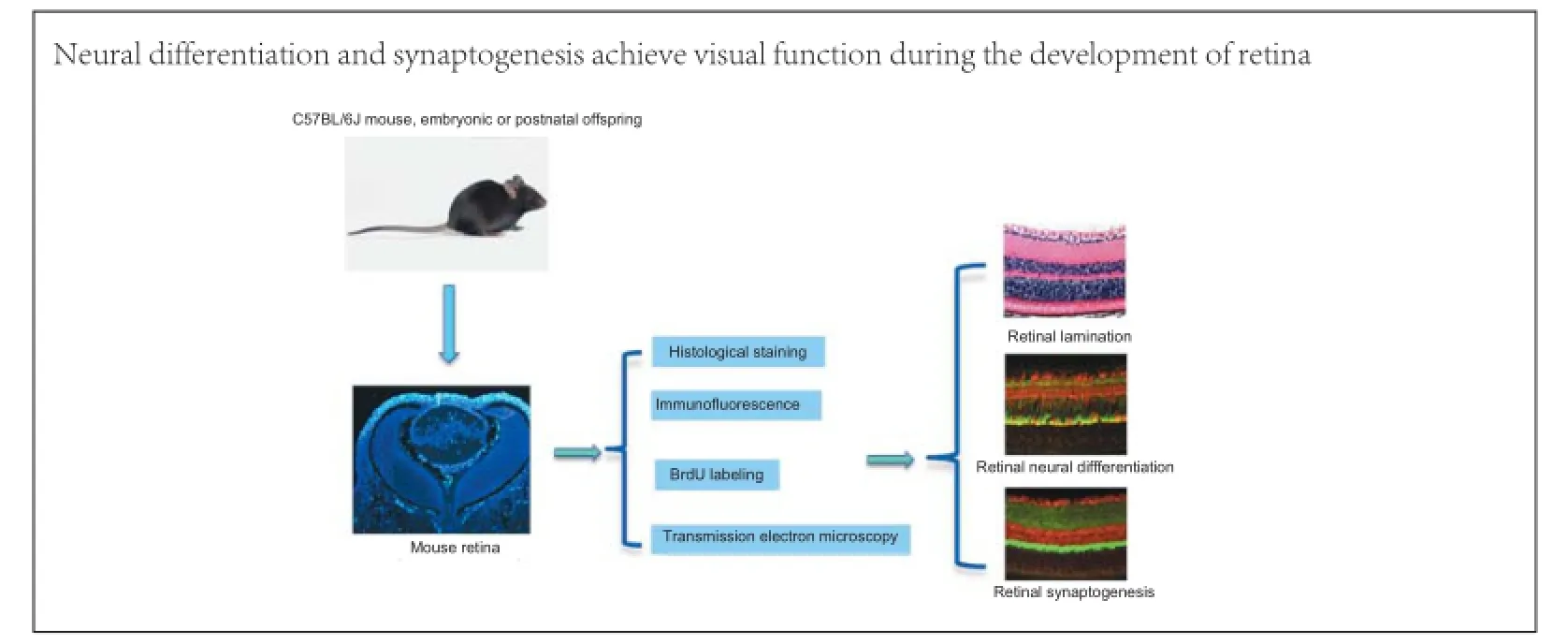
Neural differentiation and synaptogenesis in retinal development
Wen-juan Fan, Xue Li, Huan-ling Yao, Jie-xin Deng, Hong-liang Liu, Zhan-jun Cui, Qiang Wang, Ping Wu, Jin-bo Deng,
Institute of Neurobiology, School of Life Science, Henan University, Kaifeng, Henan Province, China
RESEARCH
Neural differentiation and synaptogenesis in retinal development
Wen-juan Fan#, Xue Li, Huan-ling Yao, Jie-xin Deng, Hong-liang Liu, Zhan-jun Cui, Qiang Wang, Ping Wu*, Jin-bo Deng*,#
Institute of Neurobiology, School of Life Science, Henan University, Kaifeng, Henan Province, China
Graphical Abstract
#These authors contributed equally to this article.
orcid: 0000-0002-3166-2686 (Jin-bo Deng) 0000-0003-0507-5119 (Ping Wu)
To investigate the pattern of neural differentiation and synaptogenesis in the mouse retina, immunolabeling, BrdU assay and transmission electron microscopy were used. We show that the neuroblastic cell layer is the germinal zone for neural differentiation and retinal lamination. Ganglion cells differentiated initially at embryonic day 13 (E13), and at E18 horizontal cells appeared in the neuroblastic cell layer. Neural stem cells in the outer neuroblastic cell layer differentiated into photoreceptor cells as early as postnatal day 0 (P0), and neural stem cells in the inner neuroblastic cell layer differentiated into bipolar cells at P7. Synapses in the retina were mainly located in the outer and inner plexiform layers. At P7, synaptophysin immunostaining appeared in presynaptic terminals in the outer and inner plexiform layers with button-like structures. After P14, presynaptic buttons were concentrated in outer and inner plexiform layers with strong staining. These data indicate that neural differentiation and synaptogenesis in the retina play important roles in the formation of retinal neural circuitry. Our study showed that the period before P14, especially between P0 and P14, represents a critical period during retinal development. Mouse eye opening occurs during that period, suggesting that cell differentiation and synaptic formation lead to the attainment of visual function.
nerve regeneration; neural stem cells; neural differentiation; retinal development; synaptogenesis; neural regeneration
Introduction
The retina is regarded as a part of the peripheral brain with a complex structure that carries out photosensitive functions. The highly laminated retina is recognized to have 10 layers by hematoxylin and eosin staining. Within these 10 layers, the retina mainly consists of five types of neurons, including photoreceptor cells (rod and cone cells), horizontal cells, bipolar cells, amacrine cells and ganglion cells (Reese, 2011). These neurons form a complex network and function to process visual information (Jones et al., 2012). Retinal development is a complex process that involves neural differentiation and the expression of genes following strictly spatiotemporal patterns (Le et al., 2002). After neural differentiation and the formation of neural circuits, the retina can perform visual transduction; visual signals pass from photoreceptor cells to bipolar cells, and then to ganglion cells. Horizontal cells and amacrine cells function to integrate information (Collin, 2008). Conveying visual signals among neurons depends on normal synaptic functions; therefore, synaptogenesis is very important for retinal development. There are two synaptic layers in the retina, the outer plexiform layer (OPL) and inner plexiform layer (IPL). The photoreceptor cells form synapses with horizontal cells and bipolar cells in the OPL, while synapses are formed among bipolar cells, amacrine cells and ganglion cells in the IPL. The OPL is the first synaptic relay where photoreceptors, bipolarcells, and horizontal cells interact synaptically, whereas the IPL is the second synaptic relay for nerve impulses in the retina (Schiller, 2010).
The retina has been the focus of intense research efforts because of its special functions and because it is an amenable neurobiological model (Liang et al., 2009; Wei and Feller, 2011). Many studies have addressed retinal anatomy, development and pathology (Ambati and Fowler, 2012), but few studies have directly analyzed neural differentiation and synaptogenesis in the developing murine retina. In this study, we utilized 5-bromo-2′-deoxyuridine (BrdU) assays, electron microscopy and immunofluorescence labeling to investigate neural differentiation and synaptogenesis in the developing mouse retina.
Materials and Methods
Animals
C57BL/6J mice were obtained from the Model Animal Research Center, Nanjing University, China. Adult male and female mice were placed in breeding cages in standard laboratory animal housing with a 12-hour light/dark cycle. Embryonic or postnatal offspring were produced from timed pregnancies (E, embryonic day; E0, day of vaginal plug in mated females; P, postnatal day; P0, the first 24 hours after birth). Pups were born on E19. A total of 285 embryos and postnatal pups at E8-18 and P0-180 were used in this study. All experiments were carried out in accordance with the institutional guidelines for animal welfare of Henan University, China.
To obtain embryonic mice at specific stages, pregnant dams were anesthetized (intraperitoneal sodium pentobarbital, 40 mg/ kg) and fetuses were harvested by cesarean section. From E8 to 13, the whole embryos were fixed with 4% w/v paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 2-3 days at 4°C. From day E14 onward, fetal eyeballs were carefully separated and fixed in 4% paraformaldehyde for 1-2 days at 4°C. For postnatal mice, pups were anesthetized (intraperitoneal sodium pentobarbital, 20 mg/kg) and perfused transcardially with 4% paraformaldehyde. Eyeballs were removed, and fixation with the same fixative was continued at 4°C for 1-2 days.
Histological staining
Embryos (E8-13) and eyeballs (E14 onward) were dehydrated in an ethanol gradient and embedded in paraffin. After sectioning in an anterior to posterior axis at 5-7 μm, hematoxylin-eosin staining was performed according to a standard protocol.
Immunofluorescence
Paraffin-embedded embryonic heads (E8-13) or eyeballs (E14 onward) were sectioned at 5-7 μm in an anterior to posterior direction, then mounted on poly-D-lysine-coated glass slides (P1149, Sigma) and incubated at 55°C overnight. The sections were deparaffinized by two 5-minute incubations in xylene and then serially transferred to 100%, 90% and 70% ethanol solutions for 5 minutes each. The sections were then washed in distilled water for 5 minutes. Sections were rinsed in 0.01 M phosphate buffer and preincubated in blocking solution (5% normal goat serum) for 30 minutes at room temperature before immunofluorescence labeling. SOX2, cyclin D1, proliferating cell nuclear antigen (PCNA), doublecortin (DCX), NeuN, calbindin, β-tubulin, protein kinase C-alpha (PKC-α), synaptophysin, choline acetyltransferase (ChAT) and γ-aminobutyric acid (GABA) were used as markers to visualize neural stem cells (SOX2, cyclin D1 and PCNA), newborn neurons (DCX), mature neurons (NeuN), neuronal microtubules (β-tubulin), photoreceptor cells (calbindin), bipolar cells (PKC-α), presynaptic vesicles (synaptophysin) and amacrine cells (ChAT and GABA). Sections were incubated overnight at 4° C with the indicated dilutions of the different primary antibodies: rabbit polyclonal anti SOX2 (1:600; AB97959, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-cyclin D1 antibody (1:300; IMG5703, Imgenex, Littleton, CO, USA), mouse monoclonal anti-PCNA (1:100; ZM0213, Shanghai Qikang Biological Technology, Shanghai, China), mouse polyclonal anti-DCX (1:500; ab135349, Abcam), mouse monoclonal anti-NeuN (1:500; MAB377B, Chemicon, Santa Cruz, CA, USA), mouse monoclonal anti-calbindin (1:500; ab82810, Abcam), rabbit polyclonal anti-PKC-α (1:10,000; P4334, Sigma, St. Louis, MO, USA) and rabbit anti-synaptophysin (1:500; AB9272, Abcam), rabbit polyclonal anti-ChAT (1:200; AB143, Millipore, Billerica, MA, USA) and rabbit polyclonal anti-GABA (1:1,000; AB131, Millipore). After multiple washes in 0.01 M phosphate buffer, appropriate secondary antibodies were added at the indicated dilutions and incubated at room temperature for 3 hours. The secondary antibodies were: Alexa Fluro 488 donkey anti-mouse IgG (1:300; A21202, Invitrogen, Carlsbad, CA, USA), Alexa Fluro 488 donkey anti-rabbit IgG (1:300; A21206, Invitrogen), Alexa Fluro 568 goat anti-mouse IgG (1:600; A11004, Invitrogen) and Alexa Fluro 568 goat anti-rabbit IgG (1:600; A11011, Invitrogen). After immunoreaction, cover-slips were mounted under 65% glycerol (in phosphate buffer) with 1:60,000 4′,6-diamidino-2-phenylindole (DAPI) (SC3598, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for counterstaining and then imaged using an epifluorescence microscope (BX61, Olympus, Tokyo, Japan) with rhodamine, fluorescein isothiocyanate or ultraviolet filter sets. High-quality sections were photographed using a laser confocal microscope (FV1000, Olympus), using separate scans at 568 nm (red) and 488 nm (green).
BrdU labeling
The incorporation of BrdU, a marker of S-phase, was used to investigate neural proliferation and neural stem cells (Remvikos et al., 1991). BrdU (B-5002, Sigma) was diluted in sterile physiological saline solution for a dose of 50-100 mg/kg, and then injected into pregnant mice at E8-17 or into postnatal mice. To intensify labeling, a second identical dose was given 3 hours later. Animals were killed 2 days after injection. Fixation and embedding were performed as described above and then eyes were sectioned at 5-7 μm using a paraffin microtome. To visualize nuclear BrdU incorporation, sections were incubated in 4 N HCl for 10 minutes to denature the DNA, then thoroughly rinsed (5×) in 0.2 M borate buffer (pH 8.0). Normal goat serum (0.5% v/v) was then added to block nonspecific binding. Sections were then incubated overnight at 4°C with mouse monoclonal anti-BrdU IgG (1:100; ZM-0013, Beijing Zhong Shan-Golden Bridge, Beijing, China). After washing, Alexa Fluro 488-donkey anti-mouse IgG (1:300;A21202, Invitrogen) was added and sections were incubated at room temperature for 3 hours. Sections were mounted on glass slides and cover-slips mounted under 65% glycerol plus 1:60,000 DAPI in phosphate-buffered saline. Sections were imaged using the fluorescence microscope, as described above.
Transmission electron microscopy
Pups at various ages were used for electron microscopy. These animals were anesthetized intraperitoneally using 1% pentobarbital sodium as described above. 4% paraformaldehyde and 1% glutaraldehyde (in 0.1 M phosphate buffer) were used for perfusion through the left ventricle. The eyeballs were then taken out, and the retinas dissected and cut radially into 2 mm × 2 mm tissue pieces using a razor blade. The samples were then fixed in 4% glutaraldehyde for 2-4 hours and washed for 15 minutes three times in 0.01 M phosphate-buffered saline. After fixing in 1% osmic acid for 30 minutes and washing with 0.01 M phosphate-buffered saline, the samples were dehydrated in an acetone gradient of increasing concentration. After embedding in Epon 812 resin, the samples were polymerized at 37°C for 3 hours, 45°C for 6 hours, and 63°C for 24 hours. Samples were then cut into 70-nm ultra-thin slices and stained with uranyl acetate and lead citrate and observed by transmission electron microscopy (S-3500N, Hitachi, Tokyo, Japan).
Results
Retinal development and lamination
Cornea, lens and retina could be identified in the eyeball as early as E13. At this time, the retina consisted of a pigmented epithelium layer and a retinal nerve layer or outer retina. Between the pigmented epithelium layer and the retinal nerve layer, there was a narrow space that would be fused at P15. The outer retina was relatively thick and comprised numerous neuroblasts or neural stem cells (Figure 1A). These neuroblasts were dense and arranged neatly to form a neuroblastic cell layer (NCL) (Figure 1A). Neural stem cells in the NCL could be visualized with cyclin D1, Sox2 and PCNA immunochemistry and the BrdU assay (Figure 1B-F). Thereafter, neural stem cells started to differentiate into neurons, which migrated into the putative ganglion cell layer (GCL) and further differentiated into ganglion cells as early as E13 (Figure 1B and D). At E17, ganglion cells in the GCL were labeled with the newborn neuron marker, DCX (Figure 1B, D and F), and the densely arranged ganglion layer was occupied with multiple layers of cells (Figure 1B, D-F). The putative IPL was seen as a narrow zone between the ganglion layer and the NCL (Figure 1D-F). The GCL thinned with age, and ganglion cells became arranged in a single layer in contrast to the widening IPL (Figure 1H and I). At E18, some neural stem cells inside the NCL began to differentiate into horizontal cells (Figure 1F), which initially did not assume their typical morphology. However, the cells later extended their interconnected T-like dendrites, which is typical morphology for horizontal cells (Figure 1G and H). At this time, the newborn horizontal cells separated the NCL into two zones: an upper zone called the inner nuclear layer (INL) and a lower zone called the outer nuclear layer (ONL). The narrow space between the INL and ONL was the OPL, which consisted of numerous axons of photoreceptor cells and dendrites of horizontal cells and bipolar cells (Figure 1G-I). Having differentiated into various types of neurons, the numbers of neural stem cells, BrdU-positive cells and Sox2-positive cells were drastically reduced in the neuroblastoma. At P7, neural stem cells in the ONL were almost absent, and only few stem cells remained in the INL (Figure 1G). The zone between the ONL and the pigmented epithelial layer was occupied by the dendrites of photoreceptor cells (rod cells and cone cells) (Figure 1H and I), thus forming the typical adult laminated retinal structure (Figure 1I).
Neural differentiation in the retina
The NCL or germinal zone consists of numerous neural stem cells. Stem cells contain various specific proteins, such as cyclin D1, Sox2 and PCNA; therefore, they can be used to mark stem cells by immunohistochemistry and BrdU assays (Figure 1B-F). Neural stem cells in the retina could also be recognized by transmission electron microscopy, and they usually looked round and oval with little cytoplasm. For their cell size, the nuclei of neural stem cells were relatively large with more heterochromatin. Some organelles were detectable inside the cytoplasm, such as mitochondria, endoplasmic reticulum and Golgi apparatus, and free ribosomes and glycogen contents were high (Figure 2D). As retinal development progressed, neural stem cells differentiated into various types of neurons, which became more and more mature. The cells that differentiated earliest were ganglion cells, which could be found in the GCL as early as E13. With NeuN and β-tubulin double immunolabeling, newborn ganglion cells were observed to extend long processes toward the pigmented epithelium to make contact. Under transmission electron microscopy, ganglion cells were observed to possess the ultrastructural characteristics of neurons (Figure 2E). The long processes of ganglion cells retreated from the pigmented epithelium, and the dendrites of ganglion cells were mainly located in the IPL after P7 (data not shown), with their unmyelinated axons located in nerve fiber layer (Figure 2E).
As early as E18, horizontal cells were DCX immunolabeled inside the NCL. Initially, DCX-positive horizontal cells were arranged in multilayers, and their arrangement was then remodeled into a monolayer (Figure 1F-H; Figure 2B). At P7, the bodies of horizontal cells were located near the edge of the INL, but their dendrites stretched into the OPL in a T shape (Figure 1G and H). Later, the dendrites of DCX-positive horizontal cells branched repeatedly and extended into the OPL, showing a fan-shape. Conversely, bipolar cells were located within the INL and could be marked with PKC-α immunolabeling after P7. Initially, cell bodies were arranged in multilayers in the ONL near the OPL. Their short dendrites extended into the OPL, and their long axons stretched into the IPL and connected with ganglion cells. After P14, bipolar cell numbers increased and were arranged densely with typical morphology (Figure 2B). At P30, the retinal lamination was formed, and the rod and cone layer was also observed (Figure 2C). Sometimes, suspected amacrine cells could be visualized by ChAT and GABA immunolabeling in the INL after P0; however, because of their unspecific shape and unsatisfactory labeling, a furtherstudy is needed to determine their identity (data not shown). Photoreceptor cells can be visualized with calbindin immunolabeling (Lin et al., 2009). Photoreceptor cells consist of three parts: outer segment, cell body and inner segment. The outer segment is located between the pigmented epithelium and the ONL to perform photoconversion. The inner segment consists of axons that stretch toward the OPL. In this study, the bodies of visual cells could be recognized by DAPI staining in the ONL as early as P0. With calbindin immunochemistry, the outer segments of visual cells could be recognized after P30 (Figure 2C), which became longer with age. Under electron microscopy, the optic discs could be seen as early as P0, and assumed typical features from P14 onward (Figure 2F).
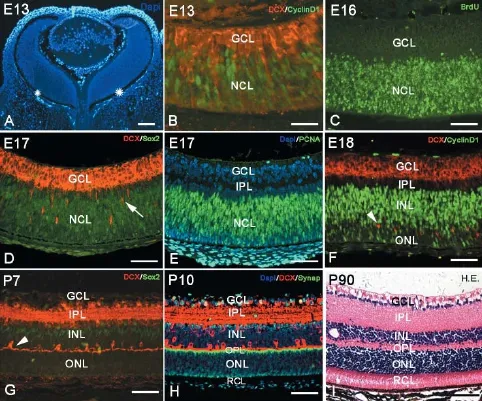
Figure 1 Retinal development and lamination in mice.
Retinal development and synaptogenesis
Our focus for this study was the characterization of synaptogenesis in the developing retina. The synapses mainly exist in two retinal zones, the OPL and IPL. In the OPL, the synapses were formed between the inner segment of photoreceptor cells and the dendrites of bipolar cells or horizontal cells. In the IPL, the synapses were formed among axons of bipolar cells, amacrine cells, and the dendrites of ganglion cells. Within the OPL and IPL, it was dense neuropil with synapses among fibers (Figure 3D). Synaptophysin is a specific protein that exists on presynaptic vesicles and can, therefore, be used as a marker for presynaptic terminals (Antonow-Schlorke et al., 2001). In our study, we used synaptophysin to investigate synaptogenesis in the OPL and IPL. In embryonic and early postnatal days before synapse formation, synaptophysin was found only in the processes of photoreceptor cells, especially in the outer segment (Figure 3A). The synaptophysin immunostaining then spread to both outer and inner segments of visual cells, appearing in parallel radial structures in rod and cone layers and the ONL (Figure 3B). At P5, synaptophysin was densely localized at the ends of inner segments of photoreceptor cells. From P10 onward, synaptophysin was no longer localized in photoreceptor cell processes, but specif-ically in presynaptic terminals. At P7, synaptic connections had formed between the inner segments and horizontal cells, marking the beginning of synapse formation (Figure 3B). At P5, synaptophysin labeling also appeared in the IPL on dense button-like structures. After P14, the synaptophysin-positive presynaptic terminals were concentrated densely in the OPL and IPL (Figure 3C), and many synapses were observed in the OPL and IPL by electron microscopy (Figure3F).
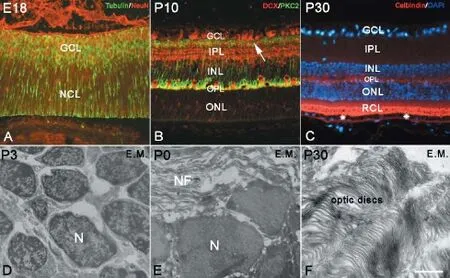
Figure 2 Neural differentiation in the retina.
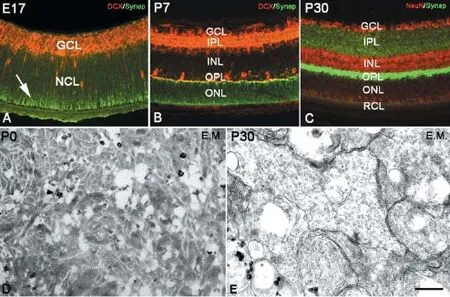
Figure 3 Synaptogenesis in retina.
Discussion
The NCL serves as an anlage for retinal development
Like the brain, the retina also originates from neuroepithelium. The outer retina or the neuronal cell layer is a continuum of the neuroepithelium, and the NCL in the outer retina is the anlage for retinal lamination and neural differentiation. Neurogenesis in the retina is a very orderly process. The retinal neural stem cells in the neuroblast layer can differentiate into various neurons and Müller cells. The ganglion cells differentiate first, followed by amacrine, cone and horizontal cells, and rod photoreceptors shortly afterward. Bipolar cells are the last neurons to differentiate (Guduric-Fuchs et al., 2009; Bejarano-Escobar et al., 2012). In the mouse retina, neural differentiation begins prior to birth and is largely completed before P14. For instance, the differentiation of rods and bipolar cells starts before birth and continues for 1-2 weeks after birth (Young, 1985; Sharma et al., 2003). Photoreceptor cells were observed at approximately E18 and increased their numbers until P14. Horizontal cells were also present in E19 retinas. PKC immunoreactive bipolar cells developed postnatally and became distinguishable at P7. In cats, the chronology of neural differentiation in the retina is similar to that in mice. A distinct GCL was seen in the innermost cells of the NCL, and primitive horizontal cells appeared within the NCL, separating neuroblastic cell mass into INL and ONL (Greiner and Weidman, 1980).
In this study, neural stem cells were immunolabeled with Sox2 and PCNA, and a BrdU assay was used to study neural proliferation in the mouse retina. We observed numerous proliferating neural stem cells in the NCL during embryonic development. The NCL is a key zone for retinal development and neural differentiation. The earliest differentiated neurons were retinal ganglion cells, which appeared in the GCL as early as E13. At E18, the DCX-positive horizontal cells appeared inside the NCL, serving as a boundary marker for the putative ONL and the putative INL. Although DCX is a specific marker of newborn neurons, according to their development, location and morphological features, these DCX-positive cells were presumed to be horizontal cells. Neural stem cells in the ONL started to differentiate into photoreceptor cells. Conversely, neural stem cells in the INL differentiated into bipolar cells and amacrine cells. Amacrine cells appeared in the INL at approximately P0. In addition, bipolar cells started to differentiate after P7 and peaked in numbers at P14. These PKC-α-positive bipolar cells are usually regarded as ON-type cells (W?ssle et al., 2009). The photoreceptor cells could be recognized as early as P0. After P14, the photoreceptor cells became mature with their typical ultrastructure as observed by electron microscopy. Our data on the chronological order of neural differentiation were in line with previously reported studies (Young, 1985; Sharma et al., 2003). All neurons and glial cells differentiate from neural stem cells in the NCL (Ferri et al., 2004; Cavallaro et al., 2008; Agathocleous et al., 2009; Kihara et al., 2010; Domyan et al., 2011). Furthermore, our findings suggest that the time before P14, especially P0-14, represents a critical period during retinal development, because almost all neurons differentiate during P0-14. The retinal lamination and neural differentiation provide a morphological basis for retinal visual functions. Knowledge of the order of neural differentiation is essential for understanding eye disease etiology and for the application of stem cell therapy in the retina (Meyer et al., 2004).
Synaptogenesis plays an important role in the formation of retinal neural circuitry
The formation of synaptic connections between neural dendrites and axons is the crucial step for proper functioning of retinal neural circuits. Both axons and dendrites grow to their target neurons by responding to a large number of molecular cues (McFarlane and Lom, 2012). How a neuron establishes and modifies appropriate synaptic connectivity raises key questions in developmental neurobiology. A previous study (Tian, 2004) showed that synaptogenesis in the mouse IPL and OPL begins before eye opening and continues for several weeks, reaching its peak at P21. In cats, conventional synapses were seen in the IPL in the week prior to birth. Synaptic ribbons first appear in the newborn OPL and then in the IPL by P4 (Greiner and Weidman, 1980). The ganglion cells form synapses with bipolar and amacrine cells in the IPL to receive excitatory and inhibitory synaptic inputs, respectively. Shortly after, the horizontal cells and photoreceptors make contact with each other in the OPL, and bipolar cells eventually make connections with ganglion cells. This sequential pattern of retinal synaptogenesis is very important for functional photosensitivity (Fisher, 1979). In our study, we tried to focus on synaptogenesis during retinal development. Synaptophysin can be used to label presynaptic buttons in the retina, because it controls neurotransmitter release as a synaptic vesicle protein (Dan et al., 2008). We found that synapses mainly existed in the OPL and IPL. In the OPL, synapses formed between the inner segments of photoreceptor cells and the dendrites of bipolar cells or horizontal cells. In the IPL, synapses formed among the axons of bipolar cells, amacrine cells and the dendrites of ganglion cells. In our study, before P7, synaptophysin was mainly located in the processes of photoreceptor cells. After P7, it started to appear in the presynaptic terminals in the OPL and IPL with button-like shapes. At P14, synaptophysin was specifically localized to the OPL and IPL. In adult mice, synaptophysin-positive presynaptic buttons were concentrated strongly in the OPL and IPL. Under the electron microscope, many neuropil and synapses were found in the OPL and IPL. Photoreceptor cells, bipolar cells and ganglion cells are three types of neuron that transfer information in the retina. Furthermore, horizontal cells and amacrine cells function as integrating neurons to regulate the transduction of the visual signal. With an integrated neural circuit, the retina is able to perform its photosensitive function. Our study also showed that the development of eye function was temporally synchronized by synaptogenesis. The day of eye opening in mice is usually around P14, which is approximately when the retina starts to carry out its visual function. In our study, synaptogenesis occurred from P5 to P14, along with the development of the retinal neural circuitry. Therefore, the retinal architecture could be ready for visual function at the time of eye opening (P14). The regulation of synaptogenesis is particularly important for the formation of neural circuitry inthe retina. Certain genes are involved in the regulation of synaptogenesis. Martins et al. (2011) found that the retinoblastoma (Rb) tumor suppressor gene plays an important role in the reorganization of horizontal cell neurites. Rb-deficient horizontal cells form ectopic synapses with rods in the ONL. In addition, growth factors and the EphA-ephrinA system interact in a way that affects axon branching and synapse development (Marler et al., 2008).
In summary, the NCL was the anlage for neural differentiation and retinal lamination. The retinal neurons were derived from neural stem cells, and the crucial period for neural differentiation was the time before P14, especially from P0-14. In the retina, synapses mainly exist in the OPL and IPL. After P7, synaptophysin immunostaining started to appear in the OPL and IPL as dense button-like shapes. The OPL and IPL were strongly immunolabeled with synaptophysin. Neuronal differentiation and synaptic formation were complete before mouse eye opening, suggesting that cell differentiation and synaptic formation are prerequisites for proper visual function.
Author contributions: WJF collected the main experimental data. XL added part of the experimental data. HLY, JXD, HLL, ZJC and QW provided technical assistances. JBD and PW wrote the paper and designed the assay. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB (2009) A directional Wnt/β-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136:3289-3299.
Ambati J, Fowler BJ (2012) Mechanisms of age-related macular degeneration. Neuron 75:26-39.
Antonow-Schlorke I, Kühn B, Müller T, Schubert H, Sliwka U, Nathanielsz PW, Schwab M (2001) Antenatal betamethasone treatment reduces synaptophysin immunoreactivity in presynaptic terminals in the fetal sheep brain. Neurosci Lett 297:147-150.
Bejarano-Escobar R, Blasco M, Durán AC, Rodríguez C, Martín-Partido G, Francisco-Morcillo J (2012) Retinal histogenesis and cell differentiation in an elasmobranch species, the small-spotted catshark Scyliorhinus canicula. J Anat 220:318-335.
Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, Wanke E, Brunelli S, Favaro R, Ottolenghi S, Nicolis SK (2008) Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development 135:541-557.
Collin SP (2008) A web-based archive for topographic maps of retinal cell distribution in vertebrates. Clin Exp Optom 91:85-95.
Dan C, Jian-Bin T, Hui W, Le-Ping Z, Jin Z, Ju-Fang H, Xue-Gang L (2008) Synaptophysin expression in rat retina following acute high intraocular pressure. Acta Histochem Cytochem 41:173-178.
Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X (2011) Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138:971-981.
Ferri ALM, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131:3805-3819.
Fisher LJ (1979) Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol 187:359-372.
Greiner JV, Weidman TA (1980) Histogenesis of the cat retina. Exp Eye Res 30:439-453.
Guduric-Fuchs J, Ringland LJ, Gu P, Dellett M, Archer DB, Cogliati T (2009) Immunohistochemical study of pig retinal development. Mol Vis 15:1915-1928.
Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE (2012) Retinal remodeling. Jpn J Ophthalmol 56:289-306.
Kihara AH, Santos TO, Osuna-Melo EJ, Paschon V, Vidal KS, Akamine PS, Castro LM, Resende RR, Hamassaki DE, Britto LR (2010) Connexin-mediated communication controls cell proliferation and is essential in retinal histogenesis. Int J Dev Neurosci 28:39-52.
Le RD, Rayner K, Rex M, Wigmore PM, Scotting PJ (2002) The transcription factor cSox2 and Neuropeptide Y define a novel subgroup of amacrine cells in the retina. J Anat 200:51-56.
Liang SY, Lee GA, Shields D (2009) Self-tonometry in glaucoma management--past, present and future. Surv Ophthalmol 54:450-462.
Lin B, Masland RH, Strettoi E (2009) Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res 88:589-599.
Marler KJM, Becker-Barroso E, Martínez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U (2008) A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci 28:12700-12712.
Martins RAP, Davis D, Kerekes R, Zhang J, Bayazitov IT, Hiler D, Karakaya M, Frase S, Gleason S, Zakharenko SS, Johnson DA, Dyer MA (2011) Retinoblastoma (Rb) regulates laminar dendritic arbor reorganization in retinal horizontal neurons. Proc Natl Acad Sci U S A 108:21111-21116.
McFarlane S, Lom B (2012) The Xenopus retinal ganglion cell as a model neuron to study the establishment of neuronal connectivity. Dev Neurobiol 72:520-536.
Meyer JS, Katz ML, Maruniak JA, Kirk MD (2004) Neural differentiation of mouse embryonic stem cells in vitro and after transplantation into eyes of mutant mice with rapid retinal degeneration. Brain Res 1014:131-144.
Reese BE (2011) Development of the retina and optic pathway. Vision Res 51:613-632.
Remvikos Y, Vielh P, Padoy E, Benyahia B, Voillemot N, Magdelénat H (1991) Breast cancer proliferation measured on cytological samples: a study by flow cytometry of S-phase fractions and BrdU incorporation. Br J Cancer 64:501-507.
Schiller PH (2010) Parallel information processing channels created in the retina. Proc Natl Acad Sci U S A 107:17087-17094.
Sharma RK, O’Leary TE, Fields CM, Johnson DA (2003) Development of the outer retina in the mouse. Dev Brain Res 145:93-105.
Tian N (2004) Visual experience and maturation of retinal synaptic pathways. Vision Res 44:3307-3316.
W?ssle H, Puller C, Müller F, Haverkamp S (2009) Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 29:106-117.
Wei W, Feller MB (2011) Organization and development of direction-selective circuits in the retina. Trends Neurosci 34:638-645.
Young RW (1985) Cell proliferation during postnatal development of the retina in the mouse. Brain Res 353:229-239.
Copyedited by Allen J, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.177743 http://www.nrronline.org/
How to cite this article: Fan WJ , Li X, Yao HL, Deng JX, Liu HL, Cui ZJ, Wang Q, Wu P, Deng JB (2016) Neural differentiation and synaptogenesis in retinal development. Neural Regen Res 11(2):312-318.
Funding: This study was supported by the National Natural Science Foundation of China, No. 31070952 and U1204311; the Scientific Research Foundation of Henan University of China, No. 0000A40475 and 0000A40356.
Accepted: 2015-07-30
*Correspondence to: Jin-bo Deng, M.D. or Ping Wu, M.D., jinbo_deng@henu.edu. cn or wuping1129@126.com.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway
- Influence of immobilization and sensory re-education on the sensory recovery after reconstruction of digital nerves with direct suture or muscle-in-vein conduits
- Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury
- Neuroprotective role of (Val8)GLP-1-Glu-PAL in an in vitro model of Parkinson’s disease
- Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons