Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury
Zhi-ping Qi, Guo-xiang Wang, Peng Xia, Ting-ting Hou, Hong-li Zhou, Tie-jun Wang,, Xiao-yu Yang,
1 Department of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
RESEARCH
Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury
Zhi-ping Qi1, Guo-xiang Wang1, Peng Xia1, Ting-ting Hou1, Hong-li Zhou2, Tie-jun Wang1,*, Xiao-yu Yang1,*
1 Department of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
Graphical Abstract

orcid: 0000-0001-9388-3794 (Xiao-yu Yang) 0000-0003-4029-4202 (Tie-jun Wang)
Our preliminary proteomics analysis suggested that expression of microtubule-associated protein tau is elevated in the spinal cord after injury. Therefore, the first aim of the present study was to examine tau expression in the injured spinal cord. The second aim was to determine whether tau can regulate neural stem cell migration, a critical factor in the successful treatment of spinal cord injury. We established rat models of spinal cord injury and injected them with mouse hippocampal neural stem cells through the tail vein. We used immunohistochemistry to show that the expression of tau protein and the number of migrated neural stem cells were markedly increased in the injured spinal cord. Furthermore, using a Transwell assay, we showed that neural stem cell migration was not affected by an elevated tau concentration in the outer chamber, but it was decreased by changes in intracellular tau phosphorylation state. These results demonstrate that neural stem cells have targeted migration capability at the site of injury, and that although tau is not a chemokine for targeted migration of neural stem cells, intracellular tau phosphorylation/ dephosphorylation can inhibit cell migration.
nerve regeneration; spinal cord injury; tau protein; neural stem cells; transwell chambers; phosphatase 2A; cell transplantation; phosphorylation; migration; okadaic acid; C2-ceramide; neural regeneration
Introduction
Tau proteins belong to the microtubule-associated protein family. They are mainly expressed in axons (Kuznetsov et al., 2015), but also exist in neuronal nuclei, connecting the chromosomal scaffold (Medina et al., 2015). Tau is a multifunctional protein. It stabilizes microtubules (Dolan et al., 2010), and influences axonal formation and growth by binding to the fyn and src families of tyrosine kinases in growth cones (Lee et al., 2005). Tau is also involved in other important functions such as axonal transport (Avila et al., 2014; Saman et al., 2014), and signal transduction, binding to the cell membrane (Pooler et al., 2010), signaling molecules, cytoskeletal proteins, and ions. Furthermore, tau is associated with inflammatory gene expression (Maphis et al., 2015) and protection of neuronal DNA against oxygen free radicals (Sultan et al., 2011).
In a preliminary proteomics analysis, we examined the expression of tau in injured spinal cord (Zhu et al., 2013). The general focus of research into tau remains on its overexpression in central nervous system disease (Dolan et al., 2010; Saman et al., 2014; Krüger et al., 2015). Our observed increase in tau expression after spinal cord injury suggests that it may affect the nerve cells (Baas et al., 2005; Dixit et al., 2008; Shemesh et al., 2008). Therefore, tau expression in damaged nervous tissue, such as that arising from spinal cord injury (SCI) deserves investigation. To this end, in the present study, we used qualitative and semi-quantitative immunohistochemistry to determine tau expression in neurons in a model of SCI.
Traditional treatments for SCI have many limitations. Neural stem cells (NSCs), which have the potential to differentiate into neurons and glial cells, releasing neurotransmitters and producing neurotrophic factors, are regarded as a promising treatment for SCI (Okano et al., 2003; Bottai et al., 2008; Gincberg et al., 2012). The most important factors for the success of NSC transplantation in the treatment of SCI are the migration and differentiation abilities of the NSCs. Previous studies have shown that tau plays an important regulatory role in neuronal migration (Takei et al., 2000; Fuster-Matanzo et al., 2009; Sapir et al., 2012). Others indicated that cell migration ability could be affected by microtubule-associated protein post-translational modification, such as phosphorylation (Schober et al., 2009; Nakano et al., 2010). However, the relationship between phosphorylation of the microtubule-associated protein tau and NSC migration has not been examined to date. Here, we used a Transwell assay to explore how post-translational modification of tau (phosphorylation/dephosphorylation) affects NSC migration, and to determine whether elevated tau concentrations affect targeted migration of NSC to the injury site. The overall aim of the present study was to provide a theoretical explanation for tau expression in the injured spinal cord and determine the effect of tau on NSC migration.
Materials and Methods
Experimental animals
Five female Kunming mouse embryos at gestational day 14, weighing 35-40 g, were provided by Beijing HFK Bioscience Co., Ltd., Beijing, China (animal license No. SCXK (Jing) 2009-0004). Twenty healthy adult Sprague-Dawley rats of both sexes, weighing 280-300 g, were supplied by the Experimental Animal Center of Jilin University of China (animal license No. SCXK (Ji) 2008-0004). Rats were housed in individual cages under a 12-hour light/dark cycle and in a dry and ventilated room at 23-25°C, with free access to food and water. All surgery was performed under anesthesia, and all efforts were made to minimize pain and distress in the experiment animals. All procedures were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986). Protocols were approved by the Animal Ethics Committee of Jilin University of China.
Mouse NSC culture
Fetuses were obtained from mouse uteri. Hippocampi were isolated at embryonic day 14 under a dissecting microscope, cut into pieces, triturated mechanically, filtered with a 200-mesh stainless steel sieve, and centrifuged at 1,000 r/min for 4 minutes. After removal of the supernatant, the samples were immersed in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco BRL, Gaithersburg, MD, USA), 20 ng/mL basic fibroblast growth factor (PeproTech, Rocky Hill, CT, USA), 20 ng/ mL epidermal growth factor (Peprotech) and 2% B27 (Gibco BRL). After counting, 5 × 105cells were incubated in a culture flask at 37°C and 5% CO2. Half of the medium was replaced after 3-4 days. One week later, neurospheres were passaged in an additional culture flask by mechanical beating. The third passage of neurospheres was incubated in a 6-well plate coated with polylysine at 37°C and 5% CO2for 5 hours. All neurospheres were subjected to immunofluorescent labeling with nestin. Rabbit anti-rat nestin polyclonal antibody (1:200, Abcam, Cambridge, UK) was added to each well at 4 ° C overnight, followed by goat anti-rabbit secondary antibody IgG (1:200, Abcam). Results were observed and photographed using a laser scanning confocal microscope (BX51; Olympus Corp., Tokyo, Japan).
Establishment of rat models of SCI
Twenty adult Sprague-Dawley rats were equally and randomly assigned to an SCI group and a control group. SCI models were established in accordance with a previous study (Bowes et al., 1994). Briefly, rats were anesthetized intraperitoneally with chloral hydrate (0.3 mL/100 g). A median longitudinal incision was made on the belly and the abdominal aorta was exposed. In the SCI group, the abdominal aorta was blocked with a vascular clamp for 30 minutes; after occlusion, the left kidney had changed from bright red to dark red, and the abdominal aortic pulse had disappeared. The control group underwent the same procedure except that the abdominal aorta was exposed for 30 minutes without obstruction, before the incision was sutured.
Behavioral assessment
Animal models of SCI were evaluated by observing behavioral changes of rats in both groups. When the rats had regained consciousness, three investigators who had not participated in the model establishment performed hindlimb motor function testing using the modified Tarlov score as follows (Cheng etal., 1996): grade 0, no activity, totally paralyzed, no response to acupuncture; grade 1, no activity, totally paralyzed, response to acupuncture; grade 2, active, cannot load; grade 3, hindlimb can load, cannot walk; grade 4, can walk, but unsteady, ataxia; grade 5, can walk, but not flexible, no ataxia; grade 6, normal walking. The mean value from the three investigators was calculated and recorded. Immunohistochemical staining was then performed on segments L3-5of two rats from each group, and the remaining rats received NSC transplantation.
NSC transplantation
Neurospheres were triturated mechanically into a single cell suspension, and washed twice with DMEM. 4′,6-Diamidino-2-phenylindole (DAPI; 10 mL, 1 μg/mL) was added to the incubated culture flask for 1 hour. After removal of DAPI, cells were incubated for 24 hours, and then observed under a fluorescence microscope. Following successful labeling, 2 × 106cells/ mL were injected into rats of the SCI and control groups, through the tail vein, within 5 minutes. NSC migration in the injured spinal cord was observed 3 days later under a laser scanning confocal microscope (Zeiss, Oberkochen, Germany).
Immunohistochemical staining
L3-5spinal cord segment was obtained from rats of both groups, sectioned and collected for free-floating immunohistochemistry. These sections were incubated with 0.3% H2O2for 10 minutes, blocked with 5% bovine serum albumin for 60 minutes, incubated with rabbit anti-mouse tau-5 monoclonal antibody (1:500; Abcam, Cambridge, UK) at 37°C for 2 hours at 4°C overnight, and with biotinylated goat anti-rabbit IgG secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) at 37°C for 30 minutes. Antibodies were labeled with horseradish peroxidase, and all sections were visualized with 3,3′-diaminobenzidine (positive cytoplasm presented brown), and observed under a light microscope (BX51; Olympus, Tokyo, Japan). Using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA), integrated optical density of the stained region was measured. The area of the region of interest was measured, and the mean value of optical density of the selected region was calculated.
Transwell assay
A Transwell assay was used to study the effects of tau on NSC migration capability. Using Transwell chambers with an 8-mm pore (Costar, Cambridge, MA, USA), NSCs were isolated into a single cell suspension, and the cell concentration was adjusted to 2 × 105/mL. The first Transwell chamber was divided into five groups (four experimental and one blank control). NSCs (100 μL) were added to the upper chamber of the Transwell; NSC medium (600 μL) containing 0, 0.1, 0.5 or 1 mg/ mL tau (Sigma-Aldrich, St. Louis, MO, USA) was added separately to the lower chambers in the remaining four groups. The second Transwell chamber was divided into three experimental groups and a blank control. NSC medium (600 μL) was added to the lower chamber. NSCs pretreated with okadaic acid (OA) (10 nM; Sigma-Aldrich), NSCs pretreated with C2-ceramide (1 μM; Sigma-Aldrich) and unpretreated NSCs were added to the corresponding upper chamber, respectively. For the pretreatment process, OA or C2-ceramide were mixed and incubated with NSC solution for 24 hours at 37°C and 5% CO2. All chambers were incubated for 24 hours. Remaining NSCs in the upper chambers were removed using a cotton ball. The chambers were stained with 0.1% cresyl violet for 5 minutes, and observed under a light microscope. After decolorizing with 33% acetic acid, optical density values were measured at 573 nm using a microplate reader (Gene Company Limited, Hong Kong, China).
Statistical analysis
Measurement data are expressed as the mean, and analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Intergroup data were compared with one-way analysis of variance and Tukey-Kramer test. A value of P < 0.05 was considered statistically significant.
Results
NSC culture identification
Neurospheres were observed after primary cell culture, proliferation and passage (Figure 1). As time in culture increased, the neurospheres grew, refractive index decreased, and boundaries became clearer. No growth of processes was observed. The cells grew well and met the requirements of subsequent experiments.
Behavioral changes in rat models of SCI
When the rats regained consciousness, hindlimb motor function was evaluated using the Tarlov scoring system. Hindlimb function was normal in the control group (6.00 ± 0.00 points), but markedly impaired in the SCI group (2.60 ± 0.25 points), indicating success of the model.
NSC migration and tau protein immunoreactivity in the spinal cord after transplantation
DAPI-labeled NSCs were implanted into the rats. Three days later, fluorescent labeling revealed that many migrated NSCs were visible in the SCI group, but no labeled cells were found in the identical segments in the control group (Figure 2A, B). Immunohistochemistry revealed swollen and deformed cells in the SCI group, with dark stained cytoplasm. In the control group, cells had a normal shape, and tau was observed in the cytoplasm and axon. Average optical density value was significantly higher in the SCI group (14.5 ± 4.6) than in the control group (8.6 ± 2.2; P < 0.05; Figure 2C, D).
Effects of high tau concentration on targeted migration of NSCs in the Transwell migratory assay
We varied the concentration of tau in the lower chamber of the Transwell to determine whether extracellular tau affected targeted migration in the NSCs. The optical density of cresyl violet did not change as tau concentration increased (P >0.05), indicating that tau does not affect NSC migration. We then used PP2A agonists and inhibitors to alter intracellular tau phosphorylation, and found that optical density was significantly higher in the unpretreated NSCs than in the groupsexposed to OA and C2-ceramide (P < 0.05; Figure 3). These findings indicate that altered tau phosphorylation impairs NSC migration.
Discussion
Tau expression can be studied at the site of injury using qualitative/semi-quantitative immunohistochemistry. In the present study, we examined the morphology and staining intensity of transplanted NSCs in a model of SCI, and explored the effect of different concentrations and phosphorylation states of tau on NSC migration in vitro. Control animals showed normal neuronal morphology, uniformly weak tau staining in the cytoplasm and axon, and low optical density values. In the SCI group, however, swollen neurons, dark staining, and high optical density values were observed.
Previous studies have focused more on the relationship between tau and nerve injury, demonstrating that tau expression is high in cerebrospinal fluid during attacks of transient brain ischemia, acute brain injury (Shultz et al., 2015) and SCI (Yokobori et al., 2015). Those reports suggested that SCI not only caused tau upregulation in cerebrospinal fluid, but also resulted in high tau expression in tissues. In the present study, the low expression of tau in the control group indicated that tau was not needed in normal cell physiological activity such as microtubule formation and signal transduction. At this point, tau was mainly distributed in the cytoplasm and axon, promoting microtubule formation and extension by binding to tubulin, stabilizing microtubules, and contributing to the transport of materials between the cell body and axon. Tau reduces oxidative stress in nerve cells (Maier et al., 2008; Violet et al., 2014), and also resists cell apoptosis (Duan et al., 2013).
We observed NSC migration after SCI in the model group, but not in the equivalent section of spinal cord in the control group. Transplanted NSCs are known to migrate to the site of injury (Bottai et al., 2008) and to promote the differentiation of endogenous NSCs (Okano et al., 2003). Our results confirm that NSCs are capable of targeted migration to the site of injury. Furthermore, our immunohistochemistry results show that the expression of tau and the number of NSCs were markedly increased at the injury site. Fuster-Matanzo et al. (2009) demonstrated that the differentiation and migration capabilities of nerve cells were reduced in tau knockout mice. Our aim was therefore to investigate whether elevated tau concentration is a chemotactic factor for NSC aggregation at the injury site. We observed NSC migration by changing tau concentration in the lower chamber of the Transwell assay. Our results suggested that extracellular tau concentration was not associated with NSC targeted migration, and also confirmed that a high tau concentration at the site of SCI was not a factor for targeted migration of NSCs.
Phosphorylation is the most important post-translational modification of tau, and plays essential regulatory roles in microtubule stability, protein localization and signal transduction. The alteration in tau phosphorylation can alter cell DNA (Lu et al., 2013) and cell apoptosis (Li et al., 2007). Tau contains 85 phosphorylation sites, and can be regulated by phosphokinase and phosphatase (Martin et al., 2011). PP2A is the most important phosphatase of tau. Changes in PP2A activity play a decisive role in tau phosphorylation and dephosphorylation (Yang et al., 2013; Arif et al., 2014; Wang et al., 2015). Here, we changed PP2A activity so as to modify tau phosphorylation, using the PP2A inhibitor OA (Honkanen et al., 2002) and agonist C2-ceramide (Dobrowsky et al., 1993). Our results showed that, whether tau was present in the phosphorylated or dephosphorylated form, the targeted migration capability of NSCs was decreased compared with unpretreated NSCs. During NSC migration, cells first extend the axon from the growth cone, then the cell body and nucleus move forward, and finally the process disappears. Microtubules and microtubule-associated protein play significant roles in this process (Schober et al., 2009; Nakano et al., 2010; Kaverina et al., 2011; Sapir et al., 2012). Since tau phosphorylation causes microtubule depolymerization, cytoskeletal reorganization and polarity variation, ultimately impairing migration capability, tau dephosphorylation would be expected to have the opposite effect. However, our results demonstrated that the migration capabilities were lower after both phosphorylation and dephosphorylation than in the unpretreated NSCs group. This indicates that under normal physiological conditions, tau acts on microtubule polymerization and signal transduction by maintaining a state of dynamic equilibrium between phosphorylation and dephosphorylation. If this equilibrium is broken, NSC migration capability will be reduced.
In summary, NSCs have targeted migration capability. Tau expression is upregulated at the site of SCI. The broken dynamic equilibrium of tau phosphorylation and dephosphorylation diminishes the capability of NSC migration under physiological conditions. Extracellular tau is not a chemoattractant protein for NSC targeted migration to the injury site. NSC migration is very important for therapeutic effect. During stem cell transplantation, keeping the dynamic equilibrium of tau phosphorylation and dephosphorylation is very important in maintaining stem cell migration. However, we have not discussed signal transduction pathways in the present study. Future experiments in our laboratory will explore the roles of tau in NSCs after SCI at the molecular level, to provide a theoretical basis for NSC transplantation in the treatment of SCI.
Acknowledgments: We are very grateful to the Institute of the Second Hospital of Jilin University of China for technical assistance.
Author contributions: ZPQ participated in NSC culture, wrote the paper and analyzed data. GXW, TTH and PX established animal models. HLZ was in charge of statistical analysis. XYY and TJW conceived and designed the study, obtained the funding, provided technical and material support. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.

Figure 1 Culture and morphology of mouse NSCs.
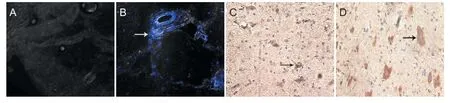
Figure 2 NSC migration and tau protein immunoreactivity in SCI rats.
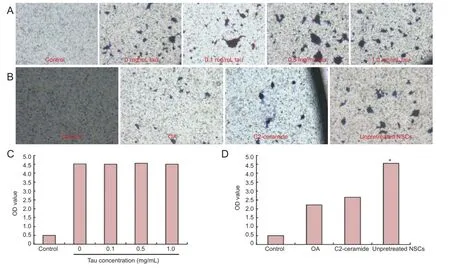
Figure 3 Effects of high tau protein concentration on targeted migration of NSCs in the injured spinal cord of rats (Transwell assay).
Arif M, Kazim SF, Grundke-Iqbal I, Garruto RM, Iqbal K (2014) Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc Natl Acad Sci U S A 111:1144-1149.
Avila J, Simon D, Diaz-Hernandez M, Pintor J, Hernandez F (2014) Sources of extracellular tau and its signaling. J Alzheimers Dis 40:S7-S15.
Baas PW, Qiang L (2005) Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol 15:183-187.
Bottai D, Madaschi L, Di Giulio AM, Gorio A (2008) Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol Med 14:634-644.
Bowes MP, Masliah E, Otero DA, Zivin JA, Saitoh T (1994) Reduction of neurological damage by a peptide segment of the amyloid beta/A4 protein precursor in a rabbit spinal cord ischemia model. Exp Neurol 129:112-119.
Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273:510-513.
Dixit R, Ross JL, Goldman YE, Holzbaur EL (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319:1086-1089.
Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 268:15523-15530.
Dolan PJ, Johnson GV (2010) The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Devel 13:595-603.
Duan DX, Chai GS, Ni ZF, Hu Y, Luo Y, Cheng XS, Chen NN, Wang JZ, Liu GP (2013) Phosphorylation of tau by death-associated protein kinase 1 antagonizes the kinase-induced cell apoptosis. J Alzheimers Dis 37:795-808.
Fuster-Matanzo A, de Barreda EG, Dawson HN, Vitek MP, Avila J, Hernández F (2009) Function of tau protein in adult newborn neurons. FEBS Lett 583:3063-3068.
Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI (2012) Neural stem cells: therapeutic potential for neurodegenerative diseases. Br Med Bull 104:7-19.
Honkanen RE, Golden T (2002) Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem 9:2055-2075.
Kaverina I, Straube A (2011) Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol 22:968-974.
Krüger L, Mandelkow EM (2015) Tau neurotoxicity and rescue in animal models of human Tauopathies. Curr Opin Neurobiol 36:52-58.
Kuznetsov IA, Kuznetsov AV (2015) A comparison between the diffusion-reaction and slow axonal transport models for predicting tau distribution along an axon. Math Med Biol 32:263-283.
Lee G (2005) Tau and src family tyrosine kinases. Biochim Biophys Acta 1739:323-330.
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ (2007) Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 104:3591-3596.
Louis SA, Mak CK, Reynolds BA (2013) Methods to culture, differentiate, and characterize neural stem cells from the adult and embryonic mouse central nervous system. Methods Mol Biol 946:479-506.
Lu Y, He HJ, Zhou J, Miao JY, Lu J, He YG, Pan R, Wei Y, Liu Y, He RQ (2013) Hyperphosphorylation results in tau dysfunction in DNA folding and protection. J Alzheimers Dis 37:551-563.
Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME (2008) Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci 28:9386-9403.
Maphis N, Xu G, Kokiko-Cochran ON, Cardona AE, Ransohoff RM, Lamb BT, Bhaskar K (2015) Loss of tau rescues inflammation-mediated neurodegeneration. Front Neurosci 9:196.
Martin L, Latypova X, Terro F (2011) Post-translational modifications of tau protein: implications for Alzheimer’s disease. Neurochem Int 58:458-471.
Medina M, Avila J (2015) Further understanding of tau phosphorylation: implications for therapy. Expert Rev Neurother 15:115-122.
Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y (2010) AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol 12:583-590.
Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y (2003) Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol 14:191-198.
Pooler AM, Hanger DP (2010) Functional implications of the association of tau with the plasma membrane. Biochem Soc Trans 38:1012-1015.
Saman S, Lee NC, Inoyo I, Jin J, Li Z, Doyle T, McKee AC, Hall GF (2014) Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis 40 Suppl 1:S47-70.
Sapir T, Frotscher M, Levy T, Mandelkow EM, Reiner O (2012) Tau’s role in the developing brain: implications for intellectual disability. Hum Mol Genet 21:1681-1692.
Schober JM, Cain JM, Komarova YA, Borisy GG (2009) Migration and actin protrusion in melanoma cells are regulated by EB1 protein. Cancer Lett 284:30-36.
Shemesh OA, Erez H, Ginzburg I, Spira ME (2008) Tau-induced traffic jams reflect organelles accumulation at points of microtubule polar mismatching. Traffic 9:458-471.
Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, Medcalf RL, Johnston LA, Hovens CM, Jones NC, O’Brien TJ (2015) Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138:1297-313.
Sultan A, Nesslany F, Violet M, Bégard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buée L, Galas MC (2011) Nuclear tau, a key player in neuronal DNA protection. J Biol Chem 286:4566-4575.
Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB (2002) Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Cogn Neurosci 22:6670-6681.
Takei Y, Teng J, Harada A, Hirokawa N (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol 150:989-1000.
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK (2011) A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28:1611-82.
Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, Buée L, Galas MC. (2014) A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci 8:84.
Wang X, Blanchard J, Tung YC, Grundke-Iqbal I, Iqbal K (2015) Inhibition of Protein Phosphatase-2A (PP2A) by I1PP2A leads to hyperphosphorylation of tau, neurodegeneration, and cognitive impairment in rats. J Alzheimers Dis 45:423-435.
Yang CC, Kuai XX, Li YL, Zhang L, Yu JC, Li L, Zhang L (2013) Cornel Iridoid Glycoside Attenuates Tau Hyperphosphorylation by Inhibition of PP2A Demethylation. Evid Based Complement Alternat Med 2013:108486.
Yokobori S, Zhang Z, Moghieb A, Mondello S, Gajavelli S, Dietrich WD, Bramlett H, Hayes RL, Wang M, Wang KK, Bullock MR (2015) Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg 83:867-878.
Zhu B, Li Y, Li M, Yang X, Qiu B, Gao Q, Liu J, Liu M (2013) Dynamic proteome analysis of spinal cord injury after ischemia-reperfusion in rabbits by two-dimensional difference gel electrophoresis. Spinal Cord 51:610-615.
Copyedited by Slone-Murphy J, Stow A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.177744 http://www.nrronline.org/
How to cite this article: Qi ZP, Wang GX, Xia P, Hou TT, Zhou HL, Wang TJ, Yang XY (2016) Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury. Neural Regen Res 11(2):332-337.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81250016, 31572217.
Accepted: 2015-12-22
*Correspondence to: Xiao-yu Yang, M.D. or Tie-jun Wang, M.D., yangxiaoyu88@sina.com or m13943016598@163.com.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway
- Influence of immobilization and sensory re-education on the sensory recovery after reconstruction of digital nerves with direct suture or muscle-in-vein conduits
- Neuroprotective role of (Val8)GLP-1-Glu-PAL in an in vitro model of Parkinson’s disease
- Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons
- Neural differentiation and synaptogenesis in retinal development