Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease
Jian-ming Li, Yan Zhang Liang Tang Yong-heng Chen Qian Gao Mei-hua Bao Ju Xiang De-liang Lei1 Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan Province, China2 Department of Anatomy and Neurobiology, Central South University, School of Basic Medical Science, Changsha, Hunan Province, China Neuroscience Research Center, Changsha Medical University, Changsha, Hunan Province, China Department of Anatomy, Changsha Medical University, Changsha, Hunan Province, China
Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease
Jian-ming Li1,2,3,4, Yan Zhang3, Liang Tang3, Yong-heng Chen3, Qian Gao3, Mei-hua Bao3, Ju Xiang3, De-liang Lei2,*
1 Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan Province, China
2 Department of Anatomy and Neurobiology, Central South University, School of Basic Medical Science, Changsha, Hunan Province, China
3 Neuroscience Research Center, Changsha Medical University, Changsha, Hunan Province, China
4 Department of Anatomy, Changsha Medical University, Changsha, Hunan Province, China
How to cite this article: Li JM, Zhang Y, Tang L, Chen YH, Gao Q, Bao MH, Xiang J, Lei DL (2016) Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neural Regen Res 11(9):1492-1498.
Funding: This study was supported by China Postdoctoral Science Foundation, No. 2016M590757; the Postdoctoral Science Foundation of Xiangya Hospital of Central South University of China, No. 20; the Hunan Provincial Natural Science Foundation of China, No. 2015JJ6010; a grant from the Basic Research Program of Science and Technology Commission Foundation of Hunan Province of China, No. 2015JC3059; the Project Fund of the Department of Education in Hunan Province of China, No. 15A023, 13C1107; the Scientific Research Project Fund of Health Department of Hunan Province of China, No. B2011-071, B2016096; a grant from the Construction Program of the Key Discipline in Hunan Province of China.
De-liang Lei, Ph.D.,
delianglei@csu.edu.cn.
orcid:
0000-0002-1037-7296
(De-liang Lei)
Accepted: 2016-08-16
Graphical Abstract
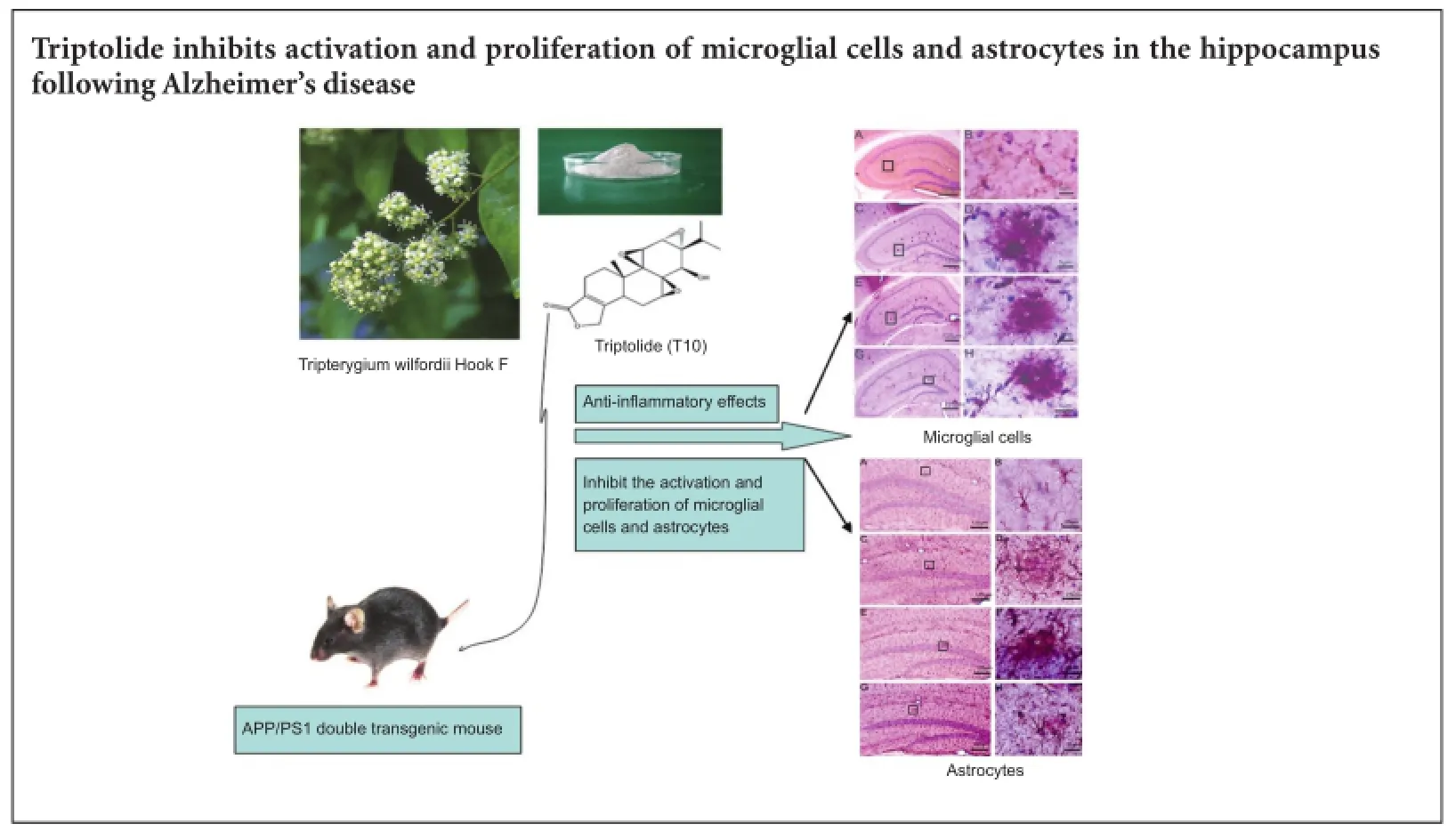
The principal pathology of Alzheimer’s disease includes neuronal extracellular deposition of amyloid-beta peptides and formation of senile pl aques, which in turn induce neuroinflammation in the brain. Triptolide, a natural extract from the vine-like herb Tripterygium wilfordii Hook F, has potent anti-inflammatory and immunosuppressive efficacy. Therefore, we determined if triptolide can inhibit activation and proliferation of microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. We used 1 or 5 μg/kg/d triptolide to treat APP/PS1 double transgenic mice (aged 4—4.5 months) for 45 days. Unbiased stereology analysis found that triptolide dose-dependently reduced the total number of microglial cells, and transformed microglial cells into the resting state. Further, triptolide (5 μg/kg/d) also reduced the total number of hippocampal astrocytes. Our in vivo test results indicate that triptolide suppresses activation and proliferation of microglial cells and astrocytes in the hippocampus of APP/PS1 double transgenic mice with Alzheimer’s disease.
nerve regeneration; neurodegenerative disease; traditional Chinese medicine; Tripterygium wilfordii Hook F; triptolide; Alzheimer’s disease; amyloid plaques; amyloid-β; amyloid precursor protein; inflammation; microglia; astrocytes; neural regeneration
Introduction
The pathological changes seen in Alzheimer’s disease (AD) brain reflect a series of chronic inflammatory processes. The chronic inflammatory response is characterized by the presence of abundant activated microglia. Moreover, reactive astrocyte proliferation is associated with deposition of amyloid-beta (Aβ), formation of amyloid plaques (senile plaques) and neurofibrillary tangles (Gil-Bea et al., 2012), and increased expression of proinflammatory cytokines and complement components (Salminen et al., 2009; Villegas-Llerena et al., 2016). Non-steroidal anti-inflammatory drugs not only reduce the risk of AD, but also delay AD progression (Pasinetti, 2002; Mcgeer and Mcgeer, 2007). Animal studies have shown that non-steroidal anti-inflammatory drugs suppress Aβ deposition and senile plaque formation, inhibit microglial activation and reactive astrocyte proliferation, and decrease expression and secretion of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β) in AD brain (Weggen et al., 2001; Smith et al., 2002). Therefore, the anti-inflammatory effects of these drugs appear to play an important role in AD. Nonetheless, there are serious and poisonous side effects associated with their use (Vane 2000), which limits their clinical application.
Triptolide (T10) compound is an active component derived from a traditional Chinese medicine extract, Tripterygium wilfordii Hook F. It possesses anti-inflammatory and immunosuppressive functionality, and has been used to treat rheumatism, rheumatism arthritis, systematic lupus erythematosus, and other autoimmune and inflammatory responsive diseases (Han et al., 2012; Ho et al., 2013; Huang et al., 2013; Peng et al., 2014). Previous in vitro research has suggested that the anti-inflammatory and immunosuppressive effects of T10 are due to suppression of expression and release of pro-inflammatory cytokines (such as IL-1β and TNF-α), as well as suppression of inducible nitric oxide synthase and cyclooxygenase-2 induction (Dai et al, 2005; Tong et al., 2007; Zhou et al., 2007; Tang et al., 2012; Wu et al., 2013; Cui et al., 2016). T10 has also been shown to have a neurotrophic function, and protects mesencephalic dopaminergic neurons from lipopolysaccharide injury (Li et al., 2004; Zhou et al., 2005). The predicted neuronal protective mechanism of T10 involves inhibition of microglial activation and suppression of the inflammatory reaction (Li et al., 2004; Zhou et al., 2005). To date, there are no reports on the effect of T10 on the inflammatory and immune cascade of AD brain. Accordingly, in the present study, we are the first to use T10 to treat the APP/PS1 double transgenic mouse model of AD (APP/PS1 dtg). Our study is preliminary, to assess the effect of T10 on APP/PS1 dtg mice. We also discuss the possibility and putative mechanism for T10 to treat or prevent AD.
Materials and Methods
Animals
Eighteen male dtg APP/PS1 mice and five C57BL/6JNNIA (B6) male wild-type control mice aged 4—4.5 months and weighing 25—30 g were obtained from the Animal Center of Xiangya School of Medicine, China (animal license No: SYXK (Xiang) 2014-0023). Two to five mice were housed in plastic cages with corncob bedding, and free access to food and filtered water in a vivarium maintained on a 12-hour light/dark cycle at 22.0 ± 0.5°C.
Animal use was in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Ethics Committee of Central South University Xiangya School of Medicine, China, for animal care and use [Ethic Committee (EC) number: EC/11/018, 6/15/2011].
Treatments
APP/PS1 dtg mice were randomly divided into three groups: high-dose T10 treatment (purity: 99%, Sigma, St. Louis, MO, USA) (T10-H group, n = 6, 5 μg/kg/d, intraperitoneal injection), low-dose T10 treatment (T10-L group, n = 6, 1 μg/kg/d, intraperitoneally), or placebo (PLC group) (intraperitoneal injection of an equivalent volume of normal saline). Triptolide was dissolved in dimethyl sulfoxide as 100 × stock solution and diluted with normal saline for the concentration used. Treatments were administered at approximately 10:00 a.m. every day for 45 days. Five wild-type male mice received no treatment and were used as controls.
Tissue preparation
After 45 days of treatment, mice were deeply anesthetized by CO2inhalation, and transcardially perfused with 0.1 M phosphate-buffered saline (PBS), followed by standard fixation using 4% paraformaldehyde in 0.1 M PBS (pH 7.4). After removal, the brain was post-fixed in 4% paraformaldehyde overnight. Next, brains were transferred to 30% sucrose in 0.1 M PBS until they sank, then frozen in dry ice/ isopentane, and stored at -80°C until sectioning. Each brain was serially sectioned along the coronal plane using a sliding freezing microtome (Walldorf, Germany). Sections were cut using an instrument setting of 50 μm and sampled in a systematic-random manner, i.e., the first five sections were randomly selected, and then every fifth section systematically cut.
Immunohistochemistry
For microglia visualization, systematic-random sections through the hippocampus (10—12 sections per brain), were collected in 12-well plates and washed in 0.1 M PBS. Sections were then incubated in 1% hydrogen peroxide for 30 minutes at room temperature, washed again in 0.1 M PBS, and incubated in 0.3% Triton X-100 for 10 minutes at room temperature. Sections were washed in 0.1 M PBS and transferred to 5% normal goat serum in 0.1 M PBS for 30 minutes at room temperature to block non-specific binding. Sections were not subjected to antigen retrieval, but incubated overnight in rat anti-mouse monoclonal antibody to mouse macrophage antigen-1 (Mac-1) (1:3,000 in 2% normal goat serum and 0.3% Triton-X-100 in PBS; CR3 receptor-CD11b;Serotec, Washington, DC, USA) at 4°C. Afterwards, sections were washed in 0.1 M PBS and incubated in biotinylated secondary goat anti-rat antibody (1:200 in normal goat serum and 0.1 M PBS; Vector Laboratories, Burlingame, CA, USA) for 90 minutes at room temperature. Sections were washed in 0.1 M PBS and re-incubated for another 90 minutes at room temperature in ABC solution from the Vectorstain Kit (Vector Laboratories, Burlingame, CA, USA). Sections were rinsed in 0.1 M PBS and reacted using 3,3′-diaminobenzidine (10 mg 3,3′-diaminobenzidine, 0.1 M PBS, 40 mL) for 6—10 minutes. On an adjacent systematic-random set of sections, astrocytes were immunostained using rabbit anti-bovine glial fibrillary acidic protein (GFAP) (1:15,000; Dako, Carpenteria, CA, USA), with goat anti-rabbit biotinylated IgG against cow GFAP as the secondary antibody. Sections were mounted on slides and dried overnight. For counterstaining, sections were dehydrated through a graded alcohol series, followed by a dH2O rinse for 1 minute. Cresyl violet (Sigma) counterstaining of basophilic structures was applied for 2 minutes, followed by color adjustment in 5% acetic acid, with a final rinse in dH2O. Sections were rehydrated using a reverse ethanol series, rinsed twice in xylene for 10 minutes each, and then coverslipped with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI, USA) (O’Neil et al. 2007).
Stereological analysis
Modern stereological methods were used to determine stereological parameters on an adjacent series of randomly sampled sections through the entire hippocampus using the Stereologer computerized stereology system (Systems Planning and Analysis, Inc., Alexandria, VA, USA). The system hardware consisted of a X-Y-Z motorized stage, color video camera interfaced to a Zeiss Axioskop(Oberkochen, Germany), high-resolution video card, and a personal computer and monitor. The stereology software allowed an outline of the region of interest (hippocampus) to be mapped while viewing the section under low magnification (4×). In addition, an estimate of the total number of immunopositive microglial cells and astrocytes was obtained using optical fractionator methods under a high magnification objective lens (60× oil with 1.4 numerical aperture) (Long et al., 1998; Mouton, 2002). Every fifth section was selected from the total number of hippocampal sections in a systematic-random manner, generating 10—12 sections per reference space. The sampling fractions included: (i) section sampling fraction (ssf): number of sections sampled divided by total number of sections for each hippocampus; (ii) area sampling fraction (asf): area of the sampling frame divided by the area of the x-y sampling step; and (iii) thickness sampling fraction (tsf): height of the dissector divided by the section thickness. A guard volume of 2.0 mm was used during cell counting to avoid sectioning artifacts (including lost caps and uneven section surfaces). Here, the sampling parameters for microglia and astrocyte counting were: (1) ssf = 1/5; (2) asf = 0.01040 {a(frame) = 0.0049 mm2]/ {[a(step) = 0.0047089 mm2]}; and (3) tsh = 0.6936 (height of the dissector (12 μm)/average post-processing section thickness (17.3 μm). N = ΣQ-·1/ssf·1/asf·1/tsf, where N is total number estimated and ΣQ- is cell number counted.
Statistical analysis
Data are expressed as the mean ± SD, and analyzed with SPSS 13.0 software (SPSS, Chicago, IL, USA). Statistical analysis was performed by one-way analysis of variance with post hoc evaluation using Scheffe’s F test. Statistical significance was set at P < 0.05.
Results
Effect of T10 on microglial cells
Immunoreactive Mac-1 microglia were recognized as small, deeply basophilic, oval-to-round shaped cell bodies, approximately 5—7 μm in diameter, with a clear halo around the cell body and immunoreactive processes that extended into the surrounding parenchyma. Immunohistochemical staining showed that Mac-1-positive microglial cells in the hippocampus of wild-type mice were characterized by small cell bodies with slender processes and multi-branched shapes. This pattern is indicative of microglial cells in the resting state. Distribution of microglial cells in the PLC, T10-L, and T10-H groups was different from wild-type mice. These three groups of mice showed clusters of microglial cells around Aβ deposits and senile plaques. The microglia had large cell bodies, short and thick processes, and some had no processes, a phenotype known as the amoeboid shape (Figure 1). These features indicate the microglial cells are in the activated state. Comparing all three groups, there were more amoeboid-shaped microglial cells in the PLC group, and more microglia with processes in the T10-H group, which tended to change into the resting state.
Quantitative analysis found that the total number of hippocampal microglial cells decreased by 30% (P < 0.01) and 18% (P < 0.05) in the T10-H and T10-L groups, respectively, compared with the PLC group. Further, the T10-H group showed a 15% reduction in total microglia number compared with the T10-L group (P < 0.05; Table 1). These data indicate that T10 reduces the total number of microglial cells and inhibits microglial proliferation.
Effect of T10 on astrocytes
GFAP is a specific marker for labeling the intermediate filament protein of astrocytes. GFAP-immunoreactive cells show typical morphological features of fibrous astrocytes. The cell bodies have variable shapes with processes that project into the parenchyma. GFAP-positive astrocytes in the hippocampus of wild-type mice were evenly scattered with small cell bodies and slender processes, indicating these astrocytes are in the static state. In the PLC, T10-L, and T10-H groups, astrocyte distribution was similar to microglial cells, and showed that activated astrocytes were distributed in senile plaque clusters with large cell bodies and thick processes (Figure 2). Compared with the PLC group, astrocytes in the T10-H group had more thin processes, which tended to change into the resting state (Figure 2).
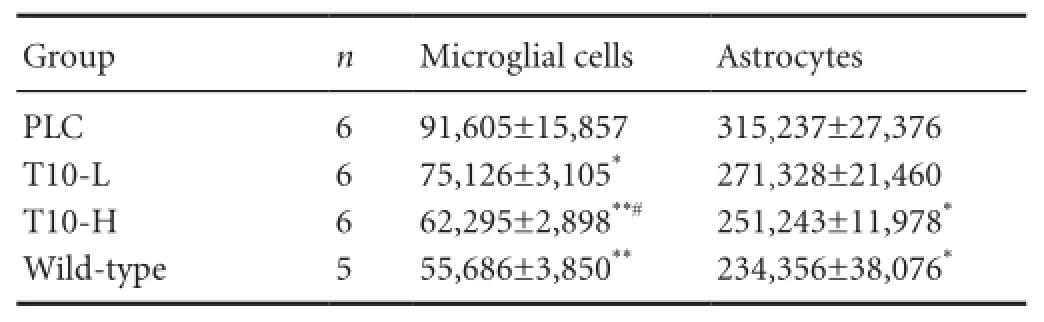
Table 1 Effect of T10 on total microglial cells and astrocyte number in the hippocampus
Quantitative analysis found that the total number of hippocampal astrocytes decreased by 20% (P < 0.05) and 13% (P > 0.05) in the T10-H and T10-L groups, respectively, compared with the PLC group. No significant difference was detected in total astrocyte number in the hippocampus between the T10-H group and wild-type mice (Table 1).
Discussion
Here, we show that T10 treatment reduces the total number of microglial cells, and inhibits microglial activation and proliferation in a dose-dependent manner. Moreover, highdose T10 treatment also reduces the total number of astrocytes, and inhibits astrocyte activation.
T10 inhibited activation and proliferation of microglial cells in the hippocampus of APP/PS1 dtg mice
Microglial cells are the macrophages of the central nervous system, and play an important role in the inflammatory and immune response in AD brain. In this study, 6-monthold APP/PS1 dtg mice showed activated microglial cells that clustered around Aβ plaque deposits and senile plaques in the hippocampus. These cells showed marked activation, including morphological characteristics of an activated state (e.g., large cell bodies, and short or no processes). Our results are consistent with other studies on transgenic mouse models of AD (Wright et al., 2013; Brendel et al., 2016). Further, we show that after 45 days of T10 treatment in 4.5-month-old APP/PS1 dtg mice, total microglial cells number in the hippocampus was reduced. Additionally, we observed that microglia tended to change to a resting state, suggesting that T10 inhibits microglial activation and proliferation in the hippocampus of the APP/PS1dtg mouse model of AD.
The effect of T10 and other components of the Chinese herb Tripterygium wilfordii on microglial cells have previously been reported. In an earlier study, lipopolysaccharide (LPS) was injected into the CA3 area of the hippocampus in rats that had been pretreated with T10 for 3 days. Observations and analysis were performed 24 hours later, and showed that T10 dose-dependently suppressed activation and proliferation of microglial cells (Dai et al., 2006). Moreover, longterm T10 administration was also shown to suppress microglial activation and improve retinal ganglion cell survival in DBA/2J mice (Yang et al., 2013). The mechanism of this protection is associated with suppression of activation and proliferation of microglial cells, together with reduction of IL-1β and TNF-α production (Zhou et al., 2005).
During the pathological process of AD, Aβ deposition and senile plaque formation from fibrotic Aβ attracts and activates microglial cells, thereby resulting in chronic inflammation (Alarcón et al., 2005; Jiang et al., 2014). In chronic inflammation in AD brain, activated microglial cells produce several proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and complements such as C1q (Alam et al., 2016). These components may act as a trigger for an inflammatory reaction in the brain, and accelerate microglial and astrocyte proliferation, together with Aβ deposition and senile plaque formation, leading to a vicious cycle. Activated microglial cells can also produce glutamate (Huang et al., 2011) and N-methyl-D-aspartate receptor 1 (Zheng et al., 2010), both of which are excitatory neurotoxins. These excitatory neurotoxins do not cause complete cell death, but may damage neural dendrites, resulting in synaptic loss, which may be associated with AD-related cognitive dysfunction. Altogether, based on these previous studies and our findings, we believe that T10 exerts its protective effect against AD by suppressing activation and proliferation of microglial cells, and reducing proinflammatory cytokine production related to activation of microglial cells.
Activated microglial cells function as phagocytic cells, which has been confirmed by in vivo and vitro experiments (Pan et al., 2011; Zhang et al., 2014). Microglial cells can engulf Aβ and digest it using scavenger receptors and advanced glycation end product receptors on the cell surface, to combine Aβ with the cell. Taken together, T10 inhibition of activated microglial cells may also suppress phagocytosis and clearance of Aβ. However, microglial phagocytosis of Aβ may launch a neurocytopathic state. Indeed, after engulfment of some foreign bodies, peripheral macrophages release certain cytotoxins that sharply increase respiratory rate in cells. Further, after the foreign body is phagocytosed, microglial cells release reactive oxygen species (Wang et al., 2013) such as TNF-α. All of these components damage nerve cells and have a close relationship with deprivation of AD.
T10 inhibited activation and proliferation of astrocytes in the hippocampus of APP/PS1 dtg mice
Our findings in the hippocampus of 6-month-old APP/PS1 dtg mice show that astrocytes are arranged around Aβ deposits and senile plaques. A similar phenomenon has been observed in other studies (Furman et al., 2012; Vromman et al. 2013; Miklossy et al., 2016). After intraperitoneal T10 injection for 45 days, activation and proliferation of astrocytes are suppressed, and total astrocyte number reduced. In our previous study in rats, we used T10 pretreatment for 3 days, and subsequently injected LPS into the hippocampus. We found that T10 inhibits astrocyte activation (Dai et al., 2005, 2006). These results suggest that T10 inhibits differentpathological conditions caused by activation of astrocytes.
Astrocytes play crucial roles in the maintenance of neuronal structure and function. As such, they respond actively to brain injury caused by trauma, stroke, or neuronal degeneration (Wilhelmsson et al., 2006; Chvatal et al., 2007; Dong et al., 2013; Johann et al., 2013; Wagner et al., 2013; Wajima et al., 2013; Torrente et al., 2014). Astrocytes that respond to injury in vivo, increase in size, and increase expression of the astrocyte specific intermediate filament protein, GFAP, i.e., become hypertrophic. These astrocytes are described as reactive and/or activated (Myer et al., 2006).
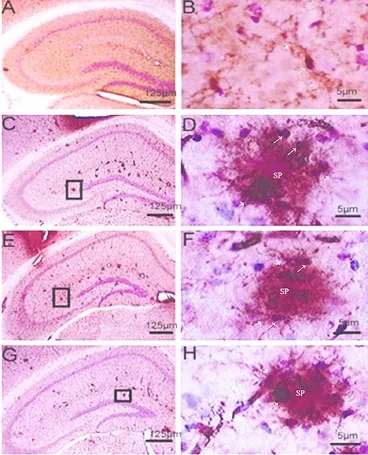
Figure 1 Effect of T10 on microglial cells in the hippocampus of APP/ PS1 double transgenic mice (immunohistochemical staining).
The hallmark of AD pathology is the presence of an increased number of reactive astrocytes near senile plaques composed of Aβ aggregates. During AD progression, astrocytes undergo both morphological and functional changes, giving rise to the term “reactive gliosis”. The function of astrocytes in the pathological process of AD is still uncertain. Further research is needed on the advantages and disadvantages of T10 suppression on astrocytes in the brain of APP/ PS1 dtg mice. Activated astrocytes are thought to provide support for damaged neural tissue through several mechanisms, including release of neurotrophic factors and degradation of amyloid deposits (Schubert et al., 2001; Burbach et al., 2004; Pihlaja et al., 2011; Thal et al. 2012; Lee et al., 2013; Wakabayashi et al., 2013; Scardigli et al., 2014; Yamamoto et al., 2014).
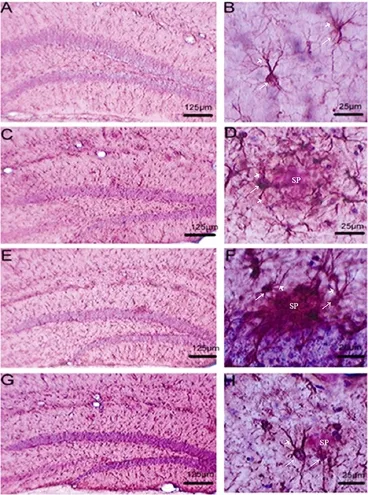
Figure 2 Effect of T10 on astrocytes in the hippocampus of APP/PS1 double transgenic mice (immunohistochemical staining).
Astrocyte activation forms a glial scar that envelopes Aβ deposits and senile plaques, forming a barrier between senile plaques and nerve cells, and preventing a toxic effect by protecting nerve cells from Aβ. As a suppressor of astrocyte activation, T10 may weaken this protective effect. However, Schubert et al. (2001) verified that reactive astrocytes contribute to thepathomechanisms underlying AD by favoring oxidative neuronal damage. Reactive astrocytes may promote transformation of Aβ into toxic forms (Abramov et al., 2004). Activated astrocytes produce several proinflammatory cytokines (e.g., IL-1) (Cekanaviciute et al., 2014; Deng et al., 2014), complement, other components, and S100β. These components accelerate not only activation of microglial cells and astrocytes, but production of proinflammatory cytokines. Such acceleration will aggravate inflammation and may even accelerate Aβ deposition and senile plaque formation. Overall, astrocyte activation causes several insalubrious effects. By suppressing astrocyte activation, T10 may reduce the damaging effect caused by activation.
In conclusion, the suppressive effect of T10 on activation and proliferation of glial cells is a double-edged sword. It can help relieve brain inflammation in the transgenic mouse model of AD and AD patients. Although the inflammatory reaction accelerates Aβ deposition and senile plaque formation, as T10 reduces Aβ deposition and senile plaque formation, this may be attributable to its suppressive effect on glial cells.
Author contributions: JML designed the project, performed experiments and wrote the paper. DLL directed the research and revised the paper writing. YZ, LT, YHC, QG, MHB, and JX were responsible for implementation of the experiment. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Abramov AY, Canevari L, Duchen MR (2004) Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742:81-87.
Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M (2016) Inflammatory process in alzheimer’s and parkinson’s diseases: central role of cytokines. Curr Pharm Des 22:541-548.
Alarcón R, Fuenzalida C, Santibá?ez M, Von BR (2005) Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem 280:30406-30415.
Brendel M, Probst F, Jaworska A, Overhoff F, Korzhova V, Albert NL, Beck R, Lindner S, Gildehaus FJ, Baumann K, Bartenstein P, Kleinberger G, Haass C, Herms J and Rominger A (2016) Glial activation and glucose metabolism in a transgenic amyloid mouse model: a triple tracer pet study. J Nucl Med 57:954-960.
Burbach GJ, Hellweg R, Haas CA, Turco DD, Deicke U, Abramowski D, Jucker M, Staufenbieland M, Deller T (2004) Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J Neurosci 24:2421-2430.
Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, gusquiza RE, Wai KM, Koshy AA and Buckwalter MS (2014) Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system toxoplasma infection. J Immunol 23:1303284.
Chvatal A, Anderova M, Kirchhoff F (2007) Three-dimensional confocal morphometry-a new approach for studying dynamic changes in cell morphology in brain slices. J Anat 210:671-683.
Cui YQ, Wang Q, Zhang DM, Wang JY, Xiao B, Zheng Y and Wang XM (2016) Triptolide rescues spatial memory deficits and amyloid-β aggregation accompanied by inhibition of inflammatory responses and MAPKs activity in APP/PS1 transgenic mice. Curr Alzheimer Res 13:288-296.
Dai YQ, Jin DZ, Zhu XZ, Lei DL (2005) Triptolide inhibits COX-2 expression via NF-kappa B pathway in astrocytes. Neurosci Res 55:154-160.
Dai YQ, Jin DZ, Wang C, Liu Z, Lei DL, Li M (2006) The inhibitory effects of triptolide on activation of astrocytes and microglia in neuroinflammation induced by LPS. Shenjing Jiepouxue Zazhi 22:648-652.
Deng YY, Xie D, Fang M, Zhu GF, Chen CB, Zeng HK, Lu J, CJ Kaur (2014) Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS One 9:e87420.
Dong QP, He JQ, Chai Z (2013) Astrocytic Ca(2+) waves mediate activation of extrasynaptic NMDA receptors in hippocampal neurons to aggravate brain damage during ischemia. Neurobiol Dis 58:68-75.
Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Eldik LJV, Norris CM (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci 32:16129-16140.
Gil-Beaa FJ, Gerenub G, Aisa B, Kirazovc LP, Schliebsd R, Ramírez MJ (2012) Cholinergic denervation exacerbates amyloid pathology and induces hippocampal atrophy in Tg2576 mice. Neurobiol Dis 48:439-446.
Han R, Rostami-Yazdi M, Gerdes S and Mrowietz U (2012) Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol 74:424-436.
Ho LJ, Chang WL, Chen A, Chao P, Lai JH (2013) Differential immunomodulatory effects by Tripterygium wilfordii Hook f-derived refined extract PG27 and its purified component PG490 (triptolide) in human peripheral blood T cells: potential therapeutics for arthritis and possible mechanisms explaining in part Chinese herbal theory“Junn-Chenn-Zuou-SS”. J Transl Med 21:294.
Huang YL, Zhao LX, Jia BB, Li YJ, Curthoys N and Zheng JC (2011) Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci 31:15195-15204.
Jiang T, Guo W, Sha S, Xing X, Guo R, Cao Y (2014) Nasal mucosal inhalation of amyloid-beta peptide 3-10 defective adenovirus attenuates cytotoxicity induced by beta-amyloid (1-42). Neural Regen Res 9:872-877.
Johann S, Beyer C (2013) Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol 137:71-81.
Mcgeer PL, Mcgeer EG (2007) NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studie. Neurobiol Aging 28:639-647.
Miklossy J, McGeer PL (2016) Common mechanisms involved in Alzheimer’s disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging (Albany NY) 8:575-888.
Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761-2772.
O’Neil J, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, Manayev KF (2007) Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat 34:102-107.
Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP, Chen XC (2011) Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer’s disease. Mol Neurodegener 6:45.
Pasinetti GM (2002) Cyclooxygenase as a target for the antiamyloidogenic activities of nonsteroidal anti-inflammatory drugs in Alzheimer’s disease. Neurosignals 11:293-297.
Peng A, Huang X, Liu R, Wang X, Zhuang J (2014) Triptolide inhibits the inflammatory response of monocytes from rheumatoid arthritis patients by regulating miR-155. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 30:635-638.
Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M (2011) Multiple cellular and molecular mechanisms are involved in human Aβ clearance by transplanted adult astrocytes. Glia 59:1643-1657.
Salminen A, Ojala J, Kauppinen A, Kai K, Suuronen T (2009) Inlammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recogni-tion receptors. Prog Neurobiol 87:181-194.
Scardigli R, Capelli P, Vignone D, Brandi R, Ceci M, Regina FL, Piras E, Cintoli S, Berardi N, Capsoni S and Cattaneo A (2014) Neutralization of nerve growth factor impairs proliferation and differentiation of adult neural progenitors in the subventricular zone. Stem Cells 32:2516-2528.
Schubert P, Ogata T, Marchini C, Ferroni S (2001) Glia-related pathomechanisms in Alzheimer’s disease: a therapeutic target? Mech Ageing Dev 123:47-57.
Smith JW, Al-Khamees O, Costall B (2002) Chronic aspirin ingestion improves spatial learning in adult and aged rats. Pharmacol Biochem Behav 71:233-238.
Tang J, Li ZH, Ge SN, Wang W (2012) The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012:185167.
Thal DR (2012) The role of astrocytes in amyloid β-protein toxicity and clearance. Exp Neurol 236:1-5.
Tong XM, Zheng S, Jin J, Zhu LF, Lou YJ and Yao HP (2007) Triptolide inhibits cyclooxygenase-2 and inducible nitric oxide synthase expression in human colon cancer and leukemia cells. Acta Biochim Biophys Sin (Shanghai) 39:89-95.
Torrente D, Cabezas R, Avila MF, García-Segur LM, Barreto GE, Guedes RCA (2014) Cortical spreading depression in traumatic brain injuries: is there a role for astrocytes? Neurosci Lett 17:2-6.
Vane J (2000) Aspirin and other anti-inflammatory drugs. Thorax 55:35-40.
Villegas-Llerena C, Phillips A, Garcia-Reitboeck P, Hardy J, Pocock JM (2016) Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr Opin Neurobiol 36:74-81.
Vromman A, Trabelsi N, Rouxel C (2013) β-Amyloid context intensifies vascular smooth muscle cells induced inflammatoryresponse and de-differentiation. Aging Cell 12:358-369.
Wagner DC, Scheibe J, Glocke I, Weise G, Deten A, Boltze J and Kranz A (2013) Object-based analysis of astroglial reaction and astrocyte subtype morphology after ischemic brain injury. Acta Neurobiol Exp (Wars) 73:79-87.
Wajima D, Nakagawa I, Nakase H, Yonezawa T (2013) Neuroprotective effect of suppression of astrocytic activation by arundic acid on brain injuries in rats with acute subdural hematomas. Brain Res 1519:127-135.
Wang J, Song N, Jiang H, Wang J, Xie J (2013) Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta 1832:618-625.
Weggen S, Eriksen JL, Das P (2001) A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414:212-216.
Wilhelmsson U, Bushong EA, Price DL (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A 103:17513-17518.
Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, Clark IA, bdipranoto AA, Vissel B (2013) Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One 8:e59586.
Wu R (2013) Triptolide ameliorates ileocolonic anastomosis inflammation in IL-10 deficient mice by mechanism involving suppression of miR-155/SHIP-1 signaling pathway. Mol Immunol 56:340-346.
Yang F, Wu L, Guo X, Wang D, Li Y (2013) Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the DBA/2J mouse model of glaucoma. Ocul Immunol Inflamm 21:378-389.
Zhang H, Su YJ, Zhou WW, Wang SW, Xu PX, Yu XL, Liu RT (2014) Activated scavenger receptor a promotes glial internalization of aβ. PLoS One 9:e94197.
Zheng H, Zhu W, Zhao H, Wang X, Wang W, Li Z (2010) Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: crucial role of interleukin-1beta. Neuroimmunomodulation 17:31-38.
Zhou GX, Ding XL, Huang JF, Zhang H, Wu SB (2007) Suppression of 5-lipoxygenase gene is involved in triptolide-induced apoptosis in pancreatic tumor cell lines. Biochim Biophys Acta 1770:1021-1027.
Zhou HF, Liu XY, Niu DB, Li FQ, He QH, Wang XM (2005) Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol Dis 18:441-449.
Copyedited by James R, Haase R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.191224
*Correspondence to:
 中國(guó)神經(jīng)再生研究(英文版)2016年9期
中國(guó)神經(jīng)再生研究(英文版)2016年9期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
- Stem Cell Ophthalmology Treatment Study (SCOTS):improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment
- Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
- Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
- Can long-term thiamine treatment improve the clinical outcomes of myotonic dystrophy type 1?
