Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxiainduced apoptosis of retinal ganglion cells
Jing Yuan, Jian-xiong Yu
1 Eye Center, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Gastrointestinal Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
RESEARCH ARTICLE
Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxiainduced apoptosis of retinal ganglion cells
Jing Yuan1, Jian-xiong Yu2,*
1 Eye Center, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Gastrointestinal Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
Graphical Abstract
orcid: 0000-0001-6757-0217 (Jing Yuan)
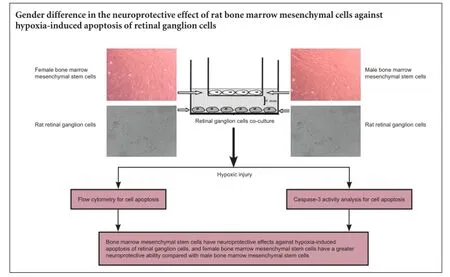
Bone marrow mesenchymal stem cells can reduce retinal ganglion cell death and effectively prevent vision loss. Previously, we found that during differentiation, female rhesus monkey bone marrow mesenchymal stem cells acquire a higher neurogenic potential compared with male rhesus monkey bone marrow mesenchymal stem cells. This suggests that female bone marrow mesenchymal stem cells have a stronger neuroprotective effect than male bone marrow mesenchymal stem cells. Here, we first isolated and cultured bone marrow mesenchymal stem cells from female and male rats by density gradient centrifugation. Retinal tissue from newborn rats was prepared by enzymatic digestion to obtain primary retinal ganglion cells. Using the transwell system, retinal ganglion cells were co-cultured with bone marrow mesenchymal stem cells under hypoxia. Cell apoptosis was detected by flow cytometry and caspase-3 activity assay. We found a marked increase in apoptotic rate and caspase-3 activity of retinal ganglion cells after 24 hours of hypoxia compared with normoxia. Moreover, apoptotic rate and caspase-3 activity of retinal ganglion cells significantly decreased with both female and male bone marrow mesenchymal stem cell co-culture under hypoxia compared with culture alone, with more significant effects from female bone marrow mesenchymal stem cells. Our results indicate that bone marrow mesenchymal stem cells exert a neuroprotective effect against hypoxia-induced apoptosis of retinal ganglion cells, and also that female cells have greater neuroprotective ability compared with male cells.
nerve regeneration; optic nerve injury; bone marrow mesenchymal stem cells; retinal ganglion cells; neuroprotection; hypoxic injury; gender difference; transwell system; co-culture; cell apoptosis; flow cytometry; caspase-3; neural regeneration
Introduction
Progressive death of retinal ganglion cells (RGCs) is a major cause of irreversible visual impairment from neurodegenerative diseases such as glaucoma (Sucher et al., 1997; Kaur et al., 2006, 2007, 2008; Abramov et al., 2007; Wang et al., 2007; Magalh?es da Silva et al., 2012). Clinically, the only available treatment is pharmacological or surgical reduction of intraocular pressure (Quigley, 2011; Mataki et al., 2014; Budenz et al., 2015; Higashide et al., 2015; Wong et al., 2015), and there are no effective treatments for recovering visual function at present. Probable approaches for maintaining vision and RGC function involve preventing RGC death and/or promoting regeneration of damaged RGCs.
Mesenchymal stem cells (MSCs) with multiple differentiation potential have been used in optic nerve regeneration due to their mesenchymal-neural transition potential in vivo and vitro (Huo et al., 2010; Moviglia et al., 2012; Haddad-Mashadrizeh et al., 2013; Xu et al., 2013). Further, there is growing interest in the development of neuroprotective therapies of MSCs for glaucoma, which might be used adjunctively with ocular hypotensive approaches to reduce RGC death and more effectively attenuate vision loss (Sensebe et al., 1997; Kinnaird et al., 2004; Ye et al., 2005; Casson et al., 2012; Chang et al., 2012; Guan et al., 2013; Huang et al., 2013; Machalińska et al., 2013; Manuguerra-Gagné et al., 2013; Ng et al., 2013, 2014; Junyi et al., 2015). Currently, most studies suggest that MSCs have neuroprotective effects in preclinical models of neurodegeneration (Bai et al., 2009; Karussis et al., 2010; Wakabayashi et al., 2010; Novikova et al., 2011; Connick et al., 2011, 2012; Auletta et al., 2012; Forostyak et al., 2013; Glavaski-Joksimovic et al., 2013; Johnson et al., 2010, 2013; Hu et al., 2013; Hao et al., 2014; Ng et al., 2014). MSC transplantation attenuates neuronal death and ensures RGC survival following ischemia/reperfusion (Li et al., 2009), optic nerve crush (Zhao et al., 2011; Mesentier-Louro et al., 2014), optic tract transaction (Zwart et al., 2009), and ocular hypertension (Yu et al., 2006; Johnson et al., 2010). However, the biological and phenotypic implications of sex-specific differences in MSCs remain unclear. Previously, we have found that female rhesus monkey bone marrow mesenchymal stem cells (BMSCs) acquire a higher neurogenic potential compared with male rhesus monkey BMSCs during differentiation (Yuan et al., 2010). Accordingly, female BMSCs may exert a stronger neuroprotective effect than male BMSCs. Here, we investigated gender differences in the neuroprotective effects of BMSCs against hypoxia-induced apoptosis of RGCs.
Materials and Methods
Materials
Ten healthy female and ten healthy male juvenile Sprague-Dawley rats (to isolate BMSCs) and ten newborn Sprague-Dawley rats (to obtain RGCs) were obtained from the Laboratory Animal Center of Renmin Hospital of Wuhan University of China. Juvenile rats were 2—6 months of age and equivalent in weight (250—300 g), while newborn rats were 1—7 days of age. Rats were housed in individual cages under a 12-hour light/dark cycle and in a dry and ventilated room at 23—25°C, with free access to food and water. All surgery was performed under anesthesia, and all efforts were made to minimize pain and distress in the experimental animals. All procedures were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1986). This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University of China.
Isolation and culture of rat BMSCs
Bone marrow cells were obtained from twenty healthy female and male rats, and characterized as previously described (Lei et al., 2007). Briefly, bone marrow aspirates were collected from the femur and tibia. Bone marrow was flushed out using Dulbecco's modified Eagle's medium with low glucose (L-DMEM) (Gibco, New York, NY, USA). Suspended cells were centrifuged at 1,000 r/min for 5 minutes. After discarding the supernatant, cells were resuspended in L-DMEM with 10% fetal bovine serum (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, 2.4 mg/mL hydroxyethyl piperazine ethanesulfonic acid, and 3.7 mg/mL NaHCO3. Next, cells were placed in 25 cm2culture flasks and incubated at 37°C in 5% CO2for 12 hours. Non-adherent cells were removed. The culture medium was replaced every 2 days. On day 12 or 13, confluent cultures (passage 0; P0) were trypsinized with 0.25% trypsin in 0.02% ethylenediaminetetraacetic acid and subcultured as P1. Acquired BMSCs were confirmed after differentiation into osteocytes and adipocytes by addition of specific differentiation media, as described previously (Wang et al., 2006). Cell morphology was observed by phase contrast microscopy (Olympus, Tokyo, Japan). Immunophenotypes were assayed by flow cytometry after co-incubation with fluorescein isothiocyanate (FITC)/phycoerythrin-conjugated monoclonal antibodies including CD29, CD34, CD44, CD45, CD80, and CD86 (BD Biosciences, Sparks, MD, USA), as described previously (Jing and Jian-Xiong, 2011). In subsequent experiments, cells at P3—6 were used for neuroprotection assays.
Purification and culture of RGCs
Primary RGCs were purified and cultured as described previously (Winzeler and Wang, 2013). Briefly, newborn rats were sacrificed, and retinae dissected and incubated for 45 minutes in Dulbecco's phosphate buffered saline supplemented with 160 U/mL papain and 200 U/mL DNase. Retinal tissue was sequentially triturated in Dulbecco's phosphate buffered saline containing 0.2% bovine serum albumin (Gibco) and 650 U/mL DNase. Cells were pelleted and resuspended in Dulbecco's phosphate buffered saline/0.2% bovine serum albumin, and then purified by a two-step immunopanning procedure. Specifically, dissociated retinal cells were incubated in plates coated with an anti-rat macrophage monoclonal antibody (1:50) to exclude macrophages, and then in plates coated with an anti-rat Thy1.1 monoclonal antibody (1:300). RGCs that adhered tothe plates were collected by centrifugation at 600 r/min for 5 minutes, and seeded onto 13 mm glass coverslips in 24-well plates coated with 50 μg/mL poly-L-lysine (Sigma-Aldrich, St. Louis, MO, USA) and 1 μg/mL laminin (Invitrogen, Carlsbad, CA, USA). Purified RGCs were plated at a density of approximately 1,000 cells per well, and cultured in neurobasal medium (Invitrogen) supplemented with 100 μg/mL bovine serum albumin, B27 (Invitrogen), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM L-glutamine, 10 μM forskolin, 40 ng/mL human recombinant brain-derived neurotrophic factor, and 40 ng/mL rat recombinant ciliary neurotrophic factor (Sigma-Aldrich) in a humidified atmosphere containing 5% CO2and 95% air at 37°C for 7 days. Cells were monitored by phase contrast microscopy (Olympus) for morphological changes during growth. Acquired retinal cells were confirmed as RGCs by immunocytochemical staining of Thy1. Coverslips containing viable RGCs were only used when there was distinct neurite sprouting, which usually appeared after 7 days of incubation. Only cultures of pure RGCs with no trace of glial contamination were selected for further study.
Immunocytochemistry
On day 7 after primary culture, RGCs plated on coverslips were fixed in 4% paraformaldehyde for 20 minutes and permeabilized with PBS supplemented with 0.1% Triton X-100 at room temperature. Subsequently, nonspecific blinding was blocked with 4% goat serum for 25 minutes at room temperature. Cells were then incubated with rat anti-mouse Thy1 monoclonal antibody (1:300; Abcam Inc., Cambridge, MA, USA) at 4°C overnight to identify neurons. Next, cells were washed with PBS and incubated with a red fluorescent protein-labeled goat anti-rabbit secondary antibody at room temperature for 2 hours (1:500; BD Biosciences, San Jose, CA, USA). Finally, cells were washed three times in PBS, with Hoechst 33342 (Vector Laboratories, Burlingame, CA, USA) used for cell nuclei staining. Images were visualized and cell numbers of positive staining and Hoechst staining counted using a confocal laser microscope (TCS, SP5; Leica, Mannheim, Germany) at 200× magnification.
RGC co-culture with BMSCs and hypoxic injury
BMSCs (P3—6) were co-cultured with RGCs. BMSCs were separated from RGCs using a 0.4 μm porous polystyrene membrane in 12-well plates with 12-mm membrane diameter transwells. This co-culture system allows cells to maintain crosstalk mediated by secretion of signaling molecules, but avoids mixing of both cell types and physical contact during the culture time prior to plating BMSC-containing inserts into plates containing RGCs. RGCs were cultured in 12-well plates for 7 days in vitro prior to hypoxic injury. BMSCs were plated in 12-well inserts and grown to 80—100% confluence in serum-containing growth medium (L-DMEM with 10% fetal bovine serum (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, 2.4 mg/mL hydroxyethyl piperazine ethanesulfonic acid, and 3.7 mg/mL NaHCO3). BMSCs were rinsed twice with PBS and the medium changed to neurobasal medium, consequently removing serum from the co-culture system. For hypoxia stress (Hong et al., 2007), the co-culture system was transferred to a controlled-atmosphere incubator containing a 5% CO2, 90% N2, and 5% O2mixture, and co-cultured for 24 hours. RGCs were divided into four groups (n = 3 for each group): normal control (normoxia group), hypoxia for 24-hours (hypoxia group), hypoxia for 24 hours in the presence of male BMSCs (MBMSCs) (hypoxia + MBMSCs group), and hypoxia for 24 hours in the presence of female BMSCs (FBMSCs) (hypoxia + FBMSCs group). In the normoxia group, RGCs were placed in an incubator at 37°C with normal humidity and 95% air and 5% CO2. Finally, RGC apoptosis was assessed by flow cytometry (FACSAria?, BD Biosciences) and caspase-3 activity assay.
Flow cytometry for cell apoptosis
After hypoxia exposure for 24 hours, the percentage of apoptotic or necrotic cells was determined by flow cytometry using an Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Becton Dickinson; Frankly Lakes, NJ, USA), according to the manufacturer's instructions. Briefly, cells (1 × 105cells/mL) attached to the bottom of plates and cells in the supernatant were collected, washed twice with ice-cold PBS, and re-suspended in 200 μL Annexin binding buffer. Subsequently, 2 μL Annexin V-FITC and 2 μL PI were added and incubated for 5—15 minutes in the dark at room temperature. After incubation, samples were analyzed by flow cytometry (FACSAria?). The percentage of apoptotic cells was directly calculated using the FACS-can flow cytometer (Becton-Dickinson).
Caspase-3 activity analysis for cell apoptosis
After hypoxia exposure for 24 hours, caspase-3 activity was determined using the CaspACETMcolorimetric assay system (Promega, Madison, WI, USA), according to the manufacturer's instructions. Briefly, cells (1 × 106cells/mL) attached to the bottom of plates and cells in the supernatant were collected, washed twice with PBS, and re-suspended in cell lysis buffer. Cells were then mixed with 32 μL assay buffer and 2 μL 10 mM DEVD-paranitroanilide substrate, and incubated for 4 hours at 37°C. After incubation, absorbance values were determined at 405 nm by subtraction of the mean absorbance of the blank from the sample using a microplate spectrofluorometer reader (Perkin Elmer, Vernon Hills, IL, USA). Caspase-3 activity was calculated as relative fluorescence units.
Statistical analysis
SPSS 18.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Measurement data results are presented as the mean ± SD. Intergroup differences in percentage of apoptosis and caspase-3 activity were performed using one-way analysis of variance and the least significant difference test. P values < 0.05 were considered statistically significant.
Results
Culture and identification of rat BMSCs
Mesenchymal stem cell populations originating from adult rat bone marrow from female and male donors were successfully isolated, purified, and expanded in monolayer cultures. After reaching confluence and subsequent sub-cultivation at an early passage, FBMSCs and MBMSCs became morphologically homogeneous fibroblast-like cells (Figure 1A, B). After long-term culture (≥ 10 passages), BMSCs began to exhibit morphological changes, and were a mixture of bipolar or dendrite-like shapes, before gradually dying as culture time extended. We observed that FBMSCs were able to maintain subculture with increased passaging compared with MBMSCs (Figure 1C, D). Flow cytometry showed expression of CD29 and CD44 but not CD34, CD45, CD80, and CD86 (data not shown). In addition, FBMSCs and MBMSCs underwent appropriate differentiation into adipocytes and osteoblasts after culture in induction media (Figure 1E—H).
Culture and identification of RGC
A RGC population originating from rat retinae was successfully isolated, purified, and expanded in monolayer culture. After 1 day of culture, the cells tended to form clusters with few micro-neurites. This tendency reached a peak at 2 days and then began to weaken at 5 days. During culture, the neurites of cells extended and connected to each other, with the cells finally forming a monolayer. Monolayer cells were a heterogeneous population that included cells with long neurites, as well as round or oval cell bodies with thin neurites (Figure 2A). Using the conditions outlined in the Materials and Methods section, we maintained RGCs in culture for as long as 2 weeks. Immunocytochemical staining demonstrated Thy1 expression, suggesting that the cells were RGCs (Figure 2B).
BMSCs protected RGCs from hypoxia-induced apoptosis and exhibited gender differences
Flow cytometry was used to examine the neuroprotective effect of BMSCs. Gender differences in their anti-apoptotic effects after hypoxia-induced apoptosis of RGCs were determined by calculating the percentage of apoptotic or necrotic cells (Figure 3A, B). Apoptotic cells include both early (AnnexinV+/PI-) and late (AnnexinV+/PI+) cells. Compared with normoxia, we found marked increases in the percentage of apoptotic cells after 24 hours of hypoxia (P < 0.05). Moreover, the percentage of apoptotic RGCs significantly decreased after co-culture with both FBMSCs and MBMSCs under hypoxia compared with culture alone (P < 0.05). Additionally, the percentage of apoptotic cells was significantly different between FBMSCs and MBMSCs (P < 0.05). These results show that BMSCs effectively inhibit hypoxia-induced apoptosis of RGCs, and the neuroprotective effects show a gender difference.
Caspase-3 activity was also used to examine the neuroprotective effect of BMSCs, and compare gender differences in their anti-apoptotic effects after hypoxia-induced apoptosis of RGCs, by measuring cleavage of the caspase-3 specific substrate, Ac-DEVD-pNA (Figure 3C). We found a marked increase in caspase-3 activity after 24 hours of hypoxia compared with normoxia (P < 0.05). Further, caspase-3 activity was significantly decreased after co-culture with both FBMSCs and MBMSCs under hypoxic conditions compared with culture alone (P < 0.05). Caspase-3 activity was significantly different between FBMSCs and MBMSCs (P < 0.05). These results show that BMSCs effectively inhibit hypoxia-induced apoptosis of RGCs, and the neuroprotective effects present a gender difference.
Discussion
The use of stem cells in neuroprotective treatments in all areas of medicine has recently been under consideration. However, gender differences in the neuroprotective effects of BMSCs have not yet been investigated. Indeed, greater understanding of sex-specific differences in the neuroprotective effects mediated by BMSCs is a prerequisite before considering their use in the treatment of neurological diseases. Accordingly here, we investigated the neuroprotective effects of BMSCs and compared the effect of each sex. Our results provide crucial information for the future application of BMSCs for successful neuroprotection of RGCs.
First, we isolated rat BMSCs from juvenile female and male rats, and established a common experimental animal model to study gender differences of BMSCs. By observing cellular morphology, we detected a uniform fibroblast-like shape after subculture at early passages. With increasing passage number, the cells gradually lost their vitality, but FBMSCs maintained longer passage numbers compared with MBMSCs, suggesting that females have increased subculture viability. Adherent cells were confirmed as BMSCs due to their ability to undergo lipogenic and osteogenic differentiation, and by representative CD34 expression. Further, we acquired purified RGCs by a two-step immunopanning procedure. However, we found it difficult to obtain uniformly shaped cells during purified RGC culture. RGCs tended to group during growth, and the high cell density led to RGC clustering and a connected network, with long neurites present as culture time continued. With increased cell density, RGC clustering and the connected network were weakened, and neurites gradually became thin and short, with round or oval shaped cells and short neurites. Cells gradually died over time. These results suggest that RGCs are highly vulnerable and sensitive to disturbances in the ambient environment.
Additionally, our RGC-BMSC co-culture model showed that BMSCs exert a protective effect on RGC viability against hypoxic injury. Using flow cytometry and caspase-3 activity assays, we found that co-culture with both FBMSCs and MBMSCs suppressed hypoxia-induced apoptosis of RGCs and improved their survival. This is in agreement with previous reports indicating that the rate of hypoxia-induced RGC loss is reduced when BMSCs are intravitreally transplanted in an experimental ocular hypertension model (Ng et al., 2014; Emre et al., 2015). Altogether, these findings suggestthat BMSCs protect RGCs from hypoxia-induced apoptosis. The mechanism of action of BMSC-mediated neuroprotection may originate from secretion of neurotrophic factors, including brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor, which play an important paracrine role in modulating inflammatory processes, regulating axonal re-growth (along with neuronal repair), and activating endogenous repair mechanisms (Kuroda et al., 2013; Shichinohe et al., 2015).
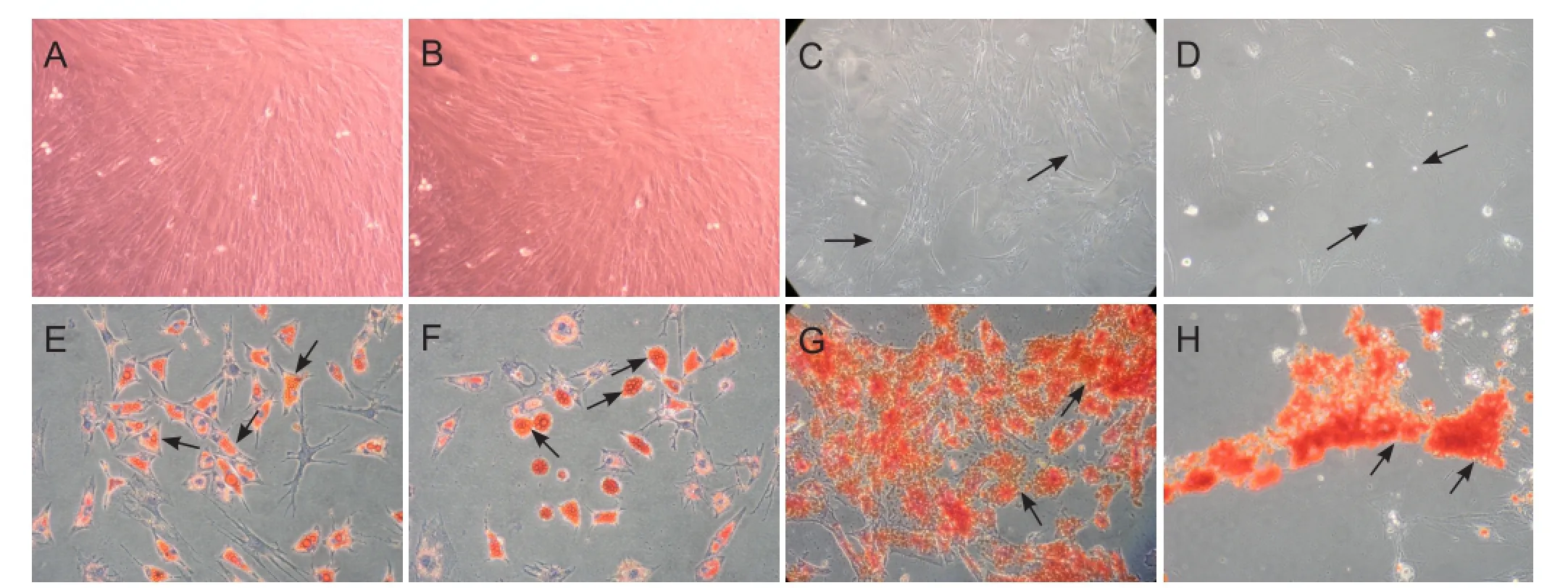
Figure 1 Culture and identification of rat BMSCs (phase contrast microscope, × 10).
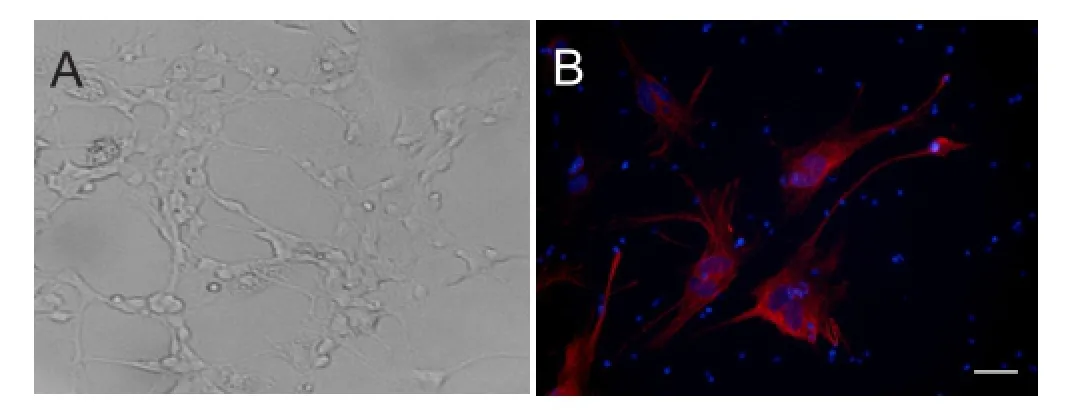
Figure 2 Culture and identification of rat RGCs (× 20).
We focused on comparing the neuroprotective properties of BMSCs due to gender. Few studies have determined if gender differences can influence stem cell function. In our previous study, we found that BMSCs from female rhesus monkeys have a higher neurogenic potential compared with MBMSCs during differentiation (Yuan et al., 2010). Here, in response to hypoxia, rat FBMSCs exhibit decreased RGC apoptosis compared with MBMSCs, which suggests that FBMSCs have a greater neuroprotective ability. This is an interesting observation that may be explained by the following: (1) FBMSCs may demonstrate improved attenuation of hypoxic injury or other insult compared with MBMSCs because of their greater inherent ability to survive (Crisostomo et al., 2007). This is supported by our results showing that FBMSCs maintain viability with increasing subculture number. (2) FBMSCs may release greater levels of protective factors and decreased inflammatory cytokines in response to hypoxia or other injury (Crisostomo et al., 2007). This and our previous study suggest that gender plays an important role in stem cell function. Gender differences may be associated with the role of estrogen. Estrogen can enhance female stem cell survival and activity (Marin-Husstege et al., 2004), which may be mediated by mitogen activated protein kinases and cyclin dependent kinases (Han et al., 2006). Exogenous estrogen, possibly via estrogen receptor alpha, increases MSC function and calcium deposition (Leskel? et al., 2006; Wang et al., 2006). Furthermore, gender differences in tumor necrosis factor-R1 signaling may account for enhanced vascular endothelial growth factor and decreased tumor necrosis factor-α expression detected in female MSCs. Tumor necrosis factor-R1 ablation also significantly reduces male MSC apoptosis after hypoxic injury. Thus, sex hormones and various intracellular signaling pathways may in part explain the observed gender differences in stem cell function. Gender differences, although observed in vitro, may provide information on the neuroprotective ability of BMSCs and their intrinsic commitment in vivo. Based on our experimental evidence, it is possible that the gender difference reflects more active neuronal growth in females compared with males. For example, this may explain the epidemiological observation that women have a lower incidence of symptomatic Parkinson's disease and a higherage of onset than men due to higher physiological neuronal expression (Haaxma et al., 2007), which may partly enable greater neuroprotection by bone marrow grafts of progenitors (e.g., BMSCs) that have migrated from the blood into the brain parenchyma across the blood-brain barrier. Although the role of host sex was not examined in our study, stem cell sex differences in neuroprotective properties may lead to different clinical treatments for neurological diseases in women and men. Nevertheless, some limitations of our study should be noted e.g., the age, low number of experimental animals, chronic injury model, and the exact mechanism underlying this protective effect. In addition, rat genes are 80% homologous with humans; therefore, human-derived cell experiments must be performed to verify gender differences that are correlated to events in humans. Thus, further studies on the neuroprotective properties and intrinsic mechanisms underlying the observed differences should be performed and accompanied by an age hierarchy of donors, more samples, model design, and mechanisms to further our understanding of the cell biology and provide improved clinical application of these cells.
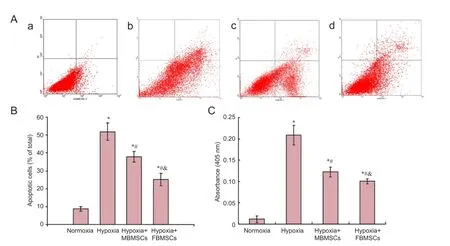
Figure 3 Co-culture effect of MBMSCs and FBMSCs on hypoxia-induced apoptosis of RGCs and caspase-3 activity.
In summary, both male and female rat BMSCs enhance RGC survival under hypoxia, although FBMSCs confer greater neuroprotective effects compared with MBMSCs after hypoxic injury. Our results promote understanding of the neuroprotective effects of BMSCs and may contribute to the development of new therapeutic strategies for neurological repair and regeneration.
Author contributions: JY designed the study, wrote the paper, provided research funding, and finalized the paper. JY and JXY carried out the laboratory experiments, interpreted the results, analyzed the data, and approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27:1129-1138.
Auletta JJ, Bartholomew AM, Maziarz RT, Deans RJ, Miller RH, Lazarus HM, Cohen JA (2012) The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy 4:529-547.
Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD,Miller RH (2009) Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 57:1192-1203.
Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group (2015) Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 122:308-316.
Casson RJ, Chidlow G, Ebneter A, Wood JP, Crowston J, Goldberg I (2012) Translational neuroprotection research in glaucoma: a review of definitions and principles. Clin Exp Ophthalmol 40:350-357.
Chang EE, Goldberg JL (2012) Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 119:979-986.
Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ,Compston A, Scott MA, Miller DH, Chandran S (2012) Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 11:150-156.
Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL, Richardson K, Barber K, Webber DJ, Wheeler-Kingshott CA, Tozer DJ, Samson RS, Thomas DL, Du MQ, Luan SL, Michell AW, Altmann DR, Thompson AJ, Miller DH, Compston A, Chandran S (2011) The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: posttest study with blinded outcome assessments. Trials 12:62.
Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR (2007) Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol 42:142-149.
Emre E, Yüksel N, Duruksu G, Pirhan D, Suba?i C, Erman G, Kara?z E (2015) Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 17:543-559.
Forostyak S, Jendelova P, Sykova E (2013) The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 95:2257-2270.
Glavaski-Joksimovic A, Bohn MC (2013) Mesenchymal stem cells and neuroregeneration in Parkinson's disease. Exp Neurol 247:25-38.
Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y, Zhang J, Gao F, Tian H, Xu L, Xu G, Li W, Jin Y, Xu GT (2013) Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med 13:1419-1431.
Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW (2007) Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 78:819-824.
Haddad-Mashadrizeh A, Bahrami AR, Matin MM, Edalatmanesh MA, Zomorodipour A, Gardaneh M, Farshchian M, Momeni-Moghaddam M (2013) Human adipose-derived mesenchymal stem cells can survive and integrate into the adult rat eye following xenotransplantation. Xenotransplantation 20:165-176.
Han HJ, Heo JS, Lee YJ (2006) Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol 290:C1067-1075.
Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L (2014) Stem cell-based therapies for ischemic stroke. Biomed Res Int 2014:468748.
Higashide T, Ohkubo S, Sugiyama K (2015) Long-term outcomes and prognostic factors of trabeculectomy following intraocular bevacizumab injection for neovascular glaucoma. PLoS One 10:e0135766.
Hong S, Lee J, Kim C, Seong GJ (2007) Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci 8:81-92.
Hu Y, Tan HB, Wang XM, Rong H, Cui HP, Cui H (2013) Bone marrow mesenchymal stem cells protect against retinal ganglion cell loss in aged rats with glaucoma. Clin Interv Aging 8:1467-1470.
Huang L, Xu W, Xu G (2013) Transplantation of CX3CL1-expressing mesenchymal stem cells provides neuroprotective and immunomodulatory effects in a rat model of retinal degeneration. Ocul Immunol Inflamm 21:276-285.
Huo DM, Dong FT, Yu WH, Gao F (2010) Differentiation of mesenchymal stem cell in the microenviroment of retinitis pigmentosa. Int J Ophthalmol 3:216-219.
Jing Y, Jian-Xiong Y (2011) 3D spheroid culture of bone marrow mesenchymal stem cell of rhesus monkey with improved muti-differentiation potential to epithelial progenitors and neuron in vitro. Clin Exp Ophthalmol 39:808-819.
Johnson TV, Martin KR (2013) Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr Opin Pharmacol 13:78-82.
Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR (2010) Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci 51:2051-2059.
Junyi L, Na L, Yan J (2015) Mesenchymal stem cells secrete brain-derived neurotrophic factor and promoteretinal ganglion cell survival after traumatic optic neuropathy. J Craniofac Surg 26:548-552.
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67:1187-1194.
Kaur C, Foulds WS, Ling EA (2008) Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol 2:879-889.
Kaur C, Sivakumar V, Foulds WS (2006) Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 47:1126-1141.
Kaur C, Sivakumar V, Yong Z, Lu J, Foulds WS, Ling EA (2007) Blood-retinal barrier disruption and ultrastructural changes in the hypoxic retina in adult rats: the beneficial effect of melatonin administration. J Pathol 212:429-439.
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S,Epstein SE (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109:1543-1549.
Kuroda S (2013) Bone marrow stromal cell transplantation for ischemic stroke-its multi-functional feature.Acta Neurobiol Exp (Wars) 73:57-65.
Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, Xinwen Z, Mingxue Z (2007) Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int 31:916-923.
Leskel? HV, Olkku A, Lehtonen S, Mahonen A, Koivunen J, Turpeinen M, Uusitalo J, Pelkonen O, Kangas L, Selander K, Lehenkari P (2006) Estrogen receptor alpha genotype confers interindividual variability of response to estrogen and testosterone in mesenchymal-stem-cell-derived osteoblasts. Bone 39:1026-1034.
Li N, Li XR, Yuan JQ (2009) Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol 247:503-514.
Machalińska A, Kawa M, Pius-Sadowska E, St?pniewski J, Nowak W, Rogińska D, Kaczyńska K, Baumert B, Wiszniewska B, Józkowicz A, Dulak J, Machaliński B (2013) Long-term neuroprotective effects of NT-4-engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Invest Ophthalmol Vis Sci 54:8292-8305.
Magalh?es da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, Tanus-Santos JE, Rios-Santos F (2012) Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk ofprimary open angle glaucoma in a Brazilian population. Gene 502:142-146.
Manuguerra-Gagné R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC (2013) Transplantation of mesenchymal stem cells promotes tissueregeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 31:1136-1148.
Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia- Bonnefil P (2004) Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci 26:245-254.
Mataki N, Murata H, Sawada A, Yamamoto T, Shigeeda T, Araie M (2014) Visual field progressive rate in normal tension glaucoma before and after trabeculectomy: a subfield-based analysis. Asia Pac J Ophthalmol (Phila) 3:263-266.
Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF (2014) Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One 9:e110722.
Moviglia GA, Blasetti N, Zarate JO, Pelayes DE (2012) In vitro differentiation of adult adipose mesenchymal stem cells into retinal progenitor cells. Ophthalmic Res 48 Suppl 1:1-5.
Ng TK, Lam DS, Cheung HS (2013) Prospects of stem cells for retinal diseases. Asia Pac J Ophthalmol 2:57-63.
Ng TK, Fortino VR, Pelaez D, Cheung HS (2014) Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells 6:111-119.
Novikova LN, Brohlin M, Kingham PJ, Novikov LN, Wiberg M (2011) Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy 13:873-887.
Quigley HA (2011) Glaucoma. Lancet 377:1367-1377.
Sensebe L, Deschaseaux M, Li J, Herve P, Charbord P (1997) The broad spectrum of cytokine gene expression by myoid cells from the human marrow microenvironment. Stem Cells 15:133-143.
Shichinohe H, Ishihara T, Takahashi K, Tanaka Y, Miyamoto M, Yamauchi T, Saito H, Takemoto H, Houkin K, Kuroda S (2015) Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil Neural Repair 29:80-89.
Sucher NJ, Lipton SA, Dreyer EB (1997) Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res 37:3483-3493.
Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S (2010) Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 88:1017-1025.
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53-68.
Wang Q, Yu JH, Zhai HH, Zhao QT, Chen JW, Shu L, Li DQ, Liu DY, Dong C, Ding Y (2006) Temporal expression of estrogen receptor alpha in rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 347:117-123.
Winzeler A, Wang JT (2013) Purification and culture of retinal ganglion cells from rodents. Cold Spring Harb Protoc 2013:643-652.
Wong MO, Lee JW, Choy BN, Chan JC, Lai JS (2015) Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol 60:36-50.
Xu Y, Gu Z, Shen B, Xu G, Zhou T, Jiang J, Xing J, Liu S, Li M, Tan W, Feng G, Sang A, Li L (2013) Roles of Wnt/β-catenin signaling in retinal neuron-like differentiation of bone marrow mesenchymal stem cells from nonobese diabetic mice. J Mol Neurosci 49:250-261.
Ye M, Chen S, Wang X, Qi C, Lu G, Liang L, Xu J (2005) Glial cell line-derived neurotrophic factor in bone marrow stromal cells of rat. Neuroreport 16:581-584.
Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N (2006) Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 344:1071-1079.
Yuan J, Yu JX, Ge J (2010) Sexual dimorphism on the neurogenic potential of rhesus monkeys mesenchymal stem cells. Biochem Biophys Res Commun 396:394-400.
Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B (2011) Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol 249:1021-1028.
Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB, Mehmet H, Navarrete R, Navarrete C, Jen LS (2009) Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol 216:439-448.
Copyedited by James R, de Souza M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182764 http://www.nrronline.org/
How to cite this article: Yuan J, Yu JX (2016) Gender difference in the neuroprotective effect of rat bone marrow mesenchymal cells against hypoxia-induced apoptosis of retinal ganglion cells. Neural Regen Res 11(5)∶846-853.
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81100664; the Open Project of the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, China, No. 303060202400306; the Wuhan Science and Technology Dawn Project of China, No. 2014070404010222; and the Independent Research Project of Wuhan University of China, No. 2042014kf0259.
Accepted: 2015-12-22
*Correspondence to: Jing Yuan, M.D., Ph.D., xyj711@163.com.
 中國(guó)神經(jīng)再生研究(英文版)2016年5期
中國(guó)神經(jīng)再生研究(英文版)2016年5期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Vitamin B complex and vitamin B12levels after peripheral nerve injury
- Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion
- A self-made, low-cost infrared system for evaluating the sciatic functional index in mice
- Methylprednisolone exerts neuroprotective effects by regulating autophagy and apoptosis
- Repetitive magnetic stimulation affects the microenvironment of nerve regeneration and evoked potentials after spinal cord injury
