiesow[29]was introduced as the extinction coeffcient of H2O2and ultimate results were expressed in IU/mg protein.
SOD activity in liver tissues
The principle of SOD activity measurement was based on the inhibition of nitrobluetetrazolium (NBT) reduction. Illumination of ribofavin in the presence of O2and methionine (electron donor) generates superoxide anions and this has been introduced as the basis of the assay of SOD. The reduction of NBT by superoxide radicals to blue colored formazan was recorded at 560 nm, as previously described by Fridovich and Beauchamp.[30]The reaction mixture contained 1.9 mL of phosphate buffer (pH 7.8), 16.8×10-5mol/L NBT, 1.17× 10-6mol/L ribofavin and 1×10-2mol/L methionine, with suitably diluted homogenized tissue in a total volume of 3 mL. The absorbance was recorded at 560 nm for 5 minutes. The rate of increase in absorbance per min was calculated from the initial linear portion of the curve. The value of 0.00436 cm-1mol-1was used as the extinction coeffcient. The values were expressed in IU/mg protein.
Western blotting analysis of liver tissue Rac1, Rac1-GTP and NOX1 levels
Liver cell protein was extracted by homogenization of tissue samples (30 mg) using phosphate saline buffer (100 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.1% SDS and 1% NP-40; pH 7.4 with protease-inhibitor cocktail, 1:100; Sigma, St Louis, MO, USA) by incubation on ice for 30 minutes and subsequent centrifugation at 15 000 g (4 ℃, 30 minutes). Protein concentrations were determined in the supernatants by the Bradford assay. Protein (100 μg) was separated on a 12% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was blocked with 3% non-fat dried milk in Trisbuffered saline (pH 7.4), with 0.05% Tween-20 (TBS/T) for 2 hours and probed with monoclonal rabbit anti-Rac1 primary antibody (Abcam, Cambridge, UK), polyclonal rabbit anti-NOX1 (Abcam), monoclonal mouse anti-Rac1-GTP (Newest biosciences) and polyclonal rabbit anti-β-actin (Abcam) as a reference at 4 ℃ overnight. The membranes were incubated with a goat anti-rabbit and anti-mouse IgG secondary antibody (1:4000) conjugated with horse radish peroxidase (Cell Signaling, Munich, Germany) for 45 minutes. The predicted sizes for Rac1 (21 kD), Rac1-GTP (22 kD), NOX1 (71 kD) and β-actin (42 kD) were checked using molecular weight markers. Specifc bands were visualized by an enhanced chemiluminescence reagent (GE) on a ChemiDoc system (Syngene GBOX, 680X) and quantifed densitometrically with the program Quantity GeneTools (SynGene, V4.1).
Gene expression analysis by quantative real-time PCR (qPCR)
Total cellular RNA was isolated from liver samples by using a Fast Pure RNA Kit from TakaRa, Japan according to the manufacturer's protocol. By measuring the absorbance at 260 nm, concentrations of RNA were identifedand its purity was evaluated by 260/280 nm absorbance ratio (Eppendorf, Hamburg, Germany). One microgram of the total RNA was reverse transcribed to cDNA using MuLV RT enzyme (Fermentas), random hexamers and dNTP in a total volume of 20 μL. The cDNA samples were diluted 1:10, and aliquots were frozen at -70 ℃until the PCRs were carried out. qPCR was performed triplicate using SYBR-green in the Rotor Gene system (Corbett Research 2004, Australia). Normalization was achieved against β-actin and relative quantity of gene expression was analyzed based on ΔCt method and the results were calculated as 2-ΔΔCt.
Oligonucleotide primer sequences used for real-time PCR were: alpha smooth muscle actin (α-SMA): forward 5'-GCT CCA TCC TGG CTT CTC TAT C-3' and reverse 5'-GGG CCA GCT TCG TCA TAC TC-3', collagen I: forward 5'-ATC AGC CCA AAC CCC AAG GAG-3' and reverse 5'-CGC AGG AAG GTC AGC TGG ATA G-3', transforming growth factor beta 1 (TGF-β1) forward 5'-AAG AAG TCA CCC GCG TGC TA-3' and reverse 5'-TGT GTG ATG TCT TTG GTT TTG TC-3', Rac1: forward 5'-GTA AAA CCT GCC TGC TCA TC-3' and reverse 5'-GCT TCA TCA AAC ACT GTC TTG-3', NOX1: forward 5'-TAC GAA GTG GCT GTA CTG GTT G-3' and reverse 5'-CTC CCA AAG GAG GTT TTC TG-3', β-actin: forward: 5'-CGT TGA CAT CCG TAA AGA CCT C-3' and reverse: 5'-AGC CAC CGA TCC ACA CAG A-3'.
Statistical analysis
Differences between obtained values (mean±SEM) were carried out by one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison using Graphpad Prism 5 software. The differences below 0.05 were considered statistically signifcant. Experiments were replicated at least two times.
Results
Histological analysis
Liver injury was frst analyzed by histology (Fig. 1). No morphological abnormalities were observed in sham and quercetin supplemented sham. They had regular morphology of liver parenchyma with intact hepatocytes, sinusoids, and portal tracts (Fig. 1A and B). BDL rats showed a loss of the normal architecture with the presence of regenerative nodules, a loss of hepatic structure in periportal areas, cellular necrosis, and fbrosis (Fig. 1C). In contrast, necrosis and fbrosis were minimal in animals from groups treated with quercetin (Fig. 1D).
Quercetin attenuated BDL-induced liver injury
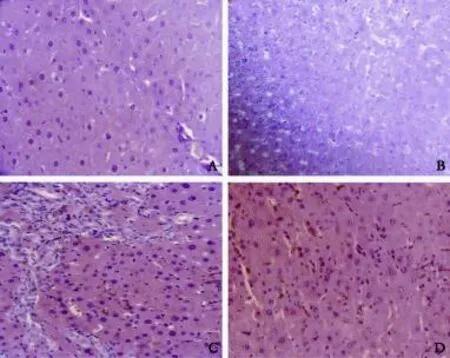
Fig. 1. Representative photomicrographs of HE staining showing: A: sham rat liver section; B: sham rat treated with quercetin (sham+Q) liver section; C: fbrotic rat (BDL) liver section; and D: fbrotic rat treated with quercetin (BDL+Q) liver section.
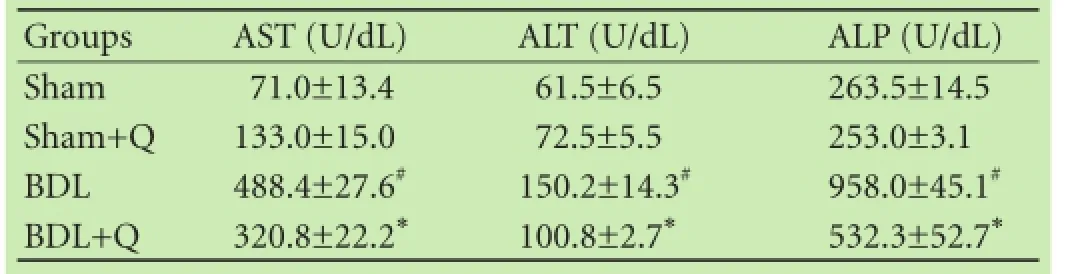
Table 1. Effect of quercetin on liver enzymes in BDL-induced hepatic injury
After 4 weeks of BDL, the rats showed signifcant alterations in enzyme markers of liver injury (Table 1). Serum levels of AST, ALT, and ALP (common biochemical indices of hepatocellular injury) were signifcantly elevated in BDL rats compared to sham animals (P<0.05). Additionally, the serum biochemical changes were improved by quercetin supplementation. Quercetin treatment signifcantly reduced these values, and attenuated BDL-induced liver injury (P<0.05).
Hydroxyproline quantifcation
Table 2 represents the results of the quercetin on hepatic level of hydroxyproline. The hydroxyproline level was signifcantly elevated in BDL rats (P<0.05). The high levels of hydroxyproline in BDL rats was signifcantly decreased after their treatment with quercetin (P<0.05).
mRNA expression of α-SMA, collagen I and TGF-β1
We measured the mRNA expression levels of α-SMA, collagen I and TGF-β1 in liver tissue by qPCR method. We observed that α-SMA, collagen I and TGF-β1 expression levels were signifcantly increased in liver tissue of BDL rats compared with those in the sham group(P<0.05). Treatment with quercetin reduced the mRNA expression of α-SMA, collagen I and TGF-β1 in the liver tissue compared with the BDL group (Table 2).
Quercetin attenuated BDL-induced oxidative stress
To evaluate the effect of quercetin on hepatic redox potential, the status of oxidative stress in liver tissues wasdetermined. As shown in Fig. 2, the level of lipid peroxidation by measurement of MDA and carbonyl group was elevated in BDL rats. Following quercetin treatment, the level of MDA and carbonyl group in BDL rats were reduced (Fig. 2A and B) indicating that quercetin possesses a biological activity to reduce oxidative stress. Besides, BDL rats showed a signifcant decrease in liver thiol group compared with sham rats. The decrease of liver thiol in the BDL group was attenuated by quercetin administration (Fig. 2C). The data suggested that quercetin can attenuate BDL-induced oxidative stress through increasing the levels of thiol groups. In contrast, the liver catalase and SOD activities signifcantly decreased in BDL rats compared to those in sham animals (P<0.05), but their activity was restored in quercetin treated BDL rats (P<0.05) (Fig. 2D and E). These fndings suggest that treatment with quercetin signifcantly reestablished the levels of the antioxidant markers.
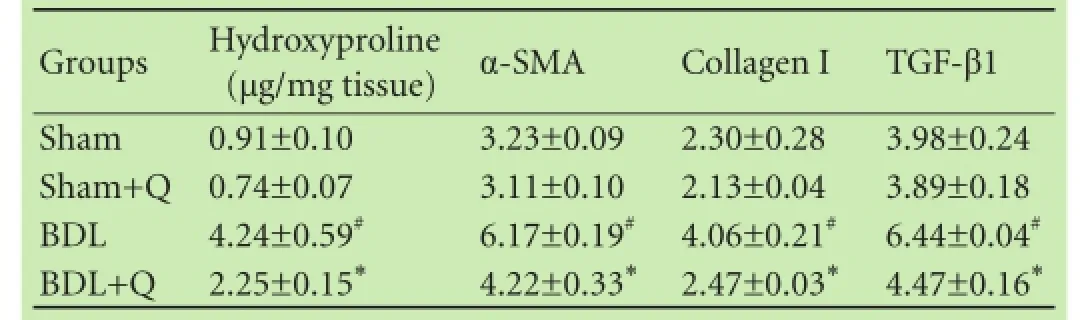
Table 2. Comparison of hydroxyproline content and the mRNA expression of α-SMA, TGF-β1 and collagen I in liver tissue of four groups
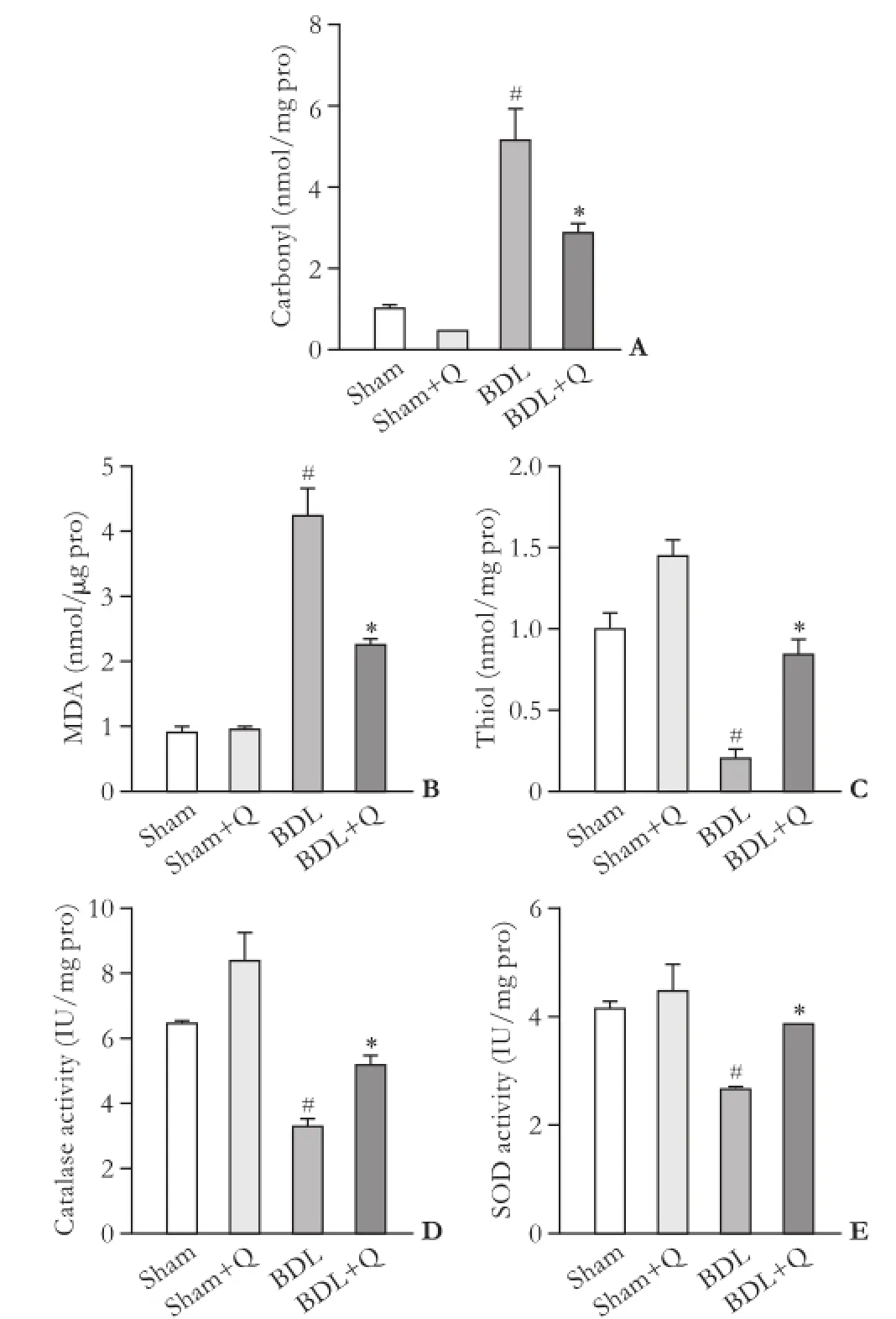
Fig. 2. Effects of quercetin on the levels of hepatic carbonyl group (A), MDA (B), thiol group (C), catalase activity (D) and SOD activity (E) in the liver tissue of the four groups. #:P<0.05, compared with the sham group; *:P<0.05, compared with the BDL group.
Protein expression of Rac1, Rac1-GTP and NOX1 reduced in quercetin administered group
To examine the anti-fbrotic effects of quercetin, three fbrogenic proteins (Rac1, Rac1-GTP and NOX1) were assayed using Western blotting method and the results were normalized to β-actin expression. Our results demonstrated that there were signifcant increases in hepatic protein expressions of Rac1, Rac1-GTP and NOX1 in BDL rats compared with sham animals (P<0.05). The expression levels of all three proteins in BDL rats weredecreased in the quercetin-treated BDL group compared to the BDL group (P<0.05), highlighting the anti-fbrotic effects of quercetin through suppression of Rac1, Rac1-GTP and NOX1 proteins (Fig. 3).
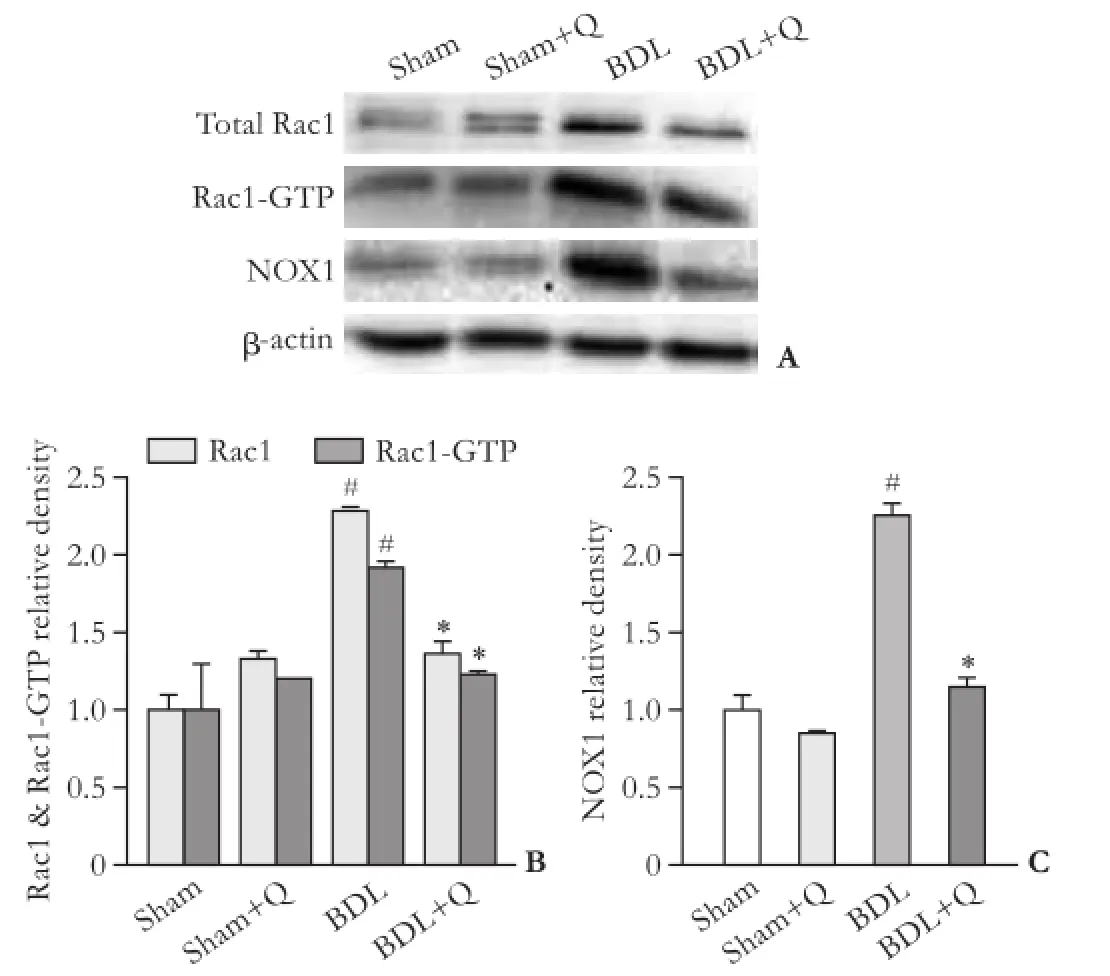
Fig. 3. Western blotting pattern of Rac1, Rac1-GTP and NOX1 proteins expression (A). The relative density of protein expression levels of Rac1 (B), Rac1-GTP (B) and NOX1 (C) in four groups. #:P<0.05, compared with the sham group; *:P<0.05, compared with the BDL group.
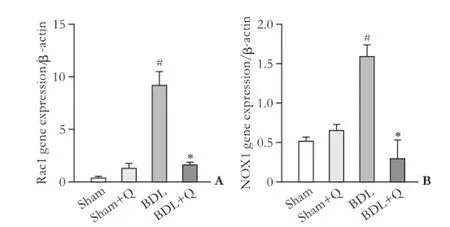
Fig. 4. Gene expression of Rac1 (A) and NOX1 (B) in four groups. #:P<0.05, compared with the sham group; *:P<0.05, compared with the BDL group.
mRNA expression of Rac1 and NOX1 reduced in quercetin administered group
mRNA expression of Rac1 and NOX1 genes (as fbrogenic agents) were analyzed by qPCR. Results were normalized with respect to housekeeping gene β-actin and were reported as expression relative units. In the fbrotic liver tissue, a signifcant increases in NOX1 and Rac1 mRNA levels was observed in the BDL group (Fig. 4). The quercetintreated BDL group displayed a lower NOX1 and Rac1 level compared with the BDL group (P<0.05).
Discussion
The present study found that quercetin treatment attenuated fbrosis progression through suppression of both mRNA and protein expression of Rac1, Rac1-GTP and NOX1. To assess the fbrosis process of liver tissue during BDL, we measured mRNA expression of hydroxyproline, α-SMA, collagen I and TGF-β1 in liver tissue of four study groups. Compared to the sham group, BDL rats demonstrated a signifcant increase in the fbrosis markers, but treatment of BDL rats with quercetin led to a reduction in liver fbrosis. Moreover, to confrm the fbrosis process of liver tissue during BDL, we measured the activity of three hepatic enzymes including AST, ALT and ALP in serum.[31]There were signifcant increases in these hepatic markers in fbrotic tissue induced by BDL compared to the sham group. To confrm the fbrosis of liver tissue, we used histological analysis of the liver tissue by HE staining. Our fndings certifed the fbrosis of the liver tissue in BDL rats compared to the sham group. Quercetin signifcantly decreased the liver enzymes indicating that liver injury was alleviated by use of quercetin.
Liver is a vital organ with key roles in metabolism, detoxifcation, and elimination of endogenous and exogenous substances.[32]Liver injury is directly related to oxidative stress. Oxidative stress is a disruption in the oxidant-antioxidant balance resulting in potential cellular damage. The imbalance can arise from an absence of antioxidant capacity caused by disturbances in production and distribution, or by an excess of ROS from other factors.[33,34]BDL is a classical model for the analysis of liver injury induced by bile duct obstruction which stimulates the production of free radicals followed by infammation and liver cirrhosis resulted from the imbalance between fbrogenesis and fbrolysis.[17,35]
Quercetin has various useful biological properties such as antioxidant, anti-infammatory, and anticarcinogenesis effects. It exerts its antioxidant impact by removing ROS during oxidative stress.[16]In recent decades, the potential application of quercetin has driven the scientists towards using this drug in treating many abnormalities including liver diseases and cancers.[18,19,36]
Recent studies have indicated that oxidative stress resulting from the metabolism of BDL plays a signifcant role in the progress of liver fbrosis.[37,38]Lipid peroxidation is an indicator of tissue damage which induces collagen synthesis by stimulating HSCs. As the main product of lipid peroxidation, MDA tissue levels have been shown to be correlated with the severity of liver fbrosis.[39]In accordance with these results, our study demonstrated that the MDA levels were signifcantly increased in BDL rats compared to the sham group; treatment of BDL rats by quercetin resulted in the reduction of the MDA levels. Our fnding confrms that MDA levels are positively associated with increased liver injury.
Among all antioxidants that are available in the body, thiols constitute the major portion of the total body antioxidants and they play a signifcant role in defense against ROS. Thiols are the organic compounds that have a sulphydryl group and they are among major plasma antioxidants with key reducing groups available in our body fuids.[7,8]Additionally, carbonylation of proteins is an irreversible oxidative damage which frequently causes a defect in protein function. It is considered a prevalent indicator of severe oxidative damage and disease-derived protein dysfunction that can be promoted by ROS.[4]Our fndings revealed that the thiol group, as the sensitive indicator of rat oxidative stress, was decreased signifcantly when exposed to oxidative stress caused by BDL. Additionally, we found that BDL-induced oxidative stress led to increased production of carbonyl group on proteins peripheral chains. These modifed levels were restored after treatment of BDL rats by quercetin. In agreement with our fndings, Dalle-Donne et al[40]have reported an increase in carbonyl protein level as an oxidative stress marker and a decrease in thiol group in BDL rats com-pared to the sham group.
Under oxidative stress conditions, the antioxidant enzymes such as catalase and SOD remove the extra ROS to maintain homeostasis. Recent studies demonstrated a signifcant reduction in the activity of catalase and SOD in BDL rats compared to the sham group.[31,35,41]Similarly, we observed that the levels of these enzymes were signifcantly decreased in the BDL group compared to the sham group. The activities of catalase and SOD were increased in BDL rats after treatment by quercetin resulting in an improvement in liver fbrosis induced by these antioxidant enzymes. Therefore, a reduction in the antioxidant defense capacity in BDL rats would possibly develop a severe liver injury.
NOX1 is a complex of several proteins which produce ROS in response to various stimuli.[14]A recent study has shown that NOX1 is more important for ROS generation in HSCs than NOX2 and NOX6.[13]Recent research certifed the crucial role of NOX1 in the HSC activity and liver fbrosis by ROS production, thus targeting NOX1 may be considered a useful therapeutic tool for liver fbrosis.[42,43]Recent observations have demonstrated that in rats with carbon tetrachloride-induced fbrosis, the maintenance of Rac1 results in the overproduction of ROS by NOX1 and increased activity of HSCs that ultimately leads to aggravation of liver fbrosis.[44]Rac1 is considered as a subunit of cytosolic NOX1. Activated Rac1 (Rac1-GTP) directly binds to NOX1 through TPR site and leads to the regulation of ROS-producer NOX1.[45,46]According to recent literature, disturbance in Rac1 leads to inhibition of the activity of NOX1 as well as reduction in oxidative stress.[47]Our experiments showed that the rate of NOX1 expression in BDL fbrotic rats was signifcantly increased compared to the sham group. This increase was in parallel to the rise in the expression rate of Rac1, and Rac1-GTP in the BDL group compared to the sham group.
According to growing evidence, the accumulation of myofbroblastic-HSC (MF-HSC) is a key factor in the occurrence of fbrosis. The required event for accumulation of MF-HSC and induction of liver injury is the transportation of HSC from the passive phenotype to the myofbroblast form. α-SMA is the marker of activated HSC, and TGF-β1 is one of the putative and strongest pro-fbrotic markers in liver fbrosis by induction of HSC activation. Our experiment revealed that quercetin signifcantly decreased TGF-β1 gene expression in BDL-induced liver fbrosis, as well as a signifcant decrease in α-SMA gene expression which is a hallmark for activation of HSC. Researchers introduced Rac1 as the key mediator for accumulation of MF-HSC, associated with progression of liver fbrosis.[44,48]Decreased oxidative stress protects liver against fbrosis through suppression of HSC cells. Quercetin via down regulation of Rac1 and NOX1 plays an important role in attenuating free radicals and reducing oxidative stress. Increased activity of Rac1 stimulates NOX1, and increase enzymatic system activity of NOX1 induces conversion of HSCs to myofbroblast forms, hence promoting liver fbrosis.[44]
In summary, our fndings demonstrated that in BDL rats, the expression levels of Rac1, Rac1-GTP and NOX1 as well as liver fbrosis markers were increased, the level of antioxidant markers were reduced. Quercetin treatment of BDL rats suppressed the expression level of Rac1, Rac1-GTP and NOX1, while it increased the antioxidant protection (catalase and SOD). Therefore, we speculate that quercetin exerts its hepatoprotective activities by reducing the expression of Rac1, Rac1-GTP and NOX1.
Contributors:KR and MA proposed the study. KR and GZ performed research and wrote the frst draft. SF and KA collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. MA is the guarantor.
Funding:None.
Ethical approval:This study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Bataller R, Brenner DA. Liver fbrosis. J Clin Invest 2005;115: 209-218.
2 Friedman SL. Liver fbrosis -- from bench to bedside. J Hepatol 2003;38:S38-53.
3 Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol 2011;25:281-290.
4 Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 2014;33:79-97.
5 Hashemi M, Hanaf Bojd H, Eskandari Nasab E, Bahari A, Hashemzehi NA, Shafeipour S, et al. Association of adiponectin rs1501299 and rs266729 gene polymorphisms with nonalcoholic fatty liver disease. Hepat Mon 2013;13:e9527.
6 Hashemi M, Eskandari-Nasab E, Fazaeli A, Bahari A, Hashemzehi NA, Shafeipour S, et al. Association of genetic polymorphisms of glutathione-S-transferase genes (GSTT1, GSTM1, and GSTP1) and susceptibility to nonalcoholic fatty liver disease in Zahedan, Southeast Iran. DNA Cell Biol 2012;31:672-677.
7 Balcerczyk A, Grzelak A, Janaszewska A, Jakubowski W, Koziol S, Marszalek M, et al. Thiols as major determinants of the total antioxidant capacity. Biofactors 2003;17:75-82.
8 Przemys?aw W, Piotr K, Gra?yna C, Danuta KP, Ma?gorzata I, Bernadeta M, et al. Total, free, and protein-bound thiols in plasma of peritoneal dialysis and predialysis patients. Int Urol Nephrol 2011;43:1201-1209.
9 Shearn CT, Orlicky DJ, Saba LM, Shearn AH, Petersen DR. Increased hepatocellular protein carbonylation in human end-stage alcoholic cirrhosis. Free Radic Biol Med 2015;89:1144-1153.
10 Gutiérrez R, Alvarado JL, Presno M, Pérez-Veyna O, Serrano CJ, Yahuaca P. Oxidative stress modulation by Rosmarinus offcinalis in CCl4-induced liver cirrhosis. Phytother Res 2010;24:595-601.
11 De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fbrogenic? Gastroenterology 2006;131:272-275.
12 Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fbrosis. J Clin Invest 2003;112:1383-1394.
13 Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fbrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 2012;56:2316-2327.
14 Choi SS, Witek RP, Yang L, Omenetti A, Syn WK, Moylan CA, et al. Activation of Rac1 promotes hedgehog-mediated acquisition of the myofbroblastic phenotype in rat and human hepatic stellate cells. Hepatology 2010;52:278-290.
15 Bopp A, Wartlick F, Henninger C, Kaina B, Fritz G. Rac1 modulates acute and subacute genotoxin-induced hepatic stress responses, fbrosis and liver aging. Cell Death Dis 2013;4:e558.
16 Ezhilarasan D, Sokal E, Karthikeyan S, Najimi M. Plant derived antioxidants and antifbrotic drugs: past, present and future. J Coast Life Med 2014;2:738-745.
17 Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399-410.
18 Vieira EK, Bona S, Di Naso FC, Porawski M, Tieppo J, Marroni NP. Quercetin treatment ameliorates systemic oxidative stress in cirrhotic rats. ISRN Gastroenterol 2011;2011:604071.
19 Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of infammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Infamm Res 2007;56:210-215.
20 Méndez L, Pazos M, Molinar-Toribio E, Sánchez-Martos V, Gallardo JM, Rosa Nogués M, et al. Protein carbonylation associated to high-fat, high-sucrose diet and its metabolic effects. J Nutr Biochem 2014;25:1243-1253.
21 Lin SY, Wang YY, Chen WY, Chuang YH, Pan PH, Chen CJ. Benefcial effect of quercetin on cholestatic liver injury. J Nutr Biochem 2014;25:1183-1195.
22 Haddadian Z, Eftekhari G, Mazloom R, Jazaeri F, Dehpour AR, Mani AR. Effect of endotoxin on heart rate dynamics in rats with cirrhosis. Auton Neurosci 2013;177:104-113.
23 Ma Z, Zhang Y, Huet PM, Lee SS. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol 1999;30:485-491.
24 Prockop DJ, Udenfriend S. A specifc method for the analysis of hydroxyproline in tissues and urine. Anal Biochem 1960;1:228-239.
25 Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302-310.
26 Lapenna D, Cuccurullo F. TBA test and “free” MDA assay in evaluation of lipid peroxidation and oxidative stress in tissue systems. Am J Physiol 1993;265:H1030-H1032.
27 Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 1994;233:380-385.
28 Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195:133-140.
29 Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coeffcients of H2O2solutions in the UV). Anal Biochem 1972;49:474-478.
30 Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44:276-287.
31 Sallie R, Tredger JM, Williams R. Drugs and the liver. Part 1: Testing liver function. Biopharm Drug Dispos 1991;12:251-259.
32 Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis 2008;28:167-174
33 Tseilikman VE, Pankov NE, Pankova NA, Filimonova TA, Sinitskii AI, Kozochkin DA, et al. Correlation between circulating corticosterone and protein carbonylation in the liver after short-term hypokinesia. Bull Exp Biol Med 2013;156:188-190.
34 De Waal EM, Liang H, Pierce A, Hamilton RT, Buffenstein R, Chaudhuri AR. Elevated protein carbonylation and oxidative stress do not affect protein structure and function in the longliving naked-mole rat: a proteomic approach. Biochem Biophys Res Commun 2013;434:815-819.
35 Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fbrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol 2010;53:702-712.
36 Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, et al. Liposomal quercetin effciently suppresses growth of solid tumors in murine models. Clin Cancer Res 2006;12:3193-3199.
37 Poli G. Pathogenesis of liver fbrosis: role of oxidative stress. Mol Aspects Med 2000;21:49-98.
38 Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stressrelated changes in the livers of bile-duct-ligated rats. J Biomed Sci 2003;10:170-178.
39 Wang L, Liu P, Wang CS. Effects of 5 classical recipes on antioxidative stress in rat liver with cirrhosis. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:435-439.
40 Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med 2003;9:169-176.
41 Eskandari-Nasab E, Kharazi-Nejad E, Nakhaee A, Afzali M, Tabatabaei SP, Tirgar-Fakheri K, et al. 50-bp Ins/Del polymorphism of SOD1 is associated with increased risk of cardiovascular disease. Acta Med Iran 2014;52:591-595.
42 Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001;269:131-140.
43 Levi M, McDonald LA, Preisig PA, Alpern RJ. Chronic K depletion stimulates rat renal brush-border membrane Na-citrate cotransporter. Am J Physiol 1991;261:F767-773.
44 Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fbrosis in mice. Hepatology 2006;44:1267-1277.
45 Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fbrosis. Antioxid Redox Signal 2014;20:2854-2872.
46 Paik YH, Brenner DA. NADPH oxidase mediated oxidative stress in hepatic fbrogenesis. Korean J Hepatol 2011;17:251-257.
47 Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Defciency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 2010;59:2033-2042.
48 Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, et al. Sonic hedgehog is an autocrine viability factor for myofbroblastic hepatic stellate cells. J Hepatol 2008;48:98-106.
Received May 11, 2016
Accepted after revision August 8, 2016
Author Affliations: Department of Biochemistry (Kabirifar R, Ghoreshi Z and Moradi A), Department of Physiology (Safari F) and Department of Pharmacology (Karimollah A), School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran; Department of Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran (Eskandari-nasab E)
Ali Moradi, PhD, Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran (Email: morady2008@gmail.com)
? 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60164-9
Published online December 28, 2016.
 Hepatobiliary & Pancreatic Diseases International2017年1期
Hepatobiliary & Pancreatic Diseases International2017年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Limitations of current liver transplant immunosuppressive regimens: renal considerations
- Editors
- Information for Readers
- The relationship between SPARC expression in primary tumor and metastatic lymph node of resected pancreatic cancer patients and patients' survival
- Prospective evaluation of the short access cholangioscopy for stone clearance and evaluation of indeterminate strictures
