Generic sustained release tablets of trimetazidine hydrochloride:Preparation and in vitro – in vivo correlation studies
The School of Pharmaceutical Science,Shandong University,44 Wenhua Xi Road,Ji’nan,China
Generic sustained release tablets of trimetazidine hydrochloride:Preparation and in vitro – in vivo correlation studies
Longmei Wang,Ruihua Feng,Jinhua Gao,Yanwei Xi,Guihua Huang*
The School of Pharmaceutical Science,Shandong University,44 Wenhua Xi Road,Ji’nan,China
A R T I C L EI N F O
Article history:
Received 15 July 2015
Received in revised form 23
September 2015
Accepted 10 October 2015
Available online 21 October 2015
Sustained release
The aim of the current work was to develop generic sustained-release tablets containing 35 mg trimetazidine dihydrochloride and to establish anin vitro–in vivocorrelation that could predict the bioavailability.The marketed sustained release tablet(Vastarel MR)used as reference,a sustained-release matrix tablet was prepared using hydroxypropyl methylcellulose (HPMC)as matrix by wet granulation and thein vitrodissolution profles of the self-made tablets were determined in four different dissolution media(0.1 M HCl,pH 4.5 PBS,pH 6.8 PBS and water).A higher similarity between prepared tablets and Vastarel MR was established,with similarity factor(f2)ranging from 60 to 75 in the four media.Thein vivopharmacokinetics was studied in six healthy beagles.Compared with Vastarel MR,theCmaxof self-made tablets was slightly decreased,while theTmaxand MRT0–twere slightly prolonged,but with no signifcant difference(P>0.05).The average of relative bioavailability (F)was 102.52%based on AUC0–t.For log-transformed AUC0–tandCmax,the upper confdence limit on the appropriate criterion is<0,indicating these two formulations were population bioequivalent.Thein vivo–in vitrocorrelation coeffcient obtained from point-to-point analysis of self-made tablets was 0.9720.In conclusion,the prepared tablets were bioequivalent to the marketed tablets,according to both thein vitrorelease rate and extent of absorption,and a goodin vivo–in vitrocorrelation was established for the self-made tablets that indicatedin vitrodissolution tests could be used as a surrogate for bioavailability studies.
?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Angina pectoris,commonly known as angina,is a clinical manifestation of ischemic heart disease,generally arising from obstruction or spasm of the coronary arteries[1,2].In patients with angina pectoris,myocardial ischemia induces lower exercise capacity,more frequent angina attacks and reduces quality of life[3].Trimetazidine dihydrochloride,1-[(2,3,4-trimethoxyphenyl)methyl]piperazin is a clinically effective antianginal agent that has been used in the prophylaxis and management of angina pectoris[4].Unlike other classical antianginal drugs,such as beta-blockers,calcium-channel antagonists,and long-acting nitrates,trimetazidine dihydrochloride displays anti-ischemic effects without inducing hemodynamic changes and thus protects the heart from the deleterious consequences of ischemia.So it is generally well tolerated and accompanied by minor side effects.Besides, trimetazidine dihydrochloride is freely soluble in water and has a relatively short half-life of 6±1.4 h.Therefore,it is considered as an ideal candidate for sustained drug delivery[5].
In the market,trimetazidine dihydrochloride is available as both immediate release oral formulations(Vastarel IR,20 mg) and modifed release tablets(Vastarel MR,35 mg).The optimal dosage regimen of IR tablets is approved three times a day while MR tablets is twice a day[6].However,repeated administration of the IR tablets leads to poor compliance for angina pectoris patients who need a long term therapy.MR tablets with sustained drug release behaviors could maintain their therapeutically effective concentrations in systemic circulation for prolonged periods of time,which decreases the number of daily administrations,minimizing local and systemic side effects. Thus it improves the patient compliance with prescribed dosage regimens[7].
Hydrophilic polymers are becoming very popular in formulating oral sustained release tablets,such as xanthan gum, cellulose derivatives,alginate sodium or carbopol[8]. Hydroxypropyl methylcellulose(HPMC)is the most commonly and successfully used hydrophilic material for sustained drug delivery[9].It possesses some important characteristics including nontoxicity,pH independence and high water swellability,which contribute to obtain a desirable drug sustained release profle.In this investigation,HPMC was used as a release retardant carrier in the design of sustained release matrix tablets for trimetazidine dihydrochloride.
Besides,a point-to-pointin vitro–in vivocorrelation was developed for relating percentage of drug dissolved to percentage of drug absorbed.The Food and Drug Administration(FDA) defnesin vitro–in vivocorrelation as a predictive mathematical model describing the relationship between anin vitroproperty of a dosage form and a relevantin vivoresponse[10]. A good correlation could predict the rate and extent of drug absorptionin vivo[11].Developing anin vitro–in vivocorrelation for a sustained release tablet is an important object to facilitate product development and serves as a quality control procedure during product manufacture.This reduces the need for expensive bioavailability testing in animals and humans [12].
Hence the objective of the work was to prepare oral administration of sustained release HPMC matrix tablets containing 35 mg trimetazidine dihydrochloride and study its bioequivalence to marketed formulations(Vastarel MR).Similar studies of the dissolutionin vitroof these two formulations(selfmade and marketed sustained release tablets)have been performed in four different dissolution media(0.1 M HCl,pH 4.5 PBS,pH 6.8 PBS and water).Their bioavailability and pharmacokinetic studies were conducted in beagles.Besides,thein vitro–in vivocorrelation of self-made tablets was developed and used as a tool for predictingin vivobioavailability based onin vitrodissolution data.
2.Materials and methods
2.1.Materials
Trimetazidine dihydrochloride was kindly supplied by Wuhan Wu Pharmaceutical Company,China.Hydroxypropyl methylcellulose(HPMC)and ethyl cellulose were purchased from Shanghai Colorcon Pharmaceutical Co.(Shanghai,China).Stearic acid was provided by Hunan Er-kang Pharmaceutical Co.,Ltd. (China).Polyvinylpyrrolidone K30(PVP K30)was purchased from Sinopharm Chemical Reagent Co.,Ltd.(China).Docosanoic acid glycerol ester was purchased from Guangzhou Tianrui Pharmaceutical Co.,Ltd.(China).Calcium hydrogen phosphate dehydrate and potassium dihydrogen phosphate were purchased from Huzhou Zhanwang Pharmaceutical Co.,Ltd. (China).Microcrystalline cellulose,starch and magnesium stearate were provided by Shandong Ahua Pharmaceutical Co.,Ltd. (China).Sodium heptane-1-sulfonate was purchased from Tianjin Kermel Company.Trichloroacetic acid and Potassium dihydrogen phosphate was purchased from Tianjin Damao Chemical Reagent Co.,Ltd.Ethanol was provided byTianjin fu yu Chemical Reagent Co.,Ltd.Acetonitrile(HPLC grade)and methanol(HPLC grade)were high-performance liquid chromatography(HPLC)grade.Double distilled water was used throughout the study.
Animal:Beagles(female or male were provided by Drug Safety Assessment Center of Shandong Institute of Materia Medica)were used for thein vivopharmacokinetic studies.
2.2.Preparation and optimization of trimetazidine dihydrochloride sustained release tablets
The sustained release tablets of trimetazidine dihydrochloride were prepared using wet granulation method.After being grinded and sifted,required quantities of drug,HPMC and excipients were mixed thoroughly,subsequently passed through an 80-mesh screen(stainless steel)to blend the ingredients uniformly.The powder blend was moistened with the required amount of wetting agent(2%HPMC E50LV in 80%ethanol solution or 8%PVP K30 in 80%ethanol solution or 80%ethanol) and then pressed through a 30-mesh screen to prepare wet granules.The granules were dried at 45–55°C for 2 h and the moisture content was controlled within 3%to 5%.The dried granules were then retained on 30-mesh screen(stainless steel), mixed with a prescribed amount of magnesium stearate as lubricant.Finally,the tablets were compressed using a fatfaced 8-mm punch in single-punch press tablet machine.Eachtablet contained 35 mg of trimetazidine dihydrochloride,keeping hardness between 5.0 and 7.0 kgf.
To study the infuences of the tablet formulation, trimetazidine dihydrochloride sustained release matrix tablets were prepared according to the orthogonal design(Table 1).The three major factors were set as follows:A,the amount of HPMC; B,the amount of blocking agents;C,the amount of loading agents.The similarity factor(f2)was employed to evaluate the release profles of various formulations compared with the release profle of marketed tablets.In the orthogonal experimental design,f2was chosen as the evaluation index.
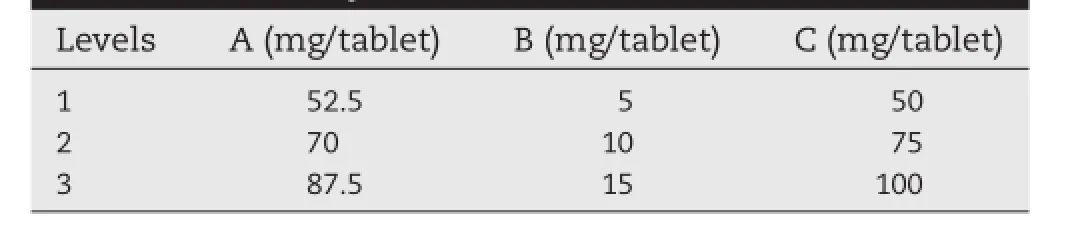
Table 1–Factors and levels of orthogonal experiment for trimetazidine dihydrochloride sustained release tablets
2.3.Tablet weight variation
Weight variation tests of marketed tablets and three batches of self-made tablets were done respectively.Twenty tablets were weighed to obtain the average tablet weight and the individual tablet weight was compared to the average.And then weight variation was evaluated for utilizing criteria based on the Chinese Pharmacopoeia(ChP).
2.4.In vitro dissolution studies
The release studies of trimetazidine dihydrochloride from the self-made and marketed sustained release tablets were performed according to the ChP paddle method.Studies were carried out at(37±0.5)°C and 50 rpm rotation speed in 900 mL of 0.1 M HCl,pH 4.5 PBS,pH 6.8 PBS and water,respectively.The amount of drug used was equivalent to 35 mg.Five milliliters of the dissolution samples were withdrawn at different time intervals(0.5,1,2,4 and 8 h)and were replaced with equal volume of fresh release medium to maintain a constant total volume[13].Samples were fltered through 0.45 μm millipore flter and assayed for drug content by a UV spectrophotometer at λmax of 231 nm after appropriate dilution.Moreover, the selectivity of the UV method was studied by comparing UV spectrum of blank excipients with those of corresponding trimetazidine dihydrochloride standard solution.As a result, no interference from excipients was observed at λmax of 231 nm(Fig.1).
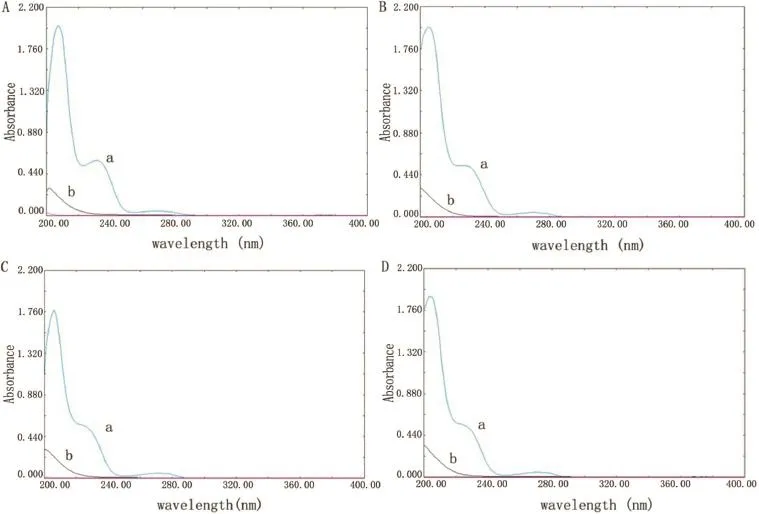
Fig.1–UV spectrums for trimetazidine dihydrochloride(a)and blank excipients(b)in 0.1 M HCl(Fig.1A),pH 4.5 PBS (Fig.1B),pH 6.8 PBS(Fig.1C)and water(Fig.1D).
Cumulative amount of trimetazidine dihydrochloride dissolved in the preparations was calculated using calibration curves.Dissolution tests were carried out in six vessels performulation(n=6).The similarity factor(f2)was used as a basis to compare the difference between self-made tablets and marketed tablets in dissolution profles,which was calculated using the following equation[14].
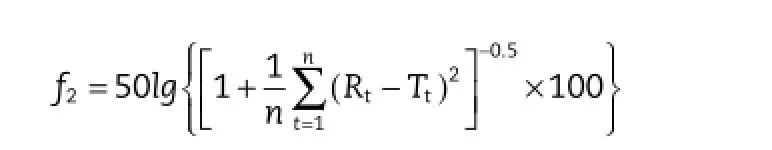
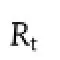
2.5.Drug release mechanism
The description ofin vitrodissolution profles was analyzed using the following mathematical models with different equations[15].

where Mtis the amount of drug released at time t,M∞the total amount of drug,k0is zero order release rate constant,k1is frst order release rate constant and kHis the Higuchi rate constant.Besides,nis the release exponent(e.g.frst order release whenn=1),describing the kinetic and the drug release operating mechanism for cylindrical shaped matrices.
In the Ritger–Peppas model,the value ofn,representing the diffusion pattern,n≤0.45,corresponds to a Fickian diffusion mechanism,0.45<n<0.89 to anomalous non-Fickian transport,n=0.89 to Case II transport,andn>0.89 to super case II transport[16].The correlation coeffcient(r)was used as an indicator of the best ftting,for each of the models considered.
2.6.In vivo pharmacokinetics study in beagles
2.6.1.Study design
The study was conducted to compare the pharmacokinetics of trimetazidine from the self-made tablets to commercially available tablets(Vastarel MR,35 mg),following the administration of single doses equivalent to 70 mg(two tablets per dose), each using a non-blind,two-treatment,two-period,randomized,crossover design.Six healthy beagle dogs of either sex were selected for experiment and divided into two groups(3 animals in each)randomly.The dogs were fasted for about 12 h before the study,while water was provided freely as much they required.
The study was performed on two phases.In Phase I,half the number of beagle dogs received the self-made tablet and the remainder received the commercially available tablet which is considered as a standard.Both treatments were ingested with 250 ml of water,while no food was allowed for 4 h after dosing. A washout period of 1 week separated the phases.In Phase II,the reverse of randomization took place.Venous blood samples(3 ml)were withdrawn and taken into heparinized tubes at 0,0.5,1,1.5,2,2.5,3,4,6,8,10,12,14,24 and 30 h after administration of each treatment.The blood samples were immediately centrifuged at 3000 rpm for 10 min,plasma obtained by and stored at?20°C until analysis by HPLC.
2.6.2.Chromatographic conditions
The quantitative determination of trimetazidine dihydrochloride in plasma was performed using a modifed HPLC method of Min Kyo Jeoung et al.[17].Chromatographic separation was performed at 30°C on reversed-phase C18column(4.6 μm×150 mm). The mobile phase consisted of potassium dihydrogen phosphate(0.05 mol/l,pH was adjusted to 4.0 with 10%phosphoric acid)and methanol(76:24,v/v)and was delivered to the system at the fow rate of 1.0 ml/min with UV detection at 210 nm.
2.6.3.Standard solutions
A 500 μl aliquot of blank plasma was accurately measured into a 5-mL centrifuge tube,spiked with appropriate standard solutions to produce concentrations of 25,50,100,200,500,1000, 2000 ng/ml of trimetazidine dihydrochloride.100 μl of 10%(w/ v)trichloroacetic acid was added to the above plasma samples, which was vortexed for 3 min and centrifuged for 12 min at 12,000 rpm[18].The resulting supernatant liquid contained 20.83,41.67,83.33,166.67,416.67,833.33,1666.67 ng/ml trimetazidine dihydrochloride in plasma,respectively.And then 20 μl of each sample was injected into the column for analysis.Under the described conditions,the retention time of trimetazidine dihydrochloride was about 12.8 min.A standard curve was established by plotting the peak area of trimetazidine dihydrochloride against its concentration in plasma.The lower limit of quantifcation was 20.83 ng/ml.The method showed good linearity(R2=0.9987)in the range of 20.83–1666.67 ng/ml in beagles plasma.The mean extraction recoveries of trimetazidine dihydrochloride were 85–115%,RSD<7%.The inter-day and intra-day relative standard deviations(RSDs)for trimetazidine were less than 12%at the above concentrations.
2.6.4.Pharmacokinetic analysis
Pharmacokinetic analysis of the two treatments was performed for each dog by using DAS2.0(drug and statistics for windows)program.Maximum drug concentration(Cmax)and the corresponding time toCmax(Tmax)were obtained from the plasma concentration-time curves directly.The area under the curves,AUC0–t(ng h/ml),was calculated by the trapezoidal rule from 0 to 30 h.On the other hand,AUC0–∞(ng h/ml),the area under the curve from zero to infnity,was calculated as AUC0–∞=AUC0–t+Ct/Ke,where,Ctis the drug plasma concentration observed at time t and Keis the apparent elimination rate constant.Half-life(t1/2)was obtained by dividing 0.693 with Ke.Mean residence time(MRT)was calculated from AUMC/ AUC.The relative bioavailability(F)of the self-made tablet was calculated by dividing its AUC0–twith that of marketed sustained release dosage form[19].
2.6.5.Statistical analysis
The results were expressed as mean±standard deviation(SD). The comparison between two groups was performed using unpaired Student’s t-test,a signifcant level of difference for the tests was considered at a level ofP<0.05.The pharmacokinetic parameters of the two groups were analyzed using the DAS 2.0 software package(Chinese Pharmacological Society).
2.7.In vitro–in vivo correlation
A point-to-pointin vitro–in vivocorrelation of self-made tablets was developed for relating percentage of drugin vitrodissolution to percentage of drugin vivoabsorbed.Based on a good correlation,measuring thein vitrodissolution rate alone is suffcient to determine the pharmacokinetic proflein vivo.It allows thein vivobioavailability test with the fewest possible trials in animals and man for the prepared formulation.
The fraction of the drug absorbed(Fa)was calculated by the Wagner–Nelson Eq.(1)or by the Loo–Riegelman Eq.(2)for both a one-compartment model and the two-compartment model [20].
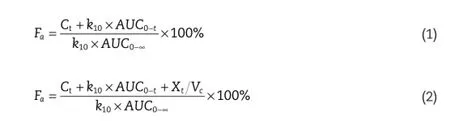
whereFais the fraction of the drug absorbed,Ctis the concentration of drug in the plasma at time point t,k10is the elimination rate constant,AUC0?tis the calculated area under the plasma concentration curve from zero to time t,andAUC0?∞is the calculated area under the plasma concentration curve from time zero to infnity.XtVCis the apparent tissue compartment concentration at the time t.The percent of drug absorbed(Fa)at the specifed time points was plotted against the percent of drug dissolvedin vitroat the same time points. The linear regression coeffcient(R2)was used to evaluate the correlation betweenin vitrorelease andin vivoabsorption.
3.Results and discussions
3.1.Optimization of formulation of trimetazidine dihydrochloride sustained release matrix tablets
3.1.1.Results of the test of orthogonal design
Based on preliminary study,three factors were chosen as research objects,including A,B and C.Furthermore,the factors affectingf2were studied in orthogonal experimental design. Table 2 showed the results of drug release and orthogonal design.The range(R)among the score averages is the difference between the highest and the lowest score average. According to Table 2,the effect of B(the amount of blocking agents)was extremely signifcant;the effect of other factors was not signifcant.The effects of each factor on thef2were as follows:B(the amount of blocking agents)>A(the amount of HPMC)>C(the amount of loading agents).K1a,K2a and K3awere the average sum scores of Level 1,Level 2 and Level 3 for each factor,respectively.Apparently,the larger the average sum score was the closer to the release profle of marketed tablets. Analytical results of three factors were A:3>2>1,B:3>2>1, C:3>2>1,so the optimal formulation was found to be A3B3C3and it was obtained as follows:HPMC,87.5 mg;blocking agents, 15 mg;loading agents,100 mg.
3.1.2.Lot reproducibility
Three batches of the trimetazidine dihydrochloride sustained release tablet were prepared by the optimal formulation and thein vitrorelease tests were performed.Batches I,II and III showed similarity factorf2values of 71.41,69.16 and 67.60, respectively.Hence,the drug release from the three batches tablets was similar to that of the marketed product(Vastarel MR,35 mg).Moreover,ANOVA test shows that batches I,II and III are not signifcantly different in their drug release profle (P>0.05 at each time point)and the values off2(P>0.05).The batch reproducibility study indicated that the formulation methodology employed was found to be suitable for trimetazidine dihydrochloride sustained release tablets.
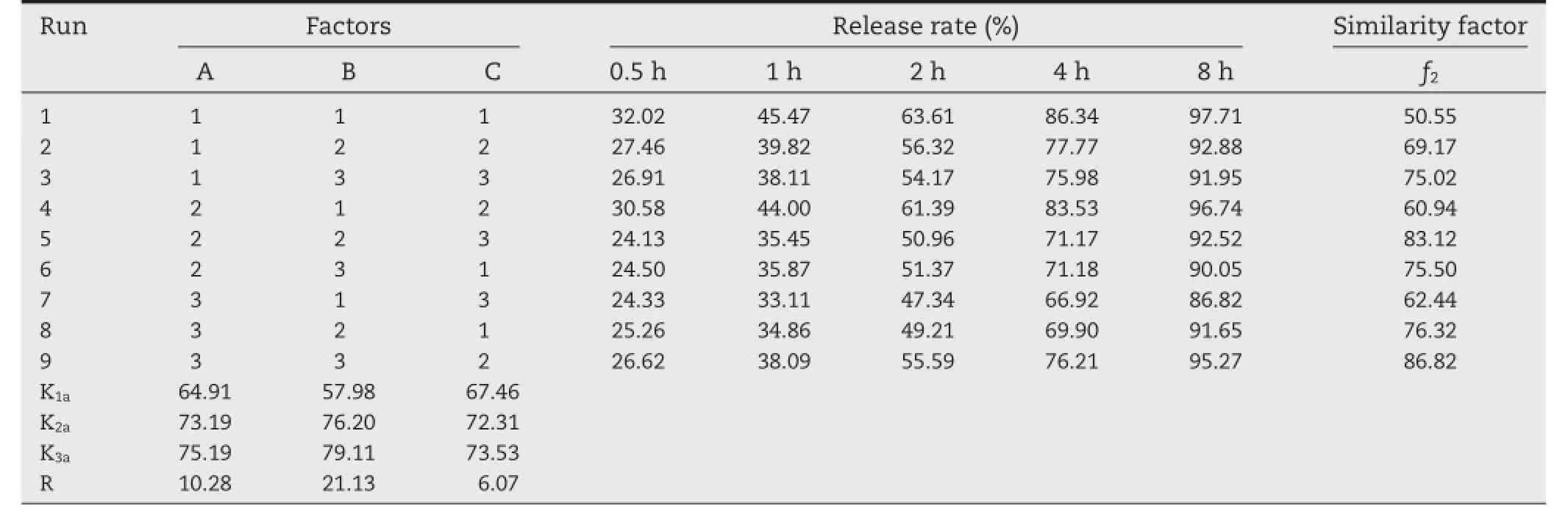
Table 2–Results of orthogonal experiment design.
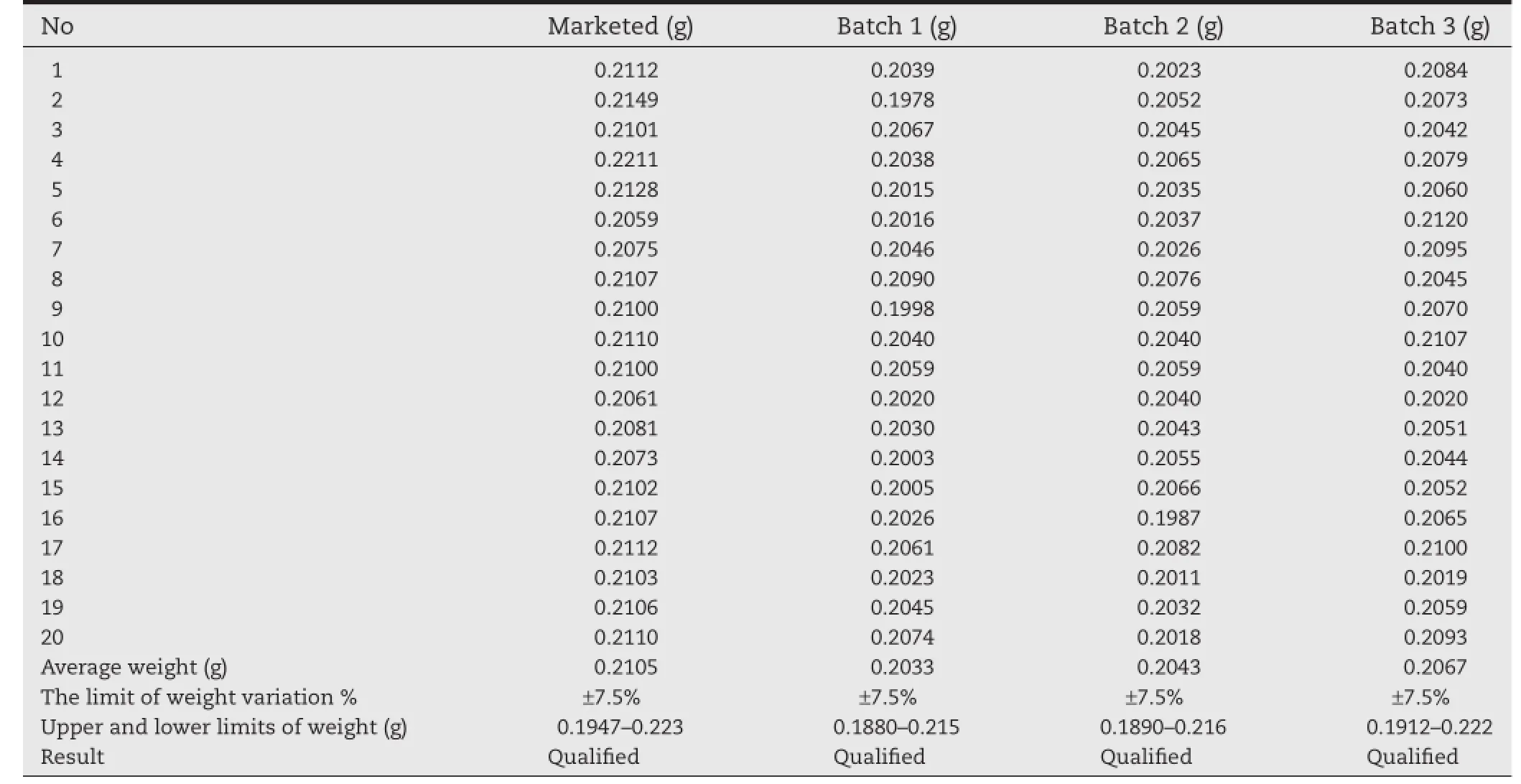
Table 3–Results of tablet weight variation.
3.2.Tablet weight variation
The results of weight variation are shown in Table 3.Average weights of marketed tablets and three batches of self-made tablets were calculated as 0.2105,0.2033,0.2043,0.2067,respectively(n=20).As shown in Table 3,no tablet deviated from the average weight by more than 7.5%and indicated that the self-made tablets were obtained of uniform weight.
3.3.In vitro dissolution studies
In the present study,thein vitrodissolution studies were carried out for the self-made sustained release tablets in four different dissolution media(0.1 M HCl,pH 4.5 PBS,pH6.8 PBS and water)and the release profles are shown in Fig.2.It is clear that the self-made sustained release tablets released about 20% of its trimetazidine dihydrochloride content in the four media after 0.5 h and the amount of the drug released in 8 h was not less than 80%of the labeled amount in the four media.Moreover,thein vitrorelease behavior of the self-made formulation was not affected by pH of dissolution medium.These results suggested that the self-made tablet exhibited a slow releasein vitrowhich is independent of the pH of dissolution media.
Besides,the results of thein vitrorelease of trimetazidine dihydrochloride from the self-made sustained release tablets in comparison with that of market product(Vastarel MR)in four media are graphically shown in Table 4 and Fig.3.As shown in Table 4,the similarity factorf2was used to compare the two dissolution profles,and the results showed thef2values ranged from 60 to 75,indicating that the self-made sustained release tablets shared a similar drugin vitrorelease behaviors with the marketed tablets.Dissolution studies revealed it is expected to provide a new excellent candidate for trimetazidine dihydrochloride sustained release tablets in the market.
3.4.Drug release mechanism studies
Thein vitrorelease studies were carried out for the marketed and self-made sustained release tablets in 0.1 M HCl,water, and phosphate buffers with pH 4.5 and 6.8 media.In order to determine the suitable drug release kinetic model,thein vitrorelease data were analyzed according to zero-order model,frstorder model,Higuchi model and Ritger–Peppas model. Correlation is higher as absolute value of the correlation coeffcient(r)is closer to 1.
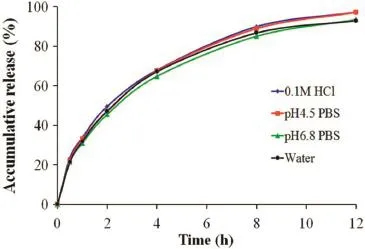
Fig.2–Dissolution profles of trimetazidine dihydrochloride from self-made sustained release tablets in 0.1 M HCl,pH 4.5 PBS,pH 6.8 PBS and water.
Firstly,analysis was performed by the zero order model,frst order model and Higuchi model equations.In case of the marketed tablets,the correlation coeffcient(r)values for Higuchi model were found to be higher in 0.1M HCl(0.9988)and watermedia(0.9974),when compared to that of frst order kinetics and zero order kinetics.However,the‘r’values for the frst order release kinetics were found to be higher in phosphate buffers with pH 4.5(0.9991)and 6.8 media(0.9987),when compared to that of zero order kinetics and Higuchi model.It indicates that the release of trimetazidine dihydrochloride from marketed sustained release tablets was ftted to Higuchi model in 0.1 M HCl and water media,while the drug release followed frst order kinetics in phosphate buffers with pH 4.5 and 6.8 media(Table 5).From Table 5,for the self-made formulation, frst-order kinetics had the higher regression value compared to the zero and Higuchi kinetics in four media,which indicates that the release of trimetazidine dihydrochloride from the prepared sustained release tablets was ftted well with the frst-order in the four media.In addition,the dissolution data of self-made and marketed tablets were ftted to the Ritger–Peppas model;the diffusion exponent values ranged between 0.45 and 0.89,but were close to 0.45,which appears to indicate trimetazidine hydrochloride was released from the two sustained-release formulations by the synergistic effect of diffusion and erosion skeleton,but with diffusion serving as the main release way.This may be attributed to the high watersolubility of the trimetazidine dihydrochloride in the sustained release matrix tablets.
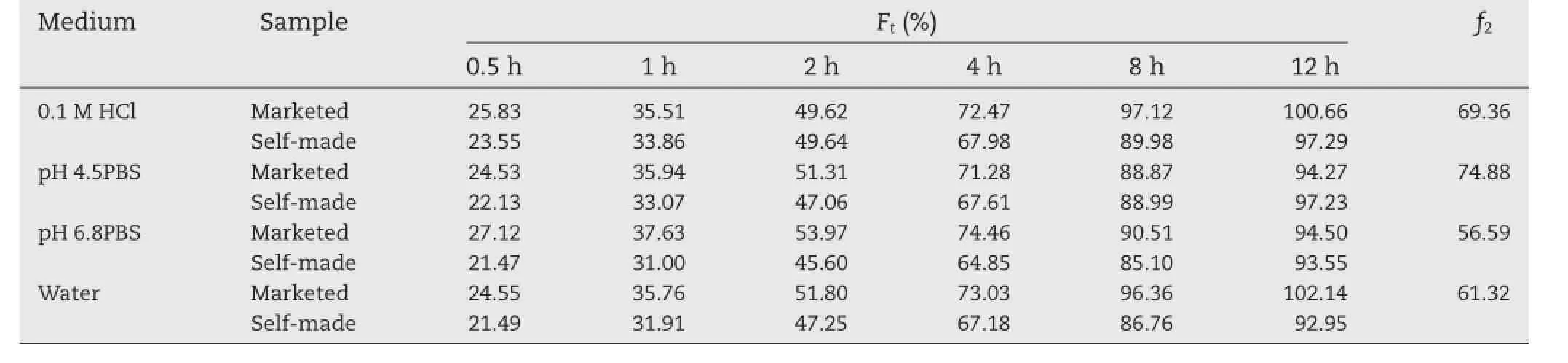
Table 4–The cumulative amount of drug release(Ft)from the self-made and marketed tablets and the calculated similarity factor(f2)for trimetazidine dihydrochloride dissolution profles obtained from self-made tablets compared to the marketed tablets.
3.5.In vivo pharmacokinetics study in beagle dogs
Pharmacokinetic studies of self-made sustained release tablets (test tablets)of trimetazidine dihydrochloride compared with commercially available sustained-release tablets(reference tablets)were investigated.The plasma drug concentrations of the two sustained-release tablets at different time intervals were determined respectively.And the mean concentration-time profles for test and reference tablets are shown in Fig.4.Similar to the commercially available products,a steady plasma concentration of trimetazidine dihydrochloride was obtained for the self-made sustained release tablets.Most importantly,theplasma concentration of trimetazidine dihydrochloride did not differ signifcantly after administration of both formulations.
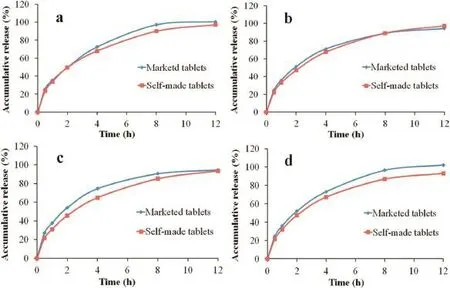
Fig.3–Comparison ofin vitrodissolution profles of self-made sustained release tablet and the marketed tablet in 0.1 M HCl(a),pH 4.5 PBS(b),pH 6.8 PBS(c)and water(d),respectively.
The parametersTmaxand AUC0–∞are related to the rate and extent of absorption,respectively,whileCmaxis related to both processes[21].The values of the pharmacokinetic parameters of the prepared and marketed tablets are given in Table 6.The meanCmaxvalues for self-made sustained release tablets and marketed product were 628.17 ng/ml and 688.00 ng/ml,respectively,reached after time(Tmax)2.6 h and 2.2 h,respectively. Besides,the values of mean residence time(MRT0–t)of trimetazidine dihydrochloride in self-made and marketed tablets were 7.682 h and 6.608 h,respectively.Compared with the marketed sustained release tablets,theCmaxof self-made tablets was slightly decreased,while theTmaxand MRT0–twere slightly prolonged.However,statistical analysis revealed that there was no signifcant difference(P>0.05)in the values ofCmax,Tmaxand MRT0–tobtained from self-made and marketed tablets.The relative bioavailability(F)of test tables compared to reference tables was calculated to be 102.52%based on AUC0–t(Table 6).
DAS 2.0 software was used to perform the population bioequivalent(PBE)statistical analyses.For log transformed observations AUC0–tandCmax,the upper confdence limit on the appropriate was<0,indicating these two formulations were population bioequivalent.Moreover,test for the analyzedTmaxshowed no signifcant differences(P=0.26>0.05).Then it could be established that the self-made tablets were bioequivalent to the marketed sustained release tablets and that both formulations could be considered equally effective and safe in therapeutics in angina pectoris patients.
Thus,the drug release behavior from self-made sustained release tabletsin vivowas similar to the commercially available products,evidenced by the similarity in their pharmacokinetic parameters;moreover,the two formulations were population bioequivalent based on AUC0–tandCmax.Therefore,the self-made tablets were bioequivalent to commercially available sustained-release tablets,and the two formulations are interchangeable.
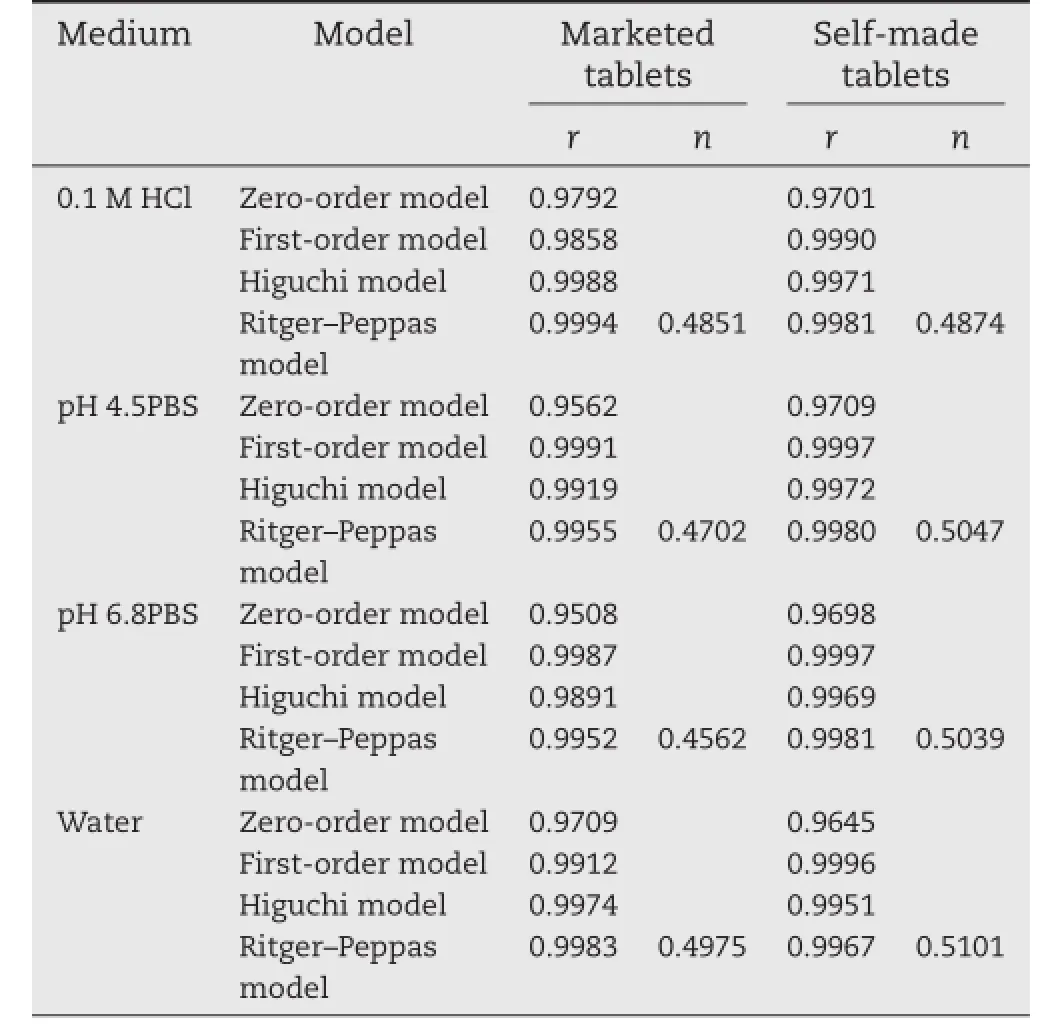
Table 5–Mathematic model ftting of release profle of self-made and marketed trimetazidine dihydrochloride sustained release tablets.
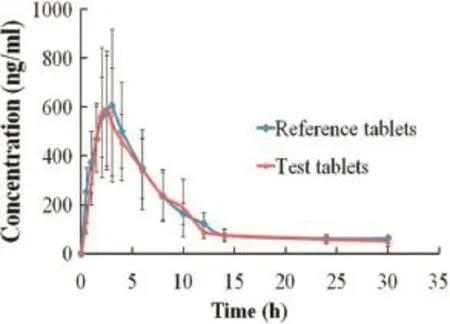
Fig.4–Plasma concentration profles of trimetazidine dihydrochloride of self-made and marketed release tablets after oral administration to six healthy beagles under fasted conditions(mean±SD).
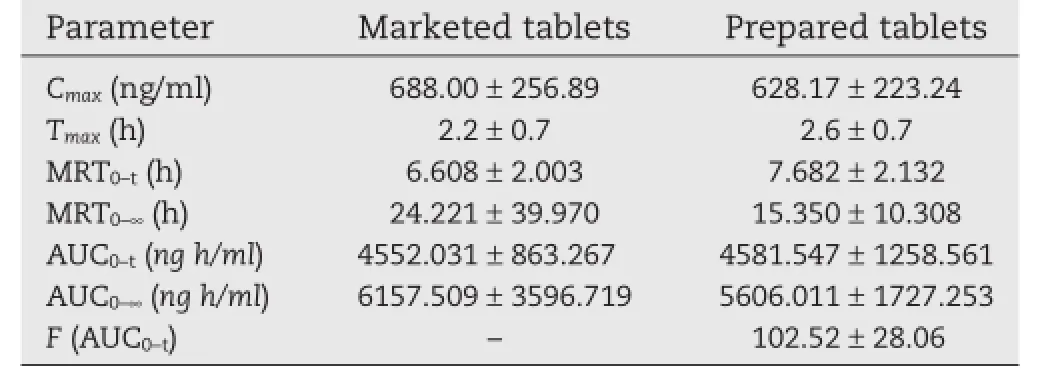
Table 6–Pharmacokinetic parameters of trimetazidine dihydrochloride in beagles(n=6)administered with marketed tablets(dose 35 mg)and prepared tablets (dose 35 mg).
3.6.In vivo–in vitro correlation
The IVIVC for trimetazidine dihydrochloride was examined.The optimization one compartment models was determined for the drug dosage forms after validation by DAS2.0(drug and statistics for windows)program.Therefore,in vivo–in vitrocorrelation was investigated using the percentage of drug release in a water medium of self-made sustained release tablets and the drug absorption fraction in beagle dogs at fasted condition.The accumulative absorption fraction of trimetazidine dihydrochloride was calculated according to the Loo–Riegelman method.As shown in Fig.5,the correlation coeffcient(R2) between the drug release from the tablets and absorption of drug was 0.9720,indicating that the drug release correlated well with the fraction absorption.Therefore,thein vivoabsorption behavior could be predicted by the test ofin vitrodrug release.
4.Conclusions
The present study was carried out for the prepared sustained release tablets of trimetazidine dihydrochloride compared withthe commercially available modifed release tablets(Vastarel MR,35 mg).For the dissolution studiesin vitro,thef2factor confrmed that the release of trimetazidine dihydrochloride from the prepared tablets was similar to that of the marketed tablets. Results fromin vivopharmacokinetics study in beagle dogs also clearly indicated that the variability in the prepared trimetazidine dihydrochloride absorption profles was equivalent to the marketed products.Thein vivocomparative study results confrmed that the pharmacokinetic parameters AUC and theCmaxof trimetazidine dihydrochloride were similar after oral administration with the self-made and the marketed sustained released tablets.Besides,compared with the marketed tablets,the relative bioavailability judged from the AUC0–twas found to be 102.52%.Moreover,the two formulations were population bioequivalent based on AUC0–tandCmax.Thus,it could be concluded the self-made sustained release tablets were bioequivalent to the branded products in the market(Vastarel MR,35 mg),which proved that the prepared tablets can be used interchangeably with Vastarel MR.Thein vitro–in vivocorrelation coeffcient(R2)was 0.9720,suggesting that the prepared tablets followed a strong correlation betweenin vitrorelease and pharmacokinetic effect;moreover,it seemed to be reasonable to predict the drug absorptionin vivothrough thein vitrorelease study.Therefore,the high correlation betweenin vitrodissolution andin vivoabsorption could be used to forecast thein vivobioavailability with allowing dosage form optimization with the fewest possible trials in animals and man.
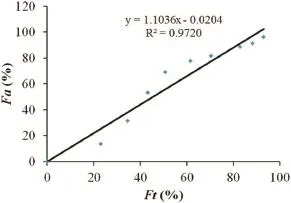
Fig.5–Percentage of fraction(Fa)of drug absorbed and percentage of drug dissolved(Ft)byin vitromethod.
Acknowledgements
The authors would like to express thanks to the School of Pharmaceutical Science,Shandong University,for providing the required infrastructure to carry out the study.
R E F E R E N C E S
[1]Fernandez SF,Tandar A,Boden WE.Emerging medical treatment for angina pectoris.Expert Opin Emerg Drugs 2010;15:283–298.
[2]Hu B,Li W,Xu T,et al.Evaluation of trimetazidine in angina pectoris by echocardiography and radionuclide angiography: a meta-analysis of randomized,controlled trials.Clin Cardiol 2011;34:395–400.
[3]Muller-Werdan U,Stockl G,Ebelt H,et al.Ivabradine in combination with beta-blocker reduces symptoms and improves quality of life in elderly patients with stable angina pectoris:age-related results from the additions study.Exp Gerontol 2014;59:34–41.
[4]MacInnes A,Fairman DA,Binding P,et al.The antianginal agent trimetazidine does not exert its functional beneft via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase.Circ Res 2003;93:e26–e32.
[5]De Robertis S,Bonferoni MC,Elviri L,et al.Advances in oral controlled drug delivery:the role of drug-polymer and interpolymer non-covalent interactions.Expert Opin Drug Deliv 2015;12:441–453.
[6]Fife KH,Ferenczy A,Douglas JM Jr,et al.Treatment of external genital warts in men using 5%imiquimod cream applied three times a week,once daily,twice daily,or three times a day.Sex Transm Dis 2001;28:226–231.
[7]He W,Wu M,Huang S,et al.Matrix tablets for sustained release of repaglinide:preparation,pharmacokinetics and hypoglycemic activity in beagle dogs.Int J Pharm 2015;478(1):297–307.
[8]Maderuelo C,Zarzuelo A,Lanao JM,et al.Critical factors in the release of drugs from sustained release hydrophilic matrices.J Control Release 2011;154:2e19.
[9]Rosenzweig O,Lavy E,Gati I,et al.Development and in vitro characterization of foating sustained-release drug delivery systems of polyphenols.Drug Deliv 2013;20:180–189.
[10]Biswas N,Sahoo RK,Guha A,et al.Chronotherapeutic delivery of hydroxypropylmethylcellulose based minitablets:an in vitro–in vivo correlation.Int J Biol Macromol 2014;66:179–185.
[11]Li X,Zhao Z,Li L,et al.Pharmacokinetics,in vitro and in vivo correlation,and effcacy of exenatide microspheres in diabetic rats.Drug Deliv 2015;22:86–93.
[12]Patel VF,Liu F,Brown MB.Modeling the oral cavity:in vitro and in vivo evaluations of buccal drug delivery systems. J Control Release 2012;161:746–756.
[13]Li L,Wang L,Shao Y,et al.Elucidation of release characteristics of highly soluble drug trimetazidine hydrochloride from chitosan-carrageenan matrix tablets. J Pharm Sci 2013;102:2644–2654.
[14]Asare-Addo K,Levina M,Rajabi-Siahboomi AR,et al.Effect of ionic strength and pH of dissolution media on theophylline release from hypromellose matrix tablets–apparatus USP III,simulated fasted and fed conditions.Carbohydr Polym 2011;86:85–93.
[15]Herrlich S,Spieth S,Messner S,et al.Osmotic micropumps for drug delivery.Adv Drug Delivery Rev 2012;64:1617–1627.
[16]Oh T-O,Kim J-Y,Ha J-M,et al.Preparation of highly porous gastroretentive metformin tablets using a sublimation method.Eur J Pharm Biopharm 2013;83:460–467.
[17]Jeoung MK,Kim KS,Kim CS,et al.An HPLC determination of trimetazidine in human plasma using liquid-liquid extraction for sample clean-up.J Liq Chromatogr Relat Technol 2005;28:1299–1309.
[18]Krishnaiah YSR,Karthikeyan RS,Bhaskar P,et al. Bioavailability studies on guar gum-based three-layer matrix tablets of trimetazidine dihydrochloride in human volunteers.J Control Release 2002;83:231–239.
[19]Agoram B,Woltosz WS,Bolger MB.Predicting the impact of physiological and biochemical processes on oral drug bioavailability.Adv Drug Delivery Rev 2001;50:S41–S67.
[20]Li Z-Q,He X,Gao X,et al.Study on dissolution and absorption of four dosage forms of isosorbide mononitrate: level A in vitro–in vivo correlation.Eur J Pharm Biopharm 2011;79:364–371.
[21]Vemula SK,Veerareddy PR.Development,evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets.Expert Opin Drug Deliv 2013;10:33–45.
*< class="emphasis_italic">Corresponding author.
.School of Pharmaceutical Science,Shandong University,44 Wenhua Xi Road,Ji’nan,China.Tel.:+86 0531 88382015; fax:+86 0531 88382548.
E-mail address:hgh2003@gmail.com(G.Huang).
The marketed sustained release tablet(Vastarel MR)used as reference,a generic sustained-release tablet of trimetazidine hydrochloride was prepared by wet granulation.The prepared tablets were bioequivalent to Vastarel MR,and a goodin vivo–in vitrocorrelation was established for the self-made tablets.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.10.001
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Generic tablets
Bioequivalence studiesIn vitro–in vivocorrelation
 Asian Journal of Pharmacentical Sciences2016年3期
Asian Journal of Pharmacentical Sciences2016年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- A novel LC–MS/MS assay for methylprednisolone in human plasma and its pharmacokinetic application
- Formulation and evaluation of co-prodrug of furbiprofen and methocarbamol
- Auricularia auricular polysaccharide-low molecular weight chitosan polyelectrolyte complex nanoparticles:Preparation and characterization
- Brain targeted delivery of paclitaxel using endogenous ligand
- Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
