Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
Guang-bin Zha, Mi Shen, Xiao-song Gu, Sheng YiJiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
Guang-bin Zha, Mi Shen, Xiao-song Gu, Sheng Yi*
Jiangsu Key Laboratory of Neuroregeneration, Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
How to cite this article: Zha GB, Shen M, Gu XS, Yi S (2016) Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration. Neural Regen Res 11(9):1506-1511.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81130080, 31300942; the National Key Basic Research Program of China (973 Program), No. 2014CB542202; the Natural Science Foundation of Jiangsu Province, China, No. BK20150409; the Natural Science Foundation of Jiangsu Higher Education Institutions of China, No. 15KJB180013; the Scientific Research Foundation of Nantong University of China, No. 14R29; the Natural Science Foundation of Nantong City in China, No. MS12015043; the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Sheng Yi, Ph.D.,
syi@ntu.edu.cn.
orcid:
0000-0003-1316-3370 (Sheng Yi)
Accepted: 2016-09-14
Graphical Abstract

Tau, a primary component of microtubule-associated protein, promotes microtubule assembly and/or disassembly and maintains the stability of the microtubule structure. Although the importance of tau in neurodegenerative diseases has been well demonstrated, whether tau is involved in peripheral nerve regeneration remains unknown. In the current study, we obtained sciatic nerve tissue from adult rats 0, 1, 4, 7, and 14 days after sciatic nerve crush and examined tau mRNA and protein expression levels and the location of tau in the sciatic nerve following peripheral nerve injury. The results from our quantitative reverse transcription polymerase chain reaction analysis showed that compared with the uninjured control sciatic nerve, mRNA expression levels for both tau and tau tubulin kinase 1, a serine/ threonine kinase that regulates tau phosphorylation, were decreased following peripheral nerve injury. Our western blot assay results suggested that the protein expression levels of tau and phosphorylated tau initially decreased 1 day post nerve injury but then gradually increased. The results of our immunohistochemical labeling showed that the location of tau protein was not altered by nerve injury. Thus, these results showed that the expression of tau was changed following sciatic nerve crush, suggesting that tau may be involved in peripheral nerve repair and regeneration.
nerve regeneration; sciatic nerve crush; microtubule-associated protein; tau; phosphorylated tau (Ser 404); tau hyper-phosphorylation; tau tubulin kinase 1; microtubule structure; microtubule assembly and disassembly; peripheral nervous system; neural regeneration
Introduction
Microtubules are components of the eukaryotic cytoskeleton that are essential for a wide variety of cellular functions, including cell motility, transport, shape, polarity, and mitosis. In the nervous system, microtubules are important for determining neuronal morphology, axonal and dendritic cellular polarity, plasticity and stability of axons and dendrites, and axonal transport (Gupta et al., 2008; Rodríguez-Martín et al., 2013; Pedersen and Sigurdsson, 2015). Microtubule-associated proteins (MAPs), as their name indicates,interact with microtubules, mediate microtubule assembly and stabilization, and influence the interactions and spatial patterns of microtubules (Black and Kurdyla, 1983). MAPs are composed of MAP1 (including MAP1a and MAP1b), MAP2, MAP4, and tau proteins. Among these MAPs, tau is ubiquitously expressed in most tissues and organs and is most abundant in the neurons of the central nervous system (Gorath et al., 2001; Kumar et al., 2015).
Due to mRNA alternative splicing and multiple posttranslational modifications (Chambers and Muma, 1997; Iqbal et al., 2005; Iqbal et al., 2009), many subtypes of tau protein exist and play different roles in neuronal growth and axonal transport (Rodríguez-Martín et al., 2013; Dai et al., 2015; Ando et al., 2016). It has been well demonstrated that tau proteins are involved in neurodegenerative diseases, such as frontotemporal lobar degeneration, chronic traumatic encephalopathy, Parkinson’s disease, and especially, Alzheimer’s disease. In the brains of patients with Alzheimer’s disease, tau protein is abnormally hyper-phosphorylated and aggregated to form neurofibrillary tangles, which then induce neuronal dysfunction and degeneration of the central nervous system (Kim et al., 2016). In addition to its involvement in neurodegenerative diseases, tau protein is also associated with the repair and regeneration of the central nervous system. Protein levels of tau are reportedly elevated in regenerating axons of the ventral fascicule (Yin et al., 1995), and tau protein has been identified and used as a biomarker of traumatic brain injury (Liao et al., 2008; K?vesdi et al., 2010).
Besides its critical role in the central nervous system, tau protein is also expressed in the peripheral nervous system (Nunez and Fischer, 1997). Wu et al. (2010) showed that recombinant human bone morphogenetic protein-2 upregulates tau protein expression and increases axonal regeneration after facial nerve injury, suggesting that tau protein may be involved in the process of peripheral nerve repair and regeneration. However, the expression pattern and the specific roles of tau protein following peripheral nerve injury have not yet been determined with certainty.
In the current study, we used a rat sciatic nerve crush model to study the mRNA and protein expression patterns of tau and to identify the contribution of tau protein to peripheral nerve repair and regeneration.
Materials and Methods
Rat sciatic nerve crush surgery and tissue preparation
Sixty clean male Sprague-Dawley rats weighing 180—200 g and aged 2.5 months were provided by the Experimental Animal Center of Nantong University of China (animal license No. SCXK [Su] 2014-0001 and SYXK [Su] 2012-0031). All animal procedures were performed in accordance with the Institutional Animal Care Guidelines of Nantong University, China, and approved by the Administration Committee of Experimental Animals, Jiangsu Province, China.
Rats were equally randomized to five groups according to the time post injury (0, 1, 4, 7, and 14 days). All rats were anesthetized with mixed narcotics, including 85 mg/kg trichloroacetaldehyde monohydrate, 42 mg/kg magnesium sulfate, and 17 mg/kg sodium pentobarbital (Ruijie, Shanghai, China). The rats underwent an operation to crush the left sciatic nerve as previously described (Yi et al., 2015). Briefly, a skin incision was made on the lateral aspect of the midthigh of the left hind limb. The sciatic nerve was exposed and then crushed three times with forceps (54 N), for 10 seconds each. The muscle and skin were sutured. At 0, 1, 4, 7, and 14 days following nerve injury, sciatic nerve segments 5 mm in length at the injury site were harvested and then stored at -80°C.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
The total RNA in stored sciatic nerve segments from four rats in each group was extracted using Trizol Reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The quality of purified RNA samples was examined using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), and the quantity of RNA was determined using a NanoDrop ND-1000 spectrophotometer (Infinigen Biotechnology Inc., City of Industry, CA, USA). Following quality and quantity determinations, extracted RNA samples were reverse-transcribed to cDNA using a PrimeScript reagent kit (TaKaRa Biotechnology Co., Ltd., Dalian, Liaoning Province, China). PCR was then conducted in triplicate for each sample with SYBR Green Premix Ex Taq (TaKaRa) using specific primer pairs with an Applied Biosystems StepOne Real-Time PCR system. The sequences of PCR primer pairs were as follows: MAP tau (MAPT) primer pairs, (forward) 5′-AAG AAG CAG GCA TCG GAG AC-3′ and (reverse) 5′-CCT TGG CTT TCT TCT CGT CA-3′; tau tubulin kinase 1 (TTBK1) primer pairs, (forward) 5′-ACT GAG TAC CAC ACT GCG TC-3′ and (reverse) 5′-CGT CCC CAG TGG TGT TAG TG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer pairs, (forward) 5′-CCT TCA TTG ACC TCA ACT ACA TG-3′ and (reverse) 5′-CTT CTC CAT GGT GGT GAA GAC-3′ The thermal cycling program consisted of 2 minutes at 95°C; 40 cycles of 30 seconds at 60°C, 45 seconds at 65°C, and 30 seconds at 72°C, followed by 5 minutes at 72°C. The quality of the PCR products was validated by the appearance of a single peaked curve, which presents a single product. The expression level of each mRNA was calculated using the threshold cycle (Ct), and the relative quantification of mRNA was conducted by the comparative 2-ΔΔCtmethod, with GAPDH as the reference gene.
Western blot assay
Protein samples of stored sciatic nerve segments from four rats in each group were extracted using the Mammalian Tissue Protein Extraction Reagent (Biocolors, Shanghai, China) and 2.0 μg/mL aprotinin. Briefly, sciatic nerve segments on ice were minced with eye scissors, homogenized in lysis buffer, and then centrifuged using a microcentrifuge for 20 minutes at 4°C. Following centrifugation, the collected supernatant was mixed with β-mercaptoethanol, glycerin, and bromophenol blue, and incubated at 100°C for 10 minutes.
The concentration of protein in the samples was determined using a modified Lowry method (Shen et al., 2013) according to the instruction manual provided for the DC Protein Assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein samples were loaded on 10% sodium dodecyl sulfate gradient gels. Following electrophoresis, proteins were transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% nonfat dry milk or bovine serum albumin for 2 hours at room temperature, probed with rabbit anti-total tau polyclonal antibody (1:500; Abcam, Cambridge, MA, USA), rabbit antip-tau (Ser 404) polyclonal antibody (1:500; Thermo Fisher Scientific, Waltham, MA, USA), or mouse anti-rat GAPDH monoclonal antibody (1:1,000; Pierce, Rockford, IL, USA) overnight at 4°C, and then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:1,500; Pierce) for 1 hour at room temperature. The blots were developed with Pierce Enhanced Chemiluminescence Western Blotting Substrate (Thermo Fisher Scientific). Quantification was performed using Quality One software (Bio-Rad). All data were derived from independent experiments in triplicate for increased reliability and accuracy.
Immunofluorescence labeling
At 0, 1, 4, 7, and 14 days post sciatic nerve crush, four rats from each group were intracardially perfused with 4% freshly depolymerized, neutral-buffered paraformaldehyde. Sciatic nerves were excised, immersed, and post-fixed in paraformaldehyde overnight at 4°C, and then dehydrated by 30% hypertonic sucrose solution for 48 hours. Following dehydration, tissues were embedded in Optimal Cutting Temperature compound and sectioned into 15-μm-thick slices on a cryostat microtome (CM3050, Leica Biosystems, Solms, Germany). Tissue slices with comparatively complete morphological structure were selected for subsequent observation. The slices were mounted on microscope slides, washed with phosphate-buffered saline, blocked with 5% goat serum for 30 minutes at room temperature, incubated with rabbit anti-total tau polyclonal antibody (1:200; Abcam) or rabbit anti-p-tau (Ser 404) polyclonal antibody (1:50; Thermo Fisher Scientific) overnight at 4°C, and then incubated with anti-rabbit Cy3 IgG (1:1,000; Sigma-Aldrich, St. Louis, MO, USA) for 2 hours at room temperature. Labeled sections were viewed with an optical and epifluorescence microscope (Axio Imager M2, Carl Zeiss Microscopy GmbH, Jena, Germany), and images were captured and analyzed using Zen 2 Pro software (Zeiss).
Statistical analysis
Statistical analysis was performed using SPSS 15.0 software (SPSS, Chicago, IL, USA). Parametric data are reported as the mean ± SEM. Differences between groups were tested using one-way analysis of variance. P values less than 0.05 were considered statistically significant.
Results
Tau mRNA expression following sciatic nerve injury
To investigate the relationship between tau and sciatic nerve regeneration, we first performed real-time quantitative RTPCR to determine the mRNA expression level of tau. The mRNA encoding tau protein is designated MAPT. Compared with that for the uninjured control (0 day), MAPT mRNA expression was significantly decreased 1, 4, and 7 days following sciatic nerve crush (P < 0.01). At 14 days post sciatic nerve injury, MAPT mRNA expression increased (P < 0.05, vs. control; Figure 1A).
Considering the importance of tau phosphorylation, in addition to MAPT mRNA expression, the expression pattern of the mRNA encoding tau tubulin kinase 1, TTBK1, was also measured. Results from real-time quantitative RT-PCR suggested that, compared with the day 0 control, the mRNA expression of TTBK-1 was significantly increased 1 day post injury (P < 0.01) and then decreased 7 and 14 days post injury (P < 0.05 and P < 0.01, respectively; Figure 1B).
Tau protein expression following sciatic nerve injury
Given the observation that MAPT mRNA expression levels were decreased post sciatic nerve crush, western blot assays were performed to detect the expression levels of phosphorylated tau (phospho-tau, Ser 404) and total tau proteins 0, 1, 4, 7, and 14 days following sciatic nerve injury. The results from these western blot assays demonstrated that both phospho-tau (Ser 404) and total tau were present in all samples, regardless of time post injury (Figure 2A).
We also determined the relative optical density of the phospho-tau (Ser 404) protein band after normalization to that for GAPDH, used as a loading control, at each time point examined following sciatic nerve crush. Compared with the day 0 control, the relative optical density of the phospho-tau (Ser 404) protein band was significantly decreased 1 day post sciatic nerve injury (P < 0.0001). At later time points, the relative optical density of phospho-tau (Ser 404) gradually increased (P < 0.05; Figure 2B). The relative optical density of the total tau protein band was also slightly decreased, although to a lesser extent, 1 day post injury (P < 0.05). Similar to the relative density of phospho-tau (Ser 404), the density of total tau protein was also increased at later time points, reaching a maximum value 14 days post injury (P < 0.0001; Figure 2C).
We also determined the ratio of phospho-tau (Ser 404) to total tau at each time point. The results shown in Figure 2D demonstrated that 1 day post injury, the ratio of phospho-tau (Ser 404) to total tau was decreased to less than 50%, while at 4, 7, and 14 days post injury, the ratio of phospho-tau (Ser 404) to total tau was elevated approximately 1.5-fold.
In addition to phospho-tau (Ser 404), we also measured the protein expression levels of other phosphorylated forms of tau protein in the sciatic nerve segment. However, we found that the amount of phospho-tau (Ser 214), phospho-tau (Ser 262), and phospho-tau (Ser 396) did not reach detectable levels (data not shown).
Tau localization following sciatic nerve injury
Immunofluorescence labeling was then performed, andthe Cy3 fluorescence signals were detected to identify immunoreactive tau protein. At each time point examined following sciatic nerve crush, we observed the presence and localization of total tau and phospho-tau (Ser 404) in cross sections of dissected rat sciatic nerve specimens (Figure 3). The localization of both total tau and phospho-tau (Ser 404) appeared to be unaltered with time following sciatic nerve injury.
The immunohistochemical signal intensity for total tau appeared unaltered 1 and 4 days post injury, but was slightly increased 7 and 14 days post injury (Figure 3), whereas that for phospho-tau (Ser 404) was more markedly increased 4, 7, and 14 days post injury (Figure 3). These results were consistent with those from our western blot assays (Figures 2B, C).
Discussion
We induced rat sciatic nerve crush and then measured the mRNA and protein expression levels of MAP tau in the sciatic nerve segments by performing real-time quantitative RT-PCR, western blot, and immunohistochemical labeling assays. Our results indicated that the expression level of the mRNA encoding tau was initially decreased following peripheral nerve injury, but then gradually increased. The tau protein expression level was also mildly decreased immediately after injury, but then later increased. The expression of the phosphorylated form of tau protein, phospho-tau (Ser 404), decreased to a level lower than that for the non-phosphorylated form 1 day post nerve injury.
Tau protein, a major MAP in the nervous system, interacts with tubulin to maintain microtubule structure stability (Gorath et al., 2001; Iqbal et al., 2009; Medina and Avila, 2015). Our results showed that, following sciatic nerve injury, expression levels of both tau mRNA and protein were first decreased and then increased. However, the expression patterns of MAPT and total tau protein were not wholly consistent with these results, suggesting the involvement of post-transcriptional modifications. The expression pattern of total tau protein was not exactly the same as that of phosphorylated tau (phospho-tau, Ser 404), indicating that the post-translational modification of tau may be critical for nerve repair and regeneration.
The phosphorylation state of tau protein is closely related to neurodegenerative diseases (Brelstaff et al., 2015). Normal tau protein phosphorylation prevents excessive assembly and maintains the stability of the microtubule structure, whereas aberrant hyper-phosphorylation of tau protein may lead to abnormal aggregation and may impair the nervous system (Iqbal et al., 2005, 2009; Kumar et al., 2015). Hyper-phosphorylation of tau may also lead to abnormal microtubule self-assembly. Kinases that phosphorylate tau protein may affect the phosphorylation state of tau and further affect the assembly and stability of microtubules. TTBK1 is a serine/ threonine kinase that regulates tau phosphorylation. TTBK1 phosphorylates tau on serine, threonine, and tyrosine residues and thus induces tau aggregation (Sato et al., 2006; Liachko et al., 2014). In addition, the microenvironment may induce neuronal degeneration by altering the level of tau phosphorylation during neuronal development (Bihaqi et al., 2014). Our present results showed that during peripheral nerve regeneration, the abundance of not only total tau but also of phosoporylated tau was altered. In the early period following sciatic nerve injury, decreased total tau and phospho-tau (Ser 404) protein expression may have induced microtubule disassembly and remodeling and subsequent nerve regeneration. Nevertheless, increased total tau and phospho-tau (Ser 404) protein expression may also prevent immoderate microtubule disassembly and help maintain the structure and stability of the cytoskeleton. To further identify the specific roles of tau expression and phosphorylation in peripheral nerve regeneration in our future experiments, we plan to treat rats undergoing sciatic nerve crush with either exogenous tau protein or an antibody against tau protein to determine whether different amounts of tau affect the speed of regeneration and the outcomes of injured peripheral nerves.
The phosphorylation states of tau protein are regulated by a series of protein kinases and phosphatases, such as glycogen synthase kinase 3 beta (GSK-3β), cyclin-dependent kinase 5, protein kinase A, Ca2+/calmodulin-dependent protein kinase II, dual specificity tyrosine-phosphorylation-regulated kinase 1A, and protein phosphatase 1, 2A, 2B, and 5 (Qian et al., 2011; Jin et al., 2015; Sun et al., 2015). The various phosphorylation sites are dependent on specific protein kinases (Yu et al., 2014). Among the numerous phosphorylation sites, the phosphorylation of tau protein at Ser 404 is mediated by the activation of GSK-3β (Annamalai et al., 2015). Therefore, the GSK-3β signaling pathway may be important for tau-mediated peripheral nerve regeneration. Future studies will be performed to determine key kinases and phosphatases of tau phoshorylation post peripheral nerve injury. Additionally, in our future studies, we plan to treat rats undergoing sciatic nerve crush with protein kinases and phosphatases to increase and reduce tau phosphorylation, respectively, and observe the effect of tau phosphorylation.
Taken together, our present results suggest that the expression and phosphorylation of tau may be involved in peripheral nerve repair, and that modulating the phosphorylated form of tau protein may contribute to peripheral nerve regeneration. Our study helps to elucidate the importance of tau protein during peripheral nerve regeneration, and may benefit the identification of novel therapeutic targets for the treatment of peripheral nerve injury.
Author contributions: GBZ, XSG and SY participated in study conception and design. GBZ, MS and SY were in charge of data collection, analysis and interpretation, and wrote the paper. XSG and SY participated in statistical expertise, obtained funding, provided administrative, technical or material support, and supervision. All authors approved the final version of the paper.
Conflicts of interest: None declared.
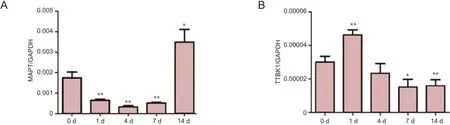
Figure 1 mRNA expression of MAPT and TTBK1 decrease following sciatic nerve injury.
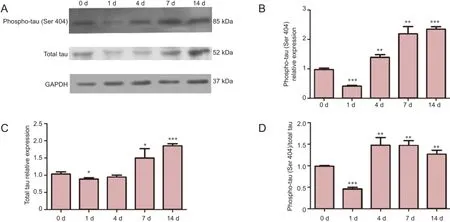
Figure 2 Changes in protein expression of phosphorylated tau (phospho-tau Ser 404) and total tau following sciatic nerve injury.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Ando K, Maruko-Otake A, Ohtake Y, Hayashishita M, Sekiya M, Iijima KM (2016) Stabilization of microtubule-unbound tau via tau phosphorylation at ser262/356 by Par-1/mark contributes to augmentation of AD-related phosphorylation and Aβ42-induced tau toxicity. PLoS Genet 12:e1005917.
Annamalai B, Won JS, Choi S, Singh I, Singh AK (2015) Role of S-nitrosoglutathione mediated mechanisms in tau hyper-phosphorylation. Biochem Biophys Res Commun 458:214-219.
Bihaqi SW, Bahmani A, Adem A, Zawia NH (2014) Infantile postnatal exposure to lead (Pb) enhances tau expression in the cerebral cortex of aged mice: relevance to AD. Neurotoxicology 0:114-120.
Black MM, Kurdyla JT (1983) Microtubule-associated proteins of neurons. J Cell Biol 97:1020-1028.
Brelstaff J, Spillantini MG, Tolkovsky AM (2015) pFTAA: a high affinity oligothiophene probe that detects filamentous tau in vivo and in cultured neurons. Neural Regen Res 10:1746-1747.
Chambers CB, Muma NA (1997) Tau mRNA isoforms following sciatic nerve axotomy with and without regeneration. Mol Brain Res 48:115-124.
Dai CL, Chen X, Kazim SF, Liu F, Gong CX, Grundke-Iqbal I, Iqbal K (2015) Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse model of Alzheimer disease and tauopathies. J Neural Transm 122:607-617.

Figure 3 Localization of total tau and phosphorylated tau (phospho-tau, Ser 404) following sciatic nerve injury.
Gorath M, Stahnke T, Mronga T, Goldbaum O, Richter-Landsberg C (2001) Developmental changes of tau protein and mRNA in cultured rat brain oligodendrocytes. Glia 36:89-101.
Gupta N, Fong J, Ang LC, Yücel YH (2008) Retinal tau pathology in human glaucomas. Can J Ophthalmol 43:53-60.
Iqbal K, Liu F, Gong CX, Alonso AdC, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118:53-69.
Iqbal K, del C. Alonso A, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739:198-210.
Jin N, Yin X, Yu D, Cao M, Gong CX, Iqbal K, Ding F, Gu X, Liu F (2015) Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci Rep 5:8187.
K?vesdi E, Lückl J, Bukovics P, Farkas O, Pál J, Czeiter E, Szellár D, Dóczi T, Komoly S, Büki A (2010) Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir (Wien) 152:1-17.
Kim Y, Choi H, Lee W, Park H, Kam TI, Hong SH, Nah J, Jung S, Shin B, Lee H, Choi TY, Choo H, Kim KK, Choi SY, Kayed R, Jung YK (2016) Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol Dis 87:19-28.
Kumar P, Jha NK, Jha SK, Ramani K, Ambasta RK (2015) Tau phosphorylation, molecular chaperones, and ubiquitin E3 ligase: clinical relevance in Alzheimer’s disease. J Alzheimers Dis 43:341-361.
Liachko NF, McMillan PJ, Strovas TJ, Loomis E, Greenup L, Murrell JR, Ghetti B, Raskind MA, Montine TJ, Bird TD, Leverenz JB, Kraemer BC (2014) The tau tubulin kinases TTBK1/2 promote accumulation of pathological TDP-43. PLoS Genet 10:e1004803.
Liao CW, Fan CK, Kao TC, Ji DD, Su KE, Lin YH, Cho WL (2008) Brain injury-associated biomarkers of TGF-beta1, S100B, GFAP, NF-L, tTG, AbetaPP, and tau were concomitantly enhanced and the UPS was impaired during acute brain injury caused by Toxocara canis in mice. BMC Infect Dis 8:84.
Medina M, Avila J (2015) Further understanding of tau phosphorylation: implications for therapy. Expert Rev Neurother 15:115-122.
Nunez J, Fischer I (1997) Microtubule-associated proteins (MAPs) in the peripheral nervous system during development and regeneration. J Mol Neurosci 8:207-222.
Pedersen JT, Sigurdsson EM (2015) Tau immunotherapy for Alzheimer’s disease. Trends Mol Med 21:394-402.
Qian W, Yin X, Hu W, Shi J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX, Liu F (2011) Activation of protein phosphatase 2B and hyperphosphorylation of Tau in Alzheimer’s disease. J Alzheimer’s Dis 23:617-627.
Rodríguez-Martín T, Cuchillo-Ibá?ez I, Noble W, Nyenya F, Anderton BH, Hanger DP (2013) Tau phosphorylation affects its axonal transport and degradation. Neurobiol Aging 34:2146-2157.
Sato S, Cerny RL, Buescher JL, Ikezu T (2006) Tau-tubulin kinase 1 (TTBK1), a neuron-specific tau kinase candidate, is involved in tau phosphorylation and aggregation. J Neurochem 98:1573-1584.
Shen YX, Xiao K, Liang P, Ma YW, Huang X (2013) Improvement on the modified Lowry method against interference of divalent cations in soluble protein measurement. Appl Microbiol Biotechnol 97:4167-4178.
Sun LH, Ban T, Liu CD, Chen QX, Wang X, Yan ML, Hu XL, Su XL, Bao YN, Sun LL, Zhao LJ, Pei SC, Jiang XM, Zong DK, Ai J (2015) Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation. J Neurochem 134:1139-1151.
Wu G, Zhu L, Jin T, Chen L, Shao L, Wang Y, Liu B (2010) Local delivery of recombinant human bone morphogenetic protein-2 increases axonal regeneration and the expression of tau protein after facial nerve injury. J Int Med Res 38:1682-1688.
Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou S, Gu X, Yu B (2015) Deep Sequencing and Bioinformatic Analysis of Lesioned Sciatic Nerves after Crush Injury. PLoS One 10:e0143491.
Yin HS, Chou HC, Chiu MM (1995) Changes in the microtubule proteins in the developing and transected spinal cords of the bullforg tadpole: Induction of microtubule-associated protein 2c and enhanced levels of Tau and tubulin in regenerating central axons. Neuroscience 67:763-775.
Yu UY, Yoo BC, Ahn JH (2014) Regulatory B subunits of protein phosphatase 2A are involved in site-specific regulation of tau protein phosphorylation. Korean J Physiol Pharmacol 18:155-161.
Copyedited by Smith T, Raye W, Yu J, Qiu Y, Li CH, Song LP, and Zhao M
10.4103/1673-5374.191227
*Correspondence to:
- 中國神經(jīng)再生研究(英文版)的其它文章
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
- Stem Cell Ophthalmology Treatment Study (SCOTS):improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment
- Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
- Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease
- Can long-term thiamine treatment improve the clinical outcomes of myotonic dystrophy type 1?