Synthesis, Crystal Structure and Fe3+ Recognition of a Fluorescent Probe Based on Quinazolinone Derivative
WANG Lin WANG Xio-Feng XUE Meng-Wei DUAN Shu-Rong LI Ting YU Zhen-Zhen ZHANG Dun-Lin①
a (School of Environmental Science, Nanjing Xiaozhuang University, Nanjing 211171, China)b (School of Food Science, Nanjing Xiaozhuang University, Nanjing 211171, China)
ABSTRACT A novel quinazolinone derivative ethyl 2-(2-methyl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl) acetate (EMOTA) was synthesized and characterized by HRMS, 1H NMR, 13C NMR spectroscopy and X-ray crystallography, and the ion recognition performance of the compound was studied by fluorescence analysis measurements. The results showed that probe EMOTA has a rapid fluorescence response, good selectivity and sensitivity to Fe3+. When the concentration of Fe3+ was in the range of 0~10.0 × 10-5 mol/L, the fluorescence quenching could be affected by the probe, and the detection limit was 1.65 × 10-6 mol/L. In addition, the identification and detection of Fe3+ in water samples were studied, and the results showed that probe EMOTA has high efficiency, significant sensitivity and high selectivity on the recognition and detection of Fe3+ in water samples, which indicates probe EMOTA has a practical application prospect.
Keywords: quinazolinone, crystal structure, fluorescent probe;
1 INTRODUCTION
With the rapid development of China's economy and the remarkable progress of science and technology, the pollution problem also appears. In recent years, although wastewater discharge and water pollution in agriculture, industry and living have been partly controlled, the situation is still severe.Part of the reason for water pollution is the excessive content of metal ions in wastewater, including Fe3+, Mn2+, Cu2+,Hg2+and other metal ions. These metal ions are difficult to be degraded and digested, which may eventually accumulate in humans through the transmission of the food chain, resulting in human regulation disorder and even various diseases[1]. Iron is one of the necessary trace elements for maintaining life and growth[2]. It is a component of hemoglobin that is involved in the transport of oxygen. Also, it is a component of myoglobin which can store oxygen. Fe3+,which can promote the activity of enzymes, is an important component of a variety of enzymes and cytochromes[3]. The imbalance of iron content in the life system will lead to cell metabolic disorder and various diseases. Once the human body is short of iron, it may cause iron deficiency anemia,resulting in metabolic disorder caused by hypoxia, slow growth and development and other diseases[4,5]. On the other hand, if the iron is excessive in human body, it will damage the function of mitochondria, cause bone loss and abnormal function of fibroblasts and other diseases[6,7].
China's health institutions have clearly stipulated that the content of iron in drinking water should not exceed 0.3 mg/L.Therefore, it is of great significance to monitor the content of iron in organisms and drinking water. In recent years, the fluorescent groups used in the reports of sensors for detecting iron elements mainly include rhodamine[8], oxadiazole[9],benzothiazole[10,11], BODIPY[12]and coumarin[13]. These fluorescent groups have the disadvantages of high toxicity,complex structure, long synthetic route or low yield.Quinazolinone is an important nitrogen-containing fused heterocyclic compound. It is well known for the biological activities such as anticancer[14], anti-inflammatory[15], antibacterial[16]and diuretic[17]. In addition, it can be used as a fluorescent group to specifically detect the presence of metal ions[18]. So far, there are studies using quinazolinone derivatives with excellent fluorescence properties as probes[19,20],but there are few reports on quinazolinone derivatives for the detection of Fe3+.
In this paper, a quinazolinone derivative was synthesized fromo-aminobenzamide and ethyl acetoacetate (Scheme 1).It was expected to affect the electron migration in the conjugated system by virtue of its electron deficiency in the benzene ring. Meanwhile, its atom N was expected to coordinate with iron ion to form the fluorescence quenching effect, so as to achieve the purpose of developing a new type of fluorescent sensor for high selective and sensitive Fe3+detection.

Scheme 1. Synthesis of EMOTA
2 EXPERIMENTAL
2. 1 Materials and methods
All starting chemicals used in this study were of analytical grade and commercially available.1H NMR and13C NMR spectra were measured with a BRUKER AVANCE AV-500 nuclear magnetic resonance (CDCl3as the solvent, and TMS as an internal-standard). Mass spectra were obtained from Agilent 1100 Capillary LC. The melting point was measured by Beijing Keyi XT-4B melting point instrument, and the melting point was not corrected. All fluorescence data were measured at room temperature by Hitachi F-4600 fluorescence spectrometer. UV spectrum was determined by Shimadzu UV2450 ultraviolet spectrophotometer. Crystal data were obtained on a Bruker P4 X-diffractometer.
2. 2 Synthesis of EMOTA
To a 50 mL round-bottom flask, 5 mL of ethyl acetoacetate and 2.72 g (0.02 mol) ofo-aminobenzamide were added with 0.1 g ofp-methylbenzenesulfonic acid as catalyst. The mixture was heated with stirring at 140 ℃ to reflux slowly for 3 h until the starting material disappeared (monitored by TLC with EtOAc/petroleum ether = 1/3). Then it was cooled to room temperature and 20 mL of water was added. After that, 30 mL of ethyl acetate was added to extract the product.The ethyl acetate layer was separated with a separating funnel and subsequently the solvent was removed by rotary evaporation to obtain the crude product. It was purified by column chromatography (petroleum ether/ethyl acetate = 1/1)to obtain a white solid product EMOTA with a yield of 80%(3.98 g). m.p.: 123~124 ℃.1H NMR (500 MHz, CDCl3):7.85 (d,J= 5Hz, 1H, Ph-H), 7.43 (s, 1H, N-H), 7.28 (m, 1H,Ph-H), 6.82 (t,J= 7.5Hz, 1H, Ph-H), 6.65 (t,J= 8.05Hz, 1H,C-H), 5.25 (s, 1H, N-H), 4.13 (s, 2H, -CH2), 2.71 (d,J=15.75Hz, 1H, -CH2), 1.63 (s, 3H, -CH3), 1.25 (t,J= 7.15Hz,3H, -CH3).13C NMR (500 MHz, CDCl3): 170.70, 164.39,145.80, 134.16, 128.13, 118.92, 114.97, 114.41, 68.06, 60.92,44.56, 27.01, 14.01. ESI-MS (ethyl acetate solution): Calcd.EMOTA [M+H]+: peak at m/z 249.1161, found 249.1238.
2. 3 X-ray structure determination
A light colourless transparent single crystal of EMOTA suitable for X-ray crystal structure determination was grown by slow evaporation of a solution of ethyl acetate at room temperature. Crystallographic data collections for compound EMOTA were carried out on a Bruker Smart APEX-II CCD with graphite-monochromated MoKαradiation (λ=0.071073 nm) at 295(2) K using theφ-ωscan mode. The data were integrated by using the SAINT program[21], which was also used for the intensity corrections for the Lorentz and polarization effects. An empirical absorption correction was applied using the SADABS program[22]. The structures were solved by direct methods using the program SHELXS-97 and all non-hydrogen atoms were refined anisotropically onF2by the full-matrix least-squares technique using the SHELXL-97 crystallographic software package.The hydrogen atoms of organic ligands were refined as rigid groups. The finalR= 0.1713 andwR= 0.3548 (w= 1/[σ2(Fo2)+ (0.0101P)2+ 24.4603P], whereP= (Fo2+ 2Fc2)/3) for 5798 observed reflections (I> 2σ(I)). (Δρ)min= -0.419 and(Δρ)max= 0.472 e·?-3.
2. 4 Determination of UV-vis absorption spectrum and fluorescence properties
Probe EMOTA, 1 mL dimethyl sulfoxide (DMSO) and finally distilled water were gradually added into a 100 mL volumetric flask to prepare 100 mL sample test solution (1.0× 10-5mol/L, V(DMSO):V(H2O) = 1:99), which was prepared for the determination of UV-vis absorption spectrum and fluorescence emission spectrum.
UV-vis absorption spectrum of the probe test solution was determined at room temperature in the range of 190~770 nm. Fluorescence properties of the probe test solution were measured at room temperature in the range of 220~770 nm and the emission width of the slit is 3 nm.
2. 5 Determination of metal ion recognition performance
Metal ion recognition performance of EMOTA was studied by fluorimetry in water solution. Fluorescence analysis measurements were carried out at excitation wavelengthλ=341 nm and the emission width of the slit is 3 nm.
3 RESULTS AND DISCUSSION
3. 1 Synthesis
Up to now, there are many synthetic methods of quinazolinone, and the main difference lies in the different raw materials which mainly includeo-aminobenzoamides,o-aminobenzoic acids,o-aminobenzonitriles ando-aminobenzoate esters. The synthetic method of synthetic method of takingo-aminobenzoylamine compounds as raw materials attracted much attention due to its high yield and high efficiency in the synthesis of building organic blocks from easily available starting materials. Therefore,o-aminobenzamide and ethyl acetoacetate were chosen as the raw materials to synthesize EMOTA. In this reaction, ethyl acetoacetate is not only a reactant but also a solvent.o-Aminobenzamide reacts with ethyl acetoacetate by heating to form a Schiff base.Then the Schiff base intermediate undergoes a cyclization reaction under the catalysis ofp-toluenesulfonic acid to form quinazolinone. The reaction is simple and convenient for operation.

Fig. 1. Structure diagram of EMOTA single crystal

Fig. 2. Space packing diagram of EMOTA

Table 1. Selected Bond Lengths (?) and Bond Angles (°)
3. 2 X-ray crystal structure
High-quality crystals of EMOTA were obtained by recrystallization from slow evaporation of EtOAc solution and analyzed by X-ray diffraction analysis. The single crystal structure of EMOTA is shown in Fig. 1 and packing diagram of compound is showed in Fig. 2. The selected bond lengths and bond angles are shown in Table 1. All non-hydrogen atoms of the compound have been anisotropically refined.The crystal is of monoclinic system, space groupP21/c, witha= 12.814(2),b= 11.8640(19),c= 18.404(3) ?,β=109.061(5)°,V= 2644.47(74) ?3andZ= 53. In the crystal structure, C(2)-O(1) and C(1)-O(2) are double bonds with the lengths of 1.255(81) and 1.184(87) ?, respectively. The six-membered ring composed of N(1), C(1), N(2), C(2), C(3)and C(4) is not coplanar but presents a torsional structure.The torsion angle is 38.535o (N(1)-C(1)-N(2)-C(2)-C(3)-C(4)). The bond length (1.34 ?) of N(2)-C(2) carbon nitrogen single bond is significantly shorter than the normal bond length, but longer than that of C=N (1.28 ?), which may be caused by electron delocalization in quinazolinone system.As can be seen from Fig. 1, compound EMOTA has a chiral configuration structure, in which C(2) is a chiral carbon. The molecules form regular chains through intermolecular hydrogen bonds and intermolecular interactions, and then these chains form a three-dimensional framework structure.
3. 3 UV-vis spectrum and fluorescence properties analysis of probe EMOTA
Probe EMOTA is very sensitive to ultraviolet light in the wavelength range of 190~250 nm due to excellent UV absorption, as shown in Fig. 3(a). The experimental spectra have two intense peaks at approximately 194 and 207 nm.
The fluorescence excitation spectrum and emission spectrum of probe EMOTA are shown in Fig. 3(b). As can be seen from the figure, the peaks of the excitation spectrum of the probe appear at 232, 341, 441 and 687 nm, respectively.When measuring the emission spectrum, we found that the fluorescence emission spectrum with different intensity and similar peak shape can be measured at 442 nm by using 232,341 and 687 nm as the excitation wavelength, respectively.Nevertheless, the intensity of the emission spectrum measured at the excitation wavelength of 232 and 441 nm was respectively too high (the peak value is more than 1000 a.u.)and too low (the peak value is less than 100 a.u.) to be suitable for the determination of ion recognition. Besides, why the emission spectrum with the peak at 442 nm can be produced at the excitation wavelength of 687 nm remains to be further studied. Therefore, the excitation wavelengthλ= 341 nm was used in this experiment as the basic condition for the determination of fluorescence emission spectrum. It can be seen from Fig. 3(b) that the fluorescence emission spectrum of probe EMOTA has peaks at 343 and 442 nm respectively.Among them, the peak at 343 nm is too low (less than 100 a.u.) and has no further research value. Therefore, in the next experiment, ion recognition measurements were carried out at the excitation wavelengthλ= 341 nm.

Fig. 3. (a) UV-vis absorption spectrum of probe EMOTA; (b) Fluorescence properties of probe EMOTA
3. 4 Determination of selective recognition ability of probe EMOTA[23]
Probe EMOTA, 2 mL dimethyl sulfoxide (DMSO) and finally distilled water were gradually added into a 100 mL volumetric flask to prepare 100 mL sample test solution (2.0× 10-5mol/L, V(DMSO):V(H2O) = 1:49). Then different inorganic salts (CoCl2, Co(NO3)2, MnCO3, CaCl2, Al2(SO4)3,BaSO4, CuCl2, NaCl, FeSO4, MnSO4, CrCl3, ZnSO4, KCl,FeCl3, Fe2(SO4)3, Ag2SO4, CuSO4, Na2SO4, NiCl2) were dissolved in distilled water to prepare solutions with a concentration of 1.0 × 10-4mol/L, respectively.
After mixing the EMOTA test solution (2.0 × 10-5mol/L)and various ionic solutions prepared above (1.0 × 10-4mol/L)evenly in equal volume, the cations in the mixed solutions are Co2+, Mn2+, Ca2+, Al3+, Ba2+, Cu2+, Na+, Fe2+, Cr3+, Zn2+,K+, Fe3+, Ag+, Ni2+and the corresponding anions are Cl-,CO33-, SO42-and NO3-. Take another EMOTA test solution and add the same volume of distilled water as the blank sample. The mixed solutions and the blank sample were stood still at room temperature for 0.5 h. Then the fluorescence emission spectra of the solutions were measured respectively, with the results shown in Fig. 4.
As can be seen from Fig. 4, the solution had obvious fluorescence quenching after adding FeCl3or Fe2(SO4)3to the EMOTA test solution, but the fluorescence intensity had not been significantly changed after adding FeSO4, ZnSO4,MnSO4, NaCl and KCl, indicating that Fe2+, SO42-and Clhave no significant effect on the fluorescence quenching, and the fluorescence intensity of the solution was not decreased significantly after adding other metal cations or anions. Even after some inorganic salts such as MnSO4, CaCl2and CuSO4were added, the fluorescence of EMOTA was slightly enhanced. Thereby, the significant fluorescence quenching after adding FeCl3or Fe2(SO4)3comes from Fe3+, which indicates that the probe EMOTA has a high selectivity for the detection of Fe3+.

Fig. 4. Fluorescence spectra of EMOTA with various cations

Fig. 5. (a) Fluorescence intensity of probe EMOTA upon addition of Fe3+ (The concentration of Fe3+ increases from top to bottom: 0, 1.0 × 10-5,2.0 × 10-5, 3.0 × 10-5, 4.0 × 10-5, 5.0 × 10-5, 6.0 × 10-5, 7.0 × 10-5, 8.0 × 10-5, 9.0 × 10-5, 10.0 × 10-5 mol/L); (b) The linear relationship between Fe3+and EMOTA fluorescence intensity in the concentration range of 0~10.0 × 10-5 mol/L (Fluorescence intensity at 442 nm)
3. 5 Fluorescence titration spectra of probe EMOTA for Fe3+
In order to further explore the detection limit of EMOTA for Fe3+, we added the EMOTA test solution (2.0 × 10-5mol/L) to the same volume of Fe3+solutions with different concentrations for fluorescence titration. It can be seen from Fig. 4 that after the probe EMOTA was mixed with FeCl3of each concentration, the fluorescence intensity gradually decreased with the gradual increase of Fe3+concentration.When Fe3+concentration is greater than 10.0 × 10-5mol/L,the fluorescence quenching degree hardly changed.
Fig. 5 showed that under the condition of equal volume of probe EMOTA and solution FeCl3, there was a certain linear relationship with the fluorescence intensity of EMOTA and the concentration of Fe3+in the range of 0~10.0 × 10-5mol/L. According to the formula:
LOD = 3σ/k[24](σis the standard error,kis the slope of the calibration curve)
The value ofkcan be calculated to be 8.63 × 104M-1. The limits of detection (LOD) for EMOTA is 1.65 × 10-6mol/L andR2= 0.9952.
3. 6 Anti interference ability of EMOTA for Fe3+ recognition
In order to study the effect of coexistence of other metal cations and anions on the recognition of Fe3+by probe EMOTA, the aqueous solutions of different inorganic salts with a concentration of 5.0 × 10-4mol/L were prepared referring to experiment 3.4 and the interference experiments were carried out. The results showed that there was no obvious quenching when other ions and anions were added to the 2.0× 10-5mol/L EMOTA test solution respectively. After adding Fe3+, the fluorescent intensity had been significantly decreased (Fig. 6). This shows that the presence of other metal ions and anions has no effect on the identification and detection of Fe3+by the probe.
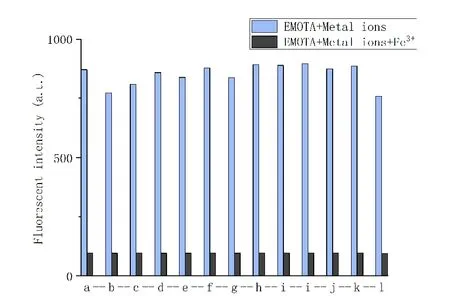
Fig. 6. Fluorescent recognition of probe EMOTA with the addition of various inorganic salts with Fe3+(a. Blank; b. Ca2+; c. Mn2+; d. Na+; e. Zn2+; f. Fe2+; g. Co2+; h. Cu2+; i. Ni2+; j. Ba2+; k. Cr3+; l. K+)

Table 2. Detection of Fe3+ in Water Samples
3. 7 Identification and detection of Fe3+ in water samples[25]
In order to evaluate the practical application value of probe EMOTA, the water samples of tap water (Laboratory) and river water (Nanjing Qinhuai River) were used for fluorescence detection of Fe3+. The samples of such water were filtered to remove impurities such as floating matter and precipitation and diluted 100 times with HEPES buffer solution (pH= 7.0). Then the probe was added. By taking 341 nm as the excitation wavelength to test the fluorescence emission intensity at the emission wavelength of 442 nm, the Fe3+concentration in the water sample was calculated, and then the standard addition recovery experiment was conducted. The results are shown in Table 2. It can be seen from the experimental results that the recovery of Fe3+in different water samples is between 98% and 108%, indicating that probe EMOTA has a good quantitative detection effect on Fe3+in actual water samples.
4 CONCLUSION
In summary, a novel quinazolinone derivative ethyl 2-(2-methyl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)acetate(EMOTA) was prepared and characterized by spectroscopy as well as by single-crystal X-ray diffraction analysis. Fluorescence analysis studies showed that the probe EMOTA has a rapid fluorescence response, good selectivity and good sensitivity to Fe3+. The detection limit of EMOTA for Fe3+is 1.65 × 10-6mol/L. Furthermore, identification and detection of Fe3+in water samples were studied, showing that probe EMOTA has high efficiency, significant sensitivity and high selectivity on the recognition and detection of Fe3+in water samples. It is expected to be applied to Fe3+related water environment monitoring.
- 結(jié)構(gòu)化學(xué)的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①

